Abstract
Objectives
The objective of this study was to estimate the association between tocilizumab or corticosteroids and the risk of intubation or death in patients with coronavirus disease 19 (COVID-19) with a hyperinflammatory state according to clinical and laboratory parameters.
Methods
A cohort study was performed in 60 Spanish hospitals including 778 patients with COVID-19 and clinical and laboratory data indicative of a hyperinflammatory state. Treatment was mainly with tocilizumab, an intermediate-high dose of corticosteroids (IHDC), a pulse dose of corticosteroids (PDC), combination therapy, or no treatment. Primary outcome was intubation or death; follow-up was 21 days. Propensity score-adjusted estimations using Cox regression (logistic regression if needed) were calculated. Propensity scores were used as confounders, matching variables and for the inverse probability of treatment weights (IPTWs).
Results
In all, 88, 117, 78 and 151 patients treated with tocilizumab, IHDC, PDC, and combination therapy, respectively, were compared with 344 untreated patients. The primary endpoint occurred in 10 (11.4%), 27 (23.1%), 12 (15.4%), 40 (25.6%) and 69 (21.1%), respectively. The IPTW-based hazard ratios (odds ratio for combination therapy) for the primary endpoint were 0.32 (95%CI 0.22–0.47; p < 0.001) for tocilizumab, 0.82 (0.71–1.30; p 0.82) for IHDC, 0.61 (0.43–0.86; p 0.006) for PDC, and 1.17 (0.86–1.58; p 0.30) for combination therapy. Other applications of the propensity score provided similar results, but were not significant for PDC. Tocilizumab was also associated with lower hazard of death alone in IPTW analysis (0.07; 0.02–0.17; p < 0.001).
Conclusions
Tocilizumab might be useful in COVID-19 patients with a hyperinflammatory state and should be prioritized for randomized trials in this situation.
Keywords: Cohort study, Corticosteroids, COVID-19, Hyperinflammatory state, Mortality, Tocilizumab
Graphical abstract
Introduction
The clinical spectrum of coronavirus disease 2019 (COVID-19) associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) varies from asymptomatic disease to severe pneumonia and death [1,2]. Increased serum concentrations of inflammatory and coagulation markers (including C-reactive protein (CRP), ferritin, and D-dimer) and proinflammatory cytokines (such as IL-2R, IL-6, IL-10, and TNF-α) have been associated with disease severity in COVID-19 [3,4]. These findings indicate that a hyperinflammatory state may play a crucial role in severe cases of COVID-19, as in other coronaviruses [5].
Regarding treatment of COVID-19, so far remdesivir is the only antiviral that has shown some efficacy [6]. Because of the dysregulated immune response characteristic of severe COVID-19, it is conceivable that immunosuppressant drugs may have some effect in selected patients. Despite the fact that some guidelines have recommended against the use of corticosteroids [7,8], dexamethasone (6 mg/day) in the RECOVERY trial reduced mortality among those receiving either invasive mechanical ventilation or oxygen alone [9]. Other host response modifiers under investigation include tocilizumab, a recombinant humanized anti-human IL-6 receptor [10], for which some comparative observational studies have been reported [[11], [12], [13], [14]].
Observational studies may help in the design of randomized trials of immunomodulatory agents for the treatment of severe COVID-19 by providing an estimation of their potential effects and identifying potential candidates for these therapies. The objective of this study was to provide an observational estimation of the association between tocilizumab/corticosteroids and outcome in non-intubated patients, specifically in those with data suggestive of a hyperinflammatory state, within a large nationwide clinical cohort of patients with COVID-19 to test the hypothesis that these drugs might be associated with a reduced risk of intubation or death.
Methods
Design, patients and procedures
The SAM-COVID study is a retrospective cohort study nested in the COVID19@Spain cohort (NCT04355871), in which consecutive patients admitted to Spanish hospitals because of COVID-19 (confirmed by PCR in nasopharyngeal swab or lower respiratory tract sample) from February 2nd to March 31st 2020 were included [15]. SAM-COVID was also registered (NCT04382781) before the analysis started.
Adult patients from the COVID19@Spain cohort were eligible for SAM-COVID if presenting on a specific date (day 0) with at least one clinical criterion and one laboratory criterion suggestive of a hyperinflammatory state. Clinical criteria were (a) temperature ≥38°C and (b) increase in oxygen support required to achieve O2 saturation >92%. Laboratory criteria were (a) ferritin >2000 ng/mL or increase >1000 ng/mL since admission, (b) D-dimers >1500 μg/mL (or doubled in 24 h), and (c) IL6 >50 pg/mL. Investigators from the COVID@Spain cohort sites were asked to further review the charts of patients by assessing daily clinical and laboratory data, and to provide additional information. Exclusion criteria were (a) being under mechanical ventilation at day 0, (b) occurrence of the primary endpoint in ≤2 day after day 0 (in order to avoid immortal time bias), (c) written decision to avoid any escalation in medical treatment before day 0, (d) previous use of systemic corticosteroids, tocilizumab, other immunomodulatory drugs or immunoglobulins, and (e) treatment with immunomodulatory drugs other than corticosteroids or tocilizumab, or with immunoglobulins during the first 48 h after day 0. In addition, day 0 must have been before March 31 to assure 21 days of follow-up when the database was locked. Sixty hospitals participated in this study. The database was monitored for missing data and inconsistencies.
Variables
The main endpoint was intubation or death, whichever happened first; follow-up was 21 days. Patients were censored on the last day of contact if discharged before day 21. Secondary outcomes were death, rates of secondary bacterial infection, digestive tract bleeding, and proportion of patients with a score of ≤3 in a seven-point ordinal scale at day 21 (1, not hospitalized; 2, hospitalized without supplemental oxygen; 3, hospitalized with supplemental oxygen; 4, hospitalized and requiring supplemental oxygen with a high nasal flow cannula or non-invasive ventilation; 5, hospitalized and requiring mechanical ventilation; 6, hospitalized and requiring extracorporeal membrane oxygenation (ECMO) or invasive mechanical ventilation with amine support; and 7, death).
The main treatments after day 0 were with tocilizumab, intermediate-high dose corticosteroids (IHDC), pulse dose corticosteroids (PDC), combination therapy with tocilizumab and corticosteroids, or no treatment. In order to try to mimic the exposure as in a randomized trial and intention-to-treat analysis, we classified exposure to treatment arms in the primary analysis as follows: patients were assigned to tocilizumab, IHDC or PDC if administered in ≤2 days after day 0; patients receiving both tocilizumab and corticosteroids in the first 2 days were assigned to the combination treatment group, while patients not receiving any of these drugs were assigned to the non-treatment arm. Patients who started treatment with the above drugs in days 3 and 4 were excluded from the primary analysis, as it would be debatable to which arm they should be assigned, and to avoid immortal time bias; however, these patients were included in a sensitivity analysis in which treatments were considered as time-dependent variables. Corticosteroid treatment was classified as PDC if ≥ 250 mg of methylprednisolone or equivalent per day were administered, or as IHDC otherwise. Other variables collected are included in Table 1 . The data were obtained from the patients' charts. An electronic case report was built using REDCap electronic data capture tools [16]. Missing values were classified as a separate category in the analyses.
Table 1.
Demographic and clinical data of patients. Data are number (proportion) of patients with known exposure to the variable except where specified
| No treatment (n = 344) | Tocilizumab (n = 88) | p valuea | Corticosteroids, intermediate–high dose (n = 117) | p valueb | Corticosteroids, pulse dose (n = 78) | p valuec | Combination therapy (n = 151) | P valued | |
|---|---|---|---|---|---|---|---|---|---|
| Age, median years (IQR) | 69 (59–76) | 66 (56–72) | 0.10 | 71 (62–76) | 0.05 | 71 (60–76) | 0.24 | 65 (58–74) | 0.01 |
| Female gender | 106/343 (30.9) | 24/64 (27.3) | 0.50 | 33/116 (28.4) | 0.61 | 21/78 (26.9) | 0.48 | 42/149 (28.1) | 0.54 |
| Caucasian ethnicity | 316/338 (93.5) | 80/87 (92.0) | 0.61 | 110/113 (97.3) | 0.12 | 75/78 (96.2) | 0.37 | 132/147 (89.8) | 0.15 |
| Comorbidities: | |||||||||
| Cardiac disease | 62/344 (18.0) | 11/88 (12.5) | 0.21 | 21/117 (17.9) | 0.98 | 11/78 (14.1) | 0.40 | 17/150 (11.3) | 0.06 |
| Hypertension | 175/344 (50.9) | 30/88 (34.1) | 0.005 | 61/117 (52.1) | 0.81 | 42/78 (53.8) | 0.63 | 73/151 (48.3) | 0.60 |
| Chronic pulmonary disease | 37 (10.8) | 6/88 (6.8) | 0.27 | 18/117 (15.4) | 0.18 | 9/78 (11.5) | 0.84 | 17/151 (11.3) | 0.86 |
| Severe chronic renal insufficiency | 13 (3.8) | 0/87 (0) | 0.08 | 3/116 (2.6) | 0.77 | 5/78 (6.4) | 0.34 | 1/151 (0.7) | 0.07 |
| Liver cirrhosis | 5/337 (1.5) | 1/87 (1.1) | 1.0 | 1/117 (0.9) | 1.0 | 1/78 (1.3) | 1.0 | 0/151 (0) | 0.33 |
| Malignancy | 15/344 (4.4) | 1/88 (1.1) | 0.09 | 4/117 (3.4) | 0.39 | 4/78 (5.1) | 0.89 | 2/151 (1.3) | 0.07 |
| HIV infection | 0/344 (0) | 1/88 (1.1) | 0.20 | 0/117 (0) | — | 0/78 (0) | — | 0/151 (0) | — |
| Obesity | 39/309 (11.4) | 12/78 (14.3) | 0.54 | 19/111 (17.1) | 0.16 | 5/68 (7.4) | 0.22 | 23/134 (17.2) | 0.20 |
| Diabetes mellitus | 72/344 (20.9) | 15/88 (17.0) | 0.41 | 29/117 (24.8) | 0.38 | 12/78 (15.4) | 0.26 | 26/151 (17.2) | 0.34 |
| Dementia | 14/344 (4.1) | 1/88 (1.1) | 0.18 | 4/117 (2.4) | 0.75 | 0 | 0.08 | 0/151 (0) | 0.01 |
| Admission data: | |||||||||
| Percentage oxygen saturation with room air, mean (SD) | 92.6 (6.0) | 92.1 (6.4) | 0.51 | 91.0 (5.1) | 0.1 | 90.0 (5.6) | 0.001 | 91.8 (5.2) | 0.19 |
| Bilateral infiltrates in thorax radiography | 235/288 (81.6) | 67/78 (85.9) | 0.37 | 91/102 (89.2) | 0.07 | 52/69 (82.6) | 0.84 | 132/131 (87.0) | 0.16 |
| Lymphocytes/μL, mean (SD) | 1069 (1049) | 989 (814) | 0.67 | 1313 (1952) | 0.09 | 1244 (1753) | 0.25 | 948 (520) | 0.17 |
| LDH in U/L, mean (SD) | 388 (158) | 392 (143) | 0.39 | 388 (152) | 0.20 | 385 (119) | 0.39 | 408 (166) | 0.73 |
| C-reactive protein in mg/L, mean (SD) | 112 (101) | 118 (100) | 0.64 | 124 (107) | 0.28 | 118 (99) | 0.63 | 112 (99) | 0.96 |
| Antiviral treatment before day 0: | |||||||||
| Lopinavir/ritonavir | 242/335 (72.2) | 71/87 (81.6) | 0.07 | 86/117 (73.5) | 0.79 | 59/78 (75.6) | 0.49 | 111/151 (73.5) | 0.77 |
| Hydroxychloroquine | 319/335 (94.4) | 86/88 (97.7) | 0.27 | 104/117 (88.9) | 0.04 | 73/78 (93.6) | 0.84 | 144/151 (95.4) | 0.65 |
| Remdesivir | 3/334 (0.9) | 0/88 (0) | 1.0 | 0/117 (0) | 0.52 | 0/78 (0) | 1.0 | 0/151 (0) | 0.55 |
| Azithromycin | 223/337 (66.2) | 65/88 (73.9) | 0.16 | 79/117 (67.5) | 0.79 | 48/78 (61.5) | 0.58 | 116/147 (78.9) | 0.005 |
| Interferon β | 71/332 (21.4) | 24/86 (27.9) | 0.19 | 25/116 (21.6) | 0.97 | 12/78 (15.4) | 0.84 | 27/151 (17.9) | 0.85 |
| Data on day 0: | |||||||||
| Median days of symptoms (IQR) | 8 (6–11) | 10 (8–13) | 0.02 | 10 (7–12) | 0.05 | 6 (9–12) | 0.22 | 11 (8–13) | <0.001 |
| Median days from admission to day 0 (IQR) | 1 (0–4) | 3 (1–5) | 0.001 | 2 (1–4) | 0.08 | 2 (1–5) | 0.21 | 3 (1–5) | 0.001 |
| Fever | 202/344 (58.7) | 42/88 (47.7) | 0.06 | 65/117 (55.6) | 0.54 | 38/78 (48.7) | 0.10 | 77/151 (51.0) | 0.11 |
| Worsening in oxygen requirements | 230/344 (66.9) | 81/88 (92.0) | <0.001 | 87/117 (74.4) | 0.13 | 70/78 (89.7) | <0.001 | 136/151 (90.1) | <0.001 |
| Ferritin >2000 ng/mL | 95/194 (49.0) | 19/59 (32.2) | 0.02 | 34/78 (43.6) | 0.42 | 29/62 (46.8) | 0.76 | 51/100 (51.0) | 0.74 |
| D-dimers >1500 μg/mL | 192/311 (61.7) | 43/82 (52.4) | 0.12 | 55/112 (49.1) | 0.02 | 40/73 (54.8) | 0.27 | 78/140 (55.7) | 0.24 |
| IL6 >50 pg/mL | 100/132 (75.8) | 57/59 (96.6) | <0.001 | 47/53 (88.7) | 0.04 | 26/37 (70.3) | 0.49 | 81/95 (85.3) | 0.07 |
| Oxygen support at day –1: | 0.001 | 0.001 | 0.001 | <0.001 | |||||
| Nasal cannula or mask | 282/340 (82.9) | 57/88 (63.6) | 82/117 (70.1) | 51/78 (65.3) | 71/149 (48.3) | ||||
| Mask with reservoir bag | 46/340 (13.5) | 26/88 (29.2) | 30/117 (25.6) | 25/78 (32.1) | 65/149 (43.0) | ||||
| High-flow nasal cannula | 10/340 (2.9) | 3/88 (3.4) | 1/117 (0.9) | 1/78 (1.3) | 5/149 (3.3) | ||||
| Non-invasive mechanical ventilation | 2/340 (0.6) | 2/88 (3.4) | 4/117 (3.4) | 1/78 (1.3) | 8/149 (5.3) | ||||
| Low-molecular-weight heparin: | |||||||||
| Prophylactic dose | 244/340 (71.8) | 69/88 (80.2) | 0.22 | 93/117 (79.5) | 0.11 | 57/78 (73.1) | 0.88 | 115/150 (76.7) | 0.27 |
| Anticoagulant dose | 36/340 (10.6) | 12/88 (14.0) | 0.44 | 17/117 (14.5) | 0.23 | 16/78 (20.5) | 0.01 | 26/150 (17.3) | 0.03 |
| Immunomodulatory drugs after day 4: | |||||||||
| Corticosteroids, low dose | 39/344 (11.3) | 11/88 (12.5) | 0.71 | — | — | 35/78 (44.9) | <0.001 | — | — |
| Corticosteroids, high dose | 26/344 (7.5) | 6/88 (6.8) | 1.0 | 0/117 (0) | <0.001 | — | — | — | — |
| Tocilizumab | 22/344 (6.5) | — | — | 7/117 (5.9) | 0.87 | 10/78 (12.8) | 0.08 | — | — |
IQR: interquartile range.
For tocilizumab versus no treatment.
For corticosteroids, high-intermediate dose versus no treatment.
For corticosteroids, pulse dose versus no treatment.
For combination versus no treatment.
The study was approved by the University hospitals Virgen Macarena and Virgen del Rocío ethic committee which waived the need to obtain written informed consent due to the observational nature of the study. The study is reported according to STROBE recommendations (Supplementary Material Table S1).
Statistical analysis
Patients classified as receiving no treatment were compared to those treated with tocilizumab, IHDC, PDC, or combination treatment for baseline variables at admission and day 0 using Student t-test or Mann–Whitney U test for continuous variables and χ2 or Fisher test for categorical variables, as appropriate. The association of treatment with time-related endpoints was analysed using Kaplan–Meier curves and Cox regression analysis. The sites were included as a random effect variable in the models. Propensity scores for receiving early treatment with tocilizumab, IHDC, PDC or combination therapy instead of no treatment were calculated by performing non-parsimonious multivariate logistic regression models by including all measured potential predictors for treatment. The ability of the propensity scores to predict the observed data was calculated by the area under the receiver operating characteristic curve (AUROC) with 95% confidence intervals (95%CIs). The propensity scores were used to calculate the inverse probability of treatment weight (IPTW) in Cox analysis, as a confounder and as a matching variable (treated/not treated, 1:2 ratio), using the nearest neighbour method with a tolerance <5%. When the proportional hazards assumptions were not fulfilled for performing Cox regression, logistic regression (conditional if matched analyses) was used. Multivariate models with forward addition of different variables to the model adjusted by the propensity score were also performed, after excluding collinearity. Sensitivity analyses were performed by including patients who started treatments on days 3 and 4, and considering exposure to study drugs as time-dependent variables, counting the days until the first dose of the drug was administered from day 0. All analyses were performed using IBM SPSS Statistics v26 and R.
Results
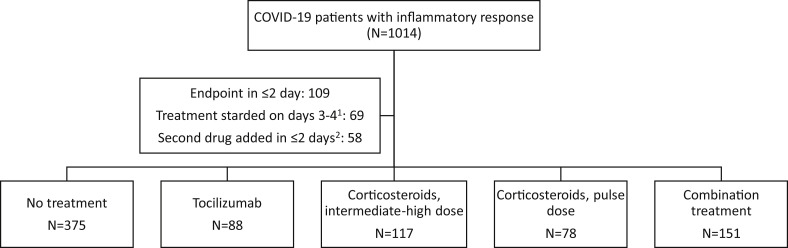
Overall, 1014 eligible patients were identified; 778 were included in the primary analysis (Fig. 1 ), including 344 in the no-treatment arm, 88 treated with tocilizumab, 117 with IHDC, 78 with PDC, and 151 with combination treatment (all received tocilizumab, 77 received IHDC and 74 PDC).
Fig. 1.
Flowchart of patients included in the primary analysis.
The features of the patients are shown in Table 1. Overall, patients in the treatment arms needed a higher level of oxygen support at day 0 than those in the no-treatment arm. The proportion of patients with measured and elevated levels of IL-6 was higher in the tocilizumab and IHDC arms; it was the only laboratory criterion for inclusion in 24.4% of the patients. By contrast, ferritin and D-dimers were less frequently elevated in the tocilizumab and IHDC arms, respectively. Details regarding the drugs dosing are shown in the Supplementary Material Table S2.
The crude outcomes of patients according to treatment arm are shown in Table 2 , and crude Kaplan–Meier curves for the primary endpoint are shown in Fig. 2 . The proportional hazard assumption was not fulfilled in the comparison of IHDC and combination versus no treatment, and logistic regression was used for these comparisons. The propensity score-adjusted associations of treatments for the primary endpoint are shown in Table 3 , which also includes the variables used for the propensity score calculation. The comparison of features of the propensity score-matched patients are shown in the Supplementary Material Table S3. The IPTW-adjusted Kaplan–Meier curves for tocilizumab and PDC are shown in the Supplementary Material Fig. S1. Overall, tocilizumab was associated with lower hazard for the primary endpoint in all adjusted analyses; the estimations for PDC were all on the protective side but were significant only in the IPTW model. IHDC and combination therapy were not associated with significant risk differences. Addition of other variables to the models and sensitivity analyses considering treatments as time-dependent variables provided no significant changes in the estimations.
Table 2.
Crude outcomes of patients in the different treatment arms. Data are number (proportion) of patients with known exposure to the variable except where specified
| No treatment (n = 344) | Tocilizumab (n = 88) | p valuea | Corticosteroids, intermediate–high dose (n = 117) | p valueb | Corticosteroids, pulse dose (n = 78) | p valuec | Combination (n = 151) | p valued | |
|---|---|---|---|---|---|---|---|---|---|
| Primary outcomee | 69/344 (20.1) | 10/88 (11.4) | 0.05 | 27/117 (23.1) | 0.57 | 12/78 (15.4) | 0.28 | 40/151 (26.5) | 0.13 |
| Median follow-up without the endpoint, days (IQR) | 20 (13–21) | 21 (16–21) | 0.01 | 21 (16–21) | 0.56 | 21 (12.21) | 0.55 | 20 (11–21) | 0.87 |
| Scale at day 21 | n = 344 | n = 88 | — | n = 117 | — | n = 78 | — | n = 151 | — |
| 1 | 253 (73.5) | 70 (79.5) | 80 (68.4) | 55 (70.5) | 100 (66.2) | ||||
| 2 | 10 (2.9) | 2 (2.3) | 4 (3.4) | 2 (2.6) | 8 (5.3) | ||||
| 3 | 16 (4.7) | 8 (9.1) | 8 (6.8) | 8 (10.3) | 14 (9.3) | ||||
| 4 | 4 (1.2) | 0 | 0 | 1 (1.3) | 1 (0.7) | ||||
| 5 | 19 (5.5) | 6 (6.8) | 2 (1.7) | 4 (5.1) | 9 (6.0) | ||||
| 6 | 1 (0.3) | 0 | 1 (0.9) | 0 | 19 (6.0) | ||||
| 7 (death) | 41 (11.9) | 2 (2.3) | 0.004 | 22 (18.8) | 0.08 | 8 (10.3) | 0.84 | 19 (12.6) | 0.88 |
| Scale ≤3 | 279 (81.1) | 80 (90.9) | 0.02 | 92 (78.6) | 0.56 | 65 (83.3) | 0.64 | 122 (80.8) | 0.93 |
| Digestive tract bleeding | 2/341 (0.6) | 1/88 (1.1) | 0.49 | 1/115 (1.4) | 1.0 | 1/74 (1.4) | 0.44 | 3/150 (2.0) | 0.16 |
| Secondary bacterial infection | 36/339 (10.3) | 11/88 (12.5) | 0.57 | 10/115 (8.7) | 0.72 | 8/75 (10.7) | 1.0 | 18/150 (12.0) | 0.64 |
IQR, interquartile range.
For tocilizumab versus no treatment.
For corticosteroids, intermediate-high dose versus no treatment.
For corticosteroids, pulse dose versus no treatment.
For combination versus no treatment.
P values obtained by univariate Cox regression except for combination therapy, for which logistic-regression was used.
Fig. 2.
Probability of remaining event-free (intubation or death) according to the different treatments used, in comparison with no treatment (crude analyses). (A) Tocilizumab. (B) Corticosteroids, intermediate-high dose. (C) Corticosteroids, pulse dose. (D) Combination therapy.
Table 3.
Estimation of the association of treatments with the primary endpoint (time until intubation or death) and with mortality in the different models. Adjusted models used specific propensity scoresa for receiving each drug
| Intubation or death | ||
| Tocilizumab versus no treatment | HR (95%CI) | p |
| Crude | 0.52 (0.27–1.01) | 0.05 |
| With propensity score | 0.32 (0.15–0.67) | 0.003 |
| Inverse probability of treatment weights | 0.32 (0.22–0.47) | <0.001 |
| Matched cases | 0.42 (0.19–0.92) | 0.03 |
| Time-dependent variable with propensity score | 0.36 (0.17–0.75) | 0.007 |
| Corticosteroids, intermediate-high dose versus no treatment | OR (95%CI) | p |
| Crude | 1.17 (0.71–1.95) | 0.52 |
| With propensity score | 0.83 (0.48–1.45) | 0.53 |
| Inverse probability of treatment weights | 1.00 (0.72–1.41) | 0.96 |
| Matched cases | 0.80 (0.42–1.41) | 0.50 |
| Time-dependent variable with propensity score | 0.95 (0.59–1.53) | 0.84 |
| Corticosteroids, pulse dose versus no treatment | HR (95%CI) | p |
| Crude | 0.71 (0.38–1.32) | 0.28 |
| With propensity score | 0.71 (0.36–1.38) | 0.31 |
| Inverse probability of treatment weights | 0.61 (0.43–0.86) | 0.006 |
| Matched cases | 0.69 (0.32–1.51) | 0.36 |
| Time-dependent variable with propensity score | 0.79 (0.41–1.53) | 0.50 |
| Combination therapy versus no treatment | OR (95%CI) | p |
| Crude | 1.41 (0.90–2.21) | 0.13 |
| With propensity score | 1.20 (0.71–2.01) | 0.48 |
| Inverse probability of treatment weights | 1.17 (0.86–1.58) | 0.30 |
| Matched cases | 1.71 (0.88–3.31) | 0.10 |
| Time-dependent variable with propensity score | 1.17 (0.74–1.84) | 0.48 |
| DEATH | ||
| Tocilizumab versus no treatment | HR (95%CI) | p |
| Crude | 0.17 (0.04–0.70) | 0.01 |
| With propensity score | 0.12 (0.02–0.56) | 0.007 |
| Inverse probability of treatment weights | 0.07 (0.02–0.17) | <0.001 |
| Matched cases | 0.22 (0.05–0.96) | 0.04 |
| Corticosteroids, intermediate-high dose versus no treatment | HR (95%CI) | p |
| Crude | 1.66 (0.99–2.79) | 0.05 |
| With propensity score | 1.16 (0.66–2.03) | 0.59 |
| Inverse probability of treatment weights | 1.21 (0.62–2.35) | 0.56 |
| Matched cases | 1.02 (0.66–1.58) | 0.90 |
| Corticosteroids, pulse dose versus no treatment | OR (95%CI) | p |
| Crude | 0.80 (0.35–1.81) | 0.59 |
| With propensity score | 0.74 (0.31–1.77) | 0.51 |
| Inverse probability of treatment weights | 0.64 (0.24–1.04) | 0.06 |
| Matched cases | 0.67 (0.24–1.84) | 0.43 |
| Combination therapy versus no treatment | OR (95%CI) | p |
| Crude | 1.03 (0.57–1.85) | 0.90 |
| With propensity score | 1.31 (0.67–2.54) | 0.42 |
| Inverse probability of treatment weights | 1.17 (0.75–1.64) | 0.57 |
| Matched cases | 1.36 (0.58–3.21) | 0.47 |
Propensity scores were calculated including age, gender, ethnicity, comorbidities (cardiac disease, hypertension, chronic pulmonary disease, chronic renal disease, liver cirrhosis, malignancy, diabetes mellitus, obesity, HIV infection), laboratory data (lymphocytes, lactate dehydrogenase, alanine aminotransferase, ferritin, D-dimers, IL-6), previous treatments, radiographic findings, 7-point scale and type of oxygen requirement. Their predictive ability for observed data are 0.79 (95%CI: 0.74–0.85) for tocilizumab, 0.72 (0.68–0.77) for corticosteroids, intermediate-high dose, 0.77 (0.71–0.82) for corticosteroids, pulse dose, and 0.81 (0.77–0.85) for combination therapy.
Regarding the secondary outcomes, the crude estimations are shown in Table 2. The proportion of patients with a score ≤3 on the 7-point scale at day 21 was higher in the tocilizumab arm. No differences were seen in the rates of secondary bacterial infection or gastrointestinal bleeding. Regarding mortality, the Kaplan–Meier curves (crude data) are shown in the Supplementary Material Fig. S2. The adjusted analyses are shown in Table 3, and the IPTW-adjusted Kaplan–Meier curves are in the Supplementary Material Fig. S3. Tocilizumab was associated with a lower hazard of death in all adjusted models. PDC was nearly associated with a lower risk of death only in the IPTW model; neither IHDC nor combination therapy could demonstrate a significant association with mortality (Table 3).
Discussion
In this observational, multicentre, propensity score-adjusted study, tocilizumab was associated with lower hazards of intubation or death in patients with COVID-19 presenting with clinical and laboratory data suggestive of a hyperinflammatory state. The association with PDC was also significant in the analysis with the IPWT but not with other adjustments, although the estimations are informative. On the other hand, we could not find a significant association between IHDC or combination therapy and outcomes.
One of the problems in observational studies is the assignment of patients to treatment arms. In this study we mimicked exposure and intention-to-treat analysis in randomized trials, in which treatments are typically started in ≤2 days, and we excluded patients for whom the endpoint was reached in such a period or patients starting treatment in days 3 and 4, in order to avoid immortal time bias. In fact, sensitivity analysis which included patients treated on days 3–4 and considered exposure to drugs as time-dependent variables did not show different results, suggesting that immortal time bias was not affecting the estimations.
We used a ‘hard’ composite primary outcome including intubation or death because some patients may be candidates for additional medical treatment but not for intubation due to their previous conditions. Anyhow, the results were similar when only mortality or the proportions of patients with a score of ≥3 in the 7-point scale were considered. Our data were not specific for adverse events, and this is a crucial aspect that should be considered in more detail in future studies.
Regarding confounders, we used propensity scores in different ways in order to control for the indication bias. Because the IPTW provides a higher weight to patients treated with the drug of interest when having a lower probability of receiving that drug, the confidence intervals are reduced, while in the case of tocilizumab all models showed a significant association with improved outcomes; it was only with this analysis that PDC showed a significant association. We hypothesize that the lack of significant association with other analysis for PDC might be due to insufficient statistical power.
We found four observational comparative studies with tocilizumab in non-intubated patients with severe COVID-19 pneumonia. In one of them, 32 patients treated with tocilizumab were compared to 33 controls; patients treated with tocilizumab showed numerically lower mortality but the differences were not significant [11]. In another, treatment with tocilizumab (62 patients) was associated with better adjusted survival and a favourable clinical course in comparison with standard treatment (23 patients) [12]. A third study compared 179 patients treated with tocilizumab (88 intravenously) with 365 receiving standard of care in three Italian centres; tocilizumab was associated with a lower adjusted risk of invasive mechanical ventilation or death [13]. Finally, a fourth study found lower mortality in non-intubated patients, but adjusted analyses were not performed [14]. Several randomized trials with tocilizumab are ongoing; a press release by the promoter of the COVACTA trial reported that it did not show superiority over placebo in the primary endpoint (data not published) [17]. However, inclusion criteria in this trial did not consider data suggestive of a hyperinflammatory state [18].
Regarding corticosteroids, recent meta-analyses showed contradictory results [19,20]. In these reviews, the dosing of corticosteroids was not specified. The results from a quasi-experimental study suggested that early administration of 0.5–1 mg/kg of methylprednisolone for 3 days is associated with a protective effect for a composite outcome including admission to ICU, mechanical ventilation or death [21], while a cohort study including 35 propensity score-matched couples of patients with and without corticosteroids (methylprednisolone, 40–50 mg/day) found no significant differences in outcomes [22]. A preliminary report of data from the RECOVERY randomized trial found that dexamethasone 6 mg/day (equivalent to methylprednisolone 30 mg) resulted in lower mortality among patients requiring oxygen or mechanical ventilation; the effect was more prominent in patients under mechanical ventilation [9]. It should be noted that corticosteroids in our study were used at higher doses in most patients, and were started only once the patients had developed a hyperinflammatory state based on clinical and laboratory data. We found no studies with pulse dose corticosteroids. While our results in this group are less clear, we think they support the development of a randomized trial in this clinical situation. We did not find any studies investigating the combination of tocilizumab and corticosteroids; the negative results in our study should be taken with caution since this was a heterogeneous group including different timing and dosing of both drugs. We could not perform more detailed analysis in this group since the numbers of patients in the subgroups were too low.
This study has several limitations. First, control for confounders in any observational study may be incomplete despite all efforts. Second, even though we registered the study design before performing any analysis, the criteria for assignment to study arms were not specified; however, they were decided before the analyses were performed. Third, a wide range of dosing regimens were used in the corticosteroid arms. Fourth, the investigators were not blinded for the exposure; however, we used hard outcomes and included consecutive cases. Fifth, the assessment of adverse events was not complete. And sixth, the study was performed during the first month of the pandemic in Spain; management may have changed afterwards.
The study also has some strengths, including the multicentre participation, the use of specific exposure definitions and advanced analyses for observational studies, and representativeness of real-life patients.
In conclusion, these findings suggest that testing tocilizumab should be prioritized for being tested in randomized trials targeting patients with data suggestive of a hyperinflammatory state, and that pending further evidence, it should be considered with caution in the treatment of this condition if participation in randomized trials is not possible. Additional data are needed for tocilizumab in patients who previously received corticosteroids, which might be the standard of care now. The results for PDC were less consistent but are also encouraging.
Transparency Declaration
IJ has received honoraria for participating in an advisory board from Gilead Sciences, and for educational activities from ViiV. JB has received research grants from AbbVie, Gilead Sciences, Merck, and ViiV, and honoraria for being a speaker or advisory board participation from AbbVie, Gilead Sciences, Janssen, Merck, and ViiV. JRA received fees for participating in an advisory board, being a speaker, and research grant support from Viiv, Janssen, Gilead, MSD, Teva, Alexa and Serono. PR is involved as speaker or advisory board participant for Gilead Sciences, AbbVie and ViiV. JR-B, JP, JC and MY have no conflicts of interest to declare. SAM-COVID was funded by Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III (COV20/01031) co-funded by European Union (ERDF/ESF, “Investing in your future”) and Fundación SEIMC/GeSIDA. In addition, Juan Berenguer, Jesús Rodríguez-Baño, Inmaculada Jarrín, Jordi Carratalá, Jerónimo Pachón, and José R Arribas received funding for research from Plan Nacional de I+D+i 2013-2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia, Innovación y Universidades – co- financed by European Development Regional Fund “A way to achieve Europe”, Operative program Intelligent Growth 2014–2020 through the networks: Spanish AIDS Research Network (RIS) [RD16/0025/0017 (JB), RD16/0025/0018 (JRA), RD16/0025/00XX (IJ)] and Spanish Network for Research in Infectious Diseases (REIPI)[RD16/0016/0001 (JRB), RD16/0016/0005 (JC), and RD16/0016/0009 (JP).
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.08.010.
Contributor Information
Fundación SEIMC-GESIDA:
Esther Aznar Muñoz, Pedro Gil Divasson, Patricia González Muñiz, and Clara Muñoz Aguirre
Hospital Universitario La Paz:
Marta Díaz Menéndez, Fernando de la Calle Prieto, Marta Arsuaga Vicente, Elena Trigo Esteban, Ignacio Pérez Valero, Rosa de Miguel Buckley, Julen Cadiñaños Loidi, Beatriz Diaz Pollan, Luz Martín Carbonero, Juan Carlos Ramos Ramos, Belén Loeches Yagüe, Rocío Montejano Sánchez, Juan González García, and Julio García Rodríguez
Hospital Universitario Gregorio Marañón:
Juan Berenguer, Margarita Ramírez, Isabel Gutiérrez, Francisco Tejerina, Teresa Aldámiz-Echevarría, Cristina Díez, Chiara Fanciulli, Leire Pérez-Latorre, Blanca Pinilla, and Juan Carlos López
Hospital Infanta Leonor:
Ana Such Diaz, Elena Álvaro Alonso, Juan Torres Macho, Guillermo Cuevas Tascon, Eva Jiménez González de Buitrago, Fátima Brañas Baztán, Jorge Valencia de la Rosa, Mario Pérez Butragueño, and Inés Fernández Jiménez
Complejo Hospitalario Virgen de la Salud:
Gemma Muñiz Nicolás, Antonia Sepúlveda Berrocal, Alberto Gato Díez, María Pilar Toledano Sierra, and María Paz García Butenegro
Hospital Universitario Rafael Méndez:
Ana Isabel Peláez Ballesta, Elena Morcillo Rodríguez, Isidoro Fernández Romero, Cristina Peláez Ballesta, and María Isabel Guirado Torrecillas
Hospital Universitario de Cruces:
Josune Goikoetxea Agirre, Elena Bereciartua Bastarrica, Laura Guio Carrión, Regino Rodríguez Álvarez, and Marta Ibarrola Hierro
Hospital de Melilla:
Isabel A. Pérez Hernández, Inés Pérez Zapata, Sergio Román Soto, Mohamed Kallouchi, and Juan Ramón Domínguez Vicent
Hospital San Eloy de Barakaldo:
Rafae Silvariño Fernández, Jon Ugalde Espiñeira, Ainhoa Sanjuan López, Silvia García Martínez, and Mikel Temprano Gogenola
Hospital Universitario Central de Asturias:
Víctor Asensi, Silvia Suárez, Lucia Suárez, Carmen Yllera, and María Rivas-Carmenado
Hospital Universitario Puerto Real:
Alberto Romero-Palacios, Jesús Ruiz Aragón, Patricia Jiménez Aguilar, Ma Luisa Fernández Ávila, and Rosario Castilla Ortiz
Hospital do Salnés:
Vanesa Alende Castro, Cristina Pérez García, Marta Fernández Morales, and María Lorena María Valle Feijoo Begoña Rodríguez Ferreira
Hospital del Mar:
Joan Gómez-Junyent, Judit Villar-García, Inmaculada López-Montesinos, Itziar Arrieta-Aldea, and Abora Rial-Villavecchia
Hospital Virgen de la Arrixaca:
Elisa García Vázquez, Aychel Elena Roura Piloto, Encarnación Moral Escudero, Alicia Hernández Torres, and Helena Albendín Iglesias
Hospital Clínico San Cecilio:
David Vinuesa García, Clara Martínez Montes, Francisco Javier De la Hera Fernández, Francisco Anguita Santos, and Andrés Ruiz Sancho
Parc Sanitari Sant Joan de Déu:
Vicens Díaz de Brito Fernández, Montserrat Sanmarti Vilamala, Sergio España Cueto, Daniel Molina Morant, and Araceli González-Cuevas
Hospital Josep Trueta:
Joel Elías Chara Cervantes, Guillem Policarpo Torres, Meritxell Ortega Montoliu, Mònica Angerri Nadal, and Ariadna De Genover Gil
Hospital Dos De Maig - Consorci Sanitari Integral:
Eleni Patera, Rita Godoy Lorenzo, Evangelia Anna María Zioga, Virginia Isern Fernández, and Carlos Enrique Sabbagh Fajardo
Hospital Clínico Universitario de Valencia:
Ana Ferrer Ribera, Carlos Bea Serrano, Rosa Oltra Sempere, Sara Vela Bernal, and Paloma Albiol Viñals
Complejo Asistencial de Ávila:
Miguel Pedromingo Kus, María Ángeles Garcinuño, Silvana Fiorante, Sergio Pérez Pinto, and Alexandra de la Vega
Hospital Universitario Marqués de Valdecilla:
María Carmen Fariñas Álvarez, Claudia González Rico, Francisco Arnaiz de las Revillas, Teresa Giménez, and Jorge Calvo
Hospital de Barcelona SCIAS:
Yolanda Meije Castillo, Alejandra Duarte Borges, Júlia Pareja Coca, Mercedes Clemente Presas, and Xavier Sanz Salvador
Hospital Álvaro Cunqueiro:
Ma Teresa Pérez Rodríguez, Adrián Sousa, Alexandre Pérez González, Rebeca Longueira, and Alejandro Araujo
Hospital Universitario Severo Ochoa:
Blanca Alonso Martínez, Laura García Escudero, Sara Lidia Kamel Rey, David Roa Alonso, and Juan Pablo Avilés Parra
Hospital CIMA-Sanitas:
Iván Pelegrín Senent, Rosana Rouco Esteves Marques, Laia Raich Montiu, Jessica Souto Higueras, and Manuel Alejandro Gálvez Bobadilla
Hospital La Inmaculada:
Jorge Parra Ruiz, Violeta Ramos Sesma, Sara Velasco Fuentes, Laura García Pereña, and Alfonso Lluna Carrascosa
Hospital de Guadalajara:
Sergio Gilaberte Reyzábal, Mónica Liébana Gómez, Juan Salillas Hernando, Alberto Serrano Martínez, and Miguel Torralba González de Suso
Hospital Universitario Infanta Sofia:
Patricia Martínez Martín, Isabel Rábago Lorite, Patricia González-Ruano Pérez, and Beatriz Pérez-Monte Mínguez
Hospital Comarcal de Blanes:
Ángeles García Flores and Pere Comas Casanova
Hospital Universitario de Gran Canaria Dr Negrín:
Andrea Martín Plata, Sergio Manuel Santana Báez, Oscar Sanz Peláez, Karim Mohamed Ramírez, and José María Robaina Bordón
Hospital Son Espases:
Helem Haydeé Vílchez Rueda, Melchor Riera Jaume, Gemma Mut Ramon, Meritxell Gavalda Manso, and Lluis Planas Bibiloni
Complejo Hospitalario Universitario A Coruña:
Laura Castelo Corral, Lucía Ramos Merino, Efrén Sánchez Vidal, María Rodríguez Mayo, and Enrique Míguez Rey
Hospital Costa del Sol:
José M. García de Lomas Guerrero, Javier De la Torre Lima, Ana Correa Ruiz, Fernando Fernández Sánchez, and Nicolás Jiménez-García
Hospital Clínico Universitario Lozano Blesa:
José Luis Sierra-Monzón, Borja Gracia-Tello, María Hernández-Bonaga, Galadriel Pellejero, and Marta Asín-Corrochano
Hospital Mutua de Terrassa:
Lucia Boix Palop, Esther Calbo, Cristina Badía, Beatriz Dietl, and Gómez Lucía
Hospital Universitario Virgen Macarena:
Ángel Domínguez-Castellano, María José Ríos-Villegas, María D. del Toro, Zaira R. Palacios Baena, Elena Salamanca-Rivera, Elena Marín, Virginia Almadana, Salvador Pérez-Galera, and Luisa González-Iglesias
Hospital Universitari de Bellvitge:
Gabriela Abelenda-Alonso, Claudia Álvarez-Pouso, Francesc Escrihuela, Carlota Gudiol, Laia Lorenzo-Esteller, Jordi Niubó, Daniel Podzamczer, Miquel Pujol, and Alexander Rombauts
Hospital Universitario y Politécnico La Fe:
Miguel Salvert Lletí, Ricardo Gil Sánchez, Marta Jiménez Escrig, Laura Parra Gómez, and Mariona Tasias Pitarch
Hospital de Sabadell (Parc Tauli):
Marta Navarro Vilasaró, María Luisa Machado Sicilia, Aina Gomila Grange, and Sonia Calzado Isbert
Hospital Fundación Jiménez Díaz:
Nerea Carrasco Antón, Elizabet Petkova-Saiz, Alfonso Cabello Úbeda, Miguel Górgolas Hernández-Mora, and Olga Sánchez-Pernaute
Hospital Clínico Universitario de Valladolid:
Carlos Dueñas Gutiérrez, Javier Martin Guerra, José Javier Castrodeza Sanz, Virginia Fernández Espinilla, and Laura Rodríguez Fernández
Hospital Son Llatzer:
Juan González-Moreno, Aroa Villoslada Gelabert, María Antonia Ribot Sanso, María Victoria Fernández-Baca, and Almudena Hernández Milian
Hospital Universitario de Álava:
Miguel Ángel Morán Rodríguez, Zuriñe Ortiz de Zárate Ibarra, José Joaquin Portu Zapirain, Ester Saez de Adana Arroniz, and Juan Carlos Gainzarain Arana
Complejo Hospitalario Universitario Santa Lucía:
Olga Meca Birlanga, Ma Jesús del Amor Espín, Montserrat Viqueira González, Josefina García García, and Onofre Martínez Madrid
Hospital General Universitario Reina Sofía:
Enrique Bernal Morell, Antonia Alcaraz, Ángeles Muñoz, Ignacio Pina, and Vicente de la Rosa
Complejo Hospitalario Universitario de Ferrol:
Tamara Caínzos Romero, Sabela Sánchez Trigo, Ana Isabel Mariño Callejo, Hortensia Álvarez Díaz, and Nieves Valcarce Pardeiro
Hospital Universitario los Arcos del Mar Menor:
Adriana Sánchez Serrano, Diana Piñar Cabezos, Eva Pilar García Villalba, Carmen Aguayo Jiménez, and María Ruíz Campuzano
Hospital Universitario de Jerez:
Virginia Naranjo Velasco, Marta Santos Peña, Juan Mora Delgado, Israel Sevilla Moreno, and Cristina Lojo Cruz
Hospital de Donostia:
Xabier Kortajarena Urkola, José Antonio Iribarren Loyarte, María Jesús Bustinduy Odriozola, Maialen Ibarguren Pinilla, and Ignacio Álvarez Rodríguez
Hospital Juan Ramón Jiménez:
Francisco Javier Martínez Marcos, Francisco Javier Rodríguez Gómez, Isabel Asschert Agüero, Francisco Muñoz Beamud, and Antonio José Ruiz Reina
Hospital Vega Baja:
Jara Llenas-García, Inmaculada González-Cuello, Elena Hellín-Valiente, Esther Martínez Birlanga, and José Manuel Tafalla Torres
Hospital Puerta de Hierro:
Jorge Calderón Parra, Gabriela Escudero López, Isabel Gutiérrez Martín, Ane Andrés Eisenhofer, and Sonia García Prieto
Hospital Universitario de Getafe:
Raquel Álvarez Franco, Daniel Roger Zapata, Blanca Martínez Cifre, Elena Aranda Rife, and Irene Martín Rubio
Hospital General de la Palma:
André Barbosa Ventura, Javier Garrido, Concepción Gonzalo, Iván Piñero, and Nieves de la Cruz Felipe
Fundación Hospital de Calahorra:
Eva Talavera García, Marta Lamata Subero, Paula Mendoza Roy, María Soledad García de Carlos, and Justo Lajusticia Aisa
Hospital Alto Deba:
Lorea Arteche Eguizabal, Ainhoa Urrutia Losada, Saioa Domingo Echaburu, Pedro Ángel Cuadros Tito, and Gurutz Orbe Narváez
Hospital Universitario de Jaén:
Ma del Carmen Liébana Martos, Carolina Roldán Fontana, Carmen Herrero Rodríguez, Gaspar Duro Ruiz, and Santiago Pérez Parra
Hospital de Palamós:
Arantzazu Mera Fidalgo, Miquel Hortos Alsina, Ana Alberich Conesa, and Lourdes Bladé Vidal
Hospital Universitario de Valme:
Nicolás Merchante Gutiérrez, Eva León Jiménez, Reinaldo Espíndola Gómez, María Erostarbe Gallardo, and Pedro Martínez Pérez-Crespo
Hospital Universitario Virgen del Rocío:
José Miguel Cisneros, Manuela Aguilar-Guisado, Teresa Aldabó, Claudio Bueno, Elisa Cordero-Matía, Ana Escoresca, Carmen Infante, Martín Guillermo, and Sonsoles Salto
Hospital Universitario Ramón y Cajal:
Francesca Gioia, Pilar Vizcarra, Jesús Fortún Abete, Pilar Martín Dávila, and Santiago Moreno Guillén
Hospital Universitario San Pedro:
José A. Oteo Revuelta, Concepción García-García, Paula Santibañez Sáenz, Cristina Cervera Acedo, and José M. Azcona Gutiérrez
Hospital Regional de Málaga:
José María Reguera Iglesias, Antonio Plata Ciezar, Lucia Valiente de Santis, Beatriz Sobrino Diaz, and Juan Diego Ruiz Mesa
Appendix.
Author contributions
Study conception and design: JR-B, JP, JC, PR, IJ, MY, JRA, JB. Acquisition, analyses and interpretation of data: JR-B, JP, JC, PR, IJ, MY, JRA, JB. Manuscript draft: JRB, JB. Manuscript critical revision: JP, JC, PR, IJ, MY, JRA. Other SAM-COVID Study Group members: Fundación SEIMC-GESIDA: Aznar Muñoz, Esther; Gil Divasson, Pedro; González Muñiz, Patricia; Muñoz Aguirre, Clara. Hospital Universitario La Paz: Díaz Menéndez, Marta, de la Calle Prieto, Fernando; Arsuaga Vicente, Marta; Trigo Esteban, Elena; Pérez Valero, Ignacio; de Miguel Buckley, Rosa; Cadiñaños Loidi, Julen; Diaz Pollan, Beatriz; Martín Carbonero, Luz; Ramos Ramos, Juan Carlos; Loeches Yagüe, Belén; Montejano Sánchez, Rocío; González García, Juan; García Rodríguez, Julio. Hospital Universitario Gregorio Marañón: Berenguer, Juan; Ramírez, Margarita; Gutiérrez, Isabel; Tejerina, Francisco; Aldámiz-Echevarría, Teresa; Díez, Cristina; Fanciulli, Chiara; Pérez-Latorre, Leire; Pinilla, Blanca; López, ; Juan Carlos. Hospital Infanta Leonor: Such Diaz, Ana; Álvaro Alonso, Elena; Torres Macho, Juan; Cuevas Tascón, Guillermo; Jiménez González de Buitrago, Eva; Brañas Baztán, Fátima; Valencia De la Rosa, Jorge; Pérez Butragueño, Mario; Fernández Jiménez, Inés. Complejo Hospitalario Virgen de la Salud: Muñiz Nicolás, Gemma; Sepúlveda Berrocal, Antonia; Gato Díez, Alberto; Toledano Sierra, María Pilar; García Butenegro, María Paz. Hospital Universitario Rafael Méndez: Peláez Ballesta, Ana I.; Morcillo Rodríguez, Elena; Fernández Romero, Isidoro; Peláez Ballesta, Cristina; Guirado Torrecillas, María Isabel, Hospital Universitario de Cruces: Goikoetxea Agirre, Josune; Bereciartua Bastarrica, Elena; Guio Carrión, Laura; Rodríguez Álvarez, Regino; Ibarrola Hierro, Marta. Hospital de Melilla: Pérez-Hernández, Isabel A.; Pérez Zapata, Inés; Román Soto, Sergio; Kallouchi, Mohamed; Domínguez Vicent, Juan Ramón. Hospital San Eloy de Barakaldo: Silvariño Fernández, Rafael; Ugalde Espiñeira, Jon; Sanjuan López, Ainhoa; García Martínez, Silvia; Temprano Gogenola, Mikel; Hospital Universitario Central de Asturias: Asensi, Víctor; Suárez, Silvia; Suárez, Lucia; Yllera, Carmen; Rivas-Carmenado, María. Hospital Universitario Puerto Real: Romero-Palacios, Alberto; Ruiz Aragón, Jesús; Jiménez Aguilar, Patricia; Fernández Ávila, Mª Luisa; Castilla Ortiz, Rosario. Hospital do Salnés: Alende Castro, Vanesa; Pérez García, Cristina; Fernández Morales, Marta; Valle Feijoo, María Begoña; Rodríguez Ferreira, Lorena María. Hospital del Mar: Gómez-Junyent, Joan; Villar-García, Judit; López-Montesinos, Inmaculada; Arrieta-Aldea, Itziar; Rial-Villavecchia, Abora. Hospital Virgen de la Arrixaca: García Vázquez, Elisa; Roura Piloto, Aychel Elena; Moral Escudero, Encarnación; Hernández Torres, Alicia; Albendín Iglesias, Helena. Hospital Clínico San Cecilio: Vinuesa García, David; Martínez Montes, Clara; De la Hera Fernández, Francisco Javier; Anguita Santos, Francisco; Ruiz Sancho, Andrés. Parc Sanitari Sant Joan de Déu: Diaz-Brito, Vicens; Sanmarti Vilamala, Montserrat; España Cueto, Sergio; Molina Morant, Daniel; González-Cuevas, Araceli. Hospital Josep Trueta: Chara Cervantes, Joel Elías; Policarpo Torres, Guillem; Ortega Montoliu, Meritxell; Angerri Nadal, Mònica; De Genover Gil, Ariadna. Hospital Dos De Maig - Consorci Sanitari Integral: Patera, Eleni; Godoy Lorenzo, Rita; Zioga, Evangelia Anna María; Isern Fernández, Virginia; Sabbagh Fajardo, Carlos Enrique. Hospital Clínico Universitario de Valencia: Ferrer Ribera, Ana; Bea Serrana, Carlos; Oltra Sempere, Rosa; Vela Bernal, Sara; Albiol Viñals, Paloma. Complejo Asistencial de ÁVILA: Pedromingo Kus, Miguel; Garcinuño, María Ángeles; Fiorante, Silvana; Pérez Pinto, Sergio; de la Vega, Alexandra. Hospital Universitario Marqués de Valdecilla: Fariñas, María Carmen; González Rico, Claudia; Arnaiz de las Revillas, Francisco; Giménez, Teresa; Calvo, Jorge; Hospital de Barcelona SCIAS: Meije Castillo, Yolanda; Duarte Borges, Alejandra; Pareja Coca, Júlia; Clemente Presas, Mercedes; Sanz Salvador, Xavier. Hospital Álvaro Cunqueiro: Pérez Rodríguez, Mª Teresa; Sousa, Adrián; Pérez González, Alexandre; Longueira, Rebeca; Araujo, Alejandro. Hospital Universitario Severo Ochoa: Alonso Martínez, Blanca; García Escudero, Laura; Lidia Kamel Rey, Sara; Roa Alonso, David; Avilés Parra, Juan Pablo; Hospital CIMA-Sanitas: Pelegrín, Iván; Rouco Esteves Marques, Rosana; Raich Montiu, Laia; Souto Higueras, Jessica; Gálvez Bobadilla, Manuel Alejandro. Hospital La Inmaculada: Parra Ruiz, Jorge; Ramos Sesma, Violeta; Velasco Fuentes, Sara; García Pereña, Laura; Lluna Carrascosa, Alfonso; Hospital de Guadalajara: Gilaberte Reyzábal, Sergio; Liébana Gómez, Mónica; Salillas Hernando, Juan; Serrano Martínez, Alberto; Torralba González de Suso, Miguel. Hospital Universitario Infanta Sofia: Martínez Martín, Patricia; Rábago Lorite, Isabel; González-Ruano Pérez, Patricia; Pérez-Monte Mínguez, Beatriz. Hospital Comarcal de Blanes: García Flores, Ángeles; Comas Casanova, Pere. Hospital Universitario de Gran Canaria Dr Negrín: Martín Plata, Andrea; Santana Báez, Sergio Manuel; Sanz Peláez, Oscar; Mohamed Ramírez, Karim; Robaina Bordón, José María. Hospital Son Espases: Vílchez Rueda, Helem Haydeé; Riera Jaume, Melchor; Mut Ramon, Gemma; Gavalda Manso, Meritxell; Planas Bibiloni, Lluis. Complejo Hospitalario Universitario A Coruña: Castelo Corral, Laura; Ramos Merino, Lucía; Sánchez Vidal, Efrén; Rodríguez Mayo, María; Míguez Rey, Enrique. Hospital Costa del Sol: García de Lomas Guerrero, José M.; De la Torre Lima, Javier; Correa Ruiz, Ana; Fernández Sánchez, Fernando; Jiménez-García, Nicolás. Hospital Clínico Universitario Lozano Blesa: Sierra-Monzón, José Luis; Gracia-Tello, Borja; Hernández-Bonaga, María; Pellejero, Galadriel; Asín-Corrochano, Marta. Hospital Mutua de Terrassa: Boix Palop, Lucia; Calbo, Esther; Badía, Cristina; Dietl, Beatriz; Gómez, Lucía. Hospital Universitario Virgen Macarena: Domínguez-Castellano, Ángel; Ríos-Villegas, María José; del Toro, María D.; Palacios Baena, Zaira R; Salamanca-Rivera, Elena; Marín Elena; Almadana, Virginia; Pérez-Galera, Salvador; González-Iglesias, Luisa. Hospital Universitari de Bellvitge: Abelenda-Alonso, Gabriela; Álvarez-Pouso, Claudia; Escrihuela, Francesc; Gudiol, Carlota; Lorenzo-Esteller, Laia; Niubó, Jordi; Podzamczer, Daniel; Pujol, Miquel; Rombauts, Alexander. Hospital Universitario y Politécnico La Fe: Salvert Lletí, Miguel; Gil Sánchez, Ricardo; Jiménez Escrig, Marta; Parra Gómez, Laura; Tasias Pitarch, Mariona. Hospital de Sabadell (Parc Tauli): Navarro Vilasaró, Marta; Machado Sicilia, María Luisa; Gomila Grange, Aina; Calzado Isbert, Sonia. Hospital Fundación Jiménez Díaz: Carrasco Antón, Nerea; Petkova-Saiz, Elizabet; Cabello Úbeda, Alfonso; Górgolas Hernández-Mora, Miguel; Sánchez-Pernaute, Olga. Hospital Clínico Universitario de Valladolid: Dueñas Gutiérrez, Carlos; Martin Guerra, Javier; Castrodeza Sanz, José Javier; Fernández Espinilla, Virginia; Rodríguez Fernández, Laura. Hospital Son Llatzer: González-Moreno, Juan; Villoslada Gelabert, Aroa; Ribot Sanso, María Antonia; Fernández-Baca, María Victoria; Hernández Milian, Almudena. Hospital Universitario de Álava: Morán Rodríguez, Miguel Ángel; Ortiz de Zárate Ibarra, Zuriñe; Portu Zapirain, José Joaquin; Saez de Adana Arroniz, Ester; Gainzarain Arana, Juan Carlos. Complejo Hospitalario Universitario Santa Lucía: Meca Birlanga, Olga; del Amor Espín, Mª Jesús; Viqueira González, Montserrat; García García, Josefina; Martínez Madrid, Onofre. Hospital General Universitario Reina Sofía: Bernal Morell, Enrique; Alcaraz, Antonia; Muñoz, Ángeles; Pina, Ignacio; de la Rosa, Vicente. Complejo Hospitalario Universitario de Ferrol: Caínzos Romero, Tamara; Sánchez Trigo, Sabela; Mariño Callejo, Ana Isabel; Álvarez Díaz, Hortensia; Valcarce Pardeiro, Nieves. Hospital Universitario los Arcos del Mar Menor: Sánchez Serrano, Adriana; Piñar Cabezos, Diana; García Villalba, Eva Pilar; Aguayo Jiménez, Carmen; Ruíz Campuzano, María. Hospital Universitario de Jerez: Naranjo Velasco, Virginia; Santos Peña, Marta; Mora Delgado, Juan; Sevilla Moreno, Israel; Lojo Cruz, Cristina. Hospital de Donostia: Kortajarena Urkola, Xabier; Iribarren Loyarte, José Antonio; Bustinduy Odriozola, María Jesús; Ibarguren Pinilla, Maialen; Álvarez Rodríguez, Ignacio. Hospital Juan Ramón Jiménez: Martínez Marcos, Francisco Javier; Rodríguez Gómez, Francisco Javier; Asschert Agüero, Isabel; Muñoz Beamud, Francisco; Ruiz Reina, Antonio José. Hospital Vega Baja: Llenas-García, Jara; González-Cuello, Inmaculada; Hellín-Valiente, Elena; Martínez Birlanga, Esther; Tafalla Torres, José Manuel. Hospital Puerta de Hierro: Calderón Parra, Jorge; Escudero López, Gabriela; Gutiérrez Martín, Isabel; Andrés Eisenhofer, Ane; García Prieto, Sonia. Hospital Universitario de Getafe: Álvarez Franco, Raquel; Roger Zapata, Daniel; Martínez Cifre, Blanca; Aranda Rife, Elena; Martín Rubio, Irene. Hospital General de la Palma: Barbosa Ventura, André; Garrido, Javier; Gonzalo, Concepción; Piñero, Iván; de la Cruz Felipe, Nieves. Fundación Hospital de Calahorra: Talavera García, Eva; Lamata Subero, Marta; Mendoza Roy, Paula; García de Carlos, María Soledad; Lajusticia Aisa, Justo. Hospital Alto Deba: Arteche Eguizabal, Lorea; Urrutia Losada, Ainhoa; Domingo Echaburu, Saioa; Cuadros Tito, Pedro Ángel; Orbe Narváez, Gurutz. Hospital Universitario de Jaén: Liébana Martos, Mª del Carmen; Roldán Fontana, Carolina; Herrero Rodríguez, Carmen; Duro Ruiz, Gaspar; Pérez Parra, Santiago. Hospital de Palamós: Mera Fidalgo, Arantzazu; Hortos Alsina, Miquel; Alberich Conesa, Ana; Bladé Vidal, Lourdes. Hospital Universitario de Valme: Merchante Gutiérrez, Nicolás; León Jiménez, Eva; Espíndola Gómez, Reinaldo; Erostarbe Gallardo, María; Martínez Pérez-Crespo, Pedro. Hospital Universitario Virgen del Rocío: Cisneros, José Miguel; Aguilar-Guisado, Manuela; Aldabó, Teresa; Bueno, Claudio; Cordero-Matía, Elisa; Escoresca, Ana; Infante, Carmen; Martín, Guillermo; Salto, Sonsoles. Hospital Universitario Ramón y Cajal: Gioia, Francesca; Vizcarra, Pilar; Fortún Abete, Jesús; Martín Dávila, Pilar; Moreno Guillén, Santiago. Hospital Universitario San Pedro: Oteo Revuelta, José A; García-García, Concepción; Santibañez Sáenz, Paula; Cervera Acedo, Cristina; Azcona Gutiérrez, José M. Hospital Regional de Málaga: Reguera Iglesias, José María; Plata Ciezar, Antonio; Valiente de Santis, Lucia; Sobrino Diaz, Beatriz; Ruiz Mesa, Juan Diego.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Liang W.H., Ou C.Q. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19 – preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. Published online May 22. [DOI] [PubMed] [Google Scholar]
- 7.Bhimraj A., Morgan R.L., Shumaker A.H., Lavergne V., Baden L., Cheng V.C. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa478. Published online April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.COVID-19 Treatment Guidelines Panel . National Institutes of Health; 2020. Coronavirus disease 2019 (COVID-19) treatment guidelines.https://www.covid19treatmentguidelines.nih.gov/ [PubMed] [Google Scholar]
- 9.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. Published online July 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le R.Q., Li L., Yuan W., Shord S.S., Nie L., Habtemariam B.A. FDA Approval Summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. 2018;23:943–947. doi: 10.1634/theoncologist.2018-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campochiaro C., Della-Torre E., Cavalli G., De Luca G., Ripa M., Boffini N. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capra R., De Rossi N., Mattioli F., Romanelli G., Scarpazza C., Sormani M.P. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur J Intern Med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guaraldi G., Meschiari M., Cozzi-Lepri A., Milic J., Tonelli R., Menozzi M. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30173-9. Published online June 24, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rojas-Marte G.R., Khalid M., Mukhtar O., Hashmi A.T., Waheed M.A., Ehrlich S. Outcomes in patients with severe COVID-19 disease treated with tocilizumab—a case-controlled study. QJM. 2020 doi: 10.1093/qjmed/hcaa206. Published online Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berenguer J., Ryan P., Rodríguez-Baño J., Jarrín I., Carratalà J., Pachón J. Characteristics and predictors of death among 4,035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020;26:1525–1536. doi: 10.1016/j.cmi.2020.07.024. Published online August 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anonymous Roche provides an update on the phase III COVACTA trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 associated pneumonia. https://www.roche.com/investors/updates/inv-update-2020-07-29.htm
- 18.Hoffmann–La Roche . 2020. A study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia (COVACTA)https://clinicaltrials.gov/ct2/show/NCT04320615 [Google Scholar]
- 19.Ye Z., Wang Y., Colunga-Lozano L.E., Prasad M., Tangamornsuksan W., Rochwerg B. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020;192:E756–E767. doi: 10.1503/cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J.J.Y., Lee K.S., Ang L.W., Leo Y.S., Young B.E. Risk factors of severe disease and efficacy of treatment in patients infected with COVID-19: a systematic review, meta-analysis and meta-regression analysis. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa576. Published online May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadel R., Morrison A.R., Vahia A., Smith Z.R., Chaudhry Z., Bhargava P. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa601. Published online May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan M., Xu X., Xia D., Tao Z., Yin W., Tan W. Effects of corticosteroid treatment for non-severe COVID-19 pneumonia: a propensity score-based analysis. Shock. 2020 doi: 10.1097/SHK.0000000000001574. Published online June 2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.