Abstract
The novel coronavirus outbreak has reported to be rapidly spreading across the countries and becomes a foremost community health alarm. At present, no vaccine or specific drug is on hand for the treatment of this infectious disease. This review investigates the drugs, which are being evaluated and found to be effective against nCOVID-19 infection. A thorough literature search was performedon the recently published research papers in between January 2020 to May 2020, through various databases like “Science Direct”, “Google Scholar”, “PubMed”,“Medline”, “Web of Science”, and “World Health Organization (WHO)”. We reviewed and documented the information related with the current and future aspects for the management and cure of COVID-19. As of 21st July 2020 a total of 14,562,550 confirmed cases of coronavirus and 607,781 deaths have been reported world-wide. The main clinical feature of COVID-19 ranges from asymptomatic disease to mild lower respiratory tract illness to severe pneumonia, acute lung injury, acute respiratory distress syndrome (ARDS), multiple organ dysfunction, and death. The drugs at present used in COVID-19 patients and ongoing clinical trials focusing on drug repurposing of various therapeutic classes of drug e.g. antiviral, anti-inflammatory and/or immunomodulatory drugs along with adjuvant/supportive care. Many drugs on clinical trials shows effective results on preliminary scale and now used currently in patients. Adjuvant/supportive care therapy are used in patients to get the best results in order to minimize the short and long-term complications. However, further studies and clinical trials are needed on large scale of population to reach any firm conclusion in terms of its efficacy and safety.
Keywords: Anti-inflammatory agents, Antiviral drugs, Corona virus, COVID-19, SARS-CoV-2
1. Introduction
Novel-coronavirus (nCOVs) is spherical in shape, enveloped with single-stranded RNA, and considered as the largest viral RNA-genome (25–32 kb). Coronaviruses (CoVs) belongs to group of zoonotic viruses having enormous diversity of animal hosts i.e. birds & humans. The infection caused by CoV varies variably and greatly from asymptomatic-symptomatic to severe disease, depending on the species of virus and the host. This virus subdivided into four families: α, β, γ, δ-coronavirus. The γ, δ-CoVs are mainly infected birds, whereas α, and β-CoVs largely infected the mammals (Yin and Wunderink, 2018).
Earlier, global spread of two β-coronavirus such as Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV), origin from Foshan (China) in November 2002 (Zhong et al., 2003) and Middle East Respiratory Syndrome Coronavirus (MERS-CoV) occurred in Saudi Arabia in June 2012 (Zaki et al., 2012). At present, a novel coronavirus (SARS-CoV-2) reported first case in China (Wuhan) on December 2019, later on February 2020, World Health Organization (WHO, 2020) given named as COVID-19 (Huids et al., 2020, WHO, 2020a). It has spread rapidly, affected many countries all over the world and declared by WHO, as COVID-19 emergency/outbreak, a pandemic (Dong et al., 2020). SARS-CoV-2 is highly infectious and transmittable diseases, highly vulnerable to entire population worldwide via a directly contact with infected person through respiratory droplets (Zheng, 2020).
According to recent evidences, this epidemic also known as zoonotic disease, a disease transmitted from animals to humans. Several genealogical analyses documented that novel CoV considerably identical to the sequence of bat SARS-like coronavirus (Benvenuto et al., 2020). The replication and transcription phase of SARS-CoV-2 starts when virus (host) enters into the cell and interacting with spike proteins (S-protein) present at the surface of target receptor. Mainly two target receptors are present within the cell to which virus binds either with angiotensin converting enzyme-2 (ACE-2) receptor or with the serine protease TMPRSS2 (Hoffmann et al., 2020). However, exact mechanism is still unknown and need to investigate whether novel coronavirus employs ACE-2 and TMPRSS2 for the entry into the cytoplasm.
Novel-coronavirus infection is a community transmission disease, primarily affected person to person, via respiratory droplets (Li et al., 2020). Sometimes these droplets can land on objects and surfaces such as tables, doorknobs and handrail. In most of the cases, person can become infect by touching these objects and then touching their eyes, nose and mouth. The most common symptoms are fever, dry cough, tiredness, nasal congestion, sore throat and develop breathlessness. In severe cases patient symptoms can be characterized to organ failure i.e. kidney & respiratory. However, geriatrics population and person with preexisting medical condition (diabetes, hypertension, heart and lung problems) chances are higher for developing symptoms (Rawat and Sharma, 2020). Apart from these symptoms this infection can be associated with lymphopenia, interstitial pneumonia and elevated levels of pro-inflammatory cytokines like as interleukins (ILs), interferons (IFNs), Granulocyte-colony stimulating factor (G-CSF), Tumor Necrosis Factor (TNF-α), etc. This condition sometimes also called “cytokine storm” can induce patient’s death (Guo et al., 2020). Currently, only symptomatic treatments are available to minimize the symptoms like self-isolation/quarantine, supplemental oxygen, mechanical ventilation, paracetamol for fever and antibiotics only for hospitalized patients for control bacterial co-infection. Till date, there are no vaccines or drug therapy approved by the U.S. Food and Drug Administration (FDA) for the treatment of this pandemic virus COVID-19 (β-coronavirus). Due to varying nature of this virus, development of novel pharmacological treatment takes several years. Many scientist or researchers work day and night and trying to focus on drug repurposing, which already are in use in some other conditions for competently control and spread of pandemic situation all over the world (Kruse, 2020).
From this review, our aim is to provide a basic overview about currently available drugs and drugs under clinical trial for COVID-19 patients. Based on many evidences from laboratory, animal and clinical studies, all are focusing on anti-viral, anti-inflammatory and immune-modulators drugs for the treatment of the novel-coronavirus. Here, we discussed all currently used treatment, under clinical development and outlook clinical trial for the treatment of COVID-19.
2. Drug therapy for COVID-19
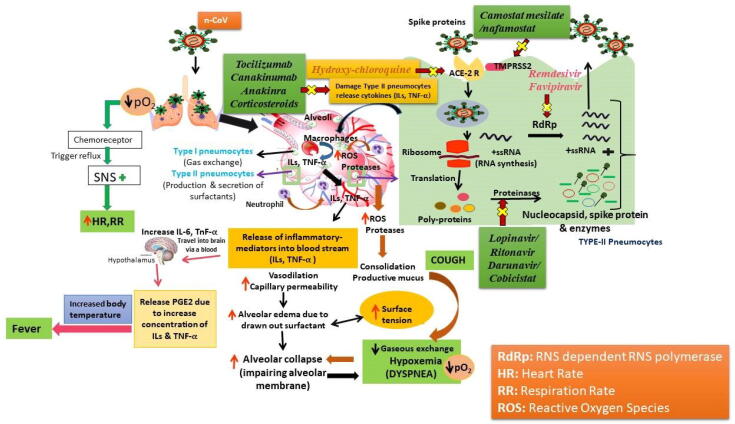
Patients with COVID-19 characterized by abnormal in respiration rate (Hypoxia), hear rate, fever, irregular neutrophil counts (neutropenia), and higher level of plasma cytokines i.e. interleukins (IL-1, IL-6, etc.), and TNF-α. All these symptoms are the reason to promote disease severity. SARS-CoV-2 virus detected in both Types I and II pneumocytes present in the ciliated epithelial cells of nasal, bronchial and bronchiolar mucosae of respiratory system. Type I pnemocytes is responsible for gaseous exchange whereas Type II has main role in the production and secretion of surfactants. Destruction of both types of pnemocytesis, resulting in hypoxia and alveolar collapse. ACE-2 act as a target receptor for the treatment of COVID-19 infection, whereas TMPRSS2 protein, enhance the transmissibility of the virus. Increase in the production of leukocytes (neutrophil) leads to the formation of highly reactive oxygen species (ROS), and oxidative stress inside alveoli, resulting in the formation of mucus (cough) and alveolar damage. Fig. 1 describes the potential targets with pathogenesis pathway for broad-spectrum antiviral agents, anti-inflammatory and immuno-modulatory agents in treatment of COVID-19.
Fig. 1.
Schematic diagram of targets with pathogenesis pathway for broad-spectrum antiviral agents, anti-inflammatory and immuno-modulatory agents in treatment of COVID-19.
3. Antiviral agents
Antiviral drugs are beneficial for several infectious diseases such as herpes, hepatitis, HIV, influenza and flu like symptoms (Razonable, 2011). Researchers and scientists are endeavoring to find drugs, which are effective in the treatment of COVID-19. Drug repurposing is widely acceptance at present, because of immediate impact of nCoVs outbreak into the society and public health emergency concern (Muralidharan et al., 2020). Many drugs that approved by USFDA for some other disease conditions are under preclinical and clinical development for the management of COVID-19. Here, we review and examine all the antiviral drug therapies and its relevance in the treatment of coronavirus as per available pharmacokinetic and pharmacodynamics data of clinical studies. Several studies has been documented that antiviral drugs delivered abruptly after beginning of symptoms and continue last up to 14 days (sputum peak around 5–6 days). These drugs minimizing the chances of infection to others by reducing release of virus from the host and replication into the respiratory secretions of infected person, and diminish risk of infection by prophylactic targeted treatment (Mitja and Clotet, 2020).
Classification, mechanism of action, adverse effects and therapeutic uses of all antiviral agents (currently used and under clinical development) against COVID-19 are shown in Table 1.
Table 1.
Classification, mechanism of action, adverse effects and therapeutic uses of Antiviral agents against COVID-19.
| Name of Drug(s) | Classification | Mechanism of action | Adverse effects | Therapeutic Uses | Safety Concern |
|---|---|---|---|---|---|
| Lopinavir/Ritonavir | HIV Protease inhibitor | Lopinavir inhibit key enzyme (Protease) of coronavirus life-cycle replication Ritonavir inhibits CYP3A4 enzyme |
Diarrhea, abdominal pain, nausea, dyslipidemias | HIV-1 &HIV-2 (anti-retroviral) | Cautiously used in cardiac arrhythmias(QT-prolongation), hepatic disease |
| Remdesivir (GS-5734) | InvestigationalNucleoside analogue | Inhibit RNA dependent RNA polymerase (RdRp) enzyme | Nausea, vomiting,liver damage | Ebola, Marburg, SARS, MERS viral infections | Caution for renal and hepatic impairment patients |
| Ribavirin | Purine nucleoside analogue | Inhibit GTP & viral RNA synthesis | Anemia, bone marrow depression, hemolysis, GIT & CNS symptoms | Chronic hepatitis C, Influenza A & B, respiratory syncytial virus bronchiolitis | Contraindicated in pregnancy due to teratogenicity |
| Favipiravir(T-705)/Umifenovir | Investigational RNA-Dependent RNA Polymerase Inhibitor | Inhibits viral RNA-synthesis | GIT and CNS symptoms, abnormal liver enzyme, elevations in serum uric acid | Influenza arenavirus, bunyavirus filovirus, Ebola &Lassa virus | Contraindicated in pregnancy due to teratogenicity |
| Darunavir/cobicistat | Protease inhibitor | Inhibit key enzyme of coronavirus life-cycle replication Protease (Mpro)/inhibition of human CYP3A4 protein | Nausea, diarrhea, vomiting, muscle ache headache, joint pain, | HIV/AIDS (used in HIV-1 type virus) | Cautiously used in liver (hepatitis B & C), kidney, hemophilia, diabetes and heart patients |
| Camostatmesilate/nafamostat | Serine protease inhibitor | Inhibits enzyme activity TMPRSS2 receptor | Oedema, urticaria, GIT-intolerance, abnormal liver enzyme | Acute Pancreatitis, postoperative reflux esophagitis | Hepatic diseases |
| Ivermectin | Antiparasitic | Inhibits RNA viral synthesis | pruritus, giddiness, nausea, abdominal pain, constipation and lethargy | Anthelmintic, Onchocerciasis, strongyloidiasis, scabies, pediculosis | Pregnant women and young children |
3.1. Lopinavir/Ritonavir
Lopinavir (LPV) and ritonavir (RTV) are effective agents that inhibit the protease activity of coronavirus. LPV and RTV act as an antiretroviral used for the treatment of AIDS/HIV virus (HIV-1& HIV-2). RTV enhances the plasma concentration of LPV through inhibition of cytochrome P450 (CYP3A)-mediated metabolism of LPV (Soliman et al., 2011). Previous studies reported that LPV blocks virus replication in the later stages steps of MERS-CoV cycle (de Wilde et al., 2014) and abate the activity of SARS-CoV by inhibition of proteinase activity (Hurst and Faulds, 2000). In a randomized, controlled and open-label trial involving 199 adults SARS-CoV2 positive patients, patients were divided into two groups (LPV-RTV treated & standard-care group). There is no remarkable changes were observed in both the groups in terms of improvement and mortality. Moreover, adverse effects were less common in LPV-RTV, as compared to standard-care group (Cao et al., 2020a, Cao et al., 2020b). Another study reported that, patients were assigned randomly in phase 2-trial and administered 14 days combination of LPV (400 mg), RTV (100 mg) and ribavirin (400 mg) in every 12 h with IFN beta-1b to improve the antiviral response. This combination seems to be better, superior and provides best output to LPV-RTV alone and also diminished the virus desquamation, reduces symptoms of patients, and often promoting discharge of patients in the mild-moderate COVID-19 (Hung et al., 2020). Another in vitro study reported that Ribavirin, analogue of guanosine nucleotide having wide spectrum of antiviral activity, used along with LPV/RTV to treat SARS-COV-2 viral infection in china (ChiCTR2000029387) (Lu et al., 2020). Earlier this combination was well-established for the treatment of SARS-CoV infection along with minimal risk of acute respiratory distress syndrome (ARDS) and mortality rate (Chu et al., 2004). Moreover, future clinical studies are needed to check this combination with other antiviral-combination against SARS-CoV-2.
3.2. Remdesivir (RDV, GS-5734)
The drug was developed in 2017, for the treatment of Ebola and Marburg virus infections. RDV is a prodrug of nucleotide (adenosine) analogue and having a broad-spectrum antiviral activity. In body, it is metabolized into active metabolite GS-441524. The antiviral mechanism of RDV is to inhibit RNA-dependent RNA polymerase (RdRp) enzyme, necessary for virus replication (Al-Tawfiq et al., 2020). Earlier invitro studies reported that RDV inhibits single stranded RNA virus replication such as SARS-CoV and MERS-CoV (Agostini et al., 2018).Another mouse model study reported that combination of RDV and IFN-beta is better than LPV& RTV for the treatment of MERS-CoV (Sheahan et al., 2020). Currently preclinical study reported that association of RDV & chloroquine is greatly efficient for preventing SARS-Cov-2 infection (Wang et al., 2020). In addition, phase 3 clinical trials conducted in 237 participants to check efficacy and safety of the RDV (loading dose, 200 mg on day 1 only followed by 100 mg maintenance dose, OD for 9 days) in COVID-19 patients (Cao, 2020). RDV shows promising output in some of the US patients with COVID-19 (Holshue et al., 2020). Furthermore, Beigel et al. (2020) reported remdesivir shows possible efficacy better as compared to placebo group in hospitalized patients for the treatment of SARS-CoV-2 virus. A randomized, double-blind and placebo-controlled clinical trial has been conducted and the patients received remdesivir i.v. {day1 loading dose (200 mg) + daily maintenance dose (100 mg) up to 9 additional days}. Patients are assigned randomly and total of, 1063 infected patients are enrolled and divided into two groups, remdesivir group: 541 patients and placebo group: 522 patients. The overall time to recovery rate is better in remdesivir and also lowers infection in respiratory system as compared to placebo. In addition, various evidences suggested serious adverse event i.e acute respiratory failure and sudden death in few patients and strict monitoring is required to manage dose at the time of treatment. From this report, we can conclude antiviral treatment always need supportive care or supplemental oxygen to prevent other negative outcomes. Recently, Hetero pharma company launched COVIFOR vials, brand name of remdesivir, a game-changer drug for treatment of COVID-19. This drug is restricted used in patients who already have kidney problems, pregnant and lactating women (https://www.pharmaceutical-technology.com/news/cipla-hetero-remdesivir-covid-19/). Hence, further research is requisite to evaluate its pharmacological and pharmacodynamics characteristics against SARS-CoV-2 virus.
3.3. Favipiravir (FPV) & Umifenovir
Favipiravir (Avigan) is an antiviral drug, firstly developed by Japan in the year 2014, for treatment of influenza virus infections (Furuta et al., 2017). It is a nucleotide (guanine) analogue, & converted into active metabolite by phosphorylation, then inhibits the synthesis of RNA (RdRp) and virus mutagenicity (Vanderlinden et al., 2016). It has been documented by invitro study that FPV inhibits SARS-CoV-2 in Vero E6 cell (EC50 = 61.88 μM) (Du and Chen, 2020). An open & controlled study designed to determine the efficacy of FPV (1600 mg BD, on day 1 followed by 600 mg BD on 2–14 days) Vs LPV/RTV (400 mg/100 mg BD) along with IFN-α1b, BD) for the treatment of COVID-19. Preliminary results showed that there is significant difference between viral clearance & chest improvement rate (Cai et al., 2020). In India, Glenmark is the first company to initiates the Phase 3 trials on FPV by enrolled 150 participants, duration of treatment maximum of 14 days and total study duration of an individual maximum upto 28 days, with mild to moderate COVID-19 under restricted emergency used. Recently, on June 22, 2020 glenmark permitted FPV, under brand name of Fabiflu tablets (1800 mg BD on first day followed by 800 mg BD from day 2 to day 14) into the market for treatment of mild to moderate cases of COVID-19 patients (Baravkar et al., 2020).
Umifenovir (Arbidol) is a dual-acting direct antiviral/host-targeting agent used as a prophylaxis in the influenza and respiratory infections. A cohort study enrolled 102 adult patients characterized by lymphopenia showed no significant difference in the results obtained between the survival and non-survival groups by receiving treatment with antiviral, antibiotic and glucocorticoid therapy. However, there is a significant data and less mortality rate for those patients who receive arbidol (Cao et al., 2020a, Cao et al., 2020b).
3.4. Darunavir/cobicistat
This combination of drug used as an antiretroviral in the treatment of HIV/AIDS. Darunavir is a HIV protease inhibitor and cobicistat enhances the plasma concentrations of darunavir by blocking its metabolism by CYP 450 enzyme (Deeks, 2018). Preclinical studies documented that this combination showed beneficial effects in the SARS-CoV-2 virus infections (Lin et al., 2020, Omotuyi et al., 2020). The effectiveness and safety concern of darunavir/cobicistat combination is being evaluated under development of clinical trials phase 3 by enrolling 30 COVID-19 patients and estimated completion of study on December 31, 2020. Lastly, the primary outcomes measures shows the clearance rate of virological salivation, less throat infections and decreases respiratory secretion at day 7 (Time frame: 7 day after first randomization) (Lu, 2020).
3.5. Camostatmesilate/nafamostat
Initially, Camostatmesilate and Nafamostatare used in postoperative reflux esophagitis and in chronic pancreatitis (Uno, 2020). Camostatmesilate and Nafamostat, both drugs act on attractive target TMPRSS2, a serine protease inhibitor and block the entry of the virus into the lung alveolar cells (Hoffmann et al., 2020). Recently, Camostat mesilate enters into the phase 2 trials by taking 114 participants (administered dose approx. 200 mg for 7 days, TID). The outcomes obtained like changes in COVID-19 symptoms (frequency and severity), body temperature and changes entry in SARS-CoV-2 viral load.
3.6. Ivermectin
Ivermectin is a FDA approved drug, used as an anti-parasitic and in the treatment of many types of virus infections such as HIV, dengue, influenza and Zika (Caly et al., 2020). Recent study showed that Ivermectin, inhibits the growing of virus in cell culture approximately for 48 hrs. Till date, test is only carried out in-vitro, for confirmation, strong clinical trials are needed to evaluate its safety and efficacy in the treatment of COVID-19 patients (Scavone et al., 2020).Clinical studies of Ivermectin in COVID-19 patients are underway by enrolling 50 confirmed cases of COVID-19, as per hospital protocol. This study is in progress and might be getting its primary outcomes about its safety and clinical efficacy by theend of July 2020 (Budhiraj and Mishra, 2020).
4. Anti-inflammatory and immunomodulatory agents
Earlier data about coronavirus (SARS & MERS) showed, cell infiltration and cytokine storm (CS) leads to acute cell lung injury, ADRS and death of many patients (Channappanavar and Perlman, 2017, Chousterman et al., 2017). Recently, COVID-19 infection, which is due to SARS-CoV-2 virus, accompanied by rapid replication of virus and a massive release of cytokine profile as same in other two reported coronavirus SARS and MERS (Teijaro, 2017). Currently, study documented that cytokines like interleukins (IL-1B, IL-6, IL-7, IL-9 & IL-10); G-CSF; IFNs; interferon-γ-inducible protein (IP10); monocyte chemoattractant protein (MCP1), tumor necrosis factor (TNFα), vascular endothelial growth factor (VEGF), platelet derived growth factors (PDGF) and other pro-inflammatory mediators are increase in COVID-19 patients(Huang et al., 2020). Another studies reported extensively elevation in the level of IL-6 in critically severe poorly in COVID-19 infected patients (Ruan et al., 2020, Zhou et al., 2020). Classification, mechanism of action, adverse effects and therapeutic uses of all anti-inflammatory and immunomodulatory drugs (currently used and under clinical development) against COVID-19 are listed in Table 2.
Table 2.
Classification, Mechanism of action, adverse effects and therapeutic uses of Anti-inflammatory &Immunomodulatory agents against COVID-19.
| Name of Drug(s) | Classification | Mechanism of action | Adverse effects | Therapeutic Uses | Safety Concern |
|---|---|---|---|---|---|
| Chloroquine/Hydroxy-chloroquine (HCQ) | 4-aminoquinolones | Inhibit viral DNA & RNA polymerase enzyme, viral protein glycosylation, ACE2 receptor, also inhibit cytokine release immune-modulation | Cardiovascular symptoms (prolongation of QT interval) | Antimalarial, Immuno-modulator | Cautiously used in liver & heart damage, GIT, retinal, neurological & hematological diseases |
| Anakinra | Biological agents | Recombinant human IL-1 receptor antagonist | Fever, chills, sweating, cough, sore throat, nausea, vomiting, diarrhea, stomach pain | Refractory rheumatoid arthritis | Caution in patients with thrombocytopenia & neutropenia, infusion related reactions |
| Canakinumab (ACZ885) | Human monoclonal antibody | IL-1β receptor antagonist | CNS (headache, vertigo), GI symptoms, musculoskeletal pain, rhinitis, bronchitis | Periodic fever syndromes, Sudden Juvenile Idiopathic Arthritis (SJIA) | Caution in patients with thrombocytopenia & neutropenia |
| Tocilizumab/Itolizumab | Humanized monoclonal antibody | IL-6 receptor antagonist | Allergic reactions, headache, hypertension, mouth ulcers, increase in hepatic enzymes | Autoimmune disorder/immunosuppressive effects (rheumatoid arthritis, giant cell arteritis) | Contraindicated in hepatic diseases, GIT perforation patients, infusion related reactions |
| Aviptadil | Vasoactive intestinal polypeptide (VIP) analogue | Inhibits production ofIL-6 and TNF-α | Alterations in blood pressure, Heart rate | Erectile dysfunction, sarcoidosis, acute lung damage | Cautiously used in cardiovascular patients |
| Eculizumab | Protein Based Therapies Monoclonal antibody (mAb) |
Inhibitor of the terminal portion of complement cascade | Allergic reactions, anemia, GIT & flu like symptoms, headache & leukopenia | Atypical hemolytic uremic syndrome, generalized myasthenia gravis | Contraindicated in patients with unresolved serious Neisseria meningitides infection |
| Baricitinib | Tyrosine protein kinase family | Janus-kinase (JAK1 & JAK 2) inhibitors | Allergic reactions, increased LDL cholesterol, nausea, trouble in breathing | Autoimmune disease (rheumatoid arthritis) | Contraindicated in kidney and liver diseases (hepatic B&C), bone-marrow depression and cancer |
4.1. Chloroquine/Hydroxychloroquine (HCQs)
It is an anti-malarial and over the counter (OTC) drug, activity similar to chloroquine. Recently, it was reported that HCQs shortened the time to clinical recovery on the SARS-CoV-2 patients. Due to its interferon blocking property, it has diminished the immune response to a viral infection. This immunomodulatory property makes its useful in autoimmune disorder such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). The safety profile of HCQ is superior as compared to chloroquine during long term used in COVID-19 patients and little concern about drug-drug interactions (Yao et al., 2020). Several in vitro studies reported CQs/HCQs are used as an effective treatment in SARS-CoV-2 virus. As they are weak bases and enhance pH of endosomal in host cell organelles, inhibit fusion of autophagosome-lysosome and inactivates enzyme require for replication of virus. They also have a potential to deactivate ACE-2 glycosylation, for which coronavirus may enters into the cell (Ferner and Aronson, 2020). Currently studies showed that HCQ administered for 3–6 days, effective in clearing viral nasopharyngeal carriage of SARS-CoV-2 in most of the COVID-19 patients and strengthen its effects along with azithromycin (Gautret et al., 2020). Azithromycin, a macrolide antibacterial drug was shown to be successful in the treatment of zika and ebola viruses by in-vitro studies (Madrid et al., 2015, Bosseboeuf et al., 2018). Previous studies reported that combination of HCQ and azithromycin showed synergistic effect and prevent respiratory tract infections when administered by viral infection patients (Bacharier et al., 2015).
Recently, World Health Organisation (WHO) updated information on 17 June 2020, about safety and efficacy of chloroquine (CQs)/Hydroxychloroquine (HCQs) and compared it with standard care treatment for COVID-19 patients. The overall outcomes showed that there is no significant difference in case of mortality when compared with standard care treatment. In addition, some reports evaluated HCQs cause’s serious adverse effects like prolongation of QT interval, ventricular fibrillation, arrhythmia and sometimes leads to abrupt death. The safety and efficacy assurance for CQs/HCQs, there is necessitate that other clinical studies are needed to be performed and exemplify with safety concern (Cochrane, 2020). Further, US FDA summarized details on July 1 with safety concern, and permitted used of CQs/HCQs is now accessible to take care of patients with COVID-19 but some strict monitoring are required while treatment as it shows various heart related problems, blood and lymph disorders, renal injury and hepatic failure (US FDA, 2020).
4.2. Interferon (IFNs) β-1b
Interferons are types of cytokines effective in the management of infections & infectious diseases (Arnaud, 2002). It is a broad-spectrum antiviral, antiproliferative & immunomodulatory drug interacting with the toll-like receptors and inhibits viral replication and protein synthesis (Uematsu and Akira, 2007). There are three types of IFN (α, β, and γ). IFN-β shows high potent specificity as compared to IFN-α in reducing the replication of MERS-CoV (Hart et al., 2014, Shalhoub, 2020). Recently, ex-vivo studies demonstrated SARS-CoV-2 triggers lower type 1 interferon than SARS-CoV in human lung tissue (Shalhoub, 2020). Further, several future studies are being conducted to determine its efficacy & safety along with other antiviral drug in treatment of COVID-19.
4.3. Interleukin antagonist
Interleukin plays a major role in the pathogenesis of all three coronavirus SARS-CoV-1, MERS & SARS-CoV-2 infections (Lau et al., 2013). Recent study reported several pro-inflammatory mediators plays a major role in the lung damage such as interleukins (IL-2, IL-6 etc) and inflammatory markers (D-dimer, ferritin, and C-reactive protein) (Conti et al., 2020, Kuppalli and Rasmussen, 2020.A). Several ILs antagonist drugs are under ongoing trials to check its safety and efficacy against SARS-CoV-2 viral infections.
4.3.1. Anakinra (IL-1 Antagonist)
Anakinra is a recombinant IL-1 receptor antagonist and may be helpful in COVID-19 patients. It prevents the binding of IL-1α and IL-1β to interleukin-1 receptors, which is used in the treatment of autoimmune disorders (Cavalli et al., 2020). Recently, retrospective cohort study showed high dose of anakinra (5 mg/kg, BD,iv) produces beneficial and efficacious effects in 72% Covid-19 infected patients associated with ARDS (Cavalli et al., 2020). In addition, anakinra alleviated hyper-inflammation and respiratory distress in SARS-CoV-2 infected patients (Tufan et al., 2020). Another studies reported anakinra (100 mg repeated after 6 h, sc for 7 days) administered along with remdesivir (200 mg LD, followed by 100 mg after 4 h, iv for 7 days) used in the treatment of respiratory dysfunction in COVID-19 patients (Franzetti et al., 2020). However, several clinical trials in large population are required for confirmation of its efficacy.
4.3.2. Canakinumab (IL-1β Antagonist)
A human monoclonal antibody particularly targets and neutralizes IL-1β thus blocking its binding with IL-1 receptors (Dhimolea, 2010). A randomized, controlled and double-blind study are underway in which 450 participants are to be enrolled, to check the efficiency and safety of canakinumab in patients with COVID-19 induced pneumonia and systemic inflammatory release syndrome (SIRS). Until date, this drug has been passed from phase 2 trials and the estimation date for the completion of clinical trials study is October 16, 2020. The dose of canakinumab administered by patients according to body weight (40–60 kg, 450 mg; 60–80 kg, 600 mg;>80 kg; 750 mg) along with 5% dextrose (iv fluid) for 2 h (Novartis Pharmaceuticals, 2020).
4.4. IL-6 receptor antagonist
These classes of drugs blocks interleukin (IL-6) mediated signaling pathways by binding competitively with soluble and membrane bound IL-6 receptors (sIL-6R and mIL-6R). IL-6 involved in various physiological process like activation of T-cells, B-cell, hematopoietic cell proliferation and differentiation, hepatic acute-phase protein synthesis initiation and also immunoglobulin secretion(Tanaka et al., 2014).
4.4.1. Tocilizumab
It is a monoclonal antibody, firstly initiates and tested by china to decrease the lung related complications in patients with COVID-19. A randomized and retrospective study showed that tocilizumab plays a beneficial role as an adjunctive therapy in COVID-19 patients. Preliminary data proved that percentage of lymphocyte, concentration of CRP, and computed-tomography clarity changes and reduces the hospitalization duration of these patients (Xu et al., 2020). At present, three clinical trials are under advancement to assess its safety and efficacy alone or in combination with some other drugs by taking 1500 participants with SARS-CoV-2 infection (Barrett, 2020, de Paris, 2020, Regeneron Pharmaceuticals, 2020). The phase 3 clinical-trial of tocilizumab approved by US-FDA in severe COVID-19 pneumonia patients. Moreover, other IL-6 inhibitors such as clazakizumab and sarilumab are underway in the clinical trials (phase 2& 3) to evaluate its efficacy in COVID-19 infected patients (Henriksen, 2020).
4.4.2. Itolizumab (ALZUMAb)
It is a humanized recombinant IgG1 anti-CD6 monoclonal antibody, used for the treatment of moderate to severe plaque psoriasis, rheumatoid arthriris and autoimmune disorders (Saavedra et al., 2020). Initially, it was developed by Biocon in Bengaluru and approved in 2013 in psoriatic patients. Recently, Biocon repositioning the drug into patients with moderate to severe COVID-19 associated with Cytokine release syndrome (CRS) and acute respiratory distress syndrome (ARDS). In addition, Drug controller General of India (DCGI) allowed use of ALZUMAb (25 mg/5ml injection) in SARS-CoV-2 patients as an emergency treatment and capable to save numerous seriously sick patients with COVID-19 (https://www.pharmaceutical-technology.com/news/biocon-itolizumab-approval/).
Itolizumab is also acts as a immunomodulator and binds with CD6 receptor along with suppress many proinflammatory mediators (cytokines) and downregulates the T-cell activation at site of inflammation (Saavedra et al., 2020). An open-label randomized and controlled study performed by Biocon, in 30 participants associated with moderate to severe symptoms of SARS-CoV-2 virus. The study being conducted in two groups, first group taken itolizumab + supportive care while second group only with supportive care. The overall outcomes showed there is no mortality rate and all patients are recovered well as compared to second group. Further, clinic-pathological observations i.e release of pro-inflammatory cytokines (IL-6 & TNF-α) significantly decrease in first group when compared to second group (Jolla, 2020, Uttamani et al., 2020). Moreover, several clinical studies are required to confirm its safety and efficacy in COVID-19 patients.
4.5. Aviptadil
It is a vasoactive intestinal polypeptide (VIP) analogue used in the treatment of erectile dysfunction, acute lung injury and Besnier-Boeck-Schaumann disease. The significance of aviptadil in ARDS, because it has been documented by animal studies that VIP is extensive concerted in the lungs and inhibits NMDA-induced caspase-3 activation, IL6 and TNF-α production and also protects against acidic (HCl)-induced lung edema. In the various animal models, aviptadil protects lungs and other organs from damage or failure by restoring barrier function at the alveolar interface (Petkov et al., 2003, Said, 2012). In a phase 2 clinical trials, conducted under European regulatory authority (ERA), patients were successfully treated with intravenous infusion of aviptadil on escalating doses and restores all the functioning of pulmonary hypertension, acute lung injury and ARDS. Few adverse effects related to cardiovascular system such as change in blood pressure, heart rate or ECG was noticed.
4.6. Eculizumab
Eculizumab is a monoclonal protein based monoclonal antibody (mAb) used to treat paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), and neuromyelitis optica (Wong and Kavanagh, 2015). The main target of eculizumab is complement protein C5, preventing cleavage to C5a and C5b, and the formation of the terminal complement complex C5b-9, which is involved in many autoimmune diseases (Gralinski et al., 2018). Based on case study of patients with SARS-CoV2 infection and also confirmed severe pneumonia and ARDS treated with i.v. infusion of eculizumab along with anticoagulant therapy (Enoxaparin 4000 IU/day s.c), antiviral therapy (LPV 800 mg/day + RTV 200 mg/day), hydroxychloroquine 400 mg/day, ceftriaxone 2 g/day IV, vitamin C 6 g/day for 4 days. All patients showing decrease in the inflammatory markers including C-reactive protein (CRP) (Diurno et al., 2020). More clinical study is needed for confirmation its safety and efficacy as ananti-inflammatory in the treatment of COVID-19.
4.7. Janus kinase (JAK) inhibitors
JAK are the intracellular enzymes located on cellular membrane, transmits signal by binding with various cytokines and growth receptors. After binding with receptor, enzymes phosphorylation occurs, which modulating the changes in the gene expression and thus, activates the signal transducers and activators of transcription proteins (STATs) (Seif et al., 2017). JAK pathway is key enzyme and plays a crucial role in the activation of immune system, hematopoiesis and inflammation. Inhibitors of JAK, prevents the enzymes phosphorylation and activation of STATs proteins (O'shea et al., 2015).
4.7.1. Baricitinib
It is reversible and competitive inhibitor of JAK1 & JAK2, and firstlyaccepted by US FDA in the treatment of autoimmune disorder i.e. rheumatoid arthritis. It also have broad immunosuppressive effect, and used in many autoimmune disorders (Winthrop, 2017). It blocks the enzymes protein kinase 1 related with AP2 (AAK1), thus inhibits the entry and binding of virus into the epithelial cell of the alveoli (Mayence and Vandeneynde, 2019). A non-randomized open label trial of baricitinib + LPV/RTV in 12 COVID-19 patients and a control group treated with HCQ + LPV/RTV. The respiratory function parameters in baricitinib-treated patients improve as compared to control group. No significant adverse effect reported in baricitinib-treated patients (Cantini et al., 2020). Further studies are needed to confirm its clinical safety and efficacy in the treatment of COVID-19 infection. Others JAK inhibitors are also under clinical development and data are being evaluated in the treatment of COVID-19 are ruxolitinib and tofacitinib (Smith and Prosser, 2020).
5. Adjuvant/supportive care
5.1. Isotretinoin
Isotretinoin,a retinoid derivative of vitamin A, is used in the treatment of severe acne. It is the strongest down-regulator of ACE-2 receptors. It has been documented that isotretinoin is a protease inhibitors and can be a taken as a target therapy in COVID-19 (Wu et al., 2020a). Based on the above mechanism, isotretinoin currently tested under phase 3 clinical trials in the adult patients with positive SARS-CoV-2 viral infections. Current outcomes showing there is large difference at the onset of clinical signs and symptoms treated patients with isotretinoin, standard therapy or combined therapy and checked by on a daily basis.
5.2. Corticosteroids
Corticosteroid therapy is only used as supportive care in patients of COVID-19 with refractory shock or ARDS (Wu et al., 2020b). According to WHO, corticosteroids are recommended only for some conditions like asthma, chronic obstructive pulmonary disease (COPD), and septic shock (WHO, 2020b). Earlier studies showed corticosteroids i.e. methyl-prednisolone directly not supported the patients having symptoms of viral-pneumonia in COVID-19 (Jin et al., 2020), only act as an anti-inflammatory and reduces inflammatory mediators i.e. interleukin (IL-7 &IL-8), MCP1, Th1 chemokine,IP10 etc. (Sung et al., 2004, Wong et al., 2004).
Dexamethasone, another cheap and widely steroid drug used in the treatment of many inflammatory disease conditions and disorders like asthma, severe skin allergies, chronic obstructive lung disease and certain cancers. Recently, it has been documented that dexamethasone could be life-saving drug in critically ill patients with COVID-19 (WHO, 2020). A retrospective cohort study showed daily administered of dexamethasone (6 mg upto 10 days) reduces the mortality rate among patients with COVID-19 who needed supplemental oxygen and mechanical ventilation. There is no advantage of dexamethasone observed in patients who did not necessitate mechanical support or/ventilation (Horby et al., 2020).
5.3. Convalescent plasma
It is an antibody-rich plasma, collected from the patients who already have recovered from diseases caused by virus e.g. polio, measles, ebola, H1N1 influenza, hepatitis B, and now currently in SARS-CoV-2 responsible for COVID-19. On May 1, 2020, US FDA permitted issued guidelines to healthcare providers, make ensure the safety and efficacy of investigational product by collecting plasma from recovered patients under demand of public health emergency (Stephen and Hahn, 2020). A newly reports showed that use of plasma therapy in five ventilated coronavirus patients, all patients improved after one week of transfusion. Moreover, this report was not a clinical trial, further research is essential to confirm its potential and managing patients in SARS-CoV-2 infected patients (https://clinicaltrials.gov/ct2/show/study/NCT04356534).
5.4. Bronchodilators
Bronchodilators are preferred only as a supportive care for COVID-19 patients associated with COPD, asthma and other respiratory disorders (Lippi and Henry, 2020). Due to concern of safety precautions, both standard nebulizers and metered dose inhalers (MDI) are risky to COVID-19 patients, as it requires opening of ventilator circuit. Regular administration of bronchodilator may be needed, to minimize the virus transmission to others multiple health care patient. In order to alleviate these hazards, Aeroneb Solo (Aerogen©, Galway, Ireland) has been successfully made for COVID-19 patients which continuously delivers medication via inhalation without circuit opening and requires minimal health-care workers for handling (Miller and Epstein, 2020).
5.5. Fibrinolytics
These are the agents that breakdown plasminogen to plasmin on the surface of thrombi, thus produced fibrinolysis (Chapin and Hajjar, 2015). A study was done in seriously unwell patients, already suffered with ARDS and respiratory collapse/failure. Patients administered with alteplase along heparin improves PaO2/FiO2 (P/F) ratio momentary approximately by 11–100% (Moore, 2020). Further, detailed studies are required to check its clinical efficacy among large population. Others supportive fibrinolytic agents are also being evaluated for COVID-19 patients e.g. defibrotide (Fabio, 2020).
5.6. Anticoagulants
Coronavirus patients have a higher risk of venous thromboembolism (VTE), deep vein thrombosis (DVT) and pulmonary embolism (Porfidia and Pola, 2020). Coagulopathy is one of the main reasons of mortality in the majority of COVID-19patients. A recent report suggested anticoagulant therapy such as low molecular weight heparin (LMWH) administered to 449 patients showed low mortality risk (Kollias et al., 2020). Aspirin is also used as a prophylaxis treatment in case of post-discharge VTE COVID-19 patients (ASH, 2020). Further, additional data needed regarding its efficacy and safety in COVID-19 patients.
6. Conclusions
Due to pandemic of COVID-19 disease, no vaccine or drug is approved to eliminate the virus. Drug development and discovery is broad and extensive process, due to utmost necessity of the treatment, drug repurposing is suitable for treatment of COVID-19 infection. At present, numerous drugs are ongoing clinical trial and tested directly in patients with COVID-19, strict monitoring is mandatory at the time of treatment of patients to assure its safety and efficacy of unknown profile of drugs. Based on the exposed and revealed structure and transmission mechanisms of novel coronavirus, the available treatment option is antiviral, anti-inflammatory & immunomodulatory drugs. This review provides detailed description of all available in-vitro & in-vivo studies, mechanism, clinical spectrum of disease, symptoms and safety concern profile of currently available treatment and concerning drugs, which are progress under clinical development. In conclusion, while waiting for the most effectual pharmacological treatment with less adverse effects, many trials are currently ongoing and will available around at the end of 2020, according to clinical trials government website. Hope, their consequences will definitely provide us good outcome and help worldwide in significant the greatest approach to cure or treat COVID-19 along with symptoms and complications.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-forprofit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agostini M.L., Andres E.L. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9(2):e00221–e00318. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Tawfiq J.A., Al-Homoud A.H. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med. Infect. Dis. 2020;5 doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud P. The interferons: pharmacology, mechanism of action, tolerance and side effects. Rev. Med. Interne. 2002;23:449s–458s. doi: 10.1016/s0248-8663(02)00659-8. [DOI] [PubMed] [Google Scholar]
- ASH, American Society of COVID-19 and VTE/anticoagulation: frequently asked questions Version 2.1. Available at. Hematology. 2020 [Google Scholar]
- Bacharier L.B., Guilbert T.W. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034–2044. doi: 10.1001/jama.2015.13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baravkar, A.A., Shende, M.V., Nalawade, N.A., Corona virus and COVID-19. Mohan, A., Cytokine storm.
- Barrett, L., 2020. Treatment of Moderate to Severe Coronavirus Disease (COVID-19) in Hospitalized Patients (https://clinicaltrials.gov/ct2/show/NCT04321993).
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D. Remdesivir for the treatment of Covid-19—preliminary report. New Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuto D., Giovanetti M. The 2019-new coronavirus epidemic: Evidence for virus evolution. J. Med. Virol. 2020;92:455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biocon’s drug Itolizumab gets approval to treat Covid-19. Updated on 13 July 2020 (https://www.pharmaceutical-technology.com/news/biocon-itolizumab-approval/).
- Bosseboeuf E., Aubry M. Azithromycin inhibits the replication of Zika virus. J. Antivir. Antiretrovir. 2018;10:6–11. [Google Scholar]
- Budhiraj, A.S., Mishra, R.S., 2020. To study the effectiveness of ivermectin with standard of care treatment versus standard of care treatment for COVID 19 cases. A pilot study (https://clinicaltrials.gov/ct2/show/record/NCT04373824).
- Cai Q., Yang M. Experimental treatment with favipiravir for COVID-19: an open-label control study. Engineering (Beijing) 2020 doi: 10.1016/j.eng.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L., Druce J.D. The FDA-approved Drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantini F., Niccoli L. Baricitinib therapy in COVID-19: A pilot study on safety and clinical impact. J. Infect. 2020;22:30228. doi: 10.1016/j.jinf.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, B., 2020. A Phase 3 Randomized, Double-blind, Placebo-controlled, Multicenter Study to Evaluate the Efficacy and Safety of Remdesivir in Hospitalized Adult Patients with Severe COVID-19(https://clinicaltrials.gov/ct2/show/NCT04257656).
- Cao B., Wang Y. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Tu W.J., Cheng W., Yu L., Liu Y.K., Hu X., Liu Q. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., De Luca G. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin J.C., Hajjar K.A. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29:17–24. doi: 10.1016/j.blre.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chousterman B.G., Swirski F.K. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- Chu C.M., Cheng V.C. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipla and Hetero launch remdesivir to treat Covid-19 in India. 2020. Pharmaceutical Technology (https://www.pharmaceutical-technology.com/news/cipla-hetero-remdesivir-covid-19/).
- Cochrane, 2020. Targeted Update: Safety and efficacy of hydroxychloroquine or chloroquine for treatment of COVID-19. Cochrane. Updated 17 June 2020. 33 (https://www.who.int/publications/m/item/targeted-update-safety-and-efficacy-of hydroxychloroquine-or-chloroquine-for-treatment-of-covid-19).
- Conti P., Ronconi G. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34(2) doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- Convalescent Plasma Trial in COVID-19 Patients. NIH U.S Library of Medicine (https://clinicaltrials.gov/ct2/show/study/NCT04356534).
- COVID-19 Treatment guidelines Panel, Coronavirus Disease 2019 (2019). The National Institutes of Health (NIH) COVID-19 Treatment Guidelines Panel Provides Recommendations for Dexamethasone in Patients with COVID-19. Updated on 2020 (available at https://www.covid19treatmentguidelines.nih.gov/dexamethasone/).
- de Paris, H., 2020. Cohort Multiple Randomized Controlled Trials Open-label of Immune Modulatory Drugs and Other Treatments in COVID-19 Patients - Sarilumab Trial - CORIMUNO-19-SARI (https://clinicaltrials.gov/ct2/show/NCT04324073).
- de Wilde A.H., Dirk J. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob. Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks E.D. Darunavir/Cobicistat/Emtricitabine/TenofovirAlafenamide: a review in HIV-1infection. Drugs. 2018;78:1013–1024. doi: 10.1007/s40265-018-0934-2. [DOI] [PubMed] [Google Scholar]
- Dhimolea E. Canakinumab. MAbs. 2010;2:3–13. doi: 10.4161/mabs.2.1.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diurno F., Numis F.G. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020;24:4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- Dong E., Du H. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Y.X., Chen, X.P., 2020. Response to“ Dose rationale for favipiravir use in patients infected with SARS-CoV-2”. Clin. Pharmacol. Ther. doi: 10.1002/cpt.1878. [DOI] [PMC free article] [PubMed]
- Fabio, C., 2020.Use of Defibrotide to Reduce Progression of Acute Respiratory Failure Rate in Patients with COVID-19 Pneumonia (https://clinicaltrials.gov/ct2/show/NCT04335201).
- Ferner R.E., Aronson J.K. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369:1432. doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
- Franzetti, M., Pozzetti, U., et al., 2020. Interleukin-1 receptor antagonist anakinra in association with remdesivir in severe Coronavirus disease 2019: a case report. Int. Infect. Dis. https://doi.org/10.1016/j.ijid.2020.05.050. [DOI] [PMC free article] [PubMed]
- Furuta Y., Komeno T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017;93:449–463. doi: 10.2183/pjab.93.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gralinski L.E., Sheahan T.P. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9:e01753–e01818. doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y.R., Cao Q.D. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak-an update on the status. Mil. Med. Res. 2020;7:1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B.J., Dyall J. Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J. Gen. Virol. 2014;95:571. doi: 10.1099/vir.0.061911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen, M., 2020. Effectiveness of Interleukin-6 Receptor Inhibitors in the Management of Patients with Severe SARS-CoV-2 Pneumonia: An Open-Label, Multicenter Sequential and Cluster Randomized Trial (https://clinicaltrials.gov/ct2/show/NCT04322773).
- Hoffmann M., Kleine-Weber H. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., Debolt C. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. MedRxiv. 2020 [Google Scholar]
- Huang C., Wang Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huids I.A.E., Madani T.A. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health: the latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F.N., Lung K.C. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst M., Faulds D. Lopinavir. Drugs. 2000;60:1371–1379. doi: 10.2165/00003495-200060060-00009. [DOI] [PubMed] [Google Scholar]
- Jin Y.H., Cai L. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolla, L., 2020. Clinical Trial Shows Itolizumab Reduces Mortality in Patients Hospitalized with COVID-19. Equillium. (https://www.globenewswire.com/news-release/2020/07/13/2060993/0/en/Clinical-Trial-Shows-Itolizumab-Reduces-Mortality-in-Patients-Hospitalized-with-COVID-19.html).
- Kollias A., Kyriakoulis K.G. Thromboembolic risk and anticoagulant therapy in COVID-19 patients: emerging evidence and call for action. Br. J. Haematol. 2020 doi: 10.1111/bjh.16727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse R.L. Therapeutic strategies in an outbreak scenario to treat the novel coronavirus originating in Wuhan, China. F1000Res. 2020;9:72. doi: 10.12688/f1000research.22211.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppalli K., Rasmussen A.L. A glimpse into the eye of the COVID-19 cytokine storm. EBioMed. 2020;55 doi: 10.1016/j.ebiom.2020.102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S.K., Lau C.C. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J. Gen. Virol. 2013;94:122679–122690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, S., Shen, R., et al., 2020. Molecular modeling evaluation of the binding effect of Ritonavir, Lopinavir and Darunavir to severe acute respiratory syndrome coronavirus 2 proteases. BioRxiv doi:https://doi.org/10.1101/2020.01.31.929695.
- Lippi G., Henry B.M. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir. Med. 2020;167 doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C.C., Chen M.Y. Potential therapeutic agents against COVID-19: What we know so far. J. Chin. Med. Assoc. 2020 doi: 10.1097/JCMA.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H., 2020. Efficacy and safety of Darunavir and Cobicistat for treatment of COVID-19 (https://clinicaltrials.gov/ct2/show/record/NCT04252274).
- Madrid P.B., Panchal R.G. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect. Dis. 2015;1:317–326. doi: 10.1021/acsinfecdis.5b00030. [DOI] [PubMed] [Google Scholar]
- Mayence A., Vandeneynde J.J. Baricitinib: a 2018 novel FDA-approved small molecule inhibiting Janus kinases. Pharmaceuticals. 2019;12:37. doi: 10.3390/ph12010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Epstein D. Safe bronchodilator treatment in mechanically ventilated COVID-19 patients: a single center experience. J. Crit. Care. 2020;58:56–57. doi: 10.1016/j.jcrc.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitja O., Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Global Health. 2020;8:639–640. doi: 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, E.E., 2020. Fibrinolytic Therapy to Treat ARDS in the Setting of COVID-19 Infection: A Phase 2a Clinical Trial (https://clinicaltrials.gov/ct2/show/NCT04357730).
- Muralidharan N., Sakthivel R. Computational studies of drug repurposing and synergism of lopinavir, oseltamivir and ritonavir binding with SARS-CoV-2 Protease against COVID-19. J. Biomol. Struct. Dyn. 2020;16:1–6. doi: 10.1080/07391102.2020.1752802. [DOI] [PubMed] [Google Scholar]
- Novartis Pharmaceuticals, 2020 Phase 3 Multicenter, Randomized, Double-blind, Placebo-controlled Study to Assess the Efficacy and Safety of Canakinumab on Cytokine Release Syndrome in Patients with COVID-19-induced Pneumonia (CAN-COVID) (https://clinicaltrials.gov/ct2/show/NCT04362813).
- Omotuyi, O.I., Nash, O., et al., 2020. Darunavir disrupts critical nodes in metastable 2019-nCoV-RBD/ACE-2 Complex. Preprints 2020030125. doi: 10.20944/preprints202003.0125.v1.
- O’shea J.J., Schwartz D.M. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkov V., Mosgoeller W. Vasoactive intestinal peptide as a new drug for treatment of primary pulmonary hypertension. J. Clin. Invest. 2003;111:1339–1346. doi: 10.1172/JCI17500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porfidia A., Pola R. Venous thromboembolism and heparin use in COVID-19 patients: juggling between pragmatic choices, suggestions of medical societies. J. Thromb. Thrombol. 2020;4:1–4. doi: 10.1007/s11239-020-02125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat, D., Sharma, S., 2020. Case Study: 60-Year-Old Female Presenting With Shortness of Breath. Stat. Pearls. Available at: https://www.ncbi.nlm.nih.gov/books/NBK499852/. [PubMed]
- Razonable R.R. Antiviral drugs for viruses other than human immunodeficiency virus. Mayo Clinic Proceedings. 2011;86:1009–1026. doi: 10.4065/mcp.2011.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regeneron Pharmaceuticals, 2020. An Adaptive Phase 2/3, Randomized, Double-Blind, Placebo-Controlled Study Assessing Efficacy and Safety of Sarilumab for Hospitalized Patients With COVID-19. (https://clinicaltrials.gov/ct2/show/NCT04315298).
- Ruan Q., Yang K. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;3:1–3. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra, D., Añé-Kourí, A.L., Sánchez, N., Filgueira, L.M., Betancourt, J., Herrera, C., Manso, L., Chavez, E., Caballero, A., Hidalgo, C., Lorenzo, G., 2020. An Anti-CD6 Monoclonal Antibody (Itolizumab) Reduces Circulating IL-6 in Severe Covid-19 Elderly Patients. [DOI] [PMC free article] [PubMed]
- Said S.I. Vasoactive intestinal peptide in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012;185:786. doi: 10.1164/ajrccm.185.7.786. [DOI] [PubMed] [Google Scholar]
- Scavone C., Brusco S. Current pharmacological treatments for COVID-19: what’s next? Br. J. Pharmacol. 2020 doi: 10.1111/bph.15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif F., Khoshmirsafa M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017;15:23. doi: 10.1186/s12964-017-0177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalhoub S. Interferon beta-1b for COVID-19. Lancet. 2020;395(10238):1670–1671. doi: 10.1016/S0140-6736(20)31101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C. Comparative therapeutic efficacy of remdesivirand combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, T., Prosser, T., 2020. COVID-19 Drug Therapy–Potential Options. Clinical Drug Information, Clinical Solutions, Elsevier.
- Soliman E.Z., Lundgren J.D. Boosted protease inhibitors and the electrocardiographic measures of QT and PR durations. AIDS. 2011;25:367–377. doi: 10.1097/QAD.0b013e328341dcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephen and Hahn, 2020. Coronavirus (COVID-19) Update: FDA Encourages Recovered Patients to Donate Plasma for Development of Blood-Related Therapies. Commissioner of Food and Drugs - Food and Drug Administration US FDA (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-encourages-recovered-patients-donate-plasma-development-blood).
- Sung J.J., Wu A. Severe acute respiratory syndrome: report of treatment and outcome after a major outbreak. Thorax. 2004;59:414–420. doi: 10.1136/thx.2003.014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Narazaki M. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014;6 doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro J.R. Cytokine storms in infectious diseases. In seminars in immunopathology. Semin. Immunopathol. 2017;39:501–503. doi: 10.1007/s00281-017-0640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufan A., AvanoğluGüler A. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J. Med. Sci. 2020;50(3):620–632. doi: 10.3906/sag-2004-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu S., Akira S. Toll-like receptors and Type I interferons. J. Biol. Chem. 2007;282:15319–15323. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- Uno Y. Camostatmesilate therapy for COVID-19. Intern. Emerg. Med. 2020;29:1–2. doi: 10.1007/s11739-020-02345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US FDA, 2020. FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems. Updated on 1 July 2020. (https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or).
- Uttamani, J., Fernandes, G., Pandey, S., Surabathula, V., Bhat, D., 2020. Therapeutic Modalities in the management of COVID-19: A worldwide landscape.
- Vanderlinden E., Vrancken B. Distinct effects of T-705 (favipiravir) and ribavirin on influenza virus replication and viral RNA synthesis. Antimicrob. Agents Chemother. 2016;60:6679–6691. doi: 10.1128/AAC.01156-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO welcomes preliminary results about dexamethasone use in treatingcritically ill COVID-19 patients 2020. Available from:https://www.who.int/news-room/detail/16-06-2020-who-welcomes-preliminary-results-about-dexamethasone-use-in-treating-critically-ill-covid-19-patients.
- WHO, World Health Organization, 2020a. DRAFT landscape of COVID-19 candidate vaccines. Available at: https://www.who.int/blueprint/priority-diseases/key-action/novel-coronavirus-landscape ncov.pdf?ua=1.
- WHO, World Health organization, 2020b Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Available at: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf.
- Winthrop K.L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 2017;13:234–243. doi: 10.1038/nrrheum.2017.23. [DOI] [PubMed] [Google Scholar]
- Wong C.K., Lam C.W. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E.K., Kavanagh D. Anticomplement C5 therapy with eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Transl. Res. 2015;165:306–320. doi: 10.1016/j.trsl.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Wu C., Chen X. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;13 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Liu Y. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Han M. Effective treatment of severe COVID-19 (2020). Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc. Natl. Acad. Sci. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Ye F. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Wunderink R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirol. 2018;23:130–137. doi: 10.1111/resp.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16(10):1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N.S., Zheng B.J. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February. Lancet. 2003;362:1353–1358. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]