Abstract
A 68-year-old man was followed up with chronic kidney disease. Follow-up CT incidentally detected a tumor at the left kidney and multiple small nodular shadows in the lungs bilaterally. The patient underwent needle biopsy and was diagnosed with Xp11.2 translocation renal cell carcinoma (RCC) pathologically. Hence, laparoscopic nephrectomy was performed. Fluorescence in situ hybridization analysis revealed a break-apart of the transcription factor E3 (TFE3) genes in the left tumor. After 2 months postoperatively, nivolumab and ipilimumab were administered thrice intravenously, considering the intermediate risk by the IMDC risk classification. However, pleural effusion occurred but was removed adequately. Lung metastasis decreased, but new metastasis occurred at the left iliopsoas muscle. Target therapy was performed with axitinib. Unfortunately, he died 6 months later postoperatively. These tumors commonly occur in children than in adults, and very rare in elderly patients. Xp11.2 translocation RCC in the elderly has a poorer prognosis than that in children. To date, no effective treatment for Xp11.2 translocation RCC has been established.
Keywords: Xp11.2 translocational renal cell carcinoma, Fluorescence in situ hybridization
Introduction
Xp11.2 translocation renal cell carcinoma (RCC) represents approximately 1% of RCC [1]. This type of RCC is generally diagnosed in pediatric RCCs. Cases of Xp11.2 translocation RCC in adults are rare and may have a poorer prognosis than in children [2]. Regarding its development mechanism, Xp11.2 breakpoints and several gene fusions clearly result in the overexpression of TFE3 proteins in RCC [3]. Recently, at least six various partner genes have been found. Alveolar soft part sarcoma critical region 1 (ASPSCR1) with der(17)t(X;17)(p11.2;q25) is a common fusion partner gene. Other common fusion genes are alveolar soft part sarcoma locus (ASPL) on 17q25 and papillary renal cell carcinoma-TFE3 (PRCC-TFE3), t(X;1)(p11.2;q21.2) and PTB-associated splicing factor-TFE3 (PSF-TFE3), t(X;1)(p11.2;p34) and clathrin heavy chain-TFE3 (CLTC-TFE3), (X;17)(p11.2;q23) and NonO-TFE3 inv.(X), (p11.2;q12) [4–7].
We present an extremely rare case of Xp11.2 translocation RCC with TFE3 gene fusion occurring in an elderly man. Herein, we discuss the corresponding histopathological and cytogenetic approaches and several treatments.
Case report
A 68-year-old man was followed up by chronic renal failure. Follow-up CT incidentally detected a solid mass (10 cm × 8 cm × 4 cm) at the left kidney and multiple small nodular shadows in the lungs bilaterally (Fig. 1a, b). Identifying whether the tumor is renal pelvic cancer or RCC was immensely difficult, even by magnetic resonance imaging (Fig. 1c). Needle biopsy was performed, and the patient was diagnosed with RCC associated with Xp11.2 translocation/TFE3 gene fusion (Fig. 2a, b). He was arranged to “ Intermediate risk” in the IMDC classification according to two factors, which were the duration from the diagnosis to the initiation of therapy and the number of neutrophil.
Fig. 1.
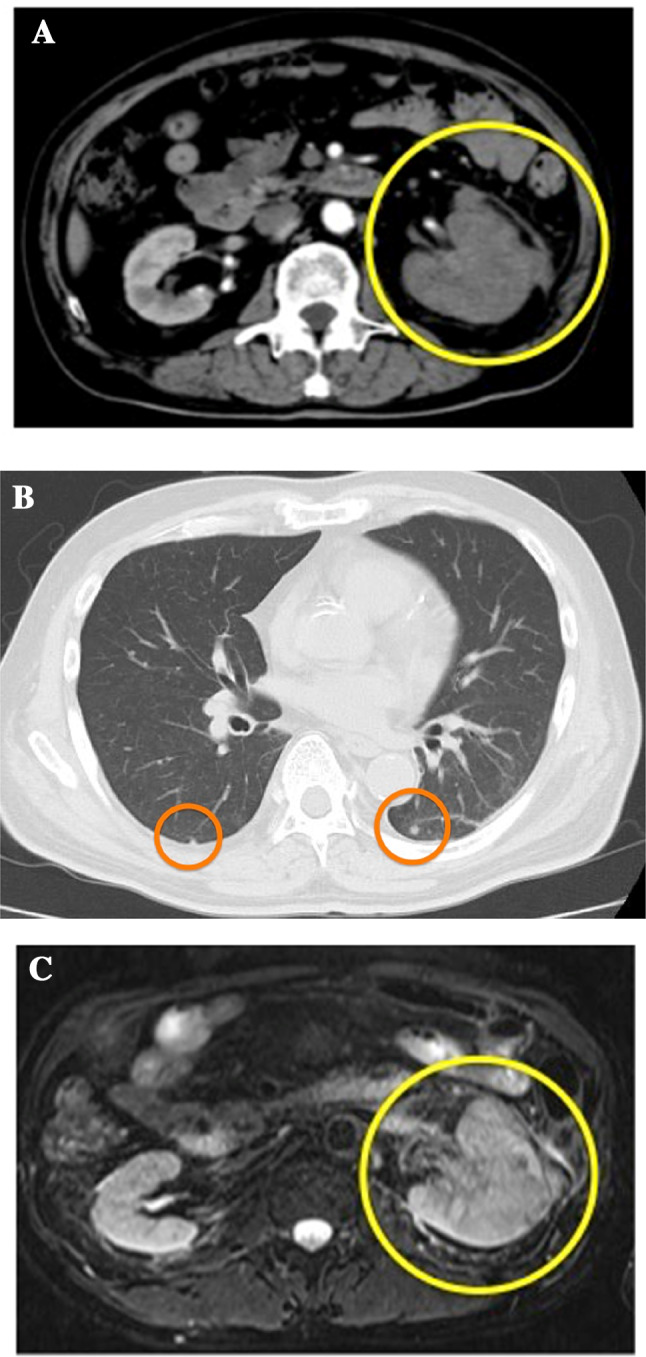
a Abdominal CT scan detected a solid mass (10 cm × 8 cm × 4 cm) at the right kidney (circle). b Chest CT revealed multiple small nodular shadows in the lungs bilaterally (circle). c Tumor diagnosis of whether renal cell carcinoma or renal pelvic cancer is difficult (circle)
Fig. 2.
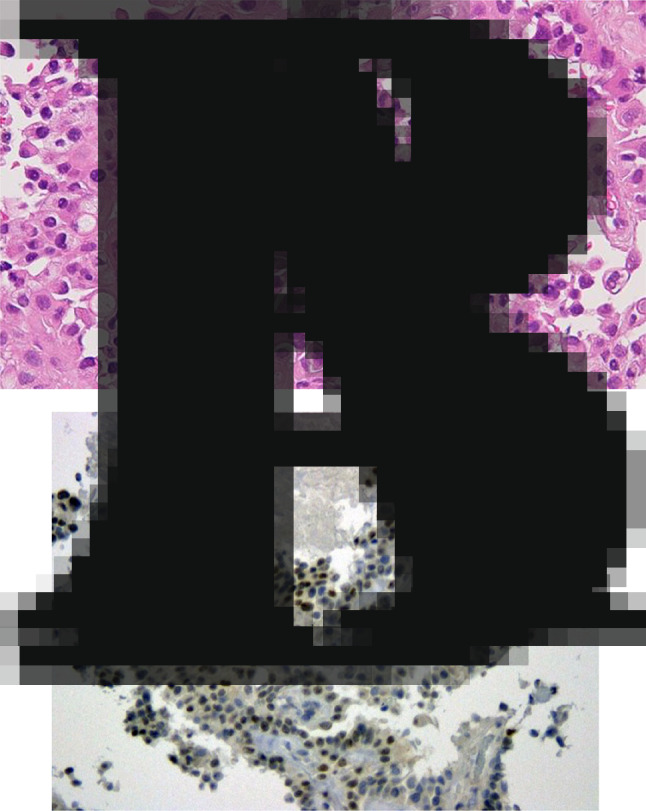
a Tumor cells were arranged in papillary formations and their cytoplasm was eosinophilic (hematoxylin and eosin staining, ×400). b Moderate-to-strong immunostaining intensity of TFE3 was found in the nuclei of tumor cells
Laparoscopic radical left nephrectomy was performed subsequently. The pathological stage was pT1b with a negative surgical margin. I thought the bilateral lung nodules would be metastases of renal cell carcinoma and judged pT1bN0M1. The tumor was a macroscopically well-circumscribed solid mass with necrotic and hemorrhagic features (Fig. 3a). Microscopically, in hematoxylin and eosin staining, non-neoplastic renal tissue showed tubulointerstitial fibrosis and interstitial expansion with an accumulation of extracellular matrix, tubular atrophy and vascular obliteration. On the other hand, the tumor showed an alveolar architecture that had mixed patterns of papillary and eosinophilic, clear, and granular cytoplasm, with hyaline nodules and psammoma bodies (Fig. 3b), presenting Fuhrman Grade 3 and partly Grade 4 of nuclear grade. Immunohistochemically, the tumor cell was diffusely positive for cluster of differentiation 10 (CD10) and cytokeratin 7 (CK7), a partial positive staining for alpha-methylacyl CoA racemase (AMACR) but negative for cathepsin K, Melan-A (Fig. 4). Almost the entire neoplastic cell nuclei stained positive for TFE3, with moderate (2+) to strong (3+) staining intensity. The chromosomal breakpoint of TFE3 was identified in a paraffin-embedded tissue by fluorescence in situ hybridization (FISH) assay (Fig. 5).
Fig. 3.
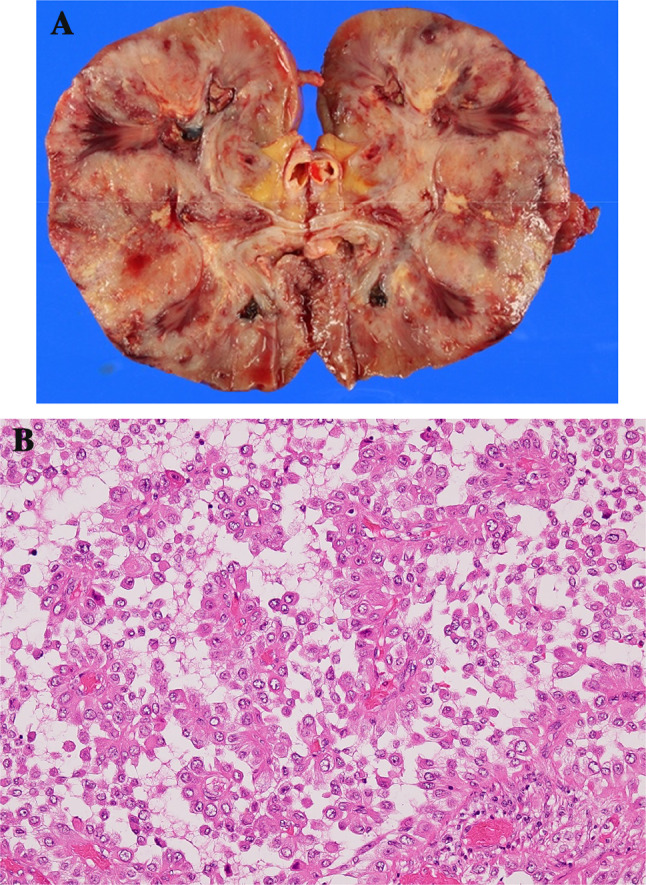
a Macroscopically, the tumor was a well-circumscribed solid mass that was necrotic and hemorrhagic. b The pathological specimen of the renal cell carcinoma showed an alveolar architecture with mixed patterns of papillary and eosinophilic cytoplasms (hematoxylin and eosin staining, ×40)
Fig. 4.
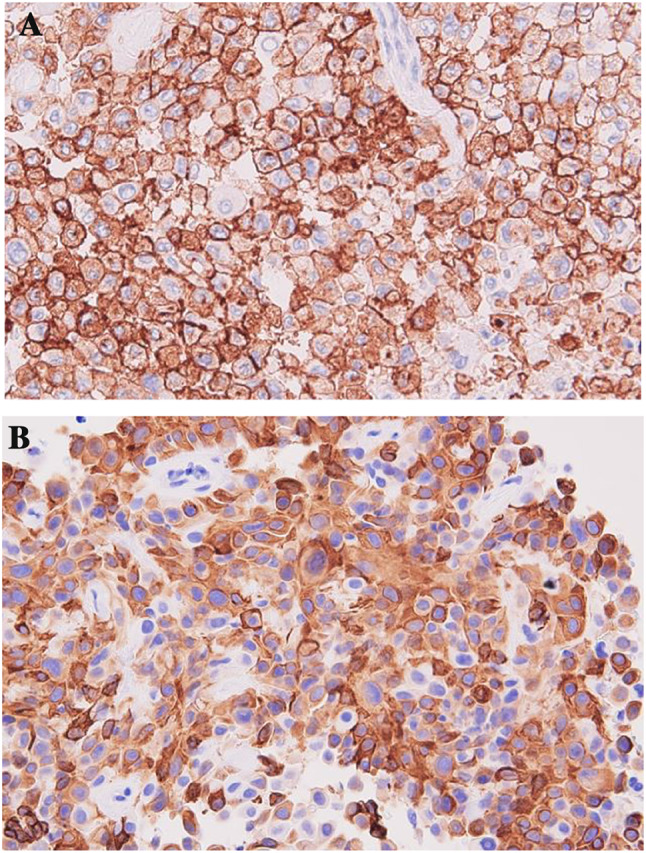
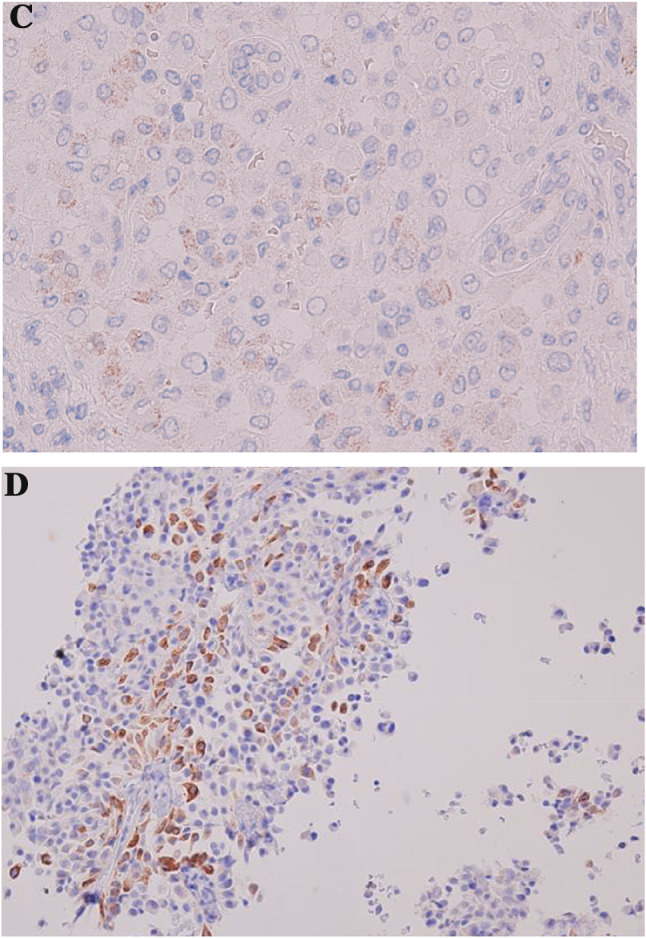
Immunohistopathologically, all tumor cells showed a strong positive staining for CD10 and CK7, a partial positive staining for AMACR (a–c) and negative for Melan A (d)
Fig. 5.
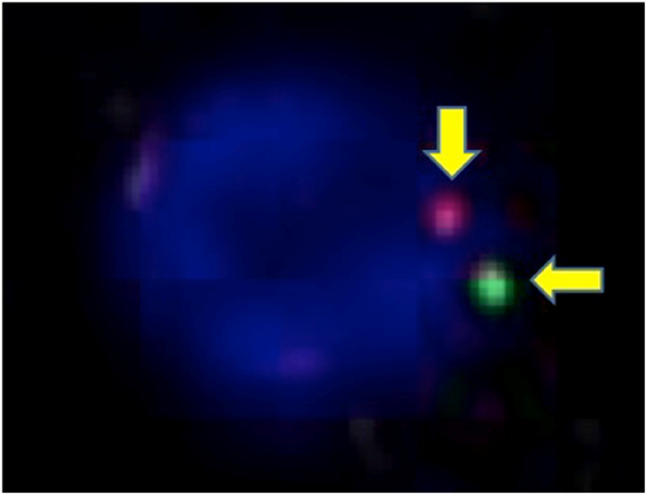
The TFE 3 break-apart probe assay identified split signals and increased TFE3 copy numbers (arrow)
He was arranged to “ Intermediate risk” in IMDC classification according to two factors which were the duration from the diagnosis to the initiation of therapy and the number of neutrophil. After 1 month postoperatively, nivolumab and ipilimumab were administered thrice intravenously. During the drug administration period, pleural effusion occurred, and needling was administered appropriately. No malignant cell was detected in the pleural area. Although the pleural effusion was almost diminished, a new metastasis occurred in the liver and left-hip hypoderm. We changed the drug therapy to another target therapy using axitinib. However, he died at 6 months postoperatively.
Discussion
Xp11.2 translocation RCC is generally a pediatric RCC, accounting for 20–40%, while only 1–1.6% in adult RCCs [8]. It is categorized as a separate entity in the 2004 World Health Organization classification of tumors of the urinary system [9]. To our knowledge, the present case is the 7th adult case of Xp11.2 translocation RCC aged over 65 years (Table 1). Regarding the prognosis, the mean survival of adult patients is up to 2 years when presenting metastases, whereas that of pediatric patients is 6.3 years [2]. Generally, having a past history of chemotherapy might cause the occurrence of Xp 11.2 translocation RCC in both pediatric and adult patients [2]. Chemotherapy might induce chronic renal failure, resulting in Xp 11.2 translocation RCC.
Table 1.
Summary of reported elderly cases of Xp11.2 translocation RCC over 65 years old
| No | Age | Gender | laterality | Diameter (cm) | AJCC | Final diagnosis | Adjuvant therapy | Authors | Year |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | M | L | 10 | pT3bN2Mx | ASPL-TFE3 type | Unknown | Argani et al. [3] | 2007 |
| 2 | 68 | F | R | 5 | pT1bNxMx | ASPL-TFE3 type | Unknown | Argani et al. [3] | 2007 |
| 3 | 77 | F | L | 5 | pT1bNxMx | Xp 11.2 RCC | Unknown | Argani et al. [3] | 2007 |
| 4 | 79 | M | L | 5 | pT1bNxMx | Xp 11.2 RCC | None | Franzini et al. [10] | 2007 |
| 5 | 72 | F | R | 5.8 | pT1bNxMx | Xp 11.2 RCC | None | Iinuma et al. [11] | 2016 |
| 6 | 70 | M | R | 8.2 | pT2aNxMx | Xp 11.2 RCC | None | Pan et al. [12] | 2017 |
| 7 | 68 | M | L | 10 | pT3aN1Mx | Xp 11.2 RCC | Immunotherapy | Present case | 2019 |
M male, F female, R right, L left, K kidney
Table 1 summarizes the background of the reported cases (including the present’s case) of elderly patients aged over 65 years with Xp 11.2 translocation RCC [3, 10–12]. The mean age was 73.1 years. Three of the patients were female. The laterality of RCC was right in three cases and left in four. The mean diameter was 7 cm. Only the present case was treated with adjuvant therapy and was the first case treated with nivolumab and ipilimumab.
Histopathologically, Xp11.2 translocation RCC has variations such as clear cell, papillary, alveolar, and nested. Most adult Xp11.2 translocation RCC cases show a clear-cell histological type, whereas pediatric cases present a papillary type [13]. However, the present case mainly consisted of clear-cell features, followed by alveolar and papillary features. These characteristic findings led to the diagnosis of adult Xp11.2 translocation RCC with ASPL-TFE3 fusion. Immunohistochemically, most previous cases of Xp11.2 translocation RCC showed a positive staining of CD10 and negative staining of cathepsin K, thereby supporting ASPL-TFE3 fusion. Meanwhile, tumors with PRCC-TFE3 fusion mostly display positive cathepsin K staining, which is also evident in RCC with papillary type [14]. In the present case, the results of positive immunostaining of CD10 and negative of cathepsin K, as well as E-cadherin, led to the diagnosis of ASPL-TFE3 fusion. Some pathologies might lead to opposite immunohistochemical results. For those with complicated pathology, identifying the correct Xp11.2 RCC type would be more difficult.
Currently, the treatment for Xp11.2 RCC is still unestablished, and no clinical studies with a large sample size are being conducted. Most cases followed the guidelines of conventional RCC. Moreover, target therapies involving the vascular endothelial growth factor receptor are still unclear, thereby requiring additional clinical studies. For localized Xp11.2 translocation RCC with positive regional lymph nodes, surgery is the optimal treatment. Recently, nivolumab plus ipilimumab, which are antibody-based immunotherapies targeting the immune checkpoint receptors, have demonstrated clinical efficacy in patients with metastatic RCC (mRCC) [15]. The combination of nivolumab and ipilimumab was effective for treatment-naïve patients with intermediate- and poor-risk mRCC with clear-cell histology [15]. When we judged the effect of immunotherapies, we needed to take care of pseudoprogression which was the brief increase in tumor size may be followed by shrinking or eradication of the tumor [16]. Therefore, it might take some months to judge whether pseudoprogression or progression disease. In the present case, immunotherapy with nivolumab plus ipilimumab was ineffective. It might be more important to change the other drugs when we found immunotherapy would not be effective in the rapid and aggressive case such as the present case.
In summary, the occurrence of elderly Xp11.2 translocation RCC over 65 years old is extremely rare. Immunohistochemical and cytogenetic findings allow the differential diagnosis of kidney neoplasms, such as Xp11.2 translocation RCC. Although the adult prognosis is severe, more cases and research are needed to detect the relationship between the effects of immunotherapy and some clinical parameters.
Funding
There is no funding source.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
The patients and/or their families were informed that data from the case would be submitted for publication and provided their consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Macher-Goeppinger S, Roth W, Wagener N, et al. Molecular heterogeneity of TFE3 activation in renal cell carcinomas. Mod Pathol. 2012;25:308–315. doi: 10.1038/modpathol.2011.169. [DOI] [PubMed] [Google Scholar]
- 2.Srigley JR, Delahunt B, Eble JN, et al. The international society of urological pathology (ISUP) vancouver classification of renal neoplasia. Am J Surg Pathol. 2013;37:1469–1489. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- 3.Argani P, Olgac S, Tickoo SK, et al. Xp11 translocation renal cell carcinoma in adults: expanded clinical, pathologic, and genetic spectrum. Am J Surg Pathol. 2007;8:1149–1160. doi: 10.1097/PAS.0b013e318031ffff. [DOI] [PubMed] [Google Scholar]
- 4.Sidhar S. The t(X;1)(p11.2;q21.2) translocation in papillary renal cell carcinoma fuses a novel gene PRCC to the TFE3 transcription factor gene. Hum Mol Genet. 1996;5:1333–1338. doi: 10.1093/hmg/5.9.1333. [DOI] [PubMed] [Google Scholar]
- 5.Mathur M, Das S, Samuels HH. PSF-TFE3 oncoprotein in papillary renal cell carcinoma inactivates TFE3 and p53 through cytoplasmic sequesteration. Oncogene. 2003;22:5031–5044. doi: 10.1038/sj.onc.1206643. [DOI] [PubMed] [Google Scholar]
- 6.Clark J, Lu YJ, Sidhar SK, et al. Fusion of splicing factor genes PSF and NonO(p54nrb) to the TFE3 gene in papillary renal cell carcinoma. Oncogene. 1997;15:2233–2239. doi: 10.1038/sj.onc.1201394. [DOI] [PubMed] [Google Scholar]
- 7.Argani P, Lui MY, Couyruier J, et al. A novel CLTC-TFE3 gene fusion in pediatric renal adenocarcinoma with t(X;17)(p11.2;q23) Oncogene. 2003;22:5374–5378. doi: 10.1038/sj.onc.1206686. [DOI] [PubMed] [Google Scholar]
- 8.Bruder E, Passera O, Harms D, et al. Morphologic and molecular characterization of renal cell carcinoma in children and young adults. Am J Surg Pahol. 2004;28:1117–1132. doi: 10.1097/01.pas.0000131558.32412.40. [DOI] [PubMed] [Google Scholar]
- 9.Chan TY. World Health Organization classification of tumours: pathology and genetics tumours of the urinary system and male genital organs. Urology. 2005;65:214–215. doi: 10.1016/j.urology.2004.09.048. [DOI] [Google Scholar]
- 10.Franzini A, Picozzi SCM, Politi PJ, et al. A case of renal cancer with TFE3 gene fusion in an elderly man. Clinical, radiological and surgical findings. Urol Int. 2007;78:179–181. doi: 10.1159/000098080. [DOI] [PubMed] [Google Scholar]
- 11.Iinuma K, Kojima K, Okamoto K, et al. A case of Xp.11.2 translocation renal cell carcinoma diagnosed by fluorescence in situ hybridization (FISH) Hinyokiyo. 2016;62:411–414. doi: 10.14989/ActaUrolJap_62_8_411. [DOI] [PubMed] [Google Scholar]
- 12.Pan X, Quan J, Zhao L, et al. Xp11.2 translocation renal cell carcinoma with TFE3 gene fusion: a case report. Mol Clin Oncol. 2017;8:83–85. doi: 10.3892/mco.2017.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karashima T, Kuno T, Kuroda N, et al. Bilateral Xp 11.2 translocation renal cell carcinoma: a case report. BMC Urol. 2018;18:106–111. doi: 10.1186/s12894-018-0419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martignoni G, Gobbo S, Camparo P, et al. Differential expression of cathepsin K in neoplasms harboring TFE3 gene fusions. Mod Pathol. 2011;24:1313–1319. doi: 10.1038/modpathol.2011.93. [DOI] [PubMed] [Google Scholar]
- 15.Gao X, McDermott DF. Ipilimumab in combination with nivolumab for the treatment of renal cell carcinoma. Expert Opin Bio Ther. 2018;18:947–957. doi: 10.1080/14712598.2018.1513485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West HJ. Immune checkpoint inhibitors. JAMA Oncol. 2015;1:115. doi: 10.1001/jamaoncol.2015.0137. [DOI] [PubMed] [Google Scholar]


