Abstract
Poly-ADP-ribose-polymerase inhibitor (PARPi) treatment is indicated for advanced-stage ovarian tumors with BRCA1/2 deficiency. The “BRCAness” status is thought to be attributed to a tumor phenotype associated with a specific epigenomic DNA methylation profile. Here, we examined the diagnostic impact of combined BRCA1/2 sequence, copy number, and promoter DNA methylation analysis, and evaluated whether genomic DNA methylation patterns can predict the BRCAness in ovarian tumors. DNA sequencing of 172 human tissue samples of advanced-stage ovarian adenocarcinoma identified 36 samples with a clinically significant tier 1/2 sequence variants (point mutations and in/dels) and 9 samples with a CNV causing a loss of function in BRCA1/2. DNA methylation analysis of the promoter of BRCA1/2 identified promoter hypermethylation of BRCA1 in two mutation-negative samples. Computational modeling of genome-wide methylation markers, measured using Infinium EPIC arrays, resulted in a total accuracy of 0.75, sensitivity: 0.83, specificity: 0.64, positive predictive value: 0.76, negative predictive value: 0.74, and area under the receiver’s operating curve (AUC): 0.77, in classifying tumors harboring a BRCA1/2 defect from the rest. These findings indicate that the assessment of CNV and promoter DNA methylation in BRCA1/2 increases the cumulative diagnostic yield by 10%, compared with the 20% yield achieved by sequence variant analysis alone. Genomic DNA methylation data can partially predict BRCAness in ovarian tumors; however, further investigation in expanded BRCA1/2 cohorts is needed, and the effect of other double strand DNA repair gene defects in these tumors warrants further investigations.
Subject terms: Diagnostic markers, Epigenomics
Introduction
The application of chemotherapy as the standard treatment for advanced-stage ovarian cancer has been associated with little progress in improving the long-term clinical outcomes [1]. This has led to more targeted therapeutic approaches in the management of ovarian cancer including the use of poly-ADP-ribose-polymerase inhibitors (PARPis). PARP inhibitors induce targeted tumor cell death in homologous recombination repair-deficient cells (e.g., those with BRCA1/2 deficiency) through the exploitation of synthetic lethality [2]. Initial Phase-II clinical trials have shown a significantly longer progression-free survival in the PARPi-treated patients as compared with those receiving placebo (8.4 vs. 4.8 months), with a more pronounced effect in subjects that had germline or somatic BRCA1/2 mutations (11.2 vs. 4.3 months) [3]. Subsequently, randomized controlled trials (RCTs) have shown improved progression-free survival in BRCA1/2 mutation carriers when PARPis are used in the upfront maintenance setting (HR 0.3, 95% CI 0.23–0.41, P < 0.0001) and when used as maintenance in the recurrent setting (19.1 vs. 5.5 months, HR 0.30, 95% CI 0.22–0.41, p < 0.0001) [4, 5]. PARPis have thus been clinically approved for use in ovarian cancer patients with either germline or somatic BRCA1/2 mutations [2, 4, 6].
Genetic testing is now being performed to detect somatic or germline mutations in BRCA1/2 to determine the eligibility for PARP inhibitors [6]. The detected variants are assessed according to the CAP/AMP classification guidelines [7], with tier 1 and tier 2 being eligible for PARPi therapy. For some patients, however, variants of uncertain clinical significance (VUS or Tier 3) are identified, which results in challenges in deciding on the appropriate therapeutic approach. In addition, some laboratories do not systematically evaluate other causes of BRCA deficiency such as copy-number variation (CNV) or aberrant promoter methylation of the BRCA1/2 genes. These events can induce an abnormal protein dosage comparable to the effect by sequence variants [8]. Therefore, a portion of the patients that benefit from PARPi therapy is not identified.
We have previously demonstrated that the implementation of copy-number variant assessment in clinical testing can significantly increase the detection yield in genetic testing [9]. We have also shown that haploinsufficiency of numerous genes in congenital disorders results in specific DNA methylation patterns across the genome, which can assist the diagnosis of the patients with an uncertain clinical diagnosis or those carrying VUSs [10–16]. DNA methylation profiling has frequently been used to classify tumor subtypes [17, 18] and prediction of treatment outcomes [19] in various cancers. In particular, a BRCA1-associated DNA methylation signature has been identified in the peripheral blood [20] and an attempt has been made to assess the pathogenicity of BRCA1 unclassified genetic variants in breast cancer using DNA methylation profiling [21]. In ovarian cancer, it is established that tumors in which homologous recombination DNA repair defect is present, including those with BRCA’s deficiency, have a relatively distinct clinical and molecular phenotype, a concept referred to as BRCAness [22]. We hypothesize that such ovarian tumors have a distinct genomic DNA methylation pattern utilizing which, in conjunction with CNV and sequence variant assessment, can assist the identification of the patients who may benefit from PARP inhibitor therapy.
Here, we describe a comprehensive clinical testing approach for the assessment of the BRCA1/2 genes in ovarian cancer specimens, which can be used to determine patients’ eligibility for PARP inhibitor therapy. We describe the overall clinical diagnostic yield as determined by sequence variant analysis and evaluate the improvements achieved by the incorporation of CNV and promoter DNA methylation evaluation of BRCA1/2. In addition, we describe a computational model for the assessment of BRCA1/2-associated genomic DNA methylation patterns in tumor tissues and examine the ability of this classifier to distinguish the patients that may benefit form PARP inhibitor therapy.
Methods
Patients and samples
Patient tissue specimens were obtained from tissue archive of deceased patients treated at the London Regional Cancer Program (LRCP, London, ON, Canada) between 2007 and 2012. All were under the age of 80 years, and treated for invasive high-grade serous, stage 3c or 4, epithelial ovarian carcinoma. Archival formalin-fixed paraffin-embedded (FFPE) slides of ovarian tissues from surgical resections were reviewed to confirm the diagnosis of high-grade serous carcinoma. Appropriate blocks with optimal tumor content were selected for analysis. This means that the cumulative tumor percentage of different blocks of each individual should be above 50%. Using a clean protocol (100% alcohol followed by DNAaway and then again with 100% alcohol), tissue blocks were cut at 20 μm sections with three sections, each placed in two tubes. Genomic DNA was then isolated using the Invitrogen RecoverAll total nucleic acid isolation kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. DNA quantification and genomic profile were then assessed with the 2200 TapeStation (Agilent Technologies, Santa Clara, CA, USA).
High-throughput DNA sequencing
Extracted genomic DNA samples were placed into three groups based on genomic profile size ranges of <700 bp, 700–1100 bp, and 1100–2500 bp, and were subject to fragmentation to 180–220 bp using a Covaris E220 Series Focused-ultrasonicator (Covaris, Inc., Woburn, MA) with recommended settings and treatment times of 20, 50, and 60 s, respectively. Libraries were then prepared with the SeqCap EZ HyperCap workflow according to manufacturer’s protocol (Roche NimbleGen, Inc., Madison, WI, USA) and captured as a 24-plex pool with a custom target design that enriched for all coding exons, as well as 20 bp of the 5′ and 3′ flanking intronic regions for 37 hereditary cancer genes. Four captured libraries (96 samples) were diluted to 4 nM each and pooled for sequencing on the NextSeq according to the manufacturer’s protocol (Illumina, San Diego, CA, USA). The libraries were sequenced using the NextSeq version 2 mid output reagent kit to generate 2 × 150 bp paired-end reads. Post-sequencing file conversion generated FASTQ files for sequence alignment with the NextGene software version 2.4.1 (SoftGenetics, LLC, State College, PA, USA) using the recommended settings. The identified variants were filtered by an allelic fraction >10% and were assessed based on CAP guidelines for pathogenicity.
Detection of copy-number variants
Base coverage distribution reports were created using NextGene software (SoftGenetics, LLC, USA) and processed through a normalization algorithm described previously [9] using a reference population of whole blood controls. Whole gene deletions and duplications for BRCA1 or BRCA2 that had at least a 30% allelic fraction were identified by concordance across four parameters: raw values (average normalized value per sample per gene), intra-sample ratio (ratio of average normalized value per gene of interest to average normalized value of the remaining genes on the panel (n = 36)), FFPE inter-sample ratio (ratio of average normalized value per gene to average normalized values of the same gene from other FFPE samples—Table S1), and whole blood inter-sample ratio (ratio of average normalized value per gene to average normalized values of the same gene from whole blood control samples). Sub-gene level events were identified by a minimum of 50% deviation from the normalized values of the remainder of the gene.
Confirmation testing for sequence variants and CNVs
Sanger sequencing was performed on selected sequence variants (minimum 20% allelic fraction, PCR fragment size <300 bp based on current in-house primer stock) with the BigDye Terminator version 1.1 cycle sequencing kit (Life Technologies, USA). Sequencing products were separated by capillary electrophoresis on ABI 3730 (Life Technologies, USA) and were analyzed with Mutation Surveyor version 4.0.7 software (SoftGenetics, LLC, USA). Multiplex ligation-dependent probe amplification (MLPA) analysis was carried out for all copy-number variants >30% according to the manufacturer’s recommendations using SALSA MLPA kits P0002-BRCA1-D1 and P090-BRCA2-A4 (MRC Holland, Amsterdam, Netherlands). PCR products were separated by capillary electrophoresis on an ABI 3730 (Life Technologies) and analyzed with WB and FFPE references with Coffalyzer. Net software version 131211.1524 (MRC Holland).
DNA methylation analysis
Of the samples processed in this study, 80 were selected for DNA methylation analysis. This included all of the samples with a clinically significant (Tier I/II) BRCA1 or BRCA2 variants together with a matching number of samples without any mutation in BRCA1 or BRCA2. Following bisulfite conversion, DNA methylation analysis of the samples was performed using the Illumina Infinium methylation EPIC bead chip arrays, according to the manufacturer’s protocol. This array includes >860,000 human genomic methylation CpG sites, including 99% of RefSeq genes, all of the known disease-associated imprinted loci in humans, and 96% of CpG islands. The resulting methylated and unmethylated signal intensity data were imported into R 3.5.2 for analysis. Normalization was performed according to the Illumina normalization method with background correction using the minfi package. The methylation level for each probe was measured as a beta value, calculated from the ratio of the methylated signal intensity versus the total sum of unmethylated and methylated signal intensities for that probe, ranging between 0 (no methylation) and 1 (complete methylation). Probes with detection p values > 0.1, those located on chromosomes X and Y, those known to contain a SNP at the CpG interrogation or single nucleotide extension, and probes known to cross-react with chromosomal locations other than their target regions were excluded from analysis. All of the samples were examined for genome-wide methylation density to ensure evidence of bimodal distribution of the genomic DNA methylation levels. The majority of the samples were analyzed in one batch and only a few of them were processed in a different batch. Factor analysis using a principal component analysis was performed to examine the batch effect and identify potential outliers. No batch effect was observed between the samples processed among the two batches. Following quality controls, the methylation levels at the promoters of BRCA1 and BRCA2 were examined for an evidence of gain of methylation in the immediate 5′ promoter region in BRCA1/2 mutation-negative specimens.
Computational modeling of BRCA1/2 positive samples
A fivefold cross validation using six different models and their ensembles was performed to examine whether the status of BRCA1/2 loss of function can be modeled using the DNA methylation data. The data were randomly divided into fivefolds. Fourfold was used for feature selection and model training while the remaining fold was used for testing the trained model. The process was repeated five times so that all of the folds were used for at least once during both testing and training. Feature selection was performed following three steps: (1) probes with a mean methylation difference of >0.05 between BRCA1/2 positive and negative samples were retained; (2) probes were sorted based on the area under the receiver’s operating characteristic’s curve (AUC) and the top 1000 with the greatest AUC were retained; (3) a pair-wise correlation coefficient was measured for every probe, separately among the cases and controls, and highly correlated features (R-squared > 0.8) were excluded. Six different models were used for training including elastic net regression, support vector machine (SVM) with linear kernel, SVM with radial basis function kernel, linear discriminant analysis, random forest, and Bayesian generalized linear model. The ensemble of these models was conducted according to the pipeline implemented in the SuperLearner R package. Using a least absolute shrinkage and selection operator, each model was assigned a coefficient to be used for combining the predictions by multiple classifiers into the final classification. After repeating this procedure for all of the fivefolds, the average prediction accuracy measures on the testing set, including the total accuracy, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and AUC were reported.
Results
Reportable sequence variants in BRCA1 and BRCA2
DNA sequence analysis for BRCA1 and BRCA2 was conducted in a cohort containing 172 samples from patients with advanced-stage high-grade serous epithelial ovarian adenocarcinoma. The samples were analyzed using a custom-designed multi-gene NGS panel for BRCA1 and BRCA2. The NGS analysis identified 36 samples with at least one clinically significant (Tier I/II) variant. Of these, 27 patients had a variant classified as Tier I (15 BRCA1 mutations and 12 BRCA2 mutations), with 1/27 having both a Tier I and a Tier II variant and 5/27 having both a Tier 1 and Tier III variant. There were also six samples with only VUS (Tier III) in BRCA1 or BRCA2 (Table 1).
Table 1.
Reportable sequence variants and CNVs in BRCA1 and BRCA2
| Sample ID | Tier 1 | Tier 2 | Tier 3 (VUS) | CNVs |
|---|---|---|---|---|
| 1105-001* | BRCA1:c.3254_3255dupGA, p.(Leu1086Aspfs*2) (57.6%) | |||
| 1105-009* | BRCA1:c.1961dupA, p.(Tyr655Valfs*18) (33.9%) | |||
| 1105-039* | BRCA1 exon13dup | |||
| 1105-054* | BRCA1:c.5266dupC, p.(Gln1756Profs*74) (58.4%) | |||
| 1105-057* | BRCA1:c.5095C>T, p.(Arg1699Trp) (44.2%) | |||
| 1105-112* | BRCA1:c.5207T>C, p.(Val1736Ala) (84.7%) | |||
| 1105-155* | BRCA1:c.4327C>T, p.(Arg1443*) (46.1%) | |||
| 1105-162* | BRCA1:c.212+3A>G (96.7%) | |||
| 1105-195* | BRCA1:c.5059delG, p.(Val1687Leufs*3) (51.3%) | |||
| 1105-207* | BRCA1del | |||
| 1105-227* | BRCA1:c.5266dupC, p.(Gln1756Profs*74) (40.6%) | |||
| 1105-270* | BRCA1:c.2891delG, p.(Gly964Aspfs*36) (58.4%) | |||
| 1105-273* | BRCA1:c.4621G>T, p.(Glu1541*) (31.7%) | |||
| 1105-024* | BRCA2:c.7615C>T, p.(Gln2539*) (11.1%) | BRCA1:c.2423_2481del, p.(Phe808Trpfs*3) (27.1%) | ||
| 1105-204* | BRCA1del | |||
| 1105-110* | BRCA1:c.1116G>A, p.(Trp372*) (80.1%) | |||
| 1105-113* | BRCA1:c.182G>C, p.(Cys61Ser) (89.2%) | |||
| 1105-119* | BRCA1:c.3967C>T, p.(Gln1323*) (93.3%) | BRCA2:c.9665G>T, p.(Cys3222Phe) (24%) | ||
| 1105-123* | BRCA1:c.5266dupC, p.(Gln1756Profs*74) (69.3%) | |||
| 1105-130* | BRCA1del | |||
| 1105-159* | BRCA1:c.5096G>A, p.(Arg1699Gln) (44.6%) | BRCA2dup | ||
| 1105-185* | BRCA1 exon13dup | |||
| 1105-192* | BRCA1:c.68_69delAG, p.(Glu23Valfs*17) (76.3%) | BRCA2dup | ||
| 1105-193* | BRCA1:c.3394_3406del, p.(Asn1132Leufs*19) (70%) | BRCA1dup | ||
| 1105-222* | BRCA1:c.709G>T, p.(Glu237*) (51.2%) | |||
| 1105-246* | BRCA1:c.5074G>A, p.(Asp1692Asn) (72.8%) | |||
| 1105-049* | BRCA2:c.7958T>C, p.(Leu2653Pro) (99.4%) | |||
| 1105-088* | BRCA2:c.3170_3174del, p.(Lys1057Thrfs*8) (71.4%) | |||
| 1105-103* | BRCA2:c.6591_6592delTG, p.(Glu2198Asnfs*4) (53.7%) | BRCA2:c.9305C>T, p.(Ala3102Val) (43.1%) | ||
| 1105-104* | BRCA2:c.7954delG, p.(Val2652Cysfs*5) (38.5%) | |||
| 1105-111* | BRCA2:c.8167G>C, p.(Asp2723His) (69.6%) | BRCA1:c.-62G>A (57.1%) | ||
| 1105-128* | BRCA2del | |||
| 1105-152# | BRCA2:c.3545_3546delTT, p.(Phe1182*) (34%) | |||
| 1105-200* | BRCA2:c.7617+2T>G (63.7%) | |||
| 1105-202* | BRCA2:c.2588dupA, p.(Asn863Lysfs*18) (67.2%) | BRCA1:c.-86C>T (83.3%) | ||
| 1105-265#* | BRCA2:c.633dupC, p.(Arg212Glnfs*3) (45.2%) | |||
| 1105-139* | BRCA2:c.3587T>A, p.(Leu1196*) (73.3%) | |||
| 1105-078* | BRCA2:c.8164dupA, p.(Thr2722Asnfs*8) (37.3%) | |||
| 1105-137* | BRCA2:c.7069_7070delCT, p.(Leu2357Valfs*2) (18.7%) | BRCA2:c.1094C>T, p.(Pro365Leu) (17.6%) | ||
| 1105-144* | BRCA2del | |||
| 1105-258* | BRCA2:c.1054dupT, p.(Tyr352Leufs*6) (72.2%) | |||
| 1105-268* | BRCA2del | |||
| 1105-041 | BRCA1:c.4258C>T, p.(Gln1420*) (11.9%) | |||
| 1105-182 | BRCA1:c.5497G>A, p.(Val1833Met) (10.3%) | BRCA2:c.10112C>T, p.(Thr3371Ile) (10.5%) | ||
| 1105-036 | BRCA1dup | |||
| 1105-018 | BRCA1dup | |||
| 1105-022 | BRCA2:c.9338T>C, p.(Ile3113Thr) (75.6%) | |||
| 1105-055 | BRCA2dup | |||
| 1105-059 | BRCA1:c.4531C>T, p.(His1511Tyr) (12.2%) | |||
| 1105-120 | BRCA2:c.44_45insATT, p.(Ile14_Phe15insLeu) (45.7%) BRCA2:c.9502–12T>G (82.5%) BRCA2:c.41_67+9del, p.(?) (12.4%) | |||
| 1105-126 | BRCA2:c.2716A>G, p.(Thr906Ala) (66.1%) | |||
| 1105-157 | BRCA2dup | |||
| 1105-174 | BRCA1:c.2212G>A, p.(Val738Ile) (10.2%) | |||
| 1105-178 | BRCA1:c.5416C>T, p.(Pro1806Ser) (14.7%) | |||
| 1105-181 | BRCA2dup | |||
| 1105-183 | BRCA1:c.2713C>G, p.(Gln905Glu) (23.9%) | BRCA2dup | ||
| 1105-197 | BRCA1dup | |||
| 1105-198 | BRCA2dup | |||
| 1105-220 | BRCA2:c.5714A>T, p.(His1905Leu) (43.1%) | |||
| 1105-229 | BRCA2dup | |||
| 1105-277 | BRCA2:c.9698G>C, p.(Cys3233Ser) (47.7%) |
Percentages in brackets indicate alternate allele fractions. Due to the presence of varying levels of normal tissue in these tumor specimens, as well as confounding factors such as chromosomal aneuploidy or copy-number alterations, the true allele fractions in the tumor may be above what is indicated. Samples with allele fractions above 40% were considered for PARPi therapy. Most of the samples had a tumor percentage >50%. It is notable that this number may not indicate a heterozygous state in the heterogenous tumors. The samples with residual or low tumor volume (10–20%) are indicated with a hash sign. The copy-number changes were confirmed with MLPA. Gene duplications are not assessed clinically, since they are not confirmed to result in recombination deficiency. Samples with a Tier 1 or Tier 2 variants or those with truncating intragenic duplications/full gene deletions are eligible for PARPi therapy. Samples used in methylation testing are indicated with an asterisk
BRCA1 and BRCA2 copy-number variants
The CNV analysis identified a total of ten subjects with a CNV involving BRCA1 (four full gene duplications, four full gene deletions, and two BRCA1 exon 13 duplications—Table 1). In addition, there were 11 cases with a CNV overlapping BRCA2, including 8 samples with duplications and 3 with deletions of the entire gene. Among none of the samples with deletions (duplications are not eligible for PARPi therapy), the primary sequence variant assessment had not identified a reportable sequence variant (Table 1).
DNA methylation analysis of the BRCA1/2 promoters
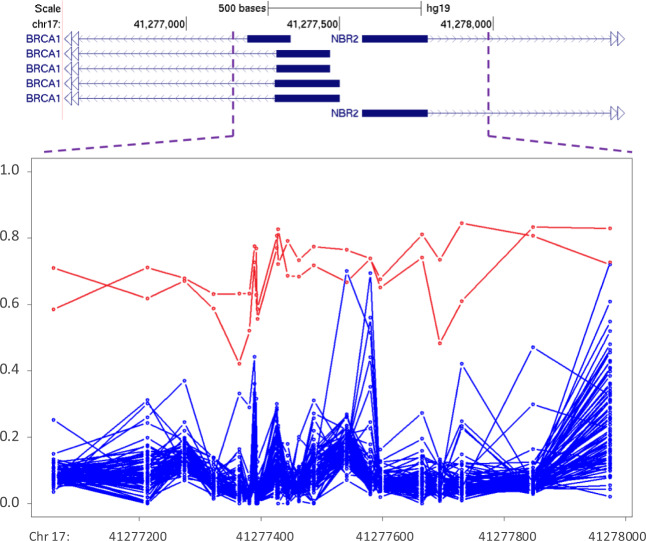
A total of 130 samples were deemed negative for any BRCA1 and BRCA2 reportable sequence variants or CNVs, among which 38 were subject to DNA methylation testing. The methylation analysis of the promoters of BRCA1 and BRCA2 identified two additional patients with increased methylation levels of up to 60% (consistent with hypermethylation of one of the two alleles) as compared with the rest of the samples, all of which were fully hypomethylated (median cross-region methylation level <15%, Fig. 1). This indicated a 5% increase in the diagnostic yield of samples negative in BRCA sequence variant testing. In addition, none of the patients with a reportable sequence variant in BRCA’s showed hypermethylation in the promoter of BRCA1 or BRCA2, indicating that the patients with BRCA’s loss of function due to pathogenic variants, do not have additional promoter hypermethylation.
Fig. 1.
DNA methylation analysis of BRCA1 promoter. The figure illustrates >500 bps annotating to the promoter of the BRCA1 gene in individuals with no sequence variant findings in BRCA1/2. The methylation levels for each CpG site in this region (0–1) is shown using a circle, connected to the adjacent CpGs of the same individual using a line. The majority of samples show a hypomethylation pattern (blue), while two samples show a gain of methylation (red)
Classification of ovarian tumors by BRCA status using DNA methylation data
We attempted, using an ensemble learning approach, to determine whether samples with and without BRCA1/2 mutations can be classified using the genome-wide DNA methylation data. DNA methylation profiling was performed on a total of 44 samples with BRCA1/2 loss of function variants and 36 samples without a reportable variant or a promoter DNA methylation aberration in BRCA1/2. Following feature selection, six different classification models were developed among which SVM with linear kernel reached the highest accuracy and was most consistently selected to be included in the final ensemble. Therefore, at the ensemble, the predictions of most models other than SVM were down-weighted. The average classification accuracies of the final model as determined during the fivefold cross-validation on the testing set—not used for feature selection or model training—were as follows: total accuracy: 0.75, sensitivity: 0.83, specificity: 0.64, PPV: 0.76, NPV: 0.74, and AUC: 0.77 (Tables 2, S2, and S3). These results indicated that DNA methylation data can partially predict the status of BRCA deficiency in ovarian cancer tumors.
Table 2.
Accuracy measures for classification of ovarian tumors by BRCA status using DNA methylation data
| Accuracy indicator | Fold 1 | Fold 2 | Fold 3 | Fold 4 | Fold 5 | Average |
|---|---|---|---|---|---|---|
| Overall accuracy | 0.69 | 0.81 | 0.81 | 0.71 | 0.73 | 0.75 |
| Sensitivity | 0.67 | 0.89 | 0.89 | 0.8 | 0.89 | 0.83 |
| Specificity | 0.71 | 0.71 | 0.71 | 0.57 | 0.5 | 0.64 |
| PPV | 0.75 | 0.8 | 0.8 | 0.73 | 0.73 | 0.76 |
| NPV | 0.62 | 0.83 | 0.83 | 0.67 | 0.75 | 0.74 |
| AUC | 0.68 | 0.79 | 0.79 | 0.8 | 0.78 | 0.77 |
Discussion
Utilization of high-throughput sequencing has become a common practice in the clinical testing of constitutional and somatic disorders. These assays enable for thorough screening of single nucleotide variants and short indels, while larger indels, extensive copy-number changes, and other causes of gene function aberrations, such as defects in DNA methylation, remain unexplored in most clinical assessments. An example of this applies to the genetic testing of BRCA1/2 in ovarian cancer. In this study, we have demonstrated that ~20% of the ovarian tumors can be identified with a pathogenic SNV/indel in BRCA1/2 while another ~10% will have a pathogenic CNV or a promoter DNA methylation defect in these genes. DNA methylation testing, despite the documented effect on PARPis response rates [23], is not being conducted in the routine assessment of drug eligibility. Thus, a multi-faceted approach can have a greater potential in detecting patients that may benefit from PARPi therapy.
Currently, there is no established guideline regarding the use of PARPis in ovarian tumors with BRCA promoter hypermethylation. However, new evidence suggests efficacy of this class of drugs on tumors with such aberrations. Swisher et al. have reported up to 50% response rate for Rucaparib, a PARP inhibitor, in ovarian samples that had BRCA1 promoter hypermethylation [23]. In their cohort of 165 ovarian samples, 12% had hypermethylation in the BRCA1 promoter, a figure comparable to those with sequence variant defects. Another study has found that none of the ovarian tumors with BRCA1 promoter hypermethylation demonstrate BRCA1 protein expression by immunohistochemistry, being consistent with the silencing of the BRCA1 gene [8]. This indicates a possible new target patient population that could benefit from PARPis. The effectiveness of PARPis in samples with BRCA1/2 promoter hypermethylation has also been documented in other tumors, including a clinical trial currently undergoing for triple-negative breast tumors with BRCA1/2 hypermethylation [24].
Another way DNA methylation testing can benefit the identification of patients eligible for PARPi’s is through the use of a DNA methylation profile associated with the BRCAness phenotype. Ovarian tumors with BRCAs mutations are known to have a distinct clinical behavior, mainly attributed to homologous recombination deficiency, leading to massive genomic instability and affecting many layers of the regulation of gene expression such as DNA methylation [25]. A DNA methylation pattern specific to BRCAness can be used to resolve uncertain cases where, for instance, a VUS is found in BRCA1/2. Our analysis shows that the status of BRCA1/2 mutations can be, to some extent, modeled using genomic DNA methylation data. The heterogeneous nature of ovarian tumors, however, may not enable a full accuracy in the classification of the samples into BRCA1/2 positive/negative profile. A previous study using DNA methylation data has also reached a comparable accuracy (~80%) in detecting breast tumors with BRCA1 mutations [26]. A BRCAness phenotype can be present in tumors with homologous recombination deficiency, but without a defect in BRCA’s expression [27]. It is possible that some of the non-mutated BRCA tumors in these studies have had a BRCAness profile, limiting the accuracy of DNA methylation classification. Consistently, the utility of PARPis is shown to be not limited to BRCA1 and BRCA2 deficient tumors. Any defective homologous recombination caused by the deficiency of other genes can be a potential target for PARPis therapy. Proteins involved in homologous recombination repair other than BRCA1 and BRCA2, including ATM, CHEK2, BARD1, BRIP1, MRE11, RAD50, NBS1, RAD51C, RAD51D, and PALB2, have been reported to be associated with increased susceptibility to ovarian cancer. Several of these have now been associated with moderate increased susceptibility to ovarian and/or breast cancer [22, 23, 27–32]. Swisher et al. have reported a 75% response rate by PARPis for RAD51 deficient ovarian tumors [23]. On a non-gynecological cancer example, there is emerging evidence that the use of PARPis can potentiate the action of alkylating agents in glioblastoma tumors that have hypermethylation of the promoter of MGMT, coding for another DNA repair protein [33].
These findings all raise the question of whether a DNA methylation signature of homologous recombination deficiency could be used to better identify patients in need of PARPi therapy than sequence variant assessment of BRCA1 and BRCA2. Further analyses on larger sample sizes attempting to map a DNA repair-associated DNA methylation signature that includes all of the involved genes beyond BRCAs, such as ATM, CHEK2, BARD1, BRIP1, MRE11, RAD50, NBS1, RAD51C, RAD51D, and PALB2, followed by clinical trials to evaluate the effectiveness of PARPis in the group of patients showing such a methylation profile are needed before the implementation of DNA methylation profiling in clinical testing of ovarian cancer patients.
A final point to consider about the PARPi eligibility is about the somatic vs. germline and zygocity status of the genetic testing findings. While it is expected that a proportion of PARP-sensitive tumors exhibit biallelic loss of BRCA1/2 that may be detectible by this analysis, in the majority of cases, the identified mutation, deletion, or methylation defects appear to occur in a single allele. Based on the recommendations by the Pan-Canadian Oncology Drug Review expert review committee, Olaparib monotherapy maintenance treatment is recommended for BRCA1/2-mutated patients with an evidence of a germline or somatic defect as detected by approved testing laboratories [34]. Therefore, while this assay is not designed to determine the germline vs. somatic status or biallelic loss of BRCA, it meets the requirements for the identification of PARP-eligible individuals.
Supplementary information
Acknowledgments
Funding
Investigator Initiated Research Grant by Astra Zeneca.
Author contributions
EA-E: bioinformatics analysis and manuscript drafting; JDM: patient recruitment and manuscript revision; VPP: summarized and drafted the results; JK: performed DNA methylation and sequencing experiment; AS, PJA, HL, CMM: manuscript revision; MV: variant classification; BS: principal investigator, project design, overview, and manuscript revision.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Research ethics approval was obtained from the Lawson Health Research Institute’s ethics committee. No consenting was required since only the anonymous post-mortem archival specimens were used. All of the samples and records were de-identified before any experimental or analytical procedures. The research was conducted in accordance with all relevant ethical regulations.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s10038-020-0780-4) contains supplementary material, which is available to authorized users.
References
- 1.Raja FA, Counsell N, Colombo N, Pfisterer J, du Bois A, Parmar MK, et al. Platinum versus platinum-combination chemotherapy in platinum-sensitive recurrent ovarian cancer: a meta-analysis using individual patient data. Ann Oncol. 2013;24:3028–34. doi: 10.1093/annonc/mdt406. [DOI] [PubMed] [Google Scholar]
- 2.Ledermann JA. PARP inhibitors in ovarian cancer. Ann Oncol. 2016;27(suppl 1):i40–4. doi: 10.1093/annonc/mdw094. [DOI] [PubMed] [Google Scholar]
- 3.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–92. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 4.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 5.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–84. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 6.Oza AM, Tinker AV, Oaknin A, Shapira-Frommer R, McNeish IA, Swisher EM, et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: integrated analysis of data from Study 10 and ARIEL2. Gynecol Oncol. 2017;147:267–75. doi: 10.1016/j.ygyno.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 7.Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60:5329–33. [PubMed] [Google Scholar]
- 9.Kerkhof J, Schenkel LC, Reilly J, McRobbie S, Aref-Eshghi E, Stuart A, et al. Clinical validation of copy number variant detection from targeted next-generation sequencing panels. J Mol Diagn. 2017;19:905–20. doi: 10.1016/j.jmoldx.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Aref-Eshghi E, Rodenhiser DI, Schenkel LC, Lin H, Skinner C, Ainsworth P, et al. Genomic DNA methylation signatures enable concurrent diagnosis and clinical genetic variant classification in neurodevelopmental syndromes. Am J Hum Genet. 2018;102:156–74. doi: 10.1016/j.ajhg.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aref-Eshghi E, Kerkhof J, Pedro VP, France GD, Barat-Houari M, Ruiz-Pallares N, et al. Evaluation of DNA methylation episignatures for diagnosis and phenotype correlations in 42 Mendelian neurodevelopmental disorders. Am J Hum Genet. 2020;106:356–70. doi: 10.1016/j.ajhg.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aref-Eshghi E, Schenkel LC, Lin H, Skinner C, Ainsworth P, Paré G, et al. The defining DNA methylation signature of Kabuki syndrome enables functional assessment of genetic variants of unknown clinical significance. Epigenetics. 2017;12:923–33. doi: 10.1080/15592294.2017.1381807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bend EG, Aref-Eshghi E, Everman DB, Rogers RC, Cathey SS, Prijoles EJ, et al. Gene domain-specific DNA methylation episignatures highlight distinct molecular entities of ADNP syndrome. Clin Epigent. 2019;11:64. doi: 10.1186/s13148-019-0658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aref-Eshghi E, Bend EG, Hood RL, Schenkel LC, Carere DA, Chakrabarti R, et al. BAFopathies’ DNA methylation epi-signatures demonstrate diagnostic utility and functional continuum of Coffin–Siris and Nicolaides–Baraitser syndromes. Nat Commun. 2018;9:4885. doi: 10.1038/s41467-018-07193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aref-Eshghi E, Bend EG, Colaiacovo S, Caudle M, Chakrabarti R, Napier M, et al. Diagnostic utility of genome-wide DNA methylation testing in genetically unsolved individuals with suspected hereditary conditions. Am J Hum Genet. 2019;104:685–700. doi: 10.1016/j.ajhg.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aref‐Eshghi E, Bourque DK, Kerkhof J, Carere DA, Ainsworth P, Sadikovic B, et al. Genome‐wide DNA methylation and RNA analyses enable reclassification of two variants of uncertain significance in a patient with clinical Kabuki syndrome. Hum Mutat. 2019;40:1684–9. doi: 10.1002/humu.23833. [DOI] [PubMed] [Google Scholar]
- 17.Aref-Eshghi E, Schenkel LC, Ainsworth P, Lin H, Rodenhiser DI, Cutz JC, et al. Genomic DNA methylation-derived algorithm enables accurate detection of malignant prostate tissues. Front Oncol. 2018;8:100. doi: 10.3389/fonc.2018.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klughammer J, Kiesel B, Roetzer T, Fortelny N, Nemc A, Nenning KH, et al. The DNA methylation landscape of glioblastoma disease progression shows extensive heterogeneity in time and space. Nat Med. 2018;24:1611. doi: 10.1038/s41591-018-0156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milani L, Lundmark A, Kiialainen A, Nordlund J, Flaegstad T, Forestier E, et al. DNA methylation for subtype classification and prediction of treatment outcome in patients with childhood acute lymphoblastic leukemia. Blood. 2010;115:1214–25. doi: 10.1182/blood-2009-04-214668. [DOI] [PubMed] [Google Scholar]
- 20.Anjum S, Fourkala EO, Zikan M, Wong A, Gentry-Maharaj A, Jones A, et al. A BRCA1-mutation associated DNA methylation signature in blood cells predicts sporadic breast cancer incidence and survival. Genome Med. 2014;6:47. doi: 10.1186/gm567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flower KJ, Shenker NS, El-Bahrawy M, Goldgar DE, Parsons MT, KConFab Investigators, et al. DNA methylation profiling to assess pathogenicity of BRCA1 unclassified variants in breast cancer. Epigenetics. 2015;10:1121–32. doi: 10.1080/15592294.2015.1111504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4:814. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 23.Swisher EM, Harrell MI, Lin K, Coleman RL, Konecny GE, Tinker AV, et al. BRCA1 and RAD51C promoter hypermethylation confer sensitivity to the PARP inhibitor rucaparib in patients with relapsed, platinum-sensitive ovarian carcinoma in ARIEL2 Part 1. Gynecol Oncol. 2017;145:5. doi: 10.1016/j.ygyno.2017.03.034. [DOI] [Google Scholar]
- 24.De La Haba J, Guerrero-Zotano A, Perez-Fidalgo JA, Gonzalez Santiago S, Muñoz M, Andres R, et al. A phase II clinical trial to analyze olaparib response in patients with BRCA1 and/or BRCA2 promoter methylation with advanced breast cancer (GEICAM/2015-06 COMETA-Breast study). https://clinicaltrials.gov/ct2/show/NCT03205761. Accessed 2 Apr 2020.
- 25.Konstantinopoulos PA, Spentzos D, Karlan BY, Taniguchi T, Fountzilas E, Francoeur N, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28:3555–61. doi: 10.1200/JCO.2009.27.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flanagan JM, Cocciardi S, Waddell N, Johnstone CN, Marsh A, Henderson S, et al. DNA methylome of familial breast cancer identifies distinct profiles defined by mutation status. Am J Hum Genet. 2010;86:420–33. doi: 10.1016/j.ajhg.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moschetta M, George A, Kaye SB, Banerjee S. BRCA somatic mutations and epigenetic BRCA modifications in serous ovarian cancer. Ann Oncol. 2016;27:1449–55. doi: 10.1093/annonc/mdw142. [DOI] [PubMed] [Google Scholar]
- 28.Loveday C, Turnbull C, Ramsay E, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43:879–84. doi: 10.1038/ng.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rafnar T, Gudbjartsson DF, Sulem P, et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nat Genet. 2011;43:1104–7. doi: 10.1038/ng.955. [DOI] [PubMed] [Google Scholar]
- 30.Nevanlinna H, Bartek J. The CHEK2 gene and inherited breast cancer susceptibility. Oncogene. 2006;25:5912–9. doi: 10.1038/sj.onc.1209877. [DOI] [PubMed] [Google Scholar]
- 31.Cunningham JM, Cicek MS, Larson NB, et al. Clinical characteristics of ovarian cancer classified by BRCA1, BRCA2, and RAD51C status. Sci Rep. 2014;4:4026. doi: 10.1038/srep04026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antoniou AC, Casadei S, Heikkinen T, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371:497–506. doi: 10.1056/NEJMoa1400382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta S, Smith E, Mladek A, Tian S, Decker P, Kizilbash S, et al. PARP inhibitors for sensitization of alkylation chemotherapy in glioblastoma: impact of blood–brain barrier and molecular heterogeneity. Front Oncol. 2019;8:670. doi: 10.3389/fonc.2018.00670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AstraZeneca Canada Inc. pCODR expert review committee (pERC) initial recommendation. https://www.cadth.ca/sites/default/files/pcodr/pcodr_olaparib_lynparza_resub_in_rec.pdf. Accessed 2 Apr 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.