Highlights
-
•
During the COVID-19 outbreak, antibiotic use has increased, especially respiratory antibiotics.
-
•
Healthcare resources are diverted to containment and management of COVID-19 cases, but the efforts of antibiotic stewardship programmes (ASPs) must run in parallel to mitigate antibiotic misuse and antimicrobial resistance.
-
•
ASPs must be operationally adaptable, and possess robust surveillance systems that detect subtle changes in prescribing and resistance trends.
-
•
This is an opportunity to execute a syndromic approach to guide antibiotic prescribing.
-
•
Continued engagement and education of prescribers to ensure appropriate prescription of antibiotics is important.
Keywords: COVID-19, Antimicrobial stewardship, ASP, ASP interventions
Abstract
Healthcare resources are being diverted for the containment and control of coronavirus disease 2019 (COVID-19). During this outbreak, it is cautioned that antibiotic misuse may be increased, especially for respiratory tract infections. With stewardship interventions, the duration of antibiotic therapy and length of stay of hospitalized patients can be reduced significantly. Antibiotic stewardship programmes should continually engage and educate prescribers to mitigate antibiotic misuse during the COVID-19 pandemic.
Brief report
In early December 2019, reports of coronavirus disease 2019 (COVID-19) first emerged in Wuhan, China [1]. On 23 January 2020, the first reported case of COVID-19 in Singapore was identified at Singapore General Hospital (SGH) [2]. The Ministry of Health, Singapore raised the Disease Outbreak Response System Condition (DORSCON) alert to orange (the second highest level of alert) on 7 February 2020, and implemented various measures nationwide, including border control, quarantine of contacts of COVID-19 cases or those who had travelled to affected areas, temperature screening, and restricting the number of hospital visitors. As of 26 July 2020, Singapore has had 50 369 confirmed cases [1].
In light of increasing numbers of cases of COVID-19 locally and internationally [2], SGH faced competing manpower needs arising from health crisis management, leading to uncertainty over manpower allocation of non-direct patient care personnel (e.g. antibiotic stewardship practitioners). This article aims to describe the impact of COVID-19 on antibiotic use and the role of an antibiotic stewardship programme (ASP) at SGH, an 1800-bed, tertiary care hospital. The multi-disciplinary ASP comprises nine trained infectious diseases (ID) pharmacists and three ID physicians. The pharmacy team performs daily electronic audits of selected broad-spectrum antibiotic prescriptions (carbapenems, piperacillin-tazobactam, ciprofloxacin and levofloxacin) for appropriateness in terms of indication, route, duration and choice, and intervenes where appropriate. The audited prescriptions of more complex cases are reviewed daily with the attending ID physician who is rostered for the day [3].
When Singapore raised the DORSCON alert to orange, SGH mandated cancellation of elective and non-urgent procedures/surgeries to ring-fence more beds for dedicated respiratory and pneumonia wards in preparation for a larger-scale outbreak. Negative pressure rooms at SGH are used solely for cases and suspected cases of COVID-19. Operationally, face-to-face meetings were discouraged within SGH, and the workforce was generally divided into small teams (e.g. five to 10 members per team) as part of the hospital's business contingency plan. Accordingly, the ASP team was divided into two pharmacy teams so that the ASP would still be operationally functional with one team if the other team had to be quarantined. Instead of face-to-face meetings, daily ASP meetings with the ID physician were conducted via teleconferencing.
The first case of COVID-19 was reported in Singapore on 23 January 2020. As the number of cases increased, a stay-at-home order and ‘cordon sanitaire’ – termed ‘circuit breaker’ – was implemented as a preventative measure on 7 April 2020 [2]. For the purpose of this article, a comparison was made between antibiotic use pre-COVID-19 (1 February–30 April 2019) and antibiotic use during the acceleration phase of the pandemic (1 February–30 April 2020).
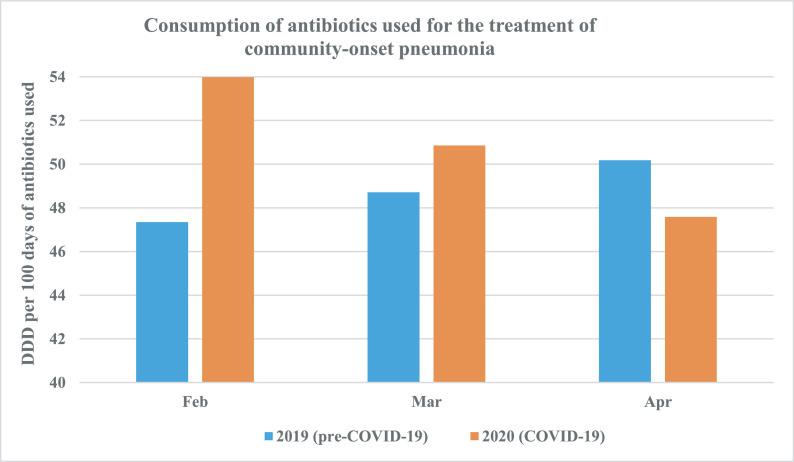
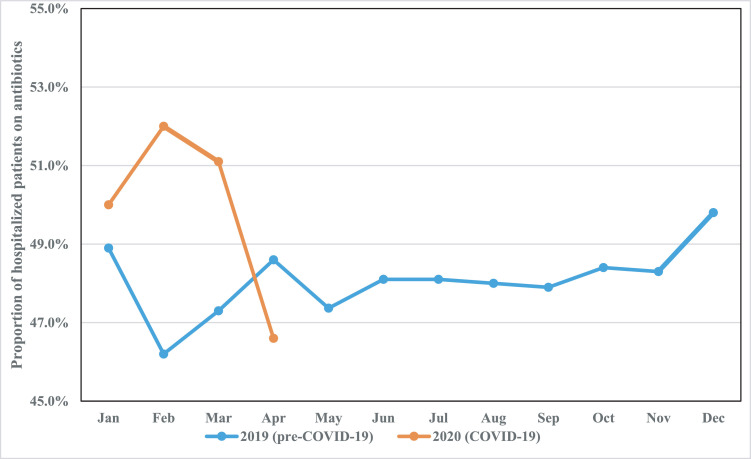
During the designated COVID-19 period, SGH saw a 16.8% year-on-year reduction in the overall number of hospital admissions (from 19 589 to 16 300). Bed occupancy was also reduced, from 130 597 bed-days pre-COVID-19 to 113 449 bed-days during COVID-19. However, there was a 25.5% increase in the utilization of broad-spectrum antibiotics such as cefepime, piperacillin/tazobactam, carbapenems and vancomycin where defined daily doses (DDD) rose from 14.92 DDD per 100 bed-days to 18.72 DDD per 100 bed-days. Similarly, the utilization of antibiotics for the treatment of community-onset pneumonia increased from 48.74 DDD per 100 bed-days to 50.81 DDD per bed-days. This increase was most marked in February 2020 during the onset of the COVID-19 pandemic, with a notable rise in month-on-month DDD of ceftriaxone, co-amoxiclav, levofloxacin, moxifloxacin, azithromycin and clarithromycin from 47.4 DDD per 100 bed-days to 54.0 DDD per 100 bed-days (see Fig. 1 ). The average monthly proportion of patients on antibiotics was also correspondingly higher (47.4% vs. 49.9%) during the COVID-19 pandemic compared with the same period of time the year before, with the highest proportion documented at the onset (see Fig. 2 ). However, as the pandemic evolved, antibiotic use gradually declined, and the authors attribute this to: (a) increased understanding of COVID-19 as international data emerged, and medical teams were more judicious with antibiotic use thereafter (per communication with Dr S.J. Chung); (b) further restrictions in elective admissions with implementation of ‘circuit breaker’ measures in April 2020; and (c) a change in demographics of admissions for respiratory viral illness, where a significant proportion were young able-bodied foreign dormitory workers with mild COVID-19, who were paucisymptomatic or asymptomatic [1], and antibiotics were not prescribed in this group of patients.
Fig. 1.
Comparison of the consumption of antibiotics used for the treatment of community-onset pneumonia pre-coronavirus disease 2019 (COVID-19) (blue bars) and during COVID-19 (orange bars). Defined daily doses (DDD) of antibiotics (ceftriaxone, co-amoxiclav, levofloxacin, moxifloxacin, azithromycin and clarithromycin) were higher in February and March during the onset of the COVID-19 pandemic.
Fig. 2.
Proportion of patients on antibiotics pre-coronavirus disease 2019 (pre-COVID-19) (blue line) and during COVID-19 (orange line). On average, approximately 48% of hospitalized patients were on antibiotics in 2019. Between January and March 2020 (during the COVID-19 pandemic), antibiotic use was proportionately higher.
Interestingly, the ASP's workload was maintained despite lower admission numbers and bed occupancy: 2542 audits were performed pre-COVID-19 and 2689 audits were performed during the COVID-19 period. The details and clinical impact of the ASP interventions are detailed in Table 1 . In essence, there was no significant difference in intervention acceptance rates between the two groups. The impact of ASP interventions was sustained during the COVID-19 period, with a significant reduction in the duration of antibiotic therapy and length of hospital stay in cases where ASP interventions were accepted. Such beneficial outcomes potentially alleviated the problem of ‘bed crunch’ during the pandemic.
Table 1.
Comparison of antibiotic stewardship programme (ASP) interventions and outcomes pre-coronavirus disease 2019 (pre-COVID-19) (1 February–30 April 2019) and during the acceleration phase of the COVID-19 pandemic (1 February–30 April 2020)
| Types of interventions | pre-COVID-19a, n (%) n=560 | COVID-19b, n (%) n=578 | P-value | |||
|---|---|---|---|---|---|---|
| ASP acceptance | 470 (83.9) | 488 (84.4) | 0.89 | |||
| Type of ASP interventions | ||||||
| Broaden empirical coverage | 6 (1.1) | 9 (1.6) | 0.87 | |||
| Narrow empirical coverage | 102 (18.2) | 102 (17.6) | 1.00 | |||
| Revision of antibiotics based on culture data | 89 (15.9) | 73 (12.6) | 1.00 | |||
| Discontinuation of antibiotics | 217 (38.8) | 181 (31.3) | 0.69 | |||
| IV to PO switch | 64 (11.4) | 79 (13.7) | 0.49 | |||
| Optimization of antibiotic doses | 24 (4.3) | 71 (12.3) | 0.56 | |||
| Trigger ID consult or ID review | 35 (6.3) | 30 (5.2) | 0.11 | |||
| Additional recommendationsc | 23 (4.1) | 33 (5.7) | 0.70 | |||
| Impact of ASP interventions | Accepted | Rejected | P-value | Accepted | Rejected | P-value |
| Median duration of antibiotic therapy (IQR), days | 3 (2–5) | 6 (4–10) | <0.05d | 4 (2-4) | 8 (3.0 – 9) | <0.05d |
| Median LOS after ASP intervention (IQR), days | 6 (2–15) | 7 (4–11) | 0.53 | 7 (4–16) | 12 (7–28) | <0.01d |
IV, intravenous; PO, per oral; IQR, interquartile range; LOS, length of stay; ID, infectious diseases.
pre-COVID-19: this refers to the period between 1 February and 30 April 2019.
COVID-19: this refers to the period between 1 February and 30 April 2020.
Recommendations for diagnostic investigations and/or infection control precautions were made to guide management of the ID issues.
P<0.05 was taken to indicate statistical significance.
This epidemic has placed enormous strain on healthcare providers and healthcare systems worldwide. Singapore has placed a high emphasis on identifying and isolating patients with COVID-19 in a bid to contain the spread of the virus. In the early weeks of the containment strategy, patients with signs and symptoms suggestive of viral illness were actively screened and isolated until results returned negative for COVID-19. Consequently, the authors believe that there were more admissions of patients with acute respiratory illness which would otherwise have been treated in the community prior to the COVID-19 pandemic. These patients were usually started empirically on antibiotics used for the treatment of community-onset pneumonia, coinciding with the increased utilization of these agents. Furthermore, the authors’ initial hypothesis that the use of broad-spectrum antibiotics should decrease together with the number of admissions was debunked. Instead, prescription numbers for these agents were observed to increase as they target serious, nosocomial infections which are often associated with urgent conditions (e.g. cancer, for which treatment cannot be delayed). Hence, in a tertiary healthcare institution such as SGH, the impact of COVID-19, as well as the continued care for hospitalized patients with complex medical issues, culminated in a higher proportion of patients on antibiotics compared with the pre-COVID period. It would be prudent to maintain the ASP even in viral epidemics/pandemics with existing manpower support [4].
At the start of the pandemic, the authors received support from hospital management to maintain the ASP as an essential service – a commitment towards the continued fight against antimicrobial resistance. The importance for ASPs to be operationally adaptable to different scenarios also became evident. Organizational restructuring and migration to telecommuting platforms for the ASP activities was crucial to maintain clinical service while observing distancing measures, thus ensuring patient and staff safety in this pandemic. It also became apparent that in addition to focusing efforts on nosocomial treatments, this was an opportunity for the authors’ team to execute a syndromic approach towards guiding antibiotic prescribing for respiratory tract infections. To do so, there is a need to employ digital platforms, and to continue to develop robust surveillance systems that are able to detect subtle changes in prescribing and resistance trends. In outbreak situations such as the COVID-19 pandemic, on-going engagement and education of prescribers to mitigate antibiotic misuse that tends to occur with viral epidemics/pandemics is extremely important to avoid undoing years of progress in antibiotic stewardship.
Given that COVID-19 is here for the long haul, the role of the ASP is vital to ensure the quality of antibiotic use at SGH is upheld because the problem of antibiotic resistance remains in the long term [5].
The Singhealth Centralised Institutional Review Board approved this retrospective review. Informed consent was not obtained from individual patients as the operations of the ASP constituted routine clinical practice, and only anonymized data were analysed.
Acknowledgements
The authors wish to acknowledge all ASP pharmacists for their contribution to the study.
Funding: None.
Competing interests: None declared.
Ethical approval: The study protocol was approved by the Singhealth Centralized Institutional Review Board (CIRB Ref: 2010/114/E).
References
- 1.World Health Organization . WHO; Geneva: 2020. Coronavirus disease 2019 (COVID-19) situation report – 41.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200301-sitrep-41-covid-19.pdf?sfvrsn=6768306d_2 Available at. [accessed 27 July 2020] [Google Scholar]
- 2.Ministry of Health Singapore . Ministry of Health Singapore; Singapore: 2020. Updates on COVID-19 (coronavirus disease 2019) local situation.https://www.moh.gov.sg/covid-19 Available at. [accessed 25 July 2020] [Google Scholar]
- 3.Loo L.W., Liew Y.X., Lee W., Lee L.W., Chlebicki P., Kwa A.L. Discontinuation of antibiotic therapy within 24 hours of treatment initiation for patients with no clinical evidence of bacterial infection: a 5-year safety and outcome study from Singapore General Hospital antimicrobial stewardship program. Int J Antimicrob Agents. 2019;53:606–611. doi: 10.1016/j.ijantimicag.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Huttner B.D., Catto G., Pano-Pardo J.R., Pulcini C., Schouten J. COVID-19: don't neglect antimicrobial stewardship principles! Clin Microbiol Infect. 2020;26:808–810. doi: 10.1016/j.cmi.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawson T.M., Moore L.S.P., Castro-Sanchez E., Charani E., Davies F., Satta G. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75:1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]