Abstract
Research, collaboration, and knowledge exchange are critical to global efforts to tackle antimicrobial resistance (AMR). Different healthcare economies are faced with different challenges in implementing effective strategies to address AMR. Building effective capacity for research to inform AMR-related strategies and policies is recognised as an important contributor to success. Interdisciplinary, intersector, as well as international collaborations are needed to span global to local efforts to tackle AMR. The development of reciprocal, long-term partnerships between collaborators in high-income and in low- and middle-income countries (LMICs) needs to be built on principles of capacity building. Using case studies spanning local and international research collaborations to codesign, implement, and evaluate strategies to tackle AMR, we have evaluated and build upon the ESSENCE criteria for capacity building in LMICs. The first case study describes the local codesign and implementation of antimicrobial stewardship (AMS) in the state of Kerala in India. The second case study describes an international research collaboration investigating AMR surgical patient pathways in India, the UK, and South Africa. We describe the steps undertaken to develop robust, agile, and flexible AMS research and implementation teams. Notably, investing in capacity building ensured that the programmes described in these case studies were sustained through the current severe acute respiratory syndrome coronavirus pandemic. Describing the strategies adopted by a local and an international collaboration to tackle AMR, we provide a model for capacity building in LMICs that can support sustainable and agile AMS programmes.
Keywords: Global health, Research partnerships, Antimicrobial resistance, Capacity building
Introduction
Antimicrobial resistance (AMR), leading to a decrease in effectiveness of antibiotics, is a major global health threat (Holmes et al., 2015). The 2014 ‘Review on antimicrobial resistance’, Chaired by Jim O’Neill, estimated that 10 million deaths could be attributed to AMR by 2050, with the majority of these deaths predicted to be in low- and middle-income countries (LMICs) (O’Neill, 2016). Healthcare-associated infections (HCAI), caused by drug-resistant pathogens, are associated with increased morbidity and mortality, length of hospital stay, and cost (Holmes et al., 2015, O’Neill, 2016). They also contribute to increased emotional and mental burdens on patients (Mo et al., 2019). Inconsistent infection prevention and control (IPC) practices and suboptimal antibiotic use remain key areas of concern across high-income countries (HICs) and LMICs (Katwyk et al., 2020). In addition to being integral to AMR containment, IPC is a universal component of all health systems, affecting the health and safety of both people who seek the healthcare services and those who provide them. This has implications for antimicrobial stewardship (AMS) at various levels. At the macro level, initiatives tackling AMR, including national action plans, have yet to fully exploit strategic approaches necessary for building the critical capacity and contingency for responsive and sustainable policies and interventions that can be transferred to multiple contexts (Ahmad et al., 2019). At the meso level, hospital-based AMS programmes are not consistently implemented, or well-integrated with IPC (Charani et al., 2019a). This is despite the fact that these programmes are proven to be effective in improving the quality and safety of patient care through increased infection cure rates, reduced treatment failures, and increased frequency of appropriate prescribing for treatment and prophylaxis (Davey et al., 2017). Reasons for this inconsistency vary according to the resources available for AMS programmes and the prevailing context in the implementation setting. The current ongoing severe acute respiratory syndrome coronavirus (SARS-CoV-2) pandemic (causing coronavirus disease-2019; COVID-19) has exposed the gaps in current IPC strategies, highlighting the need for robust investment and capacity building in IPC as as well strategies to tackle AMR. The pandemic may potentiate the long-term threat of AMR (Rawson et al., 2020). As part of efforts to control for suspected underlying bacterial infections in patients with COVID-19 infection, treating physicians may prescribe antibiotics more often, thereby unintentionally exposing the patient to selective pressure and AMR.
International partnerships in research and other collaborations are critical to global efforts to tackle AMR. Sustained efforts in strategies for IPC and AMS are essential at hospital (e.g. staff and management), national, and international levels, spanning from policy to implementation (Katwyk et al., 2020, Cox et al., 2017). The SARS-CoV-2 pandemic has changed the research landscape and the ability to deliver international research programmes. For example, since the emergence of the pandemic the majority of the UK Department for International Development (DFID) staff posted abroad have had to return to the UK, severely impeding the operational delivery of projects in partner countries (Whitehead, 2020). The UK government’s decision to merge DFID with the Foreign Office also poses threats to long-term funding and opportunities for international collaborations. This is at a time when greater global partnership and collaboration in health care had been gaining speed. At the 68th World Health Assembly held in Geneva in 2015, a Global Action Plan on AMR was adopted, in response to recognition of the need to address AMR across a One Health agenda. Later that year, AMR was included as a threat to the UN sustainable development goals (SDGs) (Børge and Bent, 2015). Although omitted from specific SDG targets, AMR is mentioned in paragraph 26, which highlights AMR as part of the problem of unattended diseases affecting developing countries (Kassebaum et al., 2016, Jasovský et al., 2016). Several of the SDGs are directly linked to AMR, including good health and wellbeing (SDG3), clean water and sanitation (SDG6), industry, innovation and infrastructure (SDG 9), and reduced inequalities (SDG10). Building research capacity—key to operationalizing strategies and interventions to tackle AMR across different countries—feeds into SDGs 9 and 10.
The need for research collaborations to tackle AMR in LMICs
In many LMICs, additional challenges impede the research, implementation and evaluation of effective strategies to tackle AMR, especially at the organisational level (Charani et al., 2019a, Cox et al., 2017, Charani et al., 2019b, Pulcini et al., 2019). These include the lack of a robust healthcare infrastructure (e.g. staff workforce, access to clinical microbiology laboratory facilities, and mechanisms for disease surveillance). Governments completely or partially control activities that promote, restore, and maintain health; in most cases, governments are the primary funders and the providers of health services. In countries where non-governmental organisations do operate, inadequate local capacity building can lead to a lack of synergy between local and international programmes. This then puts the sustainability of such programmes at risk, for example when external funding dries up or when faced with external threats such as pandemics. Health strategies in LMICs focus on promoting population-level public health through social and community mobilization, and provision of treatment through hospitals and clinics in the public sector. Due to more constrained health budgets, LMICs are expected to provide essential rather than comprehensive health services (Kruk et al., 2018). This restricts the national governments to primarily assessing the evolving trends and emerging threats of infectious diseases (Seale et al., 2017), with limited resources to further operationalize and sustain AMS programmes. While large regional AMR surveillance networks have been established in Europe (EARSNet), Latin America (Red Latinoamericana de Vigilancia de la Resistencia a los Antimicrobianos, ReLAVRA), and Central Asia and Eastern Europe (CAESAR), capacity for AMR surveillance in low-income countries is relatively limited and fragmented; this is despite evidence that AMR in low-income regions is increasing (Leung et al., 2011). Research is often hampered by inadequate investments by the government and external funders with regard to the required human resource skills and expertise, equipment, and surveillance and feedback strategies to inform practice. Coordination between researchers and policymakers remains inefficient and this can translate into inadequate evidence generation, synthesis and translation, and application to practice (May, 2013, Mannion and Davies, 2018). Significant patient load, high patient:provider ratios, and lack of sustained training in antimicrobial stewardship and pharmacotherapy can hinder sustainable and long-term improvement in care (Kakkar et al., 2020). Furthermore, the research rarely engages with patients and carers, whose perspective is often missing (Cook et al., 2019).
However, there are valuable lessons to be learnt from the innovations and resilience of healthcare systems in LMICs that have been able to implement successful change in tackling AMR, in spite of the challenges that they face. Some of these challenges remain universal. For example, the issue of access to and correct use of diagnostic laboratories is of particular concern in LMICs, and remains suboptimal in all settings. Geographical logistics can limit provision of laboratory services in both high- and low-resource settings (Skodvin et al., 2017). Where there is access to diagnostic laboratories, incidences of unnecessary testing as well as antibiotic prescribing in the absence of any microbiology tests results remain high (Skodvin et al., 2019, Charani et al., 2019c). Solutions to overcome these challenges have been reported in LMICs, an example being in Vietnam where an internationally funded and established research collaboration has been successful in delivering a sustainable AMS programme, including enhanced laboratory capacity (Wertheim et al., 2013). This is an example of how collective efforts in AMR, supported by greater international collaboration at the policy, research, synthesis and translation, and implementation levels, can lead to sustainable improvements. In the face of diminishing resources, capacity building, supported by funding, should be an integral part of AMR containment and mitigation efforts.
Capacity building in AMS and IPC research through collaboration
Building capacity for AMR research and AMS development, and its strategic implementation and adoption, requires a One World agenda and approach. Incorporating interdisciplinary, intersectoral, and international collaboration is critical. Key to spanning AMR efforts from global to local is the development of reciprocal, long-term, supported partnerships between collaborators in LMICs and HICs (Prentiss et al., 2018). Partnerships that enable learning across LMICs are needed, as solutions developed in an LMIC context are most likely to have most relevance and transferability to other LMIC settings. The ESSENCE on Health Research criteria developed by funding agencies to improve coordination of research capacity investment in LMICs provides an effective template for building research collaborations relating to AMR (ESSENCE, 2014). ESSENCE on Health Research is an initiative that allows donors and funders to increase the value of resources in LMICs, by identifying synergies and establishing coherence across human research programmes. The ESSENCE Seven Principles of capacity building for research in LMICs is the model supported by the WHO. Research capacity strengthening is a long-term process, which can often make it difficult to attribute causes and contributions. Additionally, resilience has to be built in to enable systems and collaborations to absorb the shock of unexpected events, such as pandemics. The ESSENCE Seven Principles of of capacity building for research in LMICs are summarised in Box 1, and focus on networking, communication, monitoring and evaluation, research governance, strong support and mentorship structures, and flexibility for resilience and continuity. Drawing on our collaborative research across India, South Africa, and the UK, we have evaluated our experience of capacity building for research in IPC and AMS, building on the ESSENCE principles. Developing equal partnerships is critical to fostering resilient and responsive teams, with the potential for sustainable research synthesis, implementation, and translation across international institutions.
Box 1. The ESSENCE seven principles for strengthening research capacity in LMICs (ESSENCE, 2014).
| Network, collaborate, communicate, and share experiences align="none" |
|---|
| Understand the local context and accurately evaluate existing research capacity align="none" |
| Ensure local ownership and secure active support align="none" |
| Build in monitoring, evaluation, and learning from the start align="none" |
| Establish robust research governance and support structures, and promote effective leadership align="none" |
| Embed strong support, supervision, and mentorship structures align="none" |
| Think long term, be flexible, and plan for continuity align="none" |
Alt-text: Box 1
Here we use two case studies to evaluate how the ESSENCE principles can support collaborative research in AMS and IPC at both local and international levels. The first case study is based on a public–private partnership for implementing AMS in the state of Kerala in India, and the second is based on a research partnership investigating the meso and macro drivers for implementing AMS across surgical pathways in India and South Africa. These case studies also demonstrate a commitment to delivering programmes through earmarked funding. Case study 1 highlights local commitment through providing funding for adequate staffing, including pharmacists, as part of a comprehensive AMS team, which in the long term proved to be cost-effective. Case study 2 demonstrates the added value of funding bodies assigning and supporting greater international collaborations in AMR by investing in the development of the local workforce.
Case study 1: implementing antimicrobial stewardship in a hospital in Kerala, India
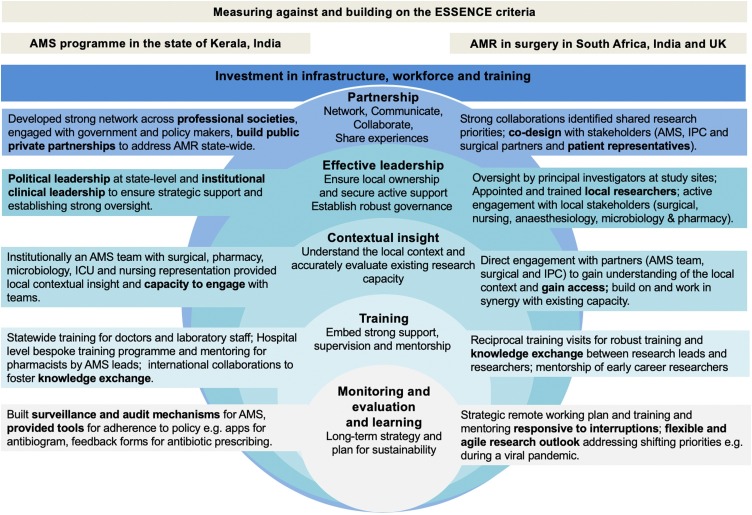
The state of Kerala, in the southern India, was the first in the country to implement a state-wide strategy for addressing AMR through AMS. The AMS programme in Kerala is an example of an effective capacity building programme for tackling AMR, demonstrating the influence of collaborative partnership and effective leadership (Figure 1). Through a 7-year campaign (beginning in 2013), led by a state-level clinical champion, an AMS program with a strong stakeholder network and robust participation was created across professional societies (Singh et al., ARIC; under review) and public–private partnerships in secondary care. At hospital level, an effective leadership drive ensured the inclusion of all stakeholders from different specialties and professional groups (including laboratory, pharmacy, and nursing) (Singh et al., 2019). Including representatives from different specialties in AMS and IPC committees assured local ownership and integration of local contexts into interventions. At the state level, capacity was developed in regional laboratories to participate in the Indian Council of Medical Research (ICMR) AMR surveillance network. Laboratory staff were trained through a bespoke programme developed in partnership with the Kerala Department of Health to ensure uniform data with respect to AMR surveillance. The initiative was incremental, beginning with stakeholder engagement and buy-in from political to clinical leadership, followed by establishment of policy and guidelines, a comprehensive state-wide post-graduate training programme for doctors, and then local capacity building for AMS within hospitals (Singh et al., ARIC; under review).
Figure 1.
Two case studies of AMS research and implementation (supported by local and international funding) measured against, and building upon, the ESSENCE principles for capacity building.
Effective training was provided at hospital and state level to all medical graduates. Additionally, at the participating private and public hospitals, a train-the-trainer model was created to assure the transfer of knowledge and skills. In the lead hospital, an effective monitoring and evaluation programme included embedding mechanisms to ensure effective tools for monitoring outcomes, comprising an antibiogram app, feedback forms for antibiotic prescribing, and pharmacist-driven reviews of prescribing practices (Singh et al., 2019). Long-term sustainability for the AMS programme was achieved through recruiting competent human resources, including trained clinical pharmacists, in the stewardship programme. Whilst this approach has proven successful in Kerala, it is important to recognize that the health system in India is diverse and there may be other successful AMS models in place.
Case study 2: an international collaboration tackling AMR across surgical pathways
The ASPIRES collaboration (Antibiotic use across Surgical Pathways—Investigating, Redesigning and Evaluating Systems; https://www.imperial.ac.uk/arc/aspires/) is an example of an externally funded partnership that was developed through smaller studies, including student exchange programmes. The collaboration aims to improve clinical outcomes by optimizing antibiotic usage along surgical pathways, working across South Africa, the UK, and India (Charani et al., 2017). The research study was codesigned with surgical, IPC, and AMS leads in each setting (Figure 1). The multidisciplinary research team includes pharmacy, nursing, and implementation science expertise. To ensure success, the leadership approach was structured to provide mentorship and support to researchers across the three countries. An initial intense phase of face-to-face training was undertaken in each setting, involving 2 months’ training for local teams by experienced researchers. This was followed with regular check-in meetings (via video link) to monitor both the development and progress of the researchers as well as the project. The mentorship mix included cross-disciplinary teams (doctors, implementation scientists, social scientists, nurses, and pharmacists) to provide a broad range of expertise. The more senior researchers also gained skills in global health research, operationalization and leadership, which allowed for the different cadres of researchers to assess and provide feedback on the skills of their colleagues. Learning from the research was shared through a codeveloped virtual learning platform (Massive Open Online Course: https://www.futurelearn.com/courses/social-science-for-tackling-antimicrobial-resistance). This model has proven to be effective and resilient to unexpected obstacles in the process, including lack of access to specific sites by some researchers due to visa restrictions and the unintended consequence of the SARS-CoV-2 pandemic on travel restrictions. Investing equally in research resources in each setting enabled a sustained research presence in South Africa and India, facilitating data gathering as well as developing long-standing professional relationships. Furthermore, international training workshops were supplemented with virtual workshops, where all researchers could participate in the implementation process in each setting. The emphasis on enabling and encouraging collaborations between early career researchers across the three sites ensured South–South and North–South knowledge transfer with regard to research methodologies and skills required to strengthen the overall capacity for AMR research. These newly gained skills also supported local AMS programs and experiential learning. This international collaboration has not been without its logistical issues, in terms of travel and language barriers; these have been overcome through greater use of technology and the invaluable input of local teams.
Investing in capacity building has assured sustained delivery of the program through the current SARS-CoV-2 pandemic, including the imposed travel restrictions, allowing the flexibility to realign the research to meet immediate needs in each setting. Furthermore, historic close collaborations between the principal investigators and country leads played a key role in delivering this project.
Conclusions
In building collaborations to address AMR, organizations need to ensure that the strengthening of research capacity remains an explicit objective, from consultations with the funders to implementation of research findings. Potential strategies that have been recognized include the promotion of good research governance, emphasis on the relevance of strategic partnership, and the fostering of good networking and collaborations between the funding agencies and the recipient organizations.
Engaging with local stakeholders (including patients and the public) as part of research synthesis, implementation, and evaluation provides a deeper understanding of the political and cultural research environment, ascertaining the potential facilitators and barriers to various initiatives for strengthening research capacity. This can be through identifying the demand for research by examining the policy process and identifying the barriers to greater use of evidence by policy makers. Such involvement will promote buy-in across the network, as well as ensuring feasibility and sustainability of necessary interventions identified from research-informed findings.
Actively seeking to build national and international collaborations that transcend traditional academic and clinical boundaries, and which recognize that little can be achieved by working in silos, will support mentorship of cadres of multi-professional researchers, adept at communicating across disciplines. Strong, self-sustaining, peer group support for early career researchers that facilitates learning in an environment characterized by openness and mutual respect is essential to developing resilient systems for research that can work in healthcare environments at increasing threat of disruption, e.g. from pandemics. In the efforts to establish AMS research and learning, it is crucial to account for multiple disciplines, and provide opportunity and mentorship both within and outside of disciplines. The case studies presented incorporate key infrastructure developments in laboratory surveillance capacities and requisite training to enhance resource personnel competence for IPC activities and AMR research, in conjunction with knowledge sharing through regional expertise networks and multinational collaborations. Our comprehensive approach of implementing, evaluating, and building upon the ESSENCE criteria for capacity building for research in the context of AMR can provide a sustainable, resilient platform for research and patient-centered care in low-resource settings.
Ethical approval
Ethical approval was not required for this research.
Conflict of interest
We declare no conflicts of interest.
Acknowledgements
This work was supported by the Economic and Social Science Research Council (ESRC) and the National Institute for Health Research, U K Department of Health [HPRU-2012-10047] in partnership with Public Health England. This study is part of the ASPIRES project (Antibiotic use across Surgical Pathways—Investigating, Redesigning and Evaluating Systems; https://www.imperial.ac.uk/arc/aspires/). ASPIRES aims to address antimicrobial resistance and improve clinical outcomes, whie optimizing antibiotic usage along surgical pathways. ECS is affiliated with the National Institute for Health Research Health Protection Research Unit in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with Public Health England. ECS is a National Institute for Health Research Senior Nurse and Midwife Research Leader, and acknowledges the support of the BRC.
References
- Ahmad R., Zhu N.J., Leather A.J.M. Strengthening strategic management approaches to address antimicrobial resistance in global human health: a scoping review. BMJ Glob Heal. 2019 doi: 10.1136/bmjgh-2019-001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børge B., Bent H. Towards evidence-based, quantitative Sustainable Development Goals for 2030. Lancet. 2015 doi: 10.1016/S0140-6736(14)61654-8. [DOI] [PubMed] [Google Scholar]
- Charani E., Ahmad R., Tarrant C. Opportunities for system level improvement in antibiotic use across the surgical pathway. Int J Infect Dis. 2017;60 doi: 10.1016/j.ijid.2017.04.020. [DOI] [PubMed] [Google Scholar]
- Charani E., Smith I., Skodvin B. Investigating the cultural and contextual determinants of antimicrobial stewardship programmes across low-, middle- and high-income countries—a qualitative study. PLoS One. 2019;14 doi: 10.1371/journal.pone.0209847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charani E., Cunnington A.J., Yousif A.E.H.A. In transition: current health challenges and priorities in Sudan. BMJ Glob Heal. 2019 doi: 10.1136/bmjgh-2019-001723. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charani E., Barra E.De, Rawson T.M. Antibiotic prescribing in general medical and surgical specialties: a prospective cohort study. Antimicrobial Resist Infect Control. 2019;6:1–10. doi: 10.1186/s13756-019-0603-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N., Siddiqi N., Twiddy M. Patient and public involvement in health research in low and middle-income countries: a systematic review. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J.A., Vlieghe E., Mendelson M. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect. 2017;23:812–818. doi: 10.1016/j.cmi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Davey P., Marwick C.A., Scott C.L. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2017. 2017 doi: 10.1002/14651858.CD003543.pub4. Art. No.: CD003543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESSENCE . 2014. Good Practice Document Series. Geneva. [Google Scholar]
- Holmes A.H., Moore L.S.P., Sundsfjord A. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2015;387 doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- Jasovský D., Littmann J., Zorzet A. Antimicrobial resistance—a threat to the world’s sustainable development. Ups J Med Sci. 2016 doi: 10.1080/03009734.2016.1195900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakkar A.K., Shafiq N., Singh G. Antimicrobial stewardship programs in resource constrained environments: understanding and addressing the need of the systems. Front Public Heal. 2020 doi: 10.3389/fpubh.2020.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassebaum N.J., Arora M., Barber R.M. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016 doi: 10.1016/S0140-6736(16)31460-X. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katwyk S.R.Van, Hoffman S.J., Mendelson M. Strengthening the science of addressing antimicrobial resistance: a framework for planning, conducting and disseminating antimicrobial resistance intervention research. Health Res Policy Syst. 2020;1:1–13. doi: 10.1186/s12961-020-00549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk M.E., Gage A.D., Arsenault C. High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Heal. 2018 doi: 10.1016/S2214-109X(18)30386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E., Weil D.E., Raviglione M. The WHO policy package to combat antimicrobial resistance. Bull World Health Organ. 2011;89:390–392. doi: 10.2471/BLT.11.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion R., Davies H. Understanding organisational culture for healthcare quality improvement. BMJ. 2018 doi: 10.1136/bmj.k4907. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C. Agency and implementation: understanding the embedding of healthcare innovations in practice. Soc Sci Med. 2013 doi: 10.1016/j.socscimed.2012.11.021. Published Online First. [DOI] [PubMed] [Google Scholar]
- Mo Y., Low I., Tambyah S.K. The socio-economic impact of multidrug-resistant nosocomial infections: a qualitative study. J Hosp Infect. 2019;102:454–460. doi: 10.1016/j.jhin.2018.08.013. [DOI] [PubMed] [Google Scholar]
- O’Neill J. 2016. Tackling Drug-Resistant Infections Globally: an Overview of Our Work. London. [Google Scholar]
- Prentiss T., Weisberg K., Zervos J. Building capacity in infection prevention and antimicrobial stewardship in low- and middle-income countries: the role of partnerships inter-countries. Curr Treat Options Infect Dis. 2018 doi: 10.1007/s40506-018-0140-5. Published Online First. [DOI] [Google Scholar]
- Pulcini C., Binda F., Lamkang A.S. Developing core elements and checklist items for global hospital antimicrobial stewardship programmes: a consensus approach. Clin Microbiol Infect. 2019;25:20–25. doi: 10.1016/j.cmi.2018.03.033. [DOI] [PubMed] [Google Scholar]
- Rawson T.M., Moore L.S.P., Castro-Sanchez E. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020 doi: 10.1093/jac/dkaa194. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale A.C., Gordon N.C., Islam J. AMR surveillance in low and middle-income settings—a roadmap for participation in the Global Antimicrobial Surveillance System (GLASS) Wellcome Open Res. 2017 doi: 10.12688/wellcomeopenres.12527.1. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Menon V.P., Mohamed Z.U. Implementation and impact of an antimicrobial stewardship program at a tertiary care center in South India. Open Forum Infect Dis. 2019 doi: 10.1093/ofid/ofy290. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodvin B., Aase K., Brekken A.L. Addressing the key communication barriers between microbiology laboratories and clinical units: a qualitative study. J Antimicrob Chemother. 2017;72:2666–2672. doi: 10.1093/jac/dkx163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodvin B., Wathne J.S., Lindemann P.C. Use of microbiology tests in the era of increasing AMR rates—a multicentre hospital cohort study. Antimicrob Resist Infect Control. 2019 doi: 10.1186/s13756-019-0480-z. Published Online First. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheim H.F.L., Chandna A., Vu P.D. Providing impetus, tools, and guidance to strengthen national capacity for antimicrobial stewardship in Viet Nam. PLoS Med. 2013;10 doi: 10.1371/journal.pmed.1001429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead A. Have DFID’s priorities changed as a result of Covid-19? Bond. 2020 [Google Scholar]