Abstract
Mutations of the Gon4l/udu gene in different organisms give rise to diverse phenotypes. Although the effects of Gon4l/Udu in transcriptional regulation have been demonstrated, they cannot solely explain the observed characteristics among species. To further understand the function of Gon4l/Udu, we used yeast two-hybrid (Y2H) screening to identify interacting proteins in zebrafish and mouse systems, confirmed the interactions by co-immunoprecipitation assay, and found four novel Gon4l-interacting proteins: BRCA1 associated protein-1 (Bap1), DNA methyltransferase 1 (Dnmt1), Tho complex 1 (Thoc1, also known as Tho1 or HPR1), and Cryptochrome circadian regulator 3a (Cry3a). Furthermore, all known Gon4l/Udu-interacting proteins—as found in this study, in previous reports, and in online resources—were investigated by Phenotype Enrichment Analysis. The most enriched phenotypes identified include increased embryonic tissue cell apoptosis, embryonic lethality, increased T cell derived lymphoma incidence, decreased cell proliferation, chromosome instability, and abnormal dopamine level, characteristics that largely resemble those observed in reported Gon4l/udu mutant animals. Similar to the expression pattern of udu, those of bap1, dnmt1, thoc1, and cry3a are also found in the brain region and other tissues. Thus, these findings indicate novel mechanisms of Gon4l/Udu in regulating CpG methylation, histone expression/modification, DNA repair/genomic stability, and RNA binding/processing/export.
Subject terms: Biochemistry, Biological techniques, Developmental biology, Molecular biology
Introduction
Gon4l is a nuclear protein conserved among species. Animal models from invertebrates to vertebrates have shown that the protein Gon4-like (Gon4l) is essential for regulating cell proliferation and differentiation. For example, the Gon4l homologous gene in Caenorhabditis elegans, gon-4, is critical for gonadogenesis. In gon-4 mutant nematodes, precursor cells for somatic gonadal tissues are unable to divide or cell division is severely delayed1. After re-expressing exogenous GON-4 protein, fertility can be rescued in gon-4 mutants. In Drosophila, the Gon4l homologous gene, muscle wasted (mute), has been proposed as one of the components of histone locus body. Loss of function in mute gene results in decreased muscle mass and defective terminal differentiation of muscle cells, therefore, suggesting a role for Mute in regulating muscle cell differentiation through controlling histone expression2.
For mammals, Justy mutant mice, which carry a point mutation within the Gon4l gene, exhibit defects in B cell development3. Detailed analysis reveals that a block in the G1/S phase transition results in severely defective differentiation of pre-pro B cells to pro-B cells in these mutant mice4. Moreover, a frameshift mutation in this gene causes proportionate dwarfism in Fleckvieh cattles5, indicating that the Gon4l protein not only affects cell proliferation/differentiation in specific types of tissues but also can impair the growth of an entire organism.
The zebrafish mutant, ugly duckling (udutu24), was first isolated from the Tübingen large-scale screen and exhibits fewer blood cells, a shorter body axis, and defective tail formation6. Another study shows that udusq3 mutants (originally named udusq1) exhibit arrested primitive hematopoiesis7. The third udu mutant allele, uduvu66, shows an irregular notochord boundary and reduced mediolateral cell polarity at early stages8. Similar to human and mouse GON4L proteins, zebrafish Udu protein is also a nuclear factor. Its N-terminus contains three conserved regions (CR1, CR2, and CR3), and its C-terminus consists of two paired PAH (amphipathic α-helix like) repeats and one SANT domain (SWI3, ADA2, N-CoR, and TFIIIB like)7. The PAH domain, which has been identified in the Saccharomyces cerevisiae SIN3 gene, has the ability to mediate protein–protein interaction9,10; and the SANT domain, which is similar to the Myb DNA-binding domain11, may be responsible for regulating chromatin remodeling and accessibility12,13. Therefore, the protein structure of human/mouse GON4L and zebrafish Udu indicates their roles in regulating gene expression through their incorporation with other proteins.
Despite the biological importance of Gon4l/Udu, only a few studies have addressed the biochemical interactions between Gon4l/Udu and other proteins, as well as how interactions among these proteins are related to its possible functions. GON4L in mouse B cells can associate with YY1, Sin3a and Hdac1 to form a complex. The interaction among these proteins correlates with the ability of GON4L to repress targeted DNA expression as determined by GAL4-UAS reporter assay14. GON4L in human and Mute in Drosophila are reported to be co-localized with NPAT (Nuclear Protein, Ataxia-Telangiectasia Locus) in the histone locus body to regulate the expression and processing of cell cycle-regulated histone transcripts15,16. Transcription levels of histones H3 and H4 are both up-regulated in mute mutant embryos, suggesting that Mute might be responsible for the repression of histone mRNA accumulation2. Additionally, a recent study using a large-scale protein–protein interaction screen in mouse T cells revealed a novel Gon4l-interacting protein, CRAMP1L, in association with the Hippo kinase, MST117. However, whether the interaction among GON4L, CRAMP1L, and MST1 affects gene transcription in regulatory T cells was not further addressed. In contrast to being a transcriptional repressor, GON4L has been shown to interact with YY1 to positively regulate CD24 expression in several human cancer cell lines18. Noteworthy, it is very interesting that Gon4l/Udu may not only exert its function as a transcriptional regulator. Expression of the PAH/SANT domain in synchronized human cells shows that it is co-localized with the BrdU signal in early S phase and with HP1b, a marker for pericentromeric heterochromatin, in late S phase19,20. These findings suggest a possible function of Gon4l/Udu for participating in DNA replication and also in protecting the epigenetic integrity.
To further understand the possible functions of Gon4l/Udu, we performed yeast two-hybrid (Y2H) screens in this study to discover novel Gon4l/Udu-interacting proteins from prey libraries of both mouse cell lines and zebrafish larvae. We have isolated 56 Gon4l/Udu-interacting candidate proteins and used co-immunoprecipitation to verify 11 of them. We have identified four proteins as novel partners for Gon4l/Udu—Bap1, Dnmt1, Thoc1, and Cry3a—which are functionally related to histone modification, maintenance of DNA methylation after replication, R-looping prevention and RNA processing during transcription, and circadian-regulated DNA repair, respectively. RNA in situ hybridization studies show that bap1, dnmt1, thoc1, and cry3a co-localize with gon4l/udu during development mainly in the brain region and some other specific tissues. Furthermore, using bioinformatic analysis reveals that enriched phenotypes in mutant animals carrying mutations at genes encoding Gon4l/Udu-interacting proteins are similar to those observed in reported Gon4l/udu mutants. These data not only indicate functional cooperation among Gon4l/Udu and its interacting proteins but also provide important links between the molecular functions of Gon4l/Udu and various kinds of reported biological effects.
Results
56 zebrafish Udu and mouse GON4L interacting candidates are identified by yeast 2-hybrid analyses
In order to understand the functions of Gon4l/Udu, we searched for novel proteins interacting with Udu in zebrafish and GON4L in mouse using the yeast two-hybrid system. Two fragments of zebrafish Udu—including the N-terminal CR1-CR3 (bait 1: 165–1,470 a.a.), which contains two putative YY1-binding domains, and the C-terminal PAH and SANT domains (bait 2: 1,523–2,054 a.a.)—were used as bait (Fig. 1a) to screen the zebrafish embryonic cDNA library. We also used two fragments for mouse GON4L, including the PAH domains (bait 3: 1,458–1,857 a.a.) and the SANT domain (bait 4: 1,858–2,260 a.a.), to screen different murine cDNA libraries originated from embryos, B lymphocytes (B cells) and cardiac myocytes (Fig. 1b). In the zebrafish screen, 15 and 11 proteins were identified by bait 1 and bait 2, respectively (Table 1). Mcm3 and Mcm4, identified by the latter, have been previously reported19. In the mouse screen, 29 proteins were identified as interacting with bait 3 (Table 2); among them, CRAMP1L has been shown to interact with GON4L in both human and mouse models21. The only protein identified by bait 4 was Npat (Table 2), which was reported as having the ability to interact with Gon4l in human and Drosophila16.
Figure 1.
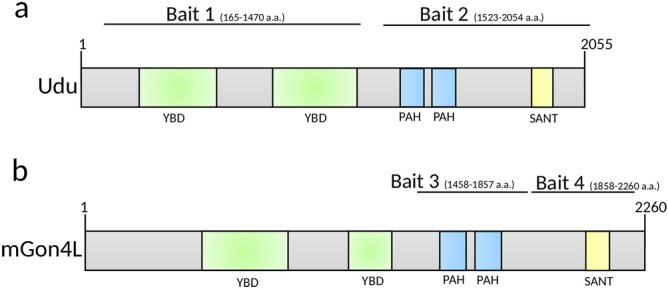
Schematic structures of the bait proteins. Domain structures of (a) zebrafish Udu and (b) mouse GON4L. Black lines indicate locations of bait used in this study.
Table 1.
Zebrafish Udu-binding proteins isolated by yeast two-hybrid analysis.
| Category | Bait | PBS* | Prey binding region | Symbol | Name (accession number) | GO terms |
|---|---|---|---|---|---|---|
| Lipid metabolism-related | 1 | D | unknown-3786 | Apobb.1 | Apolipoprotein Bb, tandem duplicate 1 (apobb.1), mRNA (NM_001030062.1 ) | Lipid transporter activity |
| 1 | D | − 1 ~ 399 | Ephx2 | Epoxide hydrolase 2, cytoplasmic, transcript variant X1, mRNA (XM_021467153.1) | Epoxide hydrolase activity | |
| Transcriptional regulation | 1 | D | 432 ~ 1,248 | Pax7b | Paired box 7b (NM_001146149.1) | Sequence-specific DNA binding |
| 2 | D | 92 ~ 652 | Hoxc4a | Homeobox C4a, mRNA (NM_131122.2) | Sequence-specific DNA binding | |
| 1 | D | 96 ~ 600 | Otx1 | Orthodenticle homeobox 1, mRNA (NM_131250.2) | Sequence-specific DNA binding | |
| 1 | D | − 1 ~ 954 | Cry3a | Cryptochrome circadian regulator 3a, transcript variant 1, mRNA (NM_001313822.1) | Negative regulation of transcription; sequence-specific DNA binding | |
| 2 | D | − 7 ~ 754 | Pelp1 | Proline, glutamate and leucine rich protein 1, mRNA (XM_001338982.7) | Transcription factor binding; chromatin binding | |
| RNA-binding/processing | 1 | D | 2094 ~ 2,781 | Heatr6 | HEAT repeat containing 6 (NM_001114313.1) | RNA binding |
| 2 | D | − 1 ~ 614 | Fbrsl1 | Fibrosin-like 1 protein variant 1a mRNA, complete cds, alternatively spliced (KY492379.1) | RNA binding | |
| 2 | D | − 1 ~ 1,026 | Rbmx2 | RNA binding motif protein X-linked 2, mRNA (NM_001025166.1) | RNA binding | |
| Apoptosis/Cell proliferation/DNA replication | 2 | D | 1,155 ~ 1893 | Mcm3 | Minichromosome maintenance complex component 3, mRNA (NM_212567.2) | DNA replication origin binding; helicase activity |
| 2 | D | 1971 ~ 2,229 | Mcm4 | minichromosome maintenance complex component 4, mRNA (NM_198913.2) | DNA replication origin binding; helicase activity | |
| 1 | D | − 1 ~ 1,355 | Bnipl | BCL2/adenovirus E1B 19kD interacting protein, like, transcript variant X1, mRNA (XM_009292526.3) | Exopolyphosphatase activity | |
| 2 | D | 270 ~ 1744 | Gulp1b | GULP, engulfment adaptor PTB domain containing 1b, transcript variant X5, mRNA (XM_001345116.6) | Apoptotic process | |
| 2 | D | 135 ~ 706 | Gpatch11 | G patch domain containing 11, mRNA; zgc92714 (NM_001002393.1) | Nucleic acid binding | |
| Signaling pathways | 1 | D | 1,356 ~ unknown | Dlc | deltaC, mRNA (NM_130944.1) | PDZ domain binding; Notch binding |
| 1 | D | Unknown ~ 1,216 | Napba | N-ethylmaleimide-sensitive factor attachment protein, beta, mRNA (NM_001020655.2) | Soluble NSF attachment protein activity; syntaxin binding | |
| 2 | D | 2,640 ~ 3,309 | Dock6 | Danio rerio dedicator of cytokinesis 6, transcript variant X1, misc_RNA (XR_660514.3) | Guanyl-nucleotide exchange factor activity | |
| Protein translocation/ processing | 1 | D | Unknown ~ 915 | Ssr1 | Signal sequence receptor, alpha, mRNA (NM_201327.1) | Cellular response to estrogen |
| 1 | D | − 1 ~ 559 | Naa15b | N(alpha)-acetyltransferase 15, NatA auxiliary subunit b, mRNA (NM_203321.2) | Peptide alpha-N-acetyltransferase activity | |
| Cytoskeleton | 1 | D | − 1 ~ 628 | Agrn | Agrin, mRNA (NM_001177452.1) | Serine-type endopeptidase inhibitor activity |
| 1 | D | − 1 ~ 608 | Pleca | plectin a, mRNA (NM_001100032.1) | Ankyrin binding; actin binding | |
| 1 | D | 147 ~ 841 | Actc1 | cardiac muscle alpha actin 1 (NM_001002066.1) | Nucleotide binding; ATP binding | |
| 2 | D | − 1 ~ unknown | Neb | Nebulin (neb), transcript variant X41, mRNA (XM_021474182.1) | Actin filament binding | |
| 2 | D | − 1 ~ unknown | Cnn3b | Calponin 3, acidic b, mRNA (NM_001024073.1) | Actin binding; calmodulin binding | |
| Others | 1 | D | 225 ~ 661 | Slc25a3b | Solute carrier family 25 (mitochondrial carrier; phosphate carrier), member 3b, mRNA (NM_213722.1) | Inorganic phosphate transmembrane transporter activity |
*PBS is a score that is automatically computed through algorithms; D means “moderate confidence in the interaction”.
Table 2.
Mouse GON4L-binding proteins isolated by yeast two-hybrid analysis.
| Category | Bait | Prey binding region | Prey library | Symbol | Name (accession number) | GO terms |
|---|---|---|---|---|---|---|
| Lipid metabolism-related | 3 | 16 ~ 240 | Mouse embryo | GC | Group specific component (NM_008096) | Vitamin D-binding; vitamin transmembrane transporter activity |
| 3 |
− 3 ~ 374 − 13 ~ 97 3 ~ 374 |
Mouse B cell/Cardiac cell | LIAS | Lipoic acid synthetase, mitochondrial protein (NM_024471) | Lipoate synthase activity; transferase activity | |
| Transcriptional regulation | 3 | 5 ~ 176 | Mouse embryo | FOXA2 | Forkhead box a2 (NM_010446) | DNA-binding transcription factor activity |
| 3 | 189 ~ 547 | Mouse B cell | HIVEP1 | Human Immunodeficiency Virus Type I Enhancer Binding Protein 1 (NM_007772) | DNA-binding transcription factor activity | |
| 3 | 259 ~ 580 | Mouse B cell | ARNTL2 | Aryl hydrocarbon receptor nuclear translocator-like 2, also known as Bmal2 (NM_172309) | DNA-binding transcription factor activity | |
| 3 | 821 ~ 1,006 | Mouse B cell | BRD4 | Bromodomain containing 4 (NM_020508) | Lysine-acetylated histone binding; chromatin binding | |
| 4 |
1,233 ~ 1,421 1,355 ~ 1,421 |
Mouse Cardiac cell | NPAT | Nuclear Protein, Ataxia-Telangiectasia Locus (NM_001081152) | Regulation of transcription involved in G1/S transition of mitotic cell cycle; transcription coactivator activity of histones | |
| 3 | 654 ~ 897 | Mouse B cell | CRAMP1L | Cramped-like, cramp1L (NM_020608) | DNA binding; nucleus; chromatin binding | |
| 3 | 591 ~ 726 | Mouse Cardiac cell | SKI | ski sarcoma viral oncogene homolog (NM_011385) | SMAD binding; transcription corepressor activity | |
| 3 | 118 ~ 296 | Mouse B cell | TLE3 | Transducin-Like Enhancer Of Split 3, Homolog Of Drosophila E (NM_009389) | Transcription corepressor activity | |
| RNA-binding/processing | 3 | 338 ~ 532 | Mouse embryo | THOC1 | Tho complex 1, also known as Tho1, HPR1 (NM_153552) | DNA binding; RNA binding; protein binding; nucleus; cytoplasm; mRNA export from nucleus; RNA splicing; replication fork processing |
| 3 |
− 6 ~ 126 − 3 ~ 126 8 ~ 97 24 ~ 118 |
Mouse Cardiac cell/Mouse embryo | RPS25 | Ribosomal Protein S25 (NM_024266) | Structural constituent of ribosome | |
| 3 |
401 ~ 655 431 ~ 664 435 ~ 711 |
Mouse B cell | DDX10 | DEAD-Box Helicase 10 (XM_284494) | RNA binding; RNA helicase activity; ATP binding | |
| 3 |
74 ~ 458 200 ~ 477 26 ~ 479 373 ~ 511 |
Mouse B cell/ Mouse embryo | CTNNBL1 | Beta-catenin-like protein 1 (NM_025680) | mRNA splicing via spliceosome; protein binding; positive regulation of apoptotic process | |
| Apoptosis/Cell proliferation/DNA replication | 3 |
625 ~ 729 545 ~ 646 |
Mouse B cell/ Mouse embryo | BAP1 | BRCA1-associated protein 1 (NM_027088) | Chromatin binding; thiol-dependent ubiquitinyl hydrolase activity |
| 3 | − 21 ~ 202 | Mouse embryo | CACYBP | Calcyclin binding protein (NM_009786) | Tubulin binding; ubiquitin protein ligase binding; S100 protein binding | |
| 3 | 423 ~ 715 | Mouse Cardiac cell | CIT | Citron Rho-interacting Kinase (NM_007708) | Protein serine/threonine kinase activity; mitotic cytokinesis | |
| 3 |
554 ~ 831 574 ~ 704 |
Mouse B cell/Cardiac cell | DNMT1 | DNA methyltransferase 1 (AF162282) | Chromatin binding; DNA (cytosine-5-)-methyltransferase activity | |
| 3 |
254 ~ 656 547 ~ 643 |
Mouse B cell/Cardiac cell | KIF20A | rabkinesin-6 (Y09632) | ATPase activity; microtubule motor activity; nucleotide binding | |
| Signaling pathways | 3 |
48 ~ 435 313 ~ 439 339 ~ 446 262 ~ 461 340 ~ 558 250 ~ 465 |
Mouse Cardiac cell/ Mouse embryo | GPC1 | Glypican-1 (NM_016696) | Fibroblast growth factor binding |
| 3 | 65 ~ 582 | Mouse embryo | ITSN1 | Intersectin 1 (NM_010587) | Guanyl-nucleotide exchange factor activity; Rho guanyl-nucleotide exchange factor activity; calcium ion binding | |
| 3 | 410 ~ 526 | Mouse embryo | PPP3CB | Protein phosphatase 3, catalytic subunit, beta isoform (NM_008914) | Calmodulin-dependent protein phosphatase activity | |
| 3 | 249 ~ 554 | Mouse B cell | FCHSD2 | FCHSD2/Carom/FCH and double SH3 domains 2/F-BAR And Double SH3 Domains Protein 2 (NM_001146010) | Phosphatidylinositol-3,4,5-trisphosphate binding | |
| 3 | 367 ~ 523 | Mouse Cardiac cell | STAM1 | Signal-Transducing Adaptor Molecule 1 (NM_011484) | Ubiquitin-like protein ligase binding | |
| 3 |
242 ~ 419 251 ~ 419 269 ~ 419 |
Mouse B cell/Mouse embryo | ALS2CR2 | STE20-related kinase adapter protein beta; STRADB (NM_172656) | Protein serine/threonine kinase activity; ATP binding | |
| 3 | 4 ~ 209 | Mouse Cardiac cell | TNIP3 | TNFAIP3 Interacting Protein 1, A20-binding inhibitor of NF-κB activation (ABIN-1) (NM_001001495) | Protein deubiquitination; identical protein binding; MAPK binding | |
| 3 | 259 ~ 554 | Mouse B cell | TNIP1 | TNFAIP3 Interacting Protein 3), A20-binding inhibitor of NF-κB activation (ABIN-3) (NM_021327) | Protein deubiquitination; identical protein binding; MAPK binding | |
| Protein processing/translocation | 3 | − 37 ~ 50 | Mouse embryo | AHSA1 | AHA1, Activator of Heat Shock 90 kDa Protein ATPase Homolog 1 (NM_146036) | ATPase activator activity; Hsp90 protein binding; extracellular exosome |
| 3 |
3 ~ 279 52 ~ 279 − 5 ~ 279 14 ~ 279 |
Mouse B cell/Cardiac cell/Mouse embryo | C1QBP | Complement component 1 Q subcomponent-binding protein, mitochondrial (NM_007573) | Mitochondrial ribosome binding; mRNA binding; translation activator activity | |
| Others | 3 |
269 ~ 376 302 ~ 383 |
Mouse embryo | KLHDC3 | Kelch domain containing 3,transcript 1 (NM_027910) | Chromatin binding; meiotic recombination |
Bap1, Dnmt1, Thoc1, Cry3a, Bnipl, Brd4, Foxa2 and Kif20a are co-localized with Udu in the nucleus
According to the categories of gene ontology (GO, ref.22,23) and the known functions of Udu, eleven genes from both mouse and zebrafish Y2H data (Table 1 and 2) were selected for co-immunoprecipitation (co-IP) analysis to confirm whether these candidate proteins are indeed Gon4l/Udu-interacting proteins. Among those selected, eight were related to replication fork processing, histone/DNA modifications, or transcription processing. The other three were chosen because of their metabolic functions that have never been connected to Gon4l or Udu.
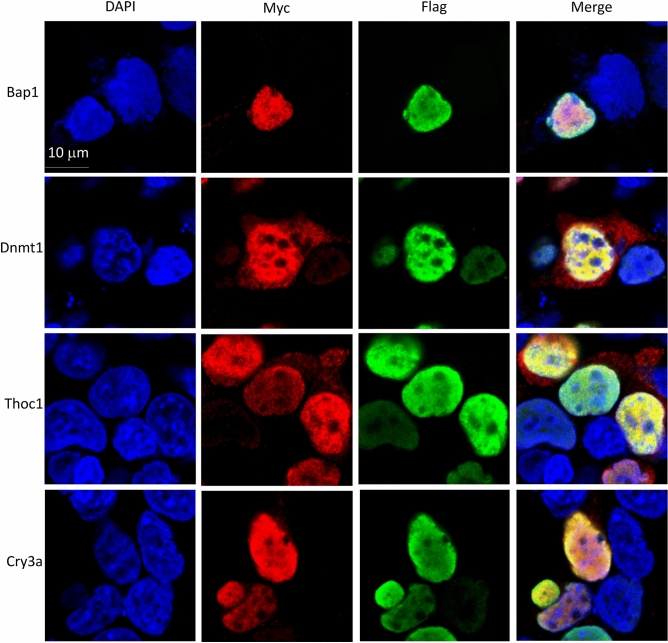
Because the important domains of mouse GON4L and zebrafish Udu are highly conserved3,7, we examined the interactions between zebrafish Udu and the zebrafish counterparts of selected proteins identified by different Y2Hs. We first cloned those candidate genes from zebrafish adult liver or embryonic cDNAs and fused them with Myc tag. Full-length zebrafish Udu (hereafter Udu-full) and Udu with only the PAH/SANT domain (hereafter Udu-P/S) were fused with Flag tag. Since Udu is known as a nuclear factor7,19, nuclear localization of Udu-full or Udu-P/S was first confirmed using immunofluorescence (Supplementary Fig. S1). Next, we co-transfected Flag-tagged Udu-full or Udu-P/S expressing constructs with Myc-tagged candidate protein constructs and performed double immunofluorescence staining and confocal microscopy to investigate their cellular localizations (Fig. 2 and Supplementary Fig. S2,S3). As compared to Udu-P/S, the transfection efficiency for Udu-full was relatively low. Therefore, we detected only a few cells co-expressing Udu-full and Myc-tagged candidate interacting proteins (Supplementary Fig. S2). We found that BRCA1-associated protein 1 (Bap1), DNA methyltransferase 1 (Dnmt1), Tho complex 1 (Thoc1), and Cryptochrome circadian regulator 3a (Cry3a) (Fig. 2 and Supplementary Fig. S2), as well as Bromodomain containing 4 (Brd4), Forkhead box a2 (Foxa2), and Rabkinesin-6 (Kif20a) were all co-localized with Udu-P/S in the nucleus (Supplementary Fig. S3). In addition to the major localization of Dnmt1 and Thoc1 in the nucleus, we also detected their lower expressions in the cytoplasm (Fig. 2), which are similar to previous studies24–26. Two alternative splice forms of human BCL2/adenovirus E1B interacting protein like (BNIPL), BNIP-Sα and BNIP-Sβ, have been reported to be expressed in the cytoplasm and nucleus, respectively27. Interestingly, we found some cells expressed both cytoplasmic and nuclear Bnipl (Supplementary Fig. S3), and therefore is co-localized with Udu in the nucleus. For those metabolism-related proteins, including Epoxide hydrolase 2 (Ephx2), Group specific component (Gc), and Lipoic acid synthetase (Lias), showed only cytoplasmic localization and are therefore unlikely to interact with Udu (Supplementary Fig. S3). Taken together, these results identified eight candidate proteins that may interact and cooperate with Udu in the nucleus.
Figure 2.
Confocal images for the cellular co-localization analysis of Udu and its interacting proteins. For co-localization analysis, Flag-tagged Udu-P/S construct was co-transfected individually with each Myc-tagged construct, including Bap1, Dnmt1, Thoc1, and Cry3a. Anti-FLAG M2 monoclonal antibody and rabbit anti-MYC antibody were used. Alexa Fluor 488-conjugated anti-mouse secondary antibody and Alexa Fluor 564-conjugated anti-rabbit secondary antibody were then applied. Green fluorescence indicates Udu-P/S, while red fluorescence indicates the expression of Myc-tagged proteins. Blue color is DAPI used for nuclear counterstain. Merged images show the nuclear co-localization of Udu and the interacting proteins.
Four newly identified interacting proteins (Bap1, Dnmt1, Thoc1, and Cry3a) indicate novel functions of Gon4l
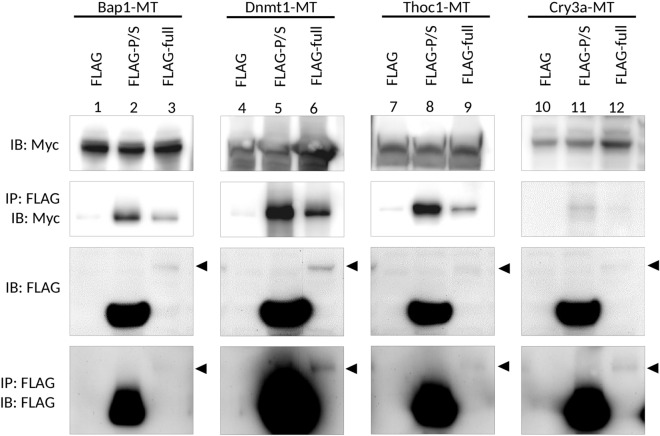
To analyze the direct interactions of these candidate proteins with Udu, the whole cell lysate was immunoprecipitated by anti-FLAG antibody and then subjected to Western blot analysis by anti-FLAG or anti-MYC antibody to detect whether the candidate proteins were co-immunoprecipitated with Udu-full or Udu-P/S. Only Bap1, Dnmt1, and Thoc1 proteins showed strong signals with Udu-P/S (Fig. 3, lane 2, 5, and 8). The Udu-P/S-binding affinity is weaker for Cry3a (Fig. 3, lane 11). Notably, although signals for both the expression level and the amount of immuoprecipitated Udu-full were very weak, milder interaction signals between Udu-full and Bap1, Dnmt1 or Thoc1 could still be detected (Fig. 3, lane 3, 6, and 9). Interaction signals for Udu-full and Cry3a were barely detectable (Fig. 3, lane 12). No co-IP signal for the other candidate proteins, including Bnipl, Brd4, Foxa2, Kif20a, Ephx2, Gc, and Lias, was detected (data not shown).
Figure 3.
Co-immunoprecipitation analysis for the interaction of Udu with Bap1, Dnmt1, Thoc1, and Cry3a. Flag-tagged control or Flag-tagged Udu-expressing constructs were co-transfected with different Myc-tagged candidate Udu-interacting proteins, including Bap1, Dnmt1, Thoc1, and Cry3a. The cell lysate was incubated with anti-FLAG M2 magnetic beads, then subjected to immunoblot with anti-MYC or anti-FLAG antibody. Arrowheads indicate the expected size of full-length Udu. Lanes 1 to 9 were cropped from the same blots, while lanes 10–12 were from the other ones. To observe the expression of full-length Udu, photographs using a longer exposure time of anti-FLAG immunoblots were taken. The original un-cropped immunoblots were shown in Supplementary Fig. S7a and photographs with shorter exposure time for anti-FLAG immunoblots were also included in Supplementary Fig. S7b as supporting information.
In addition to identifying proteins by co-IP experiments in this study, we also searched for more Gon4l-interacting proteins to get a clearer picture of multi-functional Gon4l protein. The Biological General Repository for Interaction Datasets (BioGRID) is a database that collects and archives protein, genetic, and physical interactions for model organisms, such as mice and humans (https://thebiogrid.org). Therefore, we used BioGRID to find Gon4l-interacting proteins that were identified from large-scale screenings but had never been specifically addressed in the literature. The dataset from mouse studies showed only 3 known Gon4l-interacting proteins, including YY1, Sin3A and Hdac1, all of which can co-regulate transcription of downstream genes (Supplementary Fig. S4a)14. We noticed that among ten physically interacting proteins in the datasets from humans, five belong to the histone protein family and two are related to transcriptional regulation (Supplementary Fig. S4b and Supplementary Table S1). This finding, together with our data, reinforces the role of Gon4l in regulating chromatin remodeling as well as gene expression.
Prediction for enriched phenotypes of Gon4l/Udu-interacting proteins leads to similar phenotypes observed in Gon4l/udu mutant animals
We then asked whether the phenotypic changes observed in Gon4l/udu mutant animals are correlated to the biological functions of its interacting proteins. Therefore, we applied model organism Phenotype Enrichment Analysis (modPhEA, Ref.28) to analyze phenotypes that are enriched by 20 Gon4l-interacting proteins (including those reported in the literature, ten other physically interacting proteins found in BioGRID datasets, and novel proteins identified in this study). The proteins used for modPhEA are listed in Supplementary Table S2. Among those genes input, five genes were ignored, because of the lack of annotated phenotypes in the database. According to the mouse phenome database from modPhEA (Gene Expression Database (GXD), Mouse Genome Informatics Web Site, MGI6.14, https://www.informatics.jax.org), the most enriched phenotypes are related to increased embryonic tissue cell apoptosis (p = 0.028), embryonic lethality (p = 0.014), increased T cell derived lymphoma incidence (p = 0.006), decreased cell proliferation (p = 0.012), chromosome instability (p = 0.018), and abnormal dopamine level (p = 0.014) (Table 3 and Supplementary Table S3). After focusing on Bap1, Dnmt1, Thoc1, and Cry3a identified in this study, we noticed that Bap1, Dnmt1, and Thoc1 were most related to increased embryonic tissue cell apoptosis (p = 0.001) (Supplementary Table S4). However, Cry3a did not account for the enrichment of apoptotic phenotype, indicating the specific importance of Bap1, Dnmt1, and Thoc1 proteins to cell survival during development (Supplementary Table S4). Additionally, we also analyzed the phenotype enrichment using the Caenorhabditis elegans database from modPhEA (WBPhenotype, data version: WormBase web site, https://www.wormbase.org, release WS273, November 2019). Similar to results obtained from mouse phenome, early embryonic lethal was enriched (p = 0.001) in the WBPhenotype database. Other phenotypes—such as nuclear morphology variation early emb (p = 1.2 × 10–5), sister chromatid segregation defective early emb (p = 6.3 × 10–5), embryonic cell morphology variant (p = 3.6 × 10–4), body elongation variant (p = 0.002), programmed cell death variant (p = 0.004), thin (p = 0.004), and reproductive system morphology variant (p = 0.007)—were also enriched by Gon4l-interacting proteins (Supplementary Table S5). These results were consistent with the reported phenotypes found in Gon4l/udu mutant organisms.
Table 3.
List of the most enriched phenotypes and their corresponding Udu/Gon4l-interacting genes after model organism Phenotype Enrichment Analysis of mouse phenome database.
| Phenotype name | BH FDR corrected P-value | Genes with the term |
|---|---|---|
| Embryo phenotype | 0.023 | mcm4, npat, thoc1, bap1, dnmt1, yy1, hdac1 |
| Increased embryonic tissue cell apoptosis | 0.028 | thoc1, dnmt1 |
| Mortality/aging | ||
| Prenatal lethality | 0.012 | mcm3, mcm4, thoc1, bap1, dnmt1, yy1, sin3a, hdac1 |
| Embryonic lethality, complete penetrance | 0.007 | mcm4, dnmt1, yy1, hdac1 |
| Embryonic lethality between implantation andsomite formation, complete penetrance | 0.036 | mcm4, sin3a |
| Neoplasm | ||
| Increased hemolymphoid system tumor incidence | 0.007 | mcm4, esr2, h2afx |
| Increased T cell derived lymphoma incidence | 0.006 | mcm4, h2afx |
| Cellular phenotype | ||
| Abnormal cell proliferation | 0.022 | mcm3, mcm4, dnmt1, esr2, h2afx, hdac1 |
| Decreased cell proliferation | 0.012 | mcm3, mcm4, dnmt1, h2afx, hdac1 |
| Chromosomal instability | 0.018 | mcm4, h2afx |
| Nervous system phenotype | ||
| Abnormal dopamine level | 0.014 | esr2, pnkd |
Analysis for the mRNA expression of Gon4l/Udu-interacting proteins, Bap1, Dnmt1, Thoc1, and Cry3a
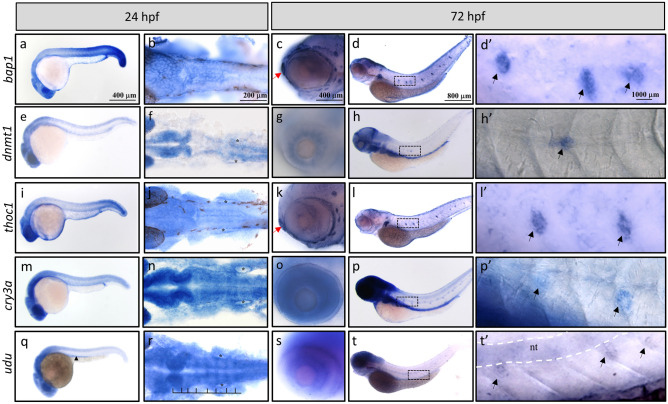
The above analyses indicated that the major biological functions of Gon4l/Udu may be mediated through these interacting proteins. For example, Bap1, Dnmt1, and Thoc1 are highly associated with increased embryonic tissue cell apoptosis and prenatal lethality (Table 3 and Supplementary Table S4). These enriched phenotypes are similar to the extensive amount of apoptotic cells and subsequent embryonic lethality observed in udutu24 mutants6,19, indicating a consequence from the loss of Udu interactions with Bap1, Dnmt1, and/or Thoc1. To further verify this hypothesis, we examined the mRNA expression patterns of bap1, dnmt1, and thoc1 during development. Due to the lack of reported phenotypes about Cry3a mutants in the modPhEA databases, we also checked the expression patterns of cry3a and compared those of four with that of udu at the same stages (Fig. 4 and Supplementary Fig. S5). We performed in situ hybridization for bap1 (Fig. 4a–d’ and Supplementary Fig. S5a–d), dnmt1 (Fig. 4e–h’ and Supplementary Fig. S5e–h), thoc1 (Fig. 4i–l’ and Supplementary Fig. S5i–l), and cry3a (Fig. 4m–p’ and Supplementary Fig. S5m–p). Surprisingly, expression patterns of these four genes were very similar to each other. All four genes were ubiquitously expressed at 6 h post fertilization (hpf) (Supplementary Fig. S5a,e,i,m), and became specifically expressed in the CNS, eyes, and tail region at 24 hpf (Fig. 4a,b,e,f,i,j,m,n). dnmt1, thoc1, and cry3a, different from bap1, are additionally expressed in the epithelium of otic vesicles (Fig. 4f,j,n). At 48 hpf, the signals in the trunk were getting weaker, while their expressions in the brain region remained strong (Supplementary Fig. S5c,g,k,o). Furthermore, bap1 and thoc1 started to be detected in the cranial neuromasts and olfactory sensory epithelium (Fig. 4c,k). All four genes were detected in eyes, pectoral fin buds, and neuromasts of the posterior lateral line at 48 hpf and 72 hpf (Fig. 4c,d’,g,h’,k,l’,o,p’, and Supplementary Fig. S5c,d,g,h,kl,o,p). Besides, strong expression signals of dnmt1 and cry3a were detected in the developing gut at 48 and 72 hpf (Fig. 4h,p, Supplementary Fig. S5g,h,o,p). Our observations for the expression patterns of dnmt1 mRNA were very similar to the previously reported expression patterns that restricted to the brain region, retina, branchial arches, pectoral fin buds, lateral line system and digestive system at later time points29,30. cry3a mRNA expression has been reported to be expressed in the brain, ganglion cell layer of retina, and the liver of zebrafish larvae31, as well as in the brain, the retina, the liver, and muscles in the adult zebrafish31,32. Our study presents here for the first time that in addition to those reported tissues, cry3a is also expressed in pectoral fin buds, the lateral line system and the digestive system (including liver and intestine) during development.
Figure 4.
Whole mount in situ hybridization analysis for temporal and spatial expression of the bap1, dnmt1, thoc1, cry3a, and udu genes during zebrafish early development. WISH of (a–d’) bap1, (e–h’) dnmt1, (i–l’) thoc1, (m–p’) cry3a, and (q–t’) udu expression at 24 hpf (a,b,e,f,i,j,m,n,q,r) and 72 hpf (c,d’,g,h’,k,l’,o,p’,s,t’) embryos. Black arrowhead indicates the pronephric duct (q). Rhombomere boundaries are indicated by black lines (r). Black asterisks indicate otic vesicles (f,j,n,r). Red arrows indicate olfactory sensory epithelium (c,k). Dashed rectangles in (d,h,l,p,t) are shown as enlarged images in (d’,h’,l’,p’,t’), respectively. Black arrows indicate neuromasts of the posterior lateral line (d’,h’,l’,p’,t’) and white dash lines indicate notochord (t’). Flat mount of WISH samples: (b,f,j,n,r). All embryos are oriented with anterior to the left.
We found that, similar to previous reported observations, udu mRNA was expressed in a ubiquitous manner at early stages (Supplementary Fig. S5q). At 24 hpf, udu expression was detected in the CNS, eyes, and pronephric duct (Fig. 4q,r). We also noticed a boundary-specific expression of udu in the rhombomeres (Fig. 4r) at 24 hpf. Similar to dnmt1, thoc1, and cry3a, udu expression was detected in the otic vesicles (Fig. 4f,j,n,r). At 48 and 72 hpf, udu started to be detected in retina and weakly expressed in the notochord (Fig. 4s,t and Supplementary Fig.q S5s,t). Moreover, udu was also detected in neuromasts of the posterior lateral line (Fig. 4t–t’). Therefore, these data further indicate that during development udu is largely co-expressed with bap1, dnmt1, thoc1, and cry3a, all of which cooperate with each other to maintain cell survival in a variety of tissues, such as the embryo proper of early stages, CNS, eyes, and neuromasts.
Discussion
The Udu protein in zebrafish, as well as its homologs among species, is a nuclear factor that possesses pleiotropic functions and influences the differentiation and proliferation of various cell types. Gon4l can collaborate with YY1, SIN3A and HDAC1 to repress gene expression and has been suggested to act as a co-regulator for transcription or as a platform for transcriptional complex formation14. In addition to direct regulating gene expression, Gon4l may also be important for pathways related to cell division and maintenance of genomic stability19, but how Gon4l/Udu exerts its function is still unclear. In this study, we used Y2H to identify novel Gon4l/Udu-interacting proteins, which is further confirmed by co-IP analysis to clarify the possible unknown functions of Gon4l/Udu. So far, we have identified four novel Gon4l/Udu-interacting proteins: Bap1, Dnmt1, Thoc1, and Cry3a. Interestingly, we perceived that zebrafish and mouse Y2Hs isolated non-overlapping Gon4l/Udu-interacting candidates. The lack of overlap may be due to the following reasons. First, the screening scale of the zebrafish Hybrigenics Y2H is about 5 to 10 folds higher than that of mouse Myriad Genetics. Second, the zebrafish prey library is made only from embryos; in contrast, three different mouse prey libraries are made from embryos, B cells, and cardiac cells, respectively. Third, and most importantly, the Hybrigenics system uses fragmented cDNAs for constructing prey library, while that of Myriad Genetics basically constructs the prey libraries with full-length cDNAs. This distinction gives rise to the differences in expressivity, toxicity, and interactivity of prey proteins.
It has been demonstrated that DNA double-strand break (DSB)-induced DNA damage response (DDR) is activated in udutu24 mutants19. However, the molecular mechanism of how Gon4l/Udu induces DDR remains unknown. The nuclear localized deubiquitylating enzyme BAP133,34, which is responsible for histone modification in transcription regulation35,36, can be phosphorylated by Ataxia telangiectasia mutated (ATM) and participates in DNA double-strand break (DSB) repair through regulating histone 2A and H2AX ubiquitylation37,38. Moreover, according to the online database BioGRID, Gon4l/Udu physically interacts with H2AX and other histone subtypes (Supplementary Table S1). Based on these data and the newly identified interaction between Bap1 and Gon4l/Udu, Bap1 may provide a key connection between Gon4l/Udu and DDR.
The most abundant DNA methyltransferase, DNMT1, is considered a major enzyme for maintaining global DNA methylation in concert with other histone modifiers and regulates gene transcription and genomic integrity39. It has been shown that lack of Dnmt1 expression leads to impaired self-renewal of hematopoietic stem cells (HSCs) in both adult mice and zebrafish models40,41. Down regulation of cebpa is crucial for the differentiation of pre-pro B cells to early pro-B cells42–44. In contrast to wild type, the mRNA level of cebpa is significantly up-regulated in the B cell progenitors of Justy mutant mice3. Further analysis using double morpholino injection revealed that Dnmt1 maintains HSCs and progenitor cells in zebrafish through regulating cebpa expression40. Similar defective hematopoietic phenotypes were also observed in Gon4l/udu mutants. The proliferation and differentiation of pre-pro B cells to early pro-B cells are impaired in Justy mutant mice3,4. In zebrafish, udutu24 mutants exhibited fewer blood cells and defective proliferation and differentiation of erythrocytes were observed in udusq3 mutants6,7. Although hematopoietic defects were not specifically described in the study using uduvu66 mutant embryos, its supplementary data using DamID-seq at the gastrula stage shows that the promoter region of cebpa was Udu-enriched, indicating that cebpa may be directly regulated by Udu8. Consistently, up-regulation of cebpa was observed in udutu24 mutants at 22 hpf (Supplementarey Fig. S6) as compared to wild type. According to these findings, we propose that the interaction between Gon4l/Udu with Dnmt1 may be responsible for regulating cebpa expression and, therefore, play an important role during hematopoiesis.
Thoc1 protein is an essential component of Tho/Trex complex to restrain harmful R-loops during transcription45,46. A study of Thoc1-interacting proteins reveals that Thoc1 restrains R-loops not only through direct RNA-binding but also through cooperation with Sin3a to promote transient histone deacetylation after transcription47, which is consistent with the fact that Gon4l associates with Sin3a, HDAC1, and YY1 as a part of a complex to suppress gene expression at the transcriptional level14. Additionally, Gon4l/Udu also interacts with Mcm3 and Mcm4 during DNA replication19. Therefore, it may be important to elucidate whether Gon4l/Udu integrates with Mcm3/Mcm4, Sin3a and Thoc1 together during transcription and DNA replication to prevent R-loop formation as well as subsequent head-on transcription-replication collisions, genomic instability, and cell death.
According to the RNA in situ hybridization data from this study and previous reports, we have found that the expression pattern of udu is mainly similar to those of dnmt1, bap1, and thoc1 genes, especially in the pectoral fin buds, eyes, CNS, and neuromasts of the posterior lateral line (Fig. 4). In addition to similar expression patterns of these genes, phenotypes including extensive apoptotic cells in head, neural tube, and tail, as well as decreased size of eyes and head have also been described in udu6,19, dnmt130,48, bap149,50, and thoc151 knockdown or mutant animals. In dnmt1 mutant zebrafish, defective lens with dysplasia have been observed30. Additionally, inherited mutations in human DNMT1 have been associated with neurodegenerative syndromes that are characterized by degeneration of the cerebellum and of the acoustic and optic nerves48. In Xenopus, expression of bap1 is restricted in the neural crest cells in early stages, and loss of bap1 results in abnormal gastrulation and malformation of ocular structure49. Zebrafish embryos injected with bap1 morpholino exhibited necrotic CNS50. Thoc1 homozygous null mouse is embryonic lethal due to higher apoptosis51. Moreover, results obtained from modPhEA analyses revealed the role of dnmt1, bap1, and thoc1 in maintenance cell survival of embryos. According to the expression pattern, phenotypic analyses, and evidence for protein interactions, we suggest that udu, dnmt1, bap1, and thoc1 may coordinately be syn-expressed, interact with each other, and function in the same processes during CNS development.
Cryptochromes (CRY) are involved in the circadian rhythms of plants and animals. In zebrafish, synchronization of circadian clock with cell cycle progression has been observed52. Compared to mammals, zebrafish retain six cryptochrome (cry) genes53. Also, it has been shown that Cry3a (also known as Cry1ba, a homolog for human CRY1) is able to negatively regulate transcriptional activity of circadian transcription factor Clock1a:Bmal1b53, indicating conserved functions for those clock genes among species. Expression of cry3a mRNA has been identified in ventral telencephalon, retina, and intestine at 5 dpf32. In adult zebrafish, it is expressed in the brain, muscle, heart, and the liver31. Although only weak protein interaction signals between Udu and Cry3a (Fig. 3) have been detected, the cellular function of circadian regulator CRY1 in mammals and the syn-expressing patterns of cry3a in zebrafish raise the necessity to investigate in the future whether Gon4l/Udu can influence cell cycle, DNA damage response, and genomic stability through interacting with Cry3a.
In addition to the genes discussed above, Cramped-like (Cramp1l or Crm) has been shown to interact with Gon4l in a large-scale human interactome study21. CRAMP1L and GON4L can also interact with MST1 when naïve CD4 + T cells differentiate into regulatory T cells (Treg) in another protein–protein interactome analysis17. Furthermore, Cramp1l is a polycomb group protein encoded gene that is dynamically regulated during cell cycle progression, peaks at S phase, and interacts with proliferating cell nuclear antigen (PCNA)54,55. In cramp1l mutants, the expression of histone H1, but not other histones, is greatly decreased54. Cramp1l has been shown to interact with another GON4L-interacting protein, NPAT (Nuclear Protein, Ataxia-Telangiectasia Locus)16. NPAT is a substrate for Cyclin E/CDK-2 and is responsible for biogenesis of histone locus body and histone gene transcription15,56. Therefore, Cramp1l and NPAT, together with Bap1, indicate that Gon4l/Udu may be essential for the connection between cell proliferation and histone expression/modification.
Gon4l has been considered as a negative transcription regulator that inhibits gene expression probably through interacting with YY1, SIN3A, and HDAC1. In this study, we have identified four novel Gon4l/Udu-interacting proteins. These newly identified proteins not only confirm the association of Gon4l/Udu with histone biogenesis, as demonstrated in previous reports, but also indicate that Gon4l/Udu can possibly regulate both histone modifications and CpG DNA methylation to influence various cellular events. Furthermore, Gon4l may also maintain genomic integrity while controlling transcription and cell proliferation. Overall, these findings provide a basis for better comprehending the pleiotropic effects of Gon4l/Udu.
Methods
Yeast two-hybrid (Y2H) screening
The yeast two-hybrid (Y2H) assays for zebrafish Udu and mouse Gon4l were performed by Hybrigenics (Paris, France) and Myriad Genetics (California, USA), respectively. Briefly, LexA and Gal4 system based on transcriptional activation of reporter genes was used to detect protein interactions. Bait fragments used for zebrafish Udu were the N-terminal CR1-CR3 (165–1,470 a.a.) and the C-terminal PAH and SANT domains (1,523–2,054 a.a.). The corresponding fragments of zebrafish Udu were cloned, checked by sequencing, and used as bait to screen for protein interactions using zebrafish embryo RP1 library (stages 18–20 hpf). A total of 56 million and 82 million colonies for Udu (165–1,470) and Udu (1,523–2,054) were analyzed for the interaction, respectively. The resulting sequences were searched against GenBank using an automated procedure. Bait fragments used for mouse Gon4l were the two PAH domains (1,458–1,857 a.a.) and SANT domain (1,858–2,261 a.a.). The two fragments were cloned and used for protein interactions with prey libraries from murine embryos, B lymphocytes (B cells), and cardiac myocytes. Around 5–10 million colonies were obtained after each mating, then picked from the selection plates and the prey inserts were identified by sequence analysis. To confirm the interactions, the bait and prey plasmid DNAs were isolated and co-transformed into a naïve yeast strain to recapitulate the interaction.
Zebrafish maintenance and embryos
Zebrafish (Danio rerio) were raised and kept under standard conditions according to a previous report57. Wild-type and udutu24 embryos were staged as described before58. All experimental procedures on zebrafish and their embryos were approved by the Institutional Animal Care and Use Committee of the National Health Research Institutes, Taiwan (NHRI-IACUC-106063-A) and carried out in accordance with the approved guidelines. Only zebrafish and no other animals were directly involved in this study.
RNA Isolation and Reverse Transcription
RNA was isolated from WT embryos harvested at 1, 2, 3, 4, and 6 dpf, as well as from adult zebrafish livers, following the instructions of the NucleoSpin RNA kit (Macherey–Nagel GmbH & Co. KG). One microgram of total RNA was used for subsequent reverse transcription using GoScript Reverse Transcriptase (Promega). The obtained cDNA was used for subsequent PCR cloning.
Plasmid construction
Full-length zebrafish udu C-terminally fused with Flag tag was PCR amplified. PCR product was subcloned into pcDNA3.1(+) at EcoRV/NotI sites. For the expression of PAH and SANT domains of Udu, pcDNA3.1(+)-flag-udu-P/S was used19. To perform co-IP analysis, candidate Udu-interacting genes were cloned from cDNA. For bnipl, brd4, cry3a, ephx2, foxa2, gc, kif20a and lias, sequence of Myc tag was added to their antisense primers. The PCR product of cry3a was digested with EcoRV/NotI and directly cloned into the corresponding sites of pcDNA3.1(+). The PCR products for bnipl, brd4, ephx2, foxa2, gc, kif20a and lias were first cloned into pJet1.2 vector and subcloned into pcDNA3.1(+) with NotI/XbaI. The PCR product for bap1 was first cloned into pJet1.2 vector and subcloned into pCS2 + MT vector with XhoI/XbaI. The PCR products for dnmt1 and thoc1 were first cloned into pJet1.2 vector and subcloned into pcDNA3.1(+)-MT vector with EcoR1/Not1 and Not1/Xba1, respectively. The accession numbers for these genes and their corresponding primer pairs used in this study are listed in Supplementary Table S6. All PCR amplification of interested genes was performed using Q5 high fidelity polymerase PCR (NEB). Sequences for all the cloned genes were confirmed by the DNA Sequencing Core Lab (NHRI, Taiwan).
Cell culture and transfection
HEK293 or COS-7 cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen). For immunofluorescent staining, 2 × 106 cells/well were seeded in a 6-well plate one day before transfection. One microgram of Myc-tagged constructs and/or one microgram Flag-tagged udu constructs were transfected into HEK293 cells using Lipofectamine 3,000 transfection reagent (Invitrogen). For co-IP analysis, 4 × 106 HEK293 cells or 2 × 106 COS-7 cells were seeded in a 60-mm dish one day before transfection. Two micrograms of Myc-tagged constructs and two micrograms Flag-tagged udu constructs were co-transfected. The pCS2-flag vector was used as a negative control for these experiments.
Immunofluorescence staining and microscopic analysis
To examine the expression pattern of Udu, Udu-P/S and all the interacting candidate proteins, immunofluorescent staining was performed 1 day after transfecting plasmids into HEK293 cells. After washed with PBS, fixed with 4% paraformaldehyde and permeabilized with PBST (0.3% TritonX-100), the cells were blocked with 5% BSA/PBST. Incubation with mouse anti-FLAG antibody (F1804, 1:100 dilution; Sigma-Aldrich) or rabbit anti-MYC antibody (sc-789, 1:100 dilutions, Santa Cruz Biotechnology) at 4 °C overnight was followed by overnight incubation with Alexa Fluor 488-conjugated anti-mouse or Alexa Fluor 564-conjugated anti-rabbit secondary antibody (Molecular Probes). SlowFad Diamond Antifade Mountant with DAPI (Thermo Fisher Scientific) was used for nuclear counterstain and mounting. The fluorescent signals were taken by Axio Imager A1 (Zeiss) or TCS SP5 confocal microscope (Leica).
Co-immunoprecipitation, SDS-PAGE and Western blot analysis
Two days after transfection, cells were harvested and lysed with Pierce IP Lysis Buffer (25 mM Tris–HCl pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 5% glycerol) with the addition of protease inhibitor cocktail (Sigma, P8340). The lysates were clarified by centrifugation and determined for their concentration using Rapid Gold BCA Protein Assay Kit (Thermo Fisher Scientific). A total of 40 μl of anti-FLAG M2 magnetic beads (Sigma-Aldrich) was washed with lysis buffer and added to 800 μg of total protein lysates. After overnight incubation at 4 °C, supernatants were removed and the magnetic beads were washed in lysis buffer for 4 times. A total of 80 μl of co-IP elution buffer (0.1 M glycine HCl, pH 3.5, buffer) was added to each sample. The samples were incubated with gentle shaking for 5 min at room temperature to elute precipitated proteins. Then the magnetic beads were spun down and the supernatants were transferred immediately to fresh Eppendorf vials containing 10 μl of neutralizing buffer (0.5 M Tris–HCl, pH 7.4, 1.5 M NaCl). SDS-PAGE and subsequent Western blot analyses were performed with mouse anti-FLAG M2 (F1804, 1:5,000 dilution, Sigma-Aldrich) or rabbit anti-MYC antibody (sc-789, 1:10,000 dilution, Santa Cruz Biotechnology). To enhance the signals, Western Blot Ag Signal Enhancer (Biomate) was used prior blocking the PVDF membranes with 5% skim milk (BD Difco) according to the manufacturer’s instructions. Mouse or rabbit secondary antibody conjugated with HRP (1:10,000 dilution; R&D Systems) was applied. The chemiluminescent signals were visualized by Immobilon HRP substrate kit (Millipore) and captured by the BioSpectrumAC Imaging System (UVP). Full-length blots are included in Supplementary Fig. S7 as supporting information. Western blots for Bap1, Dnmt1, and Thoc 1 were run in the same gel, while Cry3a was run separately.
Phenotypic enrichment analysis using model organism phenotype enrichment analysis (modPhEA)
The model organism Phenotype Enrichment Analysis (modPhEA) is a freely available tool that collects phenotypic data from both mutagenesis and knockdown animal experiments among different species, including budding yeast, roundworm, fruit fly, zebrafish, mouse, and human28. modPhEA reports enriched phenotypes that are associated with a given group of genes. Therefore, modPhEA was used to analyze phenotypes enriched by Gon4l/Udu-interacting proteins. Twenty genes encoding for Gon4l/Udu-interacting proteins with mouse Ensembl IDs were input manually as dataset 1 and compared against the rest of the genes in the genome that represent the background (dataset 2). Enrichment analyses were performed based on phenotype databases from either mouse or Caenorhabditis elegans because of their most abundant results obtained after analyses. To calculate and compare the differences between these two groups of genes (set 1 and set 2), Fisher’s Exact Test with differentially enriched hypothesis was applied. The Benjamini–Hochberg Procedure was used for correction of the p-value to decrease the false-discovery rate.
Probe preparation and in situ hybridization
For bap1 probe template preparation, an 842 bp fragment of bap1 C-terminal was PCR amplified from diluted pCS2-MT-bap1 plasmid DNA (primer pair was listed in Supplementary Table S6). The PCR product was cleaned up, and ligated to pJet1.2 vector. After checking the orientation of the bap1 insert, the pJet1.2-bap1-antisense-842 bp plasmid was linearized with Xba1 for subsequent probe synthesis using MEGAscript T7 Transcription kit (Thermo Fisher Scientific). For dnmt1 probe synthesis, a 3,197 bp fragment of dnmt1 was PCR-amplified from pcDNA3-MT-dnmt1 plasmid DNA (primer pair was listed in Supplementary Table S6). The PCR product was cleaned up, and ligated to pJet1.2 vector. After checking the orientation of the dnmt1 insert, the plasmid was linearized with EcoR1 for subsequent probe synthesis with T7 polymerase (Thermo Fisher Scientific). For thoc1 probe synthesis, we cut the ready-made pJet1.2-thoc1-antisense full-length plasmid with SacI. After enzyme digestion, a 649 nt thoc1 probe was synthesized by MEGAscript T7 Transcription kit. For cry3a probe synthesis, a 1865 bp fragment of cry3a was PCR-amplified from pcDNA3-cry3a-MT plasmid DNA (primer pair was listed in Supplementary Table S6). The PCR product was cleaned up, and ligated to pJet1.2 vector. After checking the orientation, the plasmid was linearized with Xba1 for subsequent probe synthesis with T7 polymerase (Thermo Fisher Scientific). For udu probe synthesis, we cloned full-length udu into pGEMT-easy. After checking the orientation of the insert, the plasmid was digested with BamHI and a probe around 4.1 kb was synthesized using MEGAscript SP6 Transcription kit (Thermo Fisher Scientific). The bap1 and thoc1 probes were labeled with fluorescein labeling mix (Roche), while the udu probe was labeled with digoxigenin labeling mix (Roche). Detailed methods for probe synthesis and removal of template DNA were according to the manufacturer’s protocol. To examine the expression patterns of bap1, thoc1, and udu during zebrafish development, embryos/larvae were harvested at 3, 6, 22, 24, 36, 48, and 72 hpf. Whole mount in situ hybridization was performed as described previously59. Embryos were mounted in glycerol (Sigma) or Murray’s Clear (BABB, benzyl alcohol: benzyl benzoate = 1:2), and images were taken by Zeiss Axiovision Imager A1 or Zeiss Discovery V8.
Quantitative real time PCR (qPCR) analysis
RNA was extracted from wild type and udutu24 embryos at 22 hpf as previously described. 1 μg of total RNA was reversely transcribed, and the cDNA was diluted and amplified with the respective primers listed in Supplementary Table S6. gapdh was used as an internal control. The SYBR Green-based qPCR was carried out on Roche LC480 II using SensiFAST SYBR Hi-ROX Kit (Bioline). The program for qPCR was as follows: one cycle of 95 °C for 5 min, followed by 45 cycles of 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 20 s. A program for melting curve was added to examine for the existence of primer dimers or non-specific signals. Data were expressed as fold-change compared to wild type sample (mean ± SD).
Supplementary information
Acknowledgements
We are grateful to the staff in the Zebrafish Facility of NHRI for their efforts in maintaining fish stocks and Dr. Da-Wei Liu for his technical assistance. We also thank Dr. Tzu-Min Chan and Shu-Hung Chu for cloning the Udu-P/S and Bap1-expressing constructs, respectively. This work was supported by the Agency of Science, Technology and Research (A*STAR), Singapore; by the National Health Research Institutes, Taiwan (MG-102-PP-13, MG-103-PP-13, and MG-109-PP-9); and by grants from the Ministry of Science and Technology, Taiwan (MOST 106-2311-B-400-003-MY3, MOST 107-2319-B-400-002-, MOST 108-2319-B-400-002-, and MOST 109-2740-B-400-001-).
Author contributions
Conceived and designed the experiments: Y.J.J. Performed the experiments: S.M.T., K.C.C. Analyzed the data: S.M.T, Y.J.J. Contributed reagents/materials/analysis tools: Y.J.J. Wrote the paper: S.M.T., Y.J.J.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-70855-9.
References
- 1.Friedman L, Santa Anna-Arriola S, Hodgkin J, Kimble J. gon-4, a cell lineage regulator required for gonadogenesis in Caenorhabditis elegans. Dev. Biol. 2000;228:350–362. doi: 10.1006/dbio.2000.9944. [DOI] [PubMed] [Google Scholar]
- 2.Bulchand S, Menon SD, George SE, Chia W. Muscle wasted: a novel component of the Drosophila histone locus body required for muscle integrity. J. Cell Sci. 2010;123:2697–2707. doi: 10.1242/jcs.063172. [DOI] [PubMed] [Google Scholar]
- 3.Lu P, et al. The Justy mutation identifies Gon4-like as a gene that is essential for B lymphopoiesis. J. Exp. Med. 2010;207:1359–1367. doi: 10.1084/jem.20100147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr JY, Goodfellow RX, Colgan DF, Colgan JD. Early B cell progenitors deficient for GON4L fail to differentiate due to a block in mitotic cell division. J. Immunol. 2017;198:3978–3988. doi: 10.4049/jimmunol.1602054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarzenbacher H, et al. A frameshift mutation in GON4L is associated with proportionate dwarfism in Fleckvieh cattle. Genet. Sel. Evol. 2016;48:25. doi: 10.1186/s12711-016-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammerschmidt M, et al. Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish Danio rerio. Development. 1996;123:143–151. doi: 10.1242/dev.123.1.143. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, et al. The zebrafish udu gene encodes a novel nuclear factor and is essential for primitive erythroid cell development. Blood. 2007;110:99–106. doi: 10.1182/blood-2006-11-059204. [DOI] [PubMed] [Google Scholar]
- 8.Williams MLK, et al. Gon4l regulates notochord boundary formation and cell polarity underlying axis extension by repressing adhesion genes. Nat. Commun. 2018;9:1319. doi: 10.1038/s41467-018-03715-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spronk CA, et al. The Mad1-Sin3B interaction involves a novel helical fold. Nat. Struct. Biol. 2000;7:1100–1104. doi: 10.1038/81944. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Clark I, Nicholson PR, Herskowitz I, Stillman DJ. The Saccharomyces cerevisiae SIN3 gene, a negative regulator of HO, contains four paired amphipathic helix motifs. Mol. Cell. Biol. 1990;10:5927–5936. doi: 10.1128/mcb.10.11.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aasland R, Stewart AF, Gibson T. The SANT domain: a putative DNA-binding domain in the SWI-SNF and ADA complexes, the transcriptional co-repressor N-CoR and TFIIIB. Trends Biochem. Sci. 1996;21:87–88. [PubMed] [Google Scholar]
- 12.Boyer LA, et al. Essential role for the SANT domain in the functioning of multiple chromatin remodeling enzymes. Mol. Cell. 2002;10:935–942. doi: 10.1016/s1097-2765(02)00634-2. [DOI] [PubMed] [Google Scholar]
- 13.Boyer LA, Latek RR, Peterson CL. The SANT domain: a unique histone-tail-binding module? Nat. Rev. Mol. Cell Biol. 2004;5:158–163. doi: 10.1038/nrm1314. [DOI] [PubMed] [Google Scholar]
- 14.Lu P, et al. The developmental regulator protein Gon4l associates with protein YY1, co-repressor Sin3a, and Histone Deacetylase 1 and mediates transcriptional repression. J. Biol. Chem. 2011;286:18311–18319. doi: 10.1074/jbc.M110.133603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duronio RJ, Marzluff WF. Coordinating cell cycle-regulated histone gene expression through assembly and function of the Histone Locus Body. RNA Biol. 2017;14:726–738. doi: 10.1080/15476286.2016.1265198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang XC, et al. A conserved interaction that is essential for the biogenesis of histone locus bodies. J. Biol. Chem. 2014;289:33767–33782. doi: 10.1074/jbc.M114.616466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H, et al. Hippo kinases Mst1 and Mst2 sense and amplify IL-2R-STAT5 signaling in regulatory T cells to establish stable regulatory activity. Immunity. 2018;49:899–914. doi: 10.1016/j.immuni.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal N, et al. GON4L drives cancer growth through a YY1-androgen receptor-CD24 axis. Cancer Res. 2016;76:5175–5185. doi: 10.1158/0008-5472.CAN-16-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim C-H, Chong S-W, Jiang Y-J. Udu deficiency activates DNA damage checkpoint. Mol. Biol. Cell. 2009;20:4183–4193. doi: 10.1091/mbc.e09-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang D, Wang D, Sun F. Drosophila melanogaster heterochromatin protein HP1b plays important roles in transcriptional activation and development. Chromosoma. 2011;120:97–108. doi: 10.1007/s00412-010-0294-5. [DOI] [PubMed] [Google Scholar]
- 21.Huttlin EL, et al. Architecture of the human interactome defines protein communities and disease networks. Nature. 2017;545:505–509. doi: 10.1038/nature22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashburner M, et al. Gene ontology: tool for the unification of biology The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Gene Ontology Consortium The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47:D330–D338. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honeywell RJ, Sarkisjan D, Kristensen MH, de Klerk DJ, Peters GJ. DNA methyltransferases expression in normal tissues and various human cancer cell lines, xenografts and tumors. Nucleosides Nucleotides Nucleic Acids. 2018;37:696–708. doi: 10.1080/15257770.2018.1498516. [DOI] [PubMed] [Google Scholar]
- 25.Inano K, et al. Maintenance-type DNA methyltransferase is highly expressed in post-mitotic neurons and localized in the cytoplasmic compartment. J. Biochem. 2000;128:315–321. doi: 10.1093/oxfordjournals.jbchem.a022755. [DOI] [PubMed] [Google Scholar]
- 26.Gasparri F, Sola F, Locatelli G, Muzio M. The death domain protein p84N5, but not the short isoform p84N5s, is cell cycle-regulated and shuttles between the nucleus and the cytoplasm. FEBS Lett. 2004;574:13–19. doi: 10.1016/j.febslet.2004.07.074. [DOI] [PubMed] [Google Scholar]
- 27.Zhou YT, Soh UJ, Shang X, Guy GR, Low BC. The BNIP-2 and Cdc42GAP homology/Sec14p-like domain of BNIP-Salpha is a novel apoptosis-inducing sequence. J. Biol. Chem. 2002;277:7483–7492. doi: 10.1074/jbc.M109459200. [DOI] [PubMed] [Google Scholar]
- 28.Weng MP, Liao BY. modPhEA: model organism Phenotype Enrichment Analysis of eukaryotic gene sets. Bioinformatics. 2017;33:3505–3507. doi: 10.1093/bioinformatics/btx426. [DOI] [PubMed] [Google Scholar]
- 29.Rai K, et al. Zebra fish Dnmt1 and Suv39h1 regulate organ-specific terminal differentiation during development. Mol. Cell. Biol. 2006;26:7077–7085. doi: 10.1128/MCB.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tittle RK, et al. Uhrf1 and Dnmt1 are required for development and maintenance of the zebrafish lens. Dev. Biol. 2011;350:50–63. doi: 10.1016/j.ydbio.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang M, Zhong Z, Zhong Y, Zhang W, Wang H. The zebrafish period2 protein positively regulates the circadian clock through mediation of retinoic acid receptor (RAR)-related orphan receptor alpha (Roralpha) J. Biol. Chem. 2015;290:4367–4382. doi: 10.1074/jbc.M114.605022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haug MF, Gesemann M, Lazovic V, Neuhauss SC. Eumetazoan cryptochrome phylogeny and evolution. Genome Biol Evol. 2015;7:601–619. doi: 10.1093/gbe/evv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H, et al. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol. Cell. Biol. 2010;30:5071–5085. doi: 10.1128/MCB.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dey A, et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science. 2012;337:1541–1546. doi: 10.1126/science.1221711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campagne A, et al. BAP1 complex promotes transcription by opposing PRC1-mediated H2A ubiquitylation. Nat. Commun. 2019;10:348. doi: 10.1038/s41467-018-08255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou W, Wang X, Rosenfeld MG. Histone H2A ubiquitination in transcriptional regulation and DNA damage repair. Int. J. Biochem. Cell Biol. 2009;41:12–15. doi: 10.1016/j.biocel.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Ismail IH, et al. Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res. 2014;74:4282–4294. doi: 10.1158/0008-5472.CAN-13-3109. [DOI] [PubMed] [Google Scholar]
- 38.Yu H, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc. Natl. Acad. Sci. USA. 2014;111:285–290. doi: 10.1073/pnas.1309085110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hervouet E, Peixoto P, Delage-Mourroux R, Boyer-Guittaut M, Cartron PF. Specific or not specific recruitment of DNMTs for DNA methylation, an epigenetic dilemma. Clin. Epigenet. 2018;10:17. doi: 10.1186/s13148-018-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, et al. DNA methyltransferase 1 functions through C/ebpa to maintain hematopoietic stem and progenitor cells in zebrafish. J. Hematol. Oncol. 2015;8:15. doi: 10.1186/s13045-015-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trowbridge JJ, Snow JW, Kim J, Orkin SH. DNA methyltransferase 1 is essential for and uniquely regulates hematopoietic stem and progenitor cells. Cell Stem Cell. 2009;5:442–449. doi: 10.1016/j.stem.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeKoter RP, Singh H. Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science. 2000;288:1439–1441. doi: 10.1126/science.288.5470.1439. [DOI] [PubMed] [Google Scholar]
- 43.Heavey B, Charalambous C, Cobaleda C, Busslinger M. Myeloid lineage switch of Pax5 mutant but not wild-type B cell progenitors by C/EBPalpha and GATA factors. EMBO J. 2003;22:3887–3897. doi: 10.1093/emboj/cdg380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rumfelt LL, Zhou Y, Rowley BM, Shinton SA, Hardy RR. Lineage specification and plasticity in CD19- early B cell precursors. J. Exp. Med. 2006;203:675–687. doi: 10.1084/jem.20052444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allison DF, Wang GG. R-loops: formation, function, and relevance to cell stress. Cell Stress. 2019;3:38–46. doi: 10.15698/cst2019.02.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas M, White RL, Davis RW. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc. Natl. Acad. Sci. USA. 1976;73:2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salas-Armenteros I, et al. Human THO-Sin3A interaction reveals new mechanisms to prevent R-loops that cause genome instability. EMBO J. 2017;36:3532–3547. doi: 10.15252/embj.201797208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winkelmann J, et al. Mutations in DNMT1 cause autosomal dominant cerebellar ataxia, deafness and narcolepsy. Hum. Mol. Genet. 2012;21:2205–2210. doi: 10.1093/hmg/dds035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuznetsov JN, et al. BAP1 regulates epigenetic switch from pluripotency to differentiation in developmental lineages giving rise to BAP1-mutant cancers. Sci. Adv. 2019;5:1738. doi: 10.1126/sciadv.aax1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tse WK, et al. Genome-wide loss-of-function analysis of deubiquitylating enzymes for zebrafish development. BMC Genom. 2009;10:637. doi: 10.1186/1471-2164-10-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Chang Y, Li Y, Zhang X, Goodrich DW. Thoc1/Hpr1/p84 is essential for early embryonic development in the mouse. Mol Cell Biol. 2006;26:4362–4367. doi: 10.1128/MCB.02163-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laranjeiro R, Tamai TK, Letton W, Hamilton N, Whitmore D. Circadian clock synchronization of the cell cycle in zebrafish occurs through a gating mechanism rather than a period-phase locking process. J Biol Rhythms. 2018;33:137–150. doi: 10.1177/0748730418755583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C, et al. Molecular evolution and functional divergence of zebrafish (Danio rerio) cryptochrome genes. Sci. Rep. 2015;5:8113. doi: 10.1038/srep08113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibert JM, Karch F. The Polycomb group protein CRAMPED is involved with TRF2 in the activation of the histone H1 gene. Chromosoma. 2011;120:297–307. doi: 10.1007/s00412-011-0312-2. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto Y, Girard F, Bello B, Affolter M, Gehring WJ. The cramped gene of Drosophila is a member of the Polycomb-group, and interacts with mus209, the gene encoding proliferating cell nuclear antigen. Development. 1997;124:3385–3394. doi: 10.1242/dev.124.17.3385. [DOI] [PubMed] [Google Scholar]
- 56.Sagara M, et al. Characterization of functional regions for nuclear localization of NPAT. J. Biochem. 2002;132:875–879. doi: 10.1093/oxfordjournals.jbchem.a003300. [DOI] [PubMed] [Google Scholar]
- 57.You M-S, et al. A Sketch of the Taiwan Zebrafish Core Facility. Zebrafish. 2016;13:S24–S29. doi: 10.1089/zeb.2015.1208. [DOI] [PubMed] [Google Scholar]
- 58.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 59.Hsu C-H, et al. A new mib allele with a chromosomal deletion covering foxc1a exhibits anterior somite specification defect. Sci. Rep. 2015;5:10673. doi: 10.1038/srep10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.