Abstract
Aggregation of Aβ is a pathological hallmark of Alzheimer’s disease (AD). The purpose of this study was to identify the protective roles of different polysaccharide components in Fomes officinalis Ames polysaccharides (FOAPs) against Aβ25–35-induced neurotoxicity in PC12 cells. Different doses of FOAPs components (i.e. FOAPs-a and FOAPs-b) were added to PC12 cells about 2 h before β-amyloid protein fragment 25–35 (Aβ25–35) exposure. The AD cellular model of PC12 cells was established using Aβ25–35. Then the PC12 cells were divided into 9 groups including: control group, Donepezil hydrochloride (DHCL) group, model group treated using 40 μM Aβ25–35, followed by FOAPs-a and FOAPs-b interference (50, 100 and 200 μg/mL). The mitochondrial reactive oxygen species (ROS), ATP, superoxide dismutase (SOD), malondialdehyde (MDA), lactate dehydrogenase (LDH) and mitochondrial membrane potential (MMP) were determined by commercial kits. The Cytochrome C, Bcl-2 and Bax expressions in the mitochondria and cytosol was determined by using Western blot analysis. FOAPs-a and FOAPs-b could significantly inhibit the LDH release, MDA level and the over accumulation of ROS induced by Aβ25–35 in PC12 cells in a dose-dependent manner. They could also effectively prevent Aβ25–35-stimulated cytotoxicity, which involved in attenuating cell apoptosis, increasing the ratio of Bcl-2/Bax and inhibiting Cytochrome C release from mitochondria to cytosol in PC12 cells. Moreover, FOAPs-a and FOAPs-b significantly alleviated mitochondrial dysfunction by regulating the MMP, as well as promoting the mitochondrial ATP synthesis. FOAPs-a and FOAPs-b played neuroprotective roles against Aβ25–35-induced cytotoxicity in PC12 cells through suppressing the mitochondria-mediated apoptotic pathway.
Keywords: Fomes officinalis Ames polysaccharides, PC12 cells, Aβ25–35, Mitochondrial dysfunction, Oxidative stress
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease characterized by cognitive decline and brain atrophy (Zhao et al. 2018). AD patients usually present excessive amyloid plaques and intracellular aggregation of hyper-phosphorylated Tau protein, as well as synaptic and neuronal loss (Yin et al. 2017). Meanwhile, it has been considered one of the most challenging problems among the geriatrics (Albensi 2019). Unfortunately, there is no detailed explanation for the molecular mechanisms of neurodegeneration among AD patients (Chen et al. 2017). Indeed, there is a lack of effective treatment for these patients.
Recently, β-amyloid protein (Aβ), produced by beta amyloid precursor protein (β-APP) under the circumstance of unusual pyrolysis caused by the continuous effects of β and γ enzymes, has been reported to be associated with the pathogenesis of AD (Li et al. 2019c). Over-accumulation of Aβ in brain tissues results in neuronal damages, cellular apoptosis and central neurotoxicity, which is considered the initial factors and key pathological features of AD (Kou et al. 2017; Stefanova et al. 2019). Specifically, Aβ concentration showed elevation in brain tissues of AD patients and mouse models. Meanwhile, Aβ accumulation may cause multiple neuronal damages and lead to cognitive impairments (Tobore 2019). A previous study had been focused on the target therapy of Fomes officinalis Ames polysaccharides (FOAPs) on the treatment of AD (Hu et al. 2013). In our previous study, we identified FOAPs using the DEAE-52 ion-exchange chromatography, Sepharose CL-6B and Sephadex G-100 gel chromatography, and two components of FOAPs (i.e. FOAPs-a and FOAPs-b) were purified with a molecular of 199 kD and 87 kD, respectively (Li et al. 2019a). In this study, we aimed to identify the protective roles of FOAPs-a and FOAPs-b against β-amyloid protein fragment 25–35 (Aβ25–35)-caused neurotoxicity in PC12 cells.
Materials and methods
Agents
Aβ25–35 (Genscript, China) was diluted to a final concentration of 2 mM using double-distilled deionized water. Then it was subjected to constant oscillation for 8 days at 37 °C to induce aggregation. F. officinalis Ames samples were deposited in a publicly available herbarium in Aletai Kazakh Medical Hospital (Xinjiang, China). The study protocols were approved by the Ethical Committee of Xinjiang Medical University.
Extraction of plant material
Fomes officinalis Ames, the dried fruiting body of the F. officinalis fungus, is commonly prescribed in Chinese traditional medicine. It is mainly used for the treatment of eliminating phlegm, warming lung, and relieving asthma (Kumaran et al. 2018). We isolated FOAPs by DEAE-52 cellulose ion-exchange chromatography, Sepharose CL-6B and Sephadex G-100 gel chromatography. Two components (i.e. FOAPs-a and FOAPs-b) were purified with a molecular weight of 199 kD and 87 kD, respectively. In the preliminary stage, using chemical method and spectroscopy, the two polysaccharides were composed of Arabinose, Xylose, Mannose, Glucose, and Galactose in main chains with different links between sugar sequence and glycoside type (Abuliz and Tuoheniyazi 2013; Ainiwaer and Abulizi 2016; Cong and Abulizi 2010).
Cell culture
PC12 rat pheochromocytoma cells, purchased from Cell Bank of the Chinese Academy of Science (Shanghai, China), were cultured in DMEM (Hyclone, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Hyclone, USA), 100 U/mL streptomycin and 100 U/mL penicillin (Hyclone, USA) in a 5% CO2 and 95% air incubator under appropriate humidity. The cells were passaged every 3 days. PC12 cells (1 × 104) were cultured for 24 h, and then the medium was replaced with serum-free DMEM medium.
Grouping
The PC12 cells were divided into 9 groups including: control group, Donepezil hydrochloride (DHCL) group, model group treated using 40 μM Aβ25–35, followed by FOAPs-a and FOAPs-b interference (50, 100 and 200 μg/mL). In the model group, PC12 cells were treated using Aβ25–35 (10 mM, 20 mM, 40 mM and 60 mM). Then the cells were incubated for 12 h, 24 h and 48 h until a cell viability up to 50%. PC12 cells received no pretreatment served as control. Then the cells were pretreated with 50 mM DHCL (Sigma, D6821) and different doses of FOAPs-a (50, 100 and 200 μg/mL) and FOAPs-b (50, 100 and 200 μg/mL) for 2 h, respectively, followed by Aβ25–35 (40 μM) exposure for 48 h to establish the cellular model of AD.
Cell viability analysis
The viability of PC12 cells was measured using cell counting kit-8 (Dojindo, Japan), according to the manufacturer’s instructions. Then the medium was aspirated and cells in each group were incubated with 10 μL of CCK8 at 37 °C for 2 h. Afterwards, the absorbance was determined at a wavelength of 450 nm with a microplate reader (Thermo, MuLTiSKAN MK3, USA).
Lactate dehydrogenase (LDH) release assay
Cell cytotoxicity was assessed by measuring the LDH released from the damaged cells into the medium using commercial kit (Beyotime, Shanghai, China). Briefly, the cells were centrifuged at 400 g for 5 min. Then the supernatant (120 μL) was mixed with the reaction mixture (60 μL) and were incubated for 30 min at room temperature. Finally, the absorbance was read at a wavelength of 490 nm.
Cell apoptosis analysis
Cell apoptosis was measured using an Annexin-V-FITC apoptosis detection kit (KeyGen Biotech, Jiangsu, China). PC12 cells were harvested with 0.25% trypsin, and then incubated with Annexin VFITC (5 μL) and propidium iodide (PI, 5 μL) for 15 min in dark at room temperature. The samples were then analyzed by flow cytometry (Beckman, Cytoflex, USA).
Reactive oxygen species (ROS) generation analysis
The ROS concentration was measured by dichlorodihydrofluorescein diacetate using commercial kits (Beyotime, China). PC12 cells were incubated with DCFH-DA (1 μL) and DMEM (1 mL) solution at 37 °C for 20 min. The cells were then washed with PBS to remove the non-specific staining. The DCF fluorescence intensity was measured at an excitation wavelength of 488 nm and an emission wavelength of 525 nm.
Superoxide dismutase (SOD) and malondialdehyde (MDA) assay
SOD activity and MDA level were measured using commercial assay kits (Beyotime, Shanghai, China), according to the manufacturer’s instructions. After treatment in each group, PC12 cells were homogenized and lysed on ice. Then lysates were centrifuged and the supernatant was collected to analyze the SOD activity and MDA level.
Mitochondrial membrane potential (MMP) analysis
The MMP level was monitored by using the fluorescent cationic dye 5,5′,6,6′- tetrachloro-1,1′,3,3′ -tetraethylbenzimidazolcarbocyanine iodide (JC-1) that served as a green fluorescent monomer in the presence of low MMP levels. JC-1 could form red fluorescent aggregates in the presence of higher MMP (Jeyakumar et al. 2019). In each group, PC12 cells were stained with JC-1 10 μg/mL (Beyotime, Shanghai, China) at 37 °C for 20 min in the dark. The JC-1 fluorescence was then observed under flow cytometry (Beckman, Cytoflex, USA).
ATP concentration determination
Cellular ATP concentration was determined by a commercial kit (Beyotime, China). Upon washing with PBS, the cells were lysed and centrifuged. Then ATP detection working solution (100 μL) was added to the supernatant (20 μL), and the chemiluminescence of the samples was then measured according to the manufacturer’s instructions.
Western blotting analysis
Cells in each group were lysed with ice-cold RIPA lysis buffer (Beyotime, Shanghai, China) containing protease inhibitors. Total protein concentration was measured with the BCA kit (Beyotime, China). For detection of cytochrome C, the mitochondrial and cytosolic fractions were prepared using the Mitochondria/Cytosol Fractionation kit (Abcam, Cambridge, MA, USA). The commercial cell extraction kit (Thermo Scientific, Rockford, IL, USA) was used to detect Bax and Bcl-2 concentration. The protein was separated by 10% SDS-PAGE gel, and transferred to PVDF membranes (Millipore, Bedford, MA, USA). The membranes were then blocked in 5% skim milk, followed by incubating overnight at 4 °C with primary antibodies. Afterwards, the mixture was incubated with secondary antibodies at room temperature for 1 h. All bands were visualized using the enhanced chemiluminescence kit (Cell Signaling Technology, Beverly, MA, USA), and analyzed by Image J software (NIH, USA). In order to eliminate the variations, GAPDH or COX IV was used as control.
Statistical analysis
GraphPad Prism 5.0 software was used for the statistical analysis. Data are expressed as mean ± standard deviation from at least three independent experiments. Statistical analysis was carried out using one-way analysis of variance (ANOVA) test followed by post hoc test. P < 0.05 was considered to be statistically significant.
Results
Selection of Aβ25–35 concentration and treatment duration
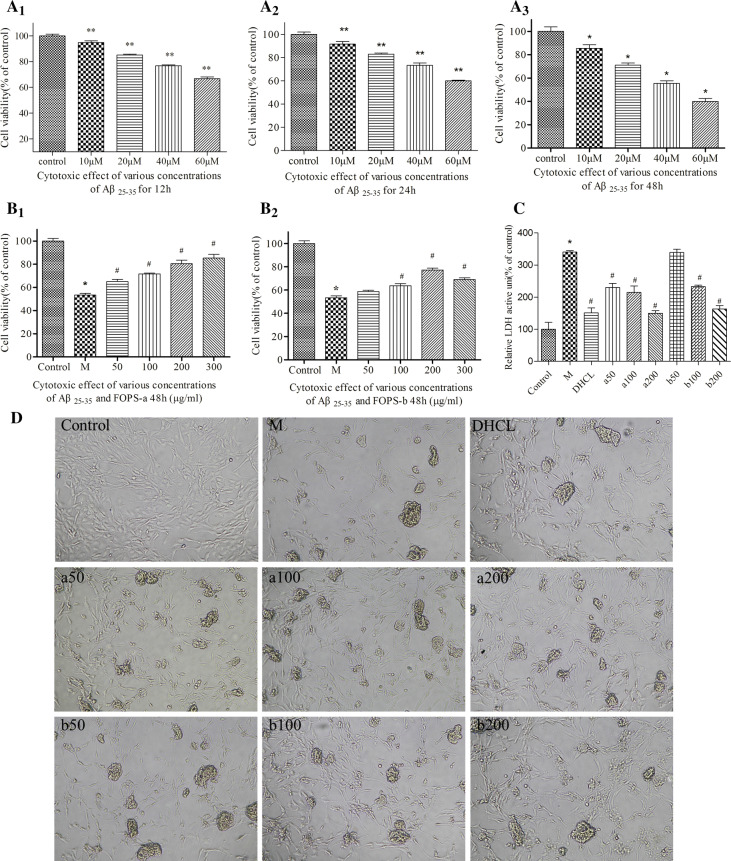
After treating with various concentrations of Aβ25–35 (10 μM, 20 μM, 40 μM, and 60 μM) for different times (12 h, 24 h and 48 h), the cell viability showed significant decline in a doze-dependent and time-dependent manner. Specifically, the cellular viability reduced by 50% upon treating with 60 μM Aβ25–35 for 48 h. For PC12 cells treated with 40 μM Aβ25–35 for 48 h, the cellular viability was about 55%. On this basis, the cell model was established by exposed with a concentration of 40 μM for 48 h (Fig. 1a1–a3).
Fig. 1.
a1–a3 Cytotoxic effects of various concentrations of Aβ25–35 for 12 h, 24 h and 48 h (10–60 μM) in PC12 cells. b1, b2 Protective effects of various concentrations of FOAPs-a and FOAPs-b (50, 100, 200 and 300 μg/mL) against Aβ25–35 induced toxicity in PC12 cells for 48 h. c Cellular injury of PC12 cells was determined by LDH release assay using the commercial assay kit. Datas are expressed as mean ± SD. *P < 0.05 versus the control cells. #P < 0.05 versus the cells treated with Aβ25–35 alone. d The morphological changes of PC12 cells under inverted phase contrast microscope (100 ×). M model group, DHCL Donepezil hydrochloride group
FOAPs-a and FOAPs-b promoted cellular viability of PC12 cells after Aβ25–35 exposure
To investigate the impact of FOAPs-a and FOAPs-b on Aβ25–35-induced cell damages, we evaluated cell viability using CCK8 assay. FOAPs-a and FOAPs-b pre-treatment at various doses (50, 100, 200 and 300 μg/mL) for 2 h prior to Aβ25–35 (40 μM, 48 h) exposure induced a dose-dependent increase in the cell viability (50,100 and 200 μg/mL) (Fig. 1b1, b2). The protective roles of FOAPs-a and FOAPs-b were also validated by the LDH release assay. Specifically, LDH release showed increase after Aβ25–35 exposure compared with the control group (P < 0.05). After treating with FOAPs-a and FOAPs-b, significant decline was noticed in the LDH activity compared with the model (P < 0.05), which was in a dose-dependent manner (Fig. 1c). These demonstrated that FOAPs-a and FOAPs-b could protect the PC12 cells against Aβ25–35 exposure induced injury. There was cellular proliferation in the PC12 cells after FOAPs-a and FOAPs-b treatment, especially the FOAPs-a under a concentration of 100 μg/mL, 200 μg/mL, and 300 μg/mL, respectively. For FOAPs-b, it showed inhibitory effects at a concentration of more than 300 μg/mL. On this basis, the concentrations of 50 μg/mL, 100 μg/mL, and 200 μg/mL were selected for the subsequent analysis.
In the blank control group, the morphology of the cells was normal. In the Aβ25–35 model group, the cells showed significant decline compared with the control group. The cells were aggregated, and adhered to the adjacent cells, which led to formation of a large space. In the FOAPs groups, the cell count showed increase, and the space showed decline, together with decline of the PC12 cells aggregation.
FOAPs-a and FOAPs-b prevented cell apoptosis after Aβ25–35 exposure in PC12 cells
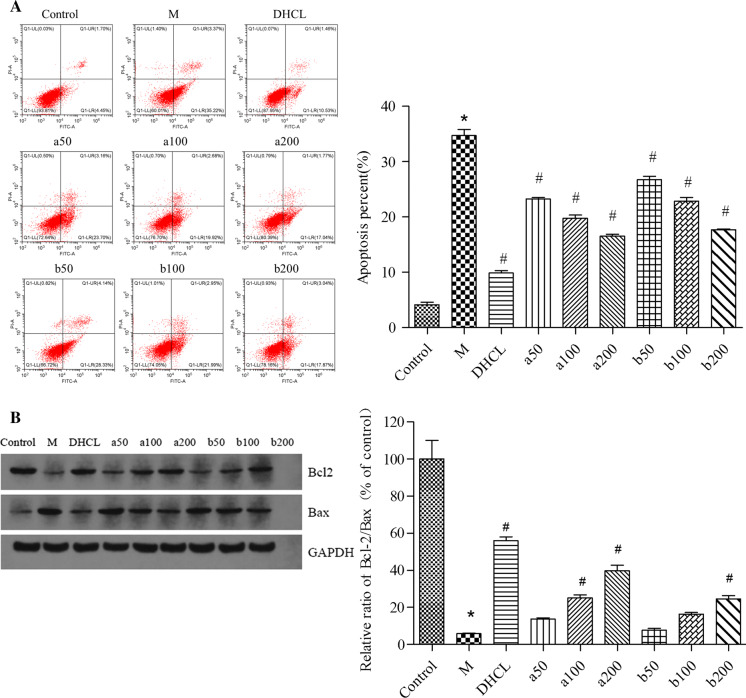
In this section, flow cytometric analysis was carried out to determine the cellular apoptosis using Annexin V/PI double staining. Pretreatment with FOAPs-a or FOAPs-b could significantly reduce the number of apoptotic PC12 cells induced by Aβ25–35 exposure in a dose-dependent manner (P < 0.05, Fig. 2a). As shown in (Fig. 2b), pretreatment with FOAPs-a or FOAPs-b increased the ratio of Bcl-2/Bax in PC12 cells treated with Aβ25–35 in a dose-dependent manner (P < 0.05). DHCL showed satisfactory anti-apoptotic effects on PC12 cells (P < 0.05). FOAPs-a and FOAPs-b (50 μg/mL, 100 μg/mL, and 200 μg/mL) showed satisfactory inhibitory effects on the apoptosis of PC12 cells (P < 0.05). However, it was lower than that of the DHCL group.
Fig. 2.
FOAPs-a and FOAPs-b prevented cell apoptosis after Aβ25–35 exposure in PC12 cells. a PC12 cells were stained with FITC-Annexin V/PI and analyzed by flow cytometry to determine the percentage of apoptotic cells. b The protein expression levels of Bcl-2 and Bax in PC12 cells were detected by Western blot analysis. Data are expressed as mean ± SD. *P < 0.05 versus the control cells. #P < 0.05 versus the cells treated with Aβ25–35 alone as model group. M model group, DHCL Donepezil hydrochloride group
FOAPs-a and FOAPs-b reduced oxidative stress after Aβ25–35 exposure in PC12 cells
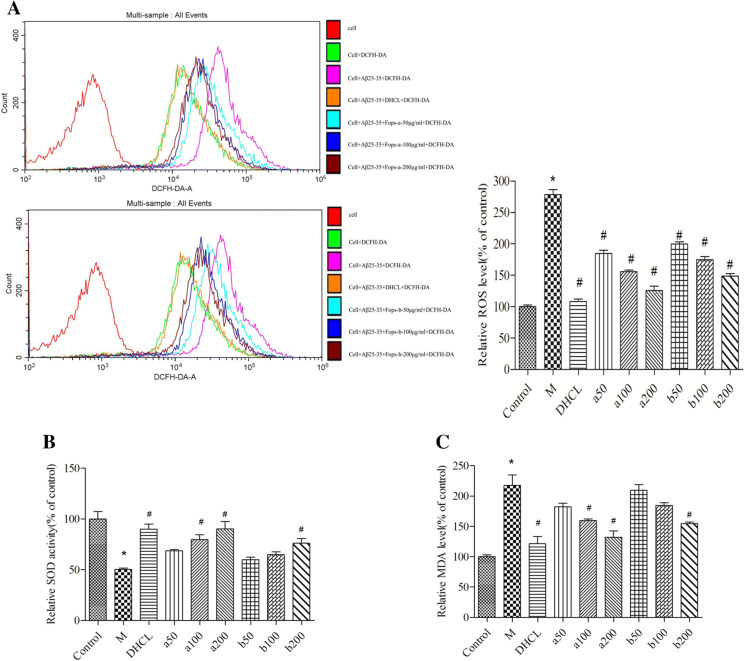
Aβ25–35 exposure led to a remarkable increase in the accumulation of ROS in PC12 cells (P < 0.05), however, ROS production was remarkably suppressed by pretreatment with FOAPs-a or FOAPs-b in a dose-dependent manner (P < 0.05, Fig. 3a). Subsequently, we tested the activity of the SOD and the level of MDA. As indicated in (Fig. 3b, c), pretreatment with FOAPs-a or FOAPs-b resulted in increase of SOD activity and decline of intracellular MDA level in Aβ25–35-treated PC12 cells (P < 0.05).
Fig. 3.
FOAPs-a or FOAPs-b reduces oxidative stress after Aβ25–35 exposure in PC12 cells. a Oxidative stress was assessed by measuring intracellular ROS generation in PC12 cells. b The SOD activity in PC12 cells was determined by the commercial assay kit. c The MDA level in PC12 cells was determined by the commercial assay kit. *P < 0.05 versus the control cells. #P < 0.05 versus the cells treated with Aβ25–35 alone as model group. M model group, DHCL Donepezil hydrochloride group
Our data showed that FOAPs-a and FOAPs-b (50 μg/mL, 100 μg/mL, and 200 μg/mL) were effective in scavenging the ROS (P < 0.05). Particularly, the clearance of ROS by FOAPs-a (200 μg/mL) was comparable to that of the DHCL. For FOAPs-b (200 μg/ml), the clearance of ROS was lower than that of the DHCL. This indicated that the inhibitory effects of FOAPs-a on ROS were significantly higher than that of FOAPs-b.
FOAPs-a and FOAPs-b alleviated mitochondrial dysfunction after Aβ25–35 exposure
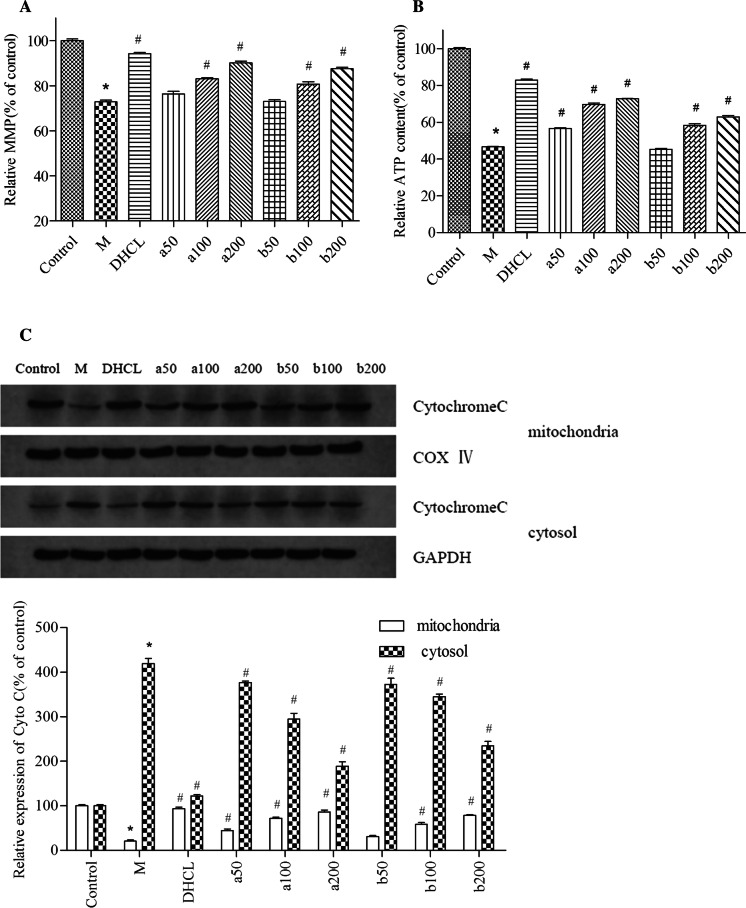
FOAPs-a or FOAPs-b prevented the loss of MMP in Aβ25–35-treated PC12 cells in a dose-dependent manner (P < 0.05, Fig. 4a). In addition, FOAPs-a and FOAPs-b partly rescued the reduction of ATP production in the Aβ25–35-treated PC12 cells (P < 0.05, Fig. 4b). Western blot analysis showed that the cytosol Cytochrome C levels were up-regulated in PC12 cells treated with Aβ25–35 (P < 0.05). However, pretreatment with FOAPs-a or FOAPs-b obviously prevented the release of Cytochrome C from mitochondria to cytosol (P < 0.05, Fig. 4c). Compared with control group, the mitochondrial Cytochrome C in the model group entered the cytoplasma, which then resulted in up-regulation of cytoplasmic Cytochrome C compared to the mitochondrion (P < 0.05). Compared with model group, the mitochondrial Cytochrome C showed significant up-regulation and the cytoplasmic Cytochrome C showed significant decline in the FOAPs groups, which was in a dose-dependent manner (P < 0.05). In cases of FOAPs-a and FOAPs-b at a concentration of 200 μg/mL, the mitochondrial Cytochrome C content was significantly higher than that of the other FOAPs groups, while the cytoplasmic Cytochrome C showed significant decline.
Fig. 4.
FOAPs-a or FOAPs-b alleviated mitochondrial dysfunction after Aβ25–35 exposure in PC12 cells. a The loss of MMP in PC12 cells was detected by JC-1 staining. b The ATP content in PC12 cells was determined by the commercial assay kit. c Western blot analysis was performed to detect cytochrome C levels in mitochondrial fraction and cytosolic fraction of PC12 cells. *P < 0.05 versus the control cells. #P < 0.05 versus the cells treated with Aβ25–35 alone as a model group. M: model group; DHCL: Donepezil hydrochloride group
Discussion
More and more data indicated that Aβ could lead to aberrant changes of mitochondrial structure and dysfunction. Aβ25–35 peptide plays a pivotal role in the pathogenesis of AD. It is a short toxic fragment consisted of 11 amino acids, which encompasses the β sheet of the full protein (Li et al. 2018). The Aβ peptide may increase oxidative stress, which is produced by reactive oxygen species (ROS) (Tan and Kim 2016). Most of the ROS was generated by the mitochondrial oxidative phosphorylation (Li et al. 2017b). Under physiological conditions, the major function of mitochondria was to maintain the ROS generation and its balance. Under pathological conditions, ROS could not be eliminated in time, which may lead to ROS deposit. The elevation of ROS concentration may affect the mitochondrial function, which then resulted in excessive oxidative stress and the subsequent neuronal injury and even death. Moreover, ROS accumulation may lead to overload of Aβ. According to the previous description (Wang et al. 2016), there was excessive oxidative stress in the damaged mitochondria, which resulted in the generation of Aβ by degrading the amyloid precursor protein (APP) and the subsequent neural damages. For the patients with early onset of AD, they were merely featured by pathological changes. Indeed, there were neurons in an overoxidative state in the brain tissues of AD patients. In this study, we demonstrated that PC12 cell viability was reduced upon exposure to Aβ25–35, together with a corresponding increase in MDA level, accumulation ROS and LDH release due to membrane damage. Our results also suggested that FOAPs-a and FOAPs-b exerts, in part, showed neuroprotective effects via inhibition of ROS, MDA and LDH productions, as well as increasing the activity of ATP thereby reducing oxidative stress after Aβ25–35 exposure. Nevertheless, pretreating with FOAPs-a and FOAPs-b could remarkably reverse these effects, which indicated that FOAPs-a and FOAPs-b exerted neuroprotective effects against Aβ25–35-induced cytotoxicity through suppression of mitochondria-mediated apoptotic pathway, especially the FOAPs-a. This indicated that FOAPs-a and FOAPs-b could improve the oxidative stress in AD models, and showed protective roles in the mitochondrial injury. Aβ accumulation was reported to trigger excessive apoptosis of neurons, which was closely related to neuronal loss in the brains of AD rats (Li et al. 2017a). Cellular apoptosis played a crucial role in the neural damages in the brain tissues of AD patients, while Aβ induced neuronal apoptosis was mainly relied on the mitochondrial pathway (Li et al. 2017b). In this study, pretreating with FOAPs-a or FOAPs-b could significantly reduce the number of apoptotic PC12 cells, together with improving the morphology and decline of space, as well as decline of the PC12 cells aggregation. In a recent report (Li et al. 2015), Bcl-2 and Bax were known to be associated with the apoptotic process. In addition, there were many manifestations of mitochondrial damages in the apoptotic cells. In this study, we also found that Bcl-2 and Bax showed aberrant expression, mitochondrial imbalance, decline of respiratory function in Aβ25–35 induced PC12 cells. All these demonstrated that mitochondrial function played important roles in the cellular apoptosis (Yao et al. 2017). FOAPs-a and FOAPs-b could lead to increase the Bcl-2/Bax ratio in PC12 cells induced by Aβ25–35 exposure in a dose-dependent manner. These indicated that FOAPs-a and FOAPs-b could attenuate the cellular apoptosis and mitochondrial dysfunction in AD.
Cytochrome C was reported to involve in cellular energy metabolism and cellular apoptosis (Briston and Hicks 2018). It was generated by damaged mitochondria, which then resulted in release of Cytochrome C from mitochondria to cytoplasma. On this basis, it could bind with the caspase-9 precursor to form the apoptosome. Afterwards, it would enhance the caspase-3 activity, which then triggered the cascade amplification. In this study, the content of Cytochrome C in the mitochondria showed elevation after FOAPs-a or FOAPs-b treatment, while the Cytochrome C in the cytoplasma showed decline. This would decrease the apoptosis through modulating the proteolysis. Besides, Cytochrome C improved the mitochondrial respiratory chain, and induced the ROS aggregation. Finally, there was abundant protein in the mitochondria that would decline cellular apoptosis, which could improve the integrity of mitochondrial membrane and its permeability. Then it decreased the cellular apoptosis, and promoted the mitochondrial function. These further indicated that FOAPs-a and FOAPs-b could attenuate the mitochondrial damages.
Mitochondrial dysfunction and oxidative stress are important pathological features of AD. To our best knowledge, oxidative stress would trigger nuclear related factor 2 (Nrf2) signaling pathway activation. In a recent study Li et al. (2019b), Nrf2 was considered a crucial transcriptional factor, which played essential roles in the anti-oxidative stress process. Under physiological conditions, Nrf2 was in an inert condition. Nevertheless, under pathological conditions, Nrf2 was activated and then bind with the antioxidant response elements, which then triggered the synthesis of components involved in the antioxidant process (Dong et al. 2020). Moreover, Heme oxygenase (HO-1) generated by Nrf2 could show neuroprotective roles to Aβ induced cytotoxicities and its catalyzed metabolites were powerful scavenger for ROS in AD (Kaidery et al. 2019). The neurons overexpressing Nrf2 would attenuate the Aβ induced toxic injuries. Up-regulation of Nrf2 target genes could attenuate the oxidative stress (Liu et al. 2019). Therefore, in this study, the FOAPs groups showed decline in the ROS. We speculated that FOAPs would improve the mitochondrial damages and attenuate the oxidative stress through modulating the Nrf2/HO-1 signaling pathway. In future, further studies are required to investigate the potential mechanism of the protective roles of FOAPs in the mitochondrial function.
In conclusion, FOAPs-a and FOAPs-b attenuated the Aβ25–35-induced cytotoxicity in PC12 cells. This phenomenon occurs through the inhibition of mitochondria-mediated apoptotic pathway. However, future studies are required before translating these experimental data into clinical application.
Abbreviations
- Aβ
Amyloid-β protein
- AD
Alzheimer’s disease
- FOAPs
Fomes officinalis Ames polysaccharides
- MMP
Mitochondrial membrane potential
- β-APP
Beta amyloid precursor protein
- Aβ25–35
β-amyloid protein fragment 25–35
- DHCL
Donepezil hydrochloride
- Nrf2
Nuclear related factor 2
Funding
This study was supported by the Natural Science Foundation of Xinjiang (2019D01C216), Funded by Scientific Research Program of Higher Education Institution of Xinjiang (XJEDU2020Y025) and National Science Natural Science Foundation of China (81760755).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayijiang Habaike, Email: ayjan_ok@126.com.
Mirensha Yakufu, Email: 1498514164@qq.com.
Yuanyuan Cong, Email: 1293649114@qq.com.
Yimin Gahafu, Email: 348025823@qq.com.
Zhen Li, Email: 1442122889@qq.com.
Palida Abulizi, Email: palida3345@163.com.
References
- Abuliz P, Tuoheniyazi R. Study on the anti-aging effect of Fomes officinalis Ames Ames polysaccharides. China J Tradit Chin Med Pharm. 2013;28:340–342. [Google Scholar]
- Ainiwaer Y, Abulizi P. Isolation, purification and basic composition analysis of polysaccharides from Fomes officinalis Ames. J Food Saf Qual. 2016;7:3992–4000. [Google Scholar]
- Albensi BC. Dysfunction of mitochondria: implications for Alzheimer’s disease. Int Rev Neurobiol. 2019;145:13–27. doi: 10.1016/bs.irn.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Briston T, Hicks AR. Mitochondrial dysfunction and neurodegenerative proteinopathies: mechanisms and prospects for therapeutic intervention. Biochem Soc Trans. 2018;46:829–842. doi: 10.1042/bst20180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GF, Xu TH, Yan Y, Zhou YR, Jiang Y, Melcher K, Xu HE. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol Sin. 2017;38:1205–1235. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y, Abulizi A. Studies on extraction and the immunity activity of polysaccharide from Fomes officinalis Ames Ames. Chin J Mod Appl Pharm. 2010;27:569–571. [Google Scholar]
- Dong Y, et al. Pathologyvia activation of Nrf2 and its targets. Theranostics. 2020;10(1):179–200. doi: 10.7150/thno.36722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, et al. Extraction of polysaccharides from Fomes officinalis Ames and their antitumor activity. Exp Ther Med. 2013;6:451–454. doi: 10.3892/etm.2013.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyakumar M, et al. Alpha-bisabolol beta-d-fucopyranoside as a potential modulator of beta-amyloid peptide induced neurotoxicity: an in vitro & in silico study. Bioorg Chem. 2019;88:102935. doi: 10.1016/j.bioorg.2019.102935. [DOI] [PubMed] [Google Scholar]
- Kaidery, et al. Crosstalk between Nrf2 signaling and mitochondrial function in Parkinson’s disease. Mol Cell Neurosci. 2019;101:103413. doi: 10.1016/j.mcn.2019.103413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou L, Du M, Zhang C, Dai Z, Li X, Zhang B, Hu X. Polysaccharide purified from Lycium barbarum protects differentiated PC12 cells against LGluinduced toxicity via the mitochondriaassociated pathway. Mol Med Rep. 2017;16:5533–5540. doi: 10.3892/mmr.2017.7289. [DOI] [PubMed] [Google Scholar]
- Kumaran A, Ho CC, Hwang LS. Protective effect of Nelumbo nucifera extracts on beta amyloid protein induced apoptosis in PC12 cells, in vitro model of Alzheimer’s disease. J Food Drug Anal. 2018;26:172–181. doi: 10.1016/j.jfda.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Du J, Zou L, Xia H, Wu T, Kim Y, Lee Y. The neuroprotective effects of decursin isolated from Angelica gigas Nakai against amyloid beta-protein-induced apoptosis in PC 12 cells via a mitochondria-related caspase pathway. Neurochem Res. 2015;40:1555–1562. doi: 10.1007/s11064-015-1623-0. [DOI] [PubMed] [Google Scholar]
- Li S, et al. Taurine inhibits 2,5-hexanedione-induced oxidative stress and mitochondria-dependent apoptosis in PC12 cells. Ind Health. 2017;55:108–118. doi: 10.2486/indhealth.2016-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, et al. Mesenchymal stem cells-conditioned medium protects PC12 cells against 2,5-hexanedione-induced apoptosis via inhibiting mitochondria-dependent caspase 3 pathway. Toxicol Ind Health. 2017;33:107–118. doi: 10.1177/0748233715598267. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang W, Chen C, Zhang C. Inotodiol protects PC12 cells against injury induced by oxygen and glucose deprivation/restoration through inhibiting oxidative stress and apoptosis. J Appl Biomed. 2018;16:126–132. doi: 10.1016/j.jab.2017.11.004. [DOI] [Google Scholar]
- Li L, et al. The first generation of iPSC line from a Korean Alzheimer’s disease patient carrying APP-V715M mutation exhibits a distinct mitochondrial dysfunction. Exp Neurobiol. 2019;28:329–336. doi: 10.5607/en.2019.28.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, et al. Activation of anti-oxidant of curcumin pyrazole derivatives through preservation of mitochondria function and Nrf2 signaling pathway. Neurochem Int. 2019;125:82–90. doi: 10.1016/j.neuint.2019.01.026. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. Inhibiting c-Jun N-terminal kinase (JNK)-mediated apoptotic signaling pathway in PC12 cells by a polysaccharide (CCP) from Coptis chinensis against Amyloid-beta (Abeta)-induced neurotoxicity. Int J Biol Macromol. 2019;134:565–574. doi: 10.1016/j.ijbiomac.2019.05.041. [DOI] [PubMed] [Google Scholar]
- Liu M, et al. Ginsenoside Re inhibits ROS/ASK-1 dependent mitochondrial apoptosis pathway and activation of Nrf2-antioxidant response in beta-amyloid-challenged SH-SY5Y cells. Molecules. 2019;24(15):2687. doi: 10.3390/molecules24152687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova NA, Ershov NI, Kolosova NG. Suppression of Alzheimer’s disease-like pathology progression by mitochondria-targeted antioxidant SkQ1: a transcriptome profiling study. Oxid Med Cell Longev. 2019;2019:3984906. doi: 10.1155/2019/3984906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JW, Kim MK. Neuroprotective effects of biochanin A against beta-amyloid-induced neurotoxicity in PC12 cells via a mitochondrial-dependent apoptosis pathway. Molecules. 2016 doi: 10.3390/molecules21050548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobore TO. On the central role of mitochondria dysfunction and oxidative stress in Alzheimer’s disease. Neurol Sci. 2019;40:1527–1540. doi: 10.1007/s10072-019-03863-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Miao J, Yan C, Ge R, Liang T, Liu E, Li Q. Chitosan attenuates dibutyltin-induced apoptosis in PC12 cells through inhibition of the mitochondria-dependent pathway. Carbohydr Polym. 2016;151:996–1005. doi: 10.1016/j.carbpol.2016.06.053. [DOI] [PubMed] [Google Scholar]
- Yao H, et al. Thevetiaflavone from Wikstroemia indica ameliorates PC12 cells injury induced by OGD/R via improving ROS mediated mitochondrial dysfunction. Mol Med Rep. 2017;16:9197–9202. doi: 10.3892/mmr.2017.7712. [DOI] [PubMed] [Google Scholar]
- Yin P, et al. Urolithin C, a gut metabolite of ellagic acid, induces apoptosis in PC12 cells through a mitochondria-mediated pathway. RSC Adv. 2017;7:17254–17263. doi: 10.1039/C7RA01548H. [DOI] [Google Scholar]
- Zhao L, Zhu L, Guo X. Valproic acid attenuates Abeta25–35-induced neurotoxicity in PC12 cells through suppression of mitochondria-mediated apoptotic pathway. Biomed Pharmacother. 2018;106:77–82. doi: 10.1016/j.biopha.2018.06.080. [DOI] [PubMed] [Google Scholar]