Abstract
There is an established, link between psychosis and metabolic abnormalities, such as altered glucose metabolism and dyslipidemia, which often precede the initiation of antipsychotic treatment. It is known that obesity-associated metabolic disorders are promoted by activation of specific cannabinoid targets (endocannabinoid system (ECS)). Our recent data suggest that there is a change in the circulating lipidome at the onset of first episode psychosis (FEP). With the aim of characterizing the involvement of the central and peripheral ECSs, and their mutual associations; here, we performed a combined neuroimaging and metabolomic study in patients with FEP and healthy controls (HC). Regional brain cannabinoid receptor type 1 (CB1R) availability was quantified in two, independent samples of patients with FEP (n = 20 and n = 8) and HC (n = 20 and n = 10), by applying three-dimensional positron emission tomography, using two radiotracers, [11C]MePPEP and [18F]FMPEP-d2. Ten endogenous cannabinoids or related metabolites were quantified in serum, drawn from these individuals during the same imaging session. Circulating levels of arachidonic acid and oleoylethanolamide (OEA) were reduced in FEP individuals, but not in those who were predominantly medication free. In HC, there was an inverse association between levels of circulating arachidonoyl glycerol, anandamide, OEA, and palmitoyl ethanolamide, and CB1R availability in the posterior cingulate cortex. This phenomenon was, however, not observed in FEP patients. Our data thus provide evidence of cross talk, and dysregulation between peripheral endocannabinoids and central CB1R availability in FEP.
Subject terms: Molecular neuroscience, Biomarkers
Introduction
Psychotic disorders are associated with a reduced life expectancy of 15–20 years1, which is in part due to the high prevalence of cardiovascular disease, type 2 diabetes (T2DM) and metabolic syndrome2,3. Unhealthy lifestyles and pharmacological side effects have been suggested to be a major cause of these mortality rates4. However, abnormal glucose homeostasis, hyperinsulinemia, dyslipidemia, and accumulation of visceral fat are already evident in drug-naive first episode psychosis (FEP) patients, independent of obesity5,6.
There are many proposed mechanisms underlying the development of metabolic comorbidities. There is evidence that antipsychotic medication, particularly clozapine, leads to rapid weight gain7, while co-treatment with metformin can help reduce this8. A recent study in FEP patients identified changes in specific circulating lipids, which are known to be associated with nonalcoholic fatty liver disease (NAFLD) and T2DM risk, prior to weight gain9.
There is emerging evidence suggesting that the endocannabinoid system (ECS) might be dysregulated in various psychiatric disorders, including schizophrenia10–15. Specifically, in the cerebrospinal fluid (CSF), anandamide (AEA), and palmitoyl ethanolamide (PEA) were found elevated in schizophrenia as compared to controls16, and AEA levels were inversely correlated to psychotic symptoms17. Furthermore, the ECS and related molecules have been shown to play a role in metabolic disorders18–20. Oleoylethanolamide (OEA) has been shown to directly alter lipid metabolism via peroxisome proliferator-activated receptor alpha (PPARα)21, resulting in anti-obesity properties, such as lower body weight, as well as decreased lipid levels in circulation and the liver22. OEA was also found to be regulated by food intake in humans23. Taken together, these findings suggest that the dysregulation of the ECS could play a role in the development or progression of metabolic comorbidities observed in psychosis.
The ECS is composed of (1) cannabinoid receptors type 1 (CB1R) and type 2 (CB2R), (2) lipid-derived endocannabinoid ligands with affinity to these or other receptors, and (3) enzymes involved in the synthesis and degradation of these ligands24,25. The CB1Rs are found in the central nervous system (CNS), as well as in the periphery, throughout the gastrointestinal tract, liver, adipose tissue, and adrenal glands26,27. In the CNS, the CB1R plays an important role in cognition28, and has been implicated in mood and anxiety disorders29,30. Recent evidence from both our group and others suggests that central CB1R availability is altered psychosis31–33. We recently showed that CB1R availability is altered in patients with psychosis, where greater reductions in CB1R levels are associated with greater symptom severity and poorer cognitive functioning33. There is also evidence that endogenous cannabinoid ligands that act as CB1R agonists are elevated in the CSF of medication-naive psychotic patients11,14,16,17,34.
Metabolomics approaches have identified systemic metabolic changes in the context of psychosis9,35–37 and neurodegenerative disorders38–40, suggesting that a strong link exists between the circulating metabolome and diseases of the CNS. Several studies have demonstrated that psychotic patients have dysregulated metabolic profiles, such as dyslipidemia and dysglycemia41,42. Intriguingly, an imbalance of peripheral endocannabinoids has also been associated with various metabolic disorders, including diabetes and obesity43,44. Furthermore, increases in AEA, OEA, and PEA serum levels are associated with improvement in symptoms of psychosis45, which could imply that endocannabinoid signaling may not only be dysregulated in the CNS of psychotic patients, but also in the periphery12,14. However, to our knowledge, there are no data investigating the link between peripheral endocannabinoid levels and brain CB1R availability in healthy individuals or in FEP.
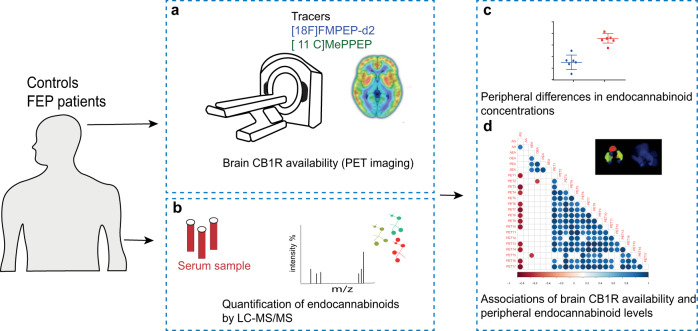
Therefore, we aimed to investigate the association between peripheral endocannabinoids and brain CB1R availability. To this end, we quantified serum endocannabinoids using liquid chromatography–triple quadrupole mass spectrometry (LC–QqQMS) and brain CB1R availability in a case-control setting, which included FEP patients and matched healthy controls (HC; Fig. 1).
Fig. 1. An overview of the experimental design to study associations between brain CB1R availability and circulating endocannabinoids.
a CB1R availability was investigated in vivo in male patients with, first episode psychosis (FEP) and healthy controls (HC) using a CB1R-selective radiotracer using positron emission tomography (PET), with arterial blood sampling. This study was performed at two study sites using two independent samples in the city of Turku, Finland, and an inner-city area of London, United Kingdom. A [18F]FMPEP-d2 PET tracer was used in Turku, while [11C]MePPEP tracer was used in London. b Quantification of circulating endocannabinoids was performed in matched FEP and HC subjects using a quantitative liquid chromatography–triple quadrupole mass spectrometry assay. c Peripheral differences in endocannabinoids between FEP and HCs were evaluated by univariate statistics. In order to assess if circulatory endocannabinoids associated with CB1R availability in the brain, we performed statistical correlation analysis between six endogenous endocannabinoids and 17 CB1R tracer distribution volumes. The correlation analysis was performed separately between FEP and HC for the two study sites.
Results
Levels of circulating endocannabinoids
We measured a total of ten endocannabinoids and related structures (Supplementary Table 1) from serum of both HC and FEP patients from the two study sites. Considerable increases in AG were observed in some samples, which can be explained by variable freezing times46. These samples were excluded from subsequent analyses, resulting in the following available sample numbers: Turku (n = 10, HC; n = 8, FEP) and London (n = 11, HC; n = 15, FEP). In the Turku study, there was a reduction in circulating levels of arachidonic acid (AA; 140.6 ± 29.9 vs. 104.1 ± 26.8 ng/mL) and OEA (3.23 ± 1.04 vs. 2.29 ± 0.50 ng/mL) in the FEP patients (Fig. 2a). AA forms the backbone of two major endocannabinoids AEA47, and is released when they are broken down by fatty acid amide hydrolase48 and monoacylglycerol lipase49. In line with this, there is a trend toward an increase in AG observed in the FEP group (2.32 ± 0.94 vs. 3.69 ± 2.02 ng/mL). Due to the use of serum, it was, however, not possible to differentiate between the levels of 1-arachidonoyl glycerol (1-AG) and 2-AG, because of their rapid isomerization at room temperature. Therefore, only the concentrations of total AG are reported.
Fig. 2. Scatter plots of the levels of circulating endocannabinoids and related structures.
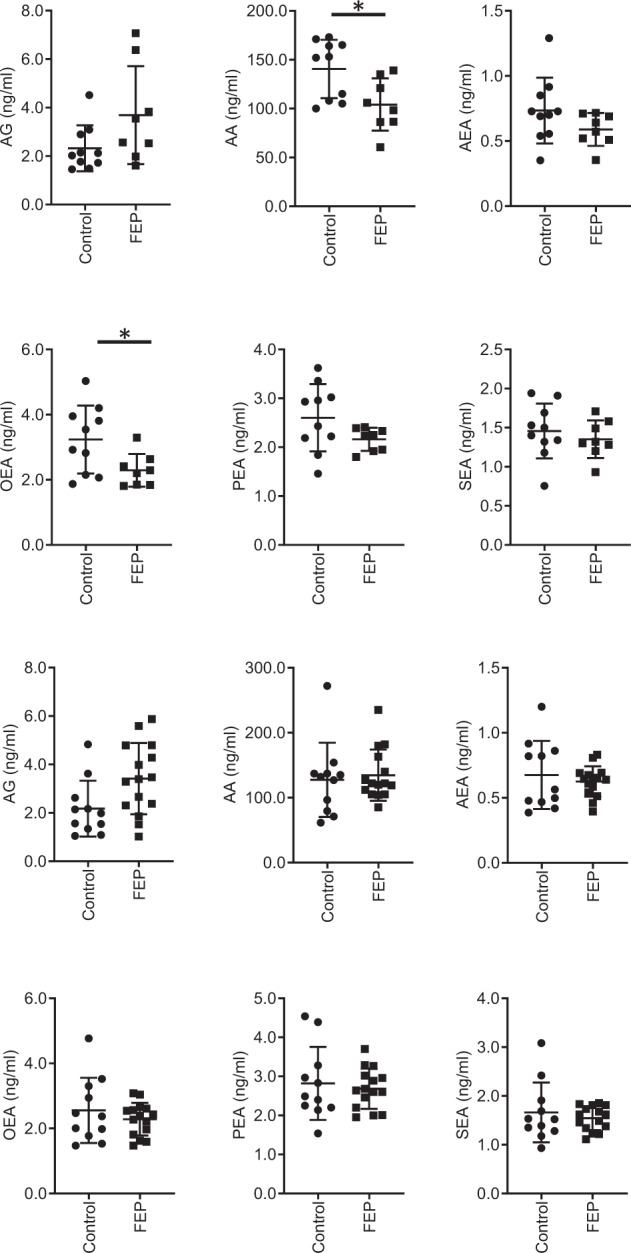
The top six show the levels from the Turku cohort and the bottom 6 show the results from the KCL cohort. Key: *p < 0.05.
The same endocannabinoids were, however, not altered in patients with FEP in the London study (Fig. 2b). In Turku, patients had already started antipsychotic medication, while in London, the patients were mostly drug naive or free of pharmacological treatments33. Previous literature has shown that endogenous central elevations in AEA (a CB1R and CB2R agonist, as well as a ligand to other proteins) found in patients with FEP14,17 are absent in patients taking typical antipsychotics, which agonize the actions of dopamine17. Thus, antipsychotic treatment in the Turku sample may account for the differences we observe. However, since the majority of the therapies involved atypical antopsychotics (Table 2), which were previously found not to normalize AEA levels17, this discrepancy could be due to other differences between the study populations, such as diagnosis, substance use, diet, or duration of illness. The full details of disease duration, medication, and diagnosis is provided in Tables 2 and 3.
Associations between central and peripheral ECSs
Given the known role of the ECS in dietary regulation50 and weight gain51, we next investigated the associations between peripheral endocannabinoid levels and CB1R availability in the ROIs described in the methods. The results from the CB1-receptor availability study have previously been published33.
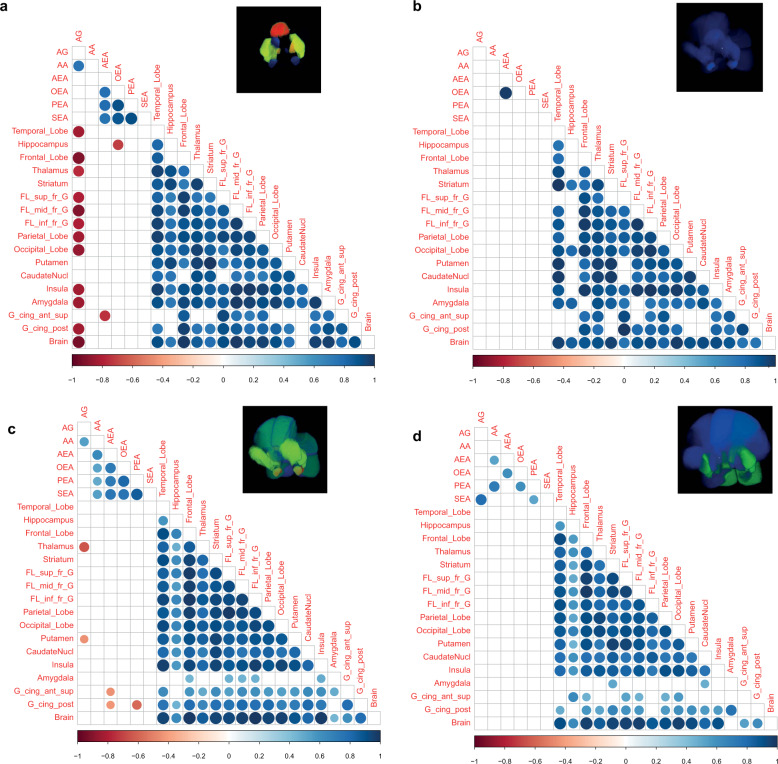
Initially, we explored how circulating levels of endocannabinoids are associated with CB1R availability in the brain in both studies using partial least squares regression (PLS-R), which allowed us to see how all of the endocannabinoids are associated with individual brain regions in one model. The R2 values were increased in HC both in the Turku (Fig. 3a) and London (Fig. 3c) cohorts, as compared to the FEP group (Fig. 3b, Turku and Fig. 3d, London). We next explored the univariate associations between levels of circulating endocannabinoids and CB1R availability in the brain. Positive correlations of CB1R availability between the brain regions were observed in HCs from both study sites (Fig. 3e). Similarly, levels of circulating endocannabinoids were also positively correlated with each other in the healthy subjects (Fig. 3e). Interestingly, the levels of peripheral endocannabinoids and central CB1R availability were, conversely, negatively correlated in HCs in both Turku and London samples (Fig. 3e). In FEP subjects, CB1R availability was also positively correlated between the different brain regions (Fig. 3f). However, the degree of positive correlation between the circulating endocannabinoids was reduced (Fig. 3f).
Fig. 3. Correlation analysis, performed between peripheral endocannabinoid concentrations and the distribution volumes of brain CB1R availability separately for patients with FEP and HC.
Correlation brain maps where the R2 values from the PLS regression analysis are projected into the 3D brain regions used for the PET analysis in the healthy controls (a, Turku, c, London) and FEP patients (b, Turku, d, London). Red shading denotes high R2, blue low R2. e Spearman correlation coefficients between CB1R availability and circulating endocannabinoids in HC from both Turku and London. The CB1R availability was combined by first autoscaling the data from each site. f Spearman correlation coefficients between CB1R availability and circulating endocannabinoids in FEP from both Turku and KCL. The correlation coefficient values revealed positive correlations between CB1R availability in different regions of the brain in both FEP and HC. There was significant negative association between peripheral AEA and PEA concentrations, and CB1R availability in certain regions of the brain among HCs, and this relationship was absent in FEP patients, at each respective site. Here, the blue gradient represents positive correlation, while the red gradient indicates negative correlation (p-values < 0.05).
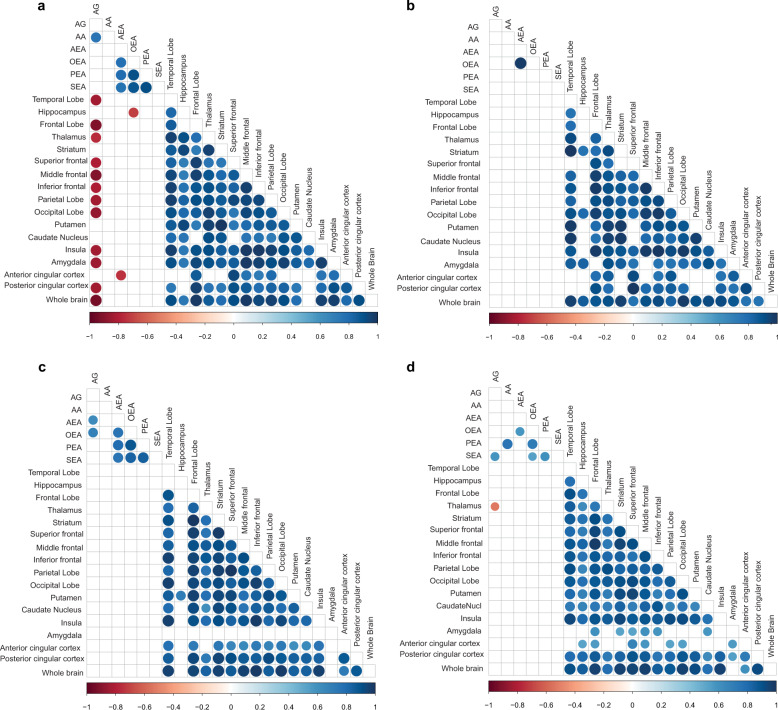
Notably, the negative correlations between CB1R availability and levels of circulating endocannabinoids, as observed in HC, were absent in FEP patients (Fig. 3e, f). The loss of this association is more pronounced in the Turku study (Fig. 4a, b) compared to the London study (Fig. 4c, d). This relative lack of association between central CB1R availability and circulating endocannabinoid levels in FEP suggests that there may normally be a functional link between these two systems, which may be dysregulated in FEP.
Fig. 4. Correlation analysis, performed between peripheral endocannabinoid concentrations and the distribution volumes of brain CB1R availability separately between FEP patients HC at the two study sites.
a Spearman correlation coefficients between CB1R availability and circulating endocannabinoids in HC from the city of Turku. b Spearman correlation coefficients between CB1R availability and circulating endocannabinoids in FEP from the city of Turku. The correlation coefficient values revealed positive correlation between CB1R availability in different regions of the brain in both FEP and HC. c Spearman correlation coefficients between CB1R availability and circulating endocannabinoids in HC from an inner-city area of London. d Spearman correlation coefficients between CB1R availability and circulating endocannabinoids in FEP from inner-city area of London. There was significant negative association between peripheral arachidonoyl glycerol (1 + 2) concentrations and CB1R availability in certain regions of the brain among HCs, which was absent in FEP patients, at different sites, respectively. Here, the blue gradient represents positive correlation, while the red gradient indicates negative correlation (p-values < 0.05).
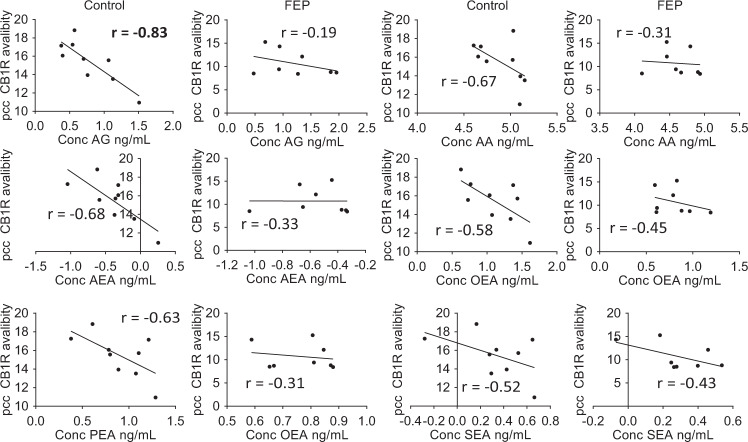
In order to further examine the associations between central CB1R availability and circulating peripheral endocannabinoids, we selected the brain region, from the available ROIs, with the highest R2 value from the PLS-R modeling, the posterior cingulate cortex (pcc; Fig. 3). The pcc has a critical role in a wide range of cognitive tasks52 and is a major node in the default mode network53. Early changes have been observed in parts of the pcc during FEP54. For the majority of serum endocannabinoids measured in FEP patients, there was a clear loss of any negative correlation with CB1R availability, with few positive associations observed in the FEP patients (Fig. 5). This effect was more pronounced in the Turku study (Supplementary Fig. 1a), with similar trends observed in the London study, though these did not reach statistical significance (Supplementary Fig. 1b).
Fig. 5. Scatter plots fitted with a linear regression model of CB1R availability in the posterior cingulate cortex (PCC), versus the log-transformed circulating levels of endocannabinoids.
The data was combined data from both studies, scaled to zero mean and unit variance within each study separately. The line shows the linear model for each dataset. Bold highlights of the r value represent significant correlations p < 0.05.
Discussion
We demonstrated that the cross talk between the endocannabinoid networks in the brain and periphery are dysregulated in FEP, as compared to HC. Reduced levels of AA and OEA in FEP subjects were observed in the Turku study, but not in London, where the patients were predominately drug naive or free from pharmacological treatments. A negative association between brain CB1R availability in the majority of the ROIs used in the positron emission tomography (PET) imaging and serum endocannabinoid levels was observed in healthy individuals, but not in FEP patients across the two independent cohorts of patients.
Endocannabinoids are lipid mediators with a vital role in the maintenance of metabolic and immune homeostasis55,56. Several studies have found that systemic lipid profiles are dysregulated in various psychotic disorders9,57. Our study could corroborate these earlier findings, suggesting that the systemic ECS is dysregulated in psychosis12,34,58. In the periphery, a significant drop in the circulatory endocannabinoid, AEA, was previously found to accompany the acute phase of schizophrenia12, although this finding could not be replicated in a larger cohort17. This is at odds with findings from the London study, as well as with the previous literature showing that patients with FEP not taking antipsychotics or using cannabis show increased levels of AEA in CSF14,17,34. However, the finding from the Turku study is consistent with previous work showing a downregulation of enzymes involved in the synthesis of endocannabinoid ligands in peripheral blood mononuclear cells of FEP patients, as compared to matched, HC58. However, small sample size in the Turku study may also be a contributing factor to the observed discrepancy. In addition to FEP, other neuroinflammatory diseases, including multiple sclerosis, Huntington’s, and Parkinson’s diseases, are characterized by ECS alterations in the periphery59.
Our previous data links rapid weight gain in FEP with elevated baseline levels of triglycerides of low carbon number and double bond count9, which are known to be associated with de novo lipogenesis, inhibition of lipolysis, and NAFLD60,61. Endocannabinoids are known to stimulate de novo lipogenesis and obesity62. In our previous hepatic venous catheterization study in humans, we found elevated liver fat to be associated both with increased levels of 2-AG, as well as higher splanchnic production of triglycerides of low carbon number and double bond count, suggesting that the ECS could contribute to the development of human NAFLD by affecting hepatic lipid metabolism60.
OEA is the monounsaturated analog of AEA, but, unlike AEA, acts independently of the cannabinoid pathway, with no reported direct activity at the two known cannabinoid receptors, CB1R and CB2R63. OEA stimulates fat utilization as a PPARα agonist64. Activation of PPARα by OEA is known to induce satiety and regulate body weight65. A study in mice also suggests that OEA inhibits food intake by recruitment of the brain histaminergic system66. Our data suggest that decreased OEA in FEP subjects is likely the effect of antipsychotic meditation, as this was not seen in the cohort that was predominately unmedicated. However, there are other factors, such as disease duration and severity, which could also be a factor in the OEA difference between the sites. Inhibition of lipolysis due to diminished OEA65 may also contribute to increased liver fat, i.e., the accumulation of triacylglycerols in the liver, as observed by lipidomics in nonobese FEP patients who subsequently rapidly gained weight9. It is thus plausible that OEA levels are an early marker of propensity for FEP patients to gain weight, possibly as a result of therapy. This hypothesis will clearly need to be examined prospectively, in a larger study setting.
Our study also shows that peripheral endocannabinoids and central CB1R availability are inversely correlated in healthy individuals, while no such association is observed in FEP patients. The CB1R modulates energy homeostasis via both central and peripheral mechanisms67. The association between peripheral and central ECS measures suggests common systemic regulation of ECS in healthy individuals, which, however, is absent in FEP. Our findings from the Turku cohort indicate that both central and peripheral measures of the ECS were altered in patients with affective/non-affective FEP who were taking antipsychotic medication. In contrast, our findings from the London cohort indicate that patients who were predominately medication naive/free from pharmacological treatments, show central alterations in CB1R availability without any alterations in peripheral levels of endocannabinoids. This suggests that peripheral measures of endocannabinoids may not be a useful biomarker for indexing central endocannabinoid dysfunction in FEP, alternatively, this negative finding may also be due to the small sample size, or differences in sample clinical characteristics between studies, including diagnosis, illness severity, therapies, and illness duration. This is in contrast to other findings in the field, where AEA has been suggested as a potential biomarker for schizophrenia16. The majority of the subjects in our study were confirmed to have a diagnosis of non-affective psychosis at 1-year follow up (Tables 2 and 3). There were, however, three cases of affective psychosis in the Turku cohort. This is too small of a group to explore if these individuals had an impact on the results.
Taken together, our data provides evidence of ECS dysregulation in FEP both centrally33 and in the periphery, in medicated patients with FEP. Our findings should be considered as preliminary due to our small sample size and partial inconsistency of results. Further studies are clearly needed in order to further examine the cross talk between the peripheral and central ECSs in health and psychosis.
Methods
Ethics statement
Ethical approvals were obtained from respective study sites in Finland (ETMK 98/180/2013) and England (14/LO/1289). Subjects’ capacities for consent were assessed and informed, written consent was obtained from all volunteers.
Study design and participants
The study setting was described in detail previously33. CB1R availability was investigated at two PET centers using independent samples; Turku (n = 11, HC; n = 8, FEP) and London (n = 23, HC; n = 20, FEP). However, some subjects were excluded due to increases in total arachidonoyl glycerol levels, caused by sample handling, resulting in the following subjects being included in the analysis: Turku (n = 10, HC; n = 8, FEP) and London (n = 15, HC; n = 11, FEP; Table 1). Given putative sex differences in CB1R availability46, we only investigated males to remove sex as a source of variability. In Turku, the FEP patients were not antipsychotic naive (Table 2). Corresponding studies in females are currently ongoing.
Table 1.
Demographic characteristics of the study populations.
| Turku | KCL | |||
|---|---|---|---|---|
| FEP | HC | FEP | HC | |
| n = 8 | n = 10 | n = 15 | n = 11 | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (years) | 26.4 (3.6) | 27.18 (5.9) | 27.1 (5.1) | 26.6 (6.9) |
| BMI (kg/m2) | 28.2 (6.9) | 25.3 (3.7) | 25.9 (5.2) | 26.3 (4.1) |
| Years of education | 13.3 (1.3) | 15.7 (3.2) | n/a | n/a |
| Illness duration (months) | 5.4 (6.8) | n/a | 27.1 (10.1) | n/a |
| BPRS total score sum | 65.3 (17.6)* | 30.6 (2.4)* | n/a | n/a |
| BPRS positive score sum | 20.0 (7.3)* | 9.2 (0.6)* | n/a | n/a |
| PANSS positive | n/a | n/a | 24.4 (5.4) | n/a |
| PANSS negative | n/a | n/a | 42.5 (9.0) | n/a |
| PANSS general | n/a | n/a | 42.53 (9.0) | n/a |
| PANSS total | n/a | n/a | 90.7 (16.6) | n/a |
*p < 0.05.
Table 2.
Disease duration and medication in the Turku cohort.
| Illness duration (mo) | Antipsychotic medication (p.o.) | CPZ eqv DDD (Leucht) | BPRS positive sum** | Diagnosis |
|---|---|---|---|---|
| 4 | Risperidone 1 mg | 60 mg | 13 | Affective psychosis |
| 2 | Aripiprazole 15 mg | 300 mg | 21 | Affective psychosis |
| 6 | Risperidone 2 mg | 120 mg | 13 | Schizophrenia* |
| 1 | Olanzapine 5 mg | 150 mg | 31 | Affective psychosis |
| 3 | Perphenazine 8 mg | 80 mg | 12 | Schizophreniform |
| 22 | Aripiprazole 15 mg | 300 mg | 26 | Schizophrenia |
| 2 | – | – | 27 | Schizophrenia |
| 3 | Aripiprazole 15 mg + olanzapine 10 mg | 600 mg | 17 | Schizophrenia |
*Cannabis dependance disorder.
**BPRS, Brief Psychiatric Rating Scale.
Data for each patient listed as separate row.
FEP patients met the following inclusion criteria: (i) DSM-IV diagnosis of a psychotic disorder, determined by the SCID-I/P; (ii) illness duration of <3 years; and (iii) male sex. In Turku, FEP volunteers were already taking antipsychotic medication prior to the start of the study and had diagnoses of affective or non-affective psychosis. In London, FEP volunteers were, with the exception of two individuals, medication free from all pharmacological treatments for at least 6 months and had diagnoses of schizophrenia or schizoaffective disorder (DSM-IV criteria; Table 3). Healthy volunteers had no current/lifetime history of any Axis-I disorder as determined by the SCID-I/P, and were matched for age (age ±3 years) and sex (male). Exclusion criteria for study and healthy volunteers were: (i) current/lifetime history of substance abuse/dependence; (ii) substance use within the last month; and (iii) positive screen on toxicology tests for cannabis and other substances.
Table 3.
Details of disease duration and medication for KCL cohort.
| Illness duration (mo) | Antipsychotic medication (p.o.) | CPZ eqv DDD (Leucht) | PANSS total | Diagnosis |
|---|---|---|---|---|
| 36 | – | 0 | 79 | Schizophrenia |
| 36 | 2 | 60 | 75 | Schizophrenia |
| 24.5 | – | 0 | 103 | Schizophrenia |
| 30 | 2 | 60 | 103 | Schizophrenia |
| 30 | – | 0 | 116 | Schizophrenia |
| 36 | – | 0 | 103 | Schizophrenia |
| 36 | – | 0 | 66 | Schizoaffective disorder |
| 12 | – | 0 | 63 | Schizophrenia |
| 24 | 2 | 60 | 90 | Schizophrenia |
| 12 | – | 0 | 107 | Schizoaffective disorder |
| 36 | – | 0 | 69 | Schizophrenia |
| 36 | – | 0 | 107 | Schizophrenia |
| 6 | – | 0 | 88 | Schizophrenia |
| 24 | – | 0 | 101 | Schizophrenia |
| 28 | – | 0 | 79 | Schizophrenia |
Data for each patient listed as separate row.
CB1R availability PET imaging
The full methods for PET CB1R imaging were described in detail previously33. However, a concise description for both studies, Turku and London, is provided here.
Turku study
[18F]FMPEP-d2 was synthesized as described previously47, with slight modifications. The radiochemical purity was >95% and the molar radioactivity was >500 GBq/μmol at the end of synthesis, resulting in an injected tracer mass <200 ng.
Positron emission scans were done in three-dimensional (3D) list mode, in increasing frame duration for a total scan range of 0–120 min, with a brain-dedicated high-resolution research tomograph (ECAT HRRT, Siemens Medical Solutions, Kemnath, Germany), after a bolus injection of [18F]FMPEP-d2 (201 ± 11.1 MBq). Attenuation correction of PET was applied using a 137Cs point source. Arterial plasma activity was measured continuously for the first 3.5 min, and discretely thereafter at 4.5, 7.5, 11,15, 20, 25, 30, 35, 40, 45, 50, and 60 min. Arterial input was corrected for tracer metabolite activity measured using thin layer chromatography and digital autoradiography. The resulting metabolite-free arterial input was corrected for temporal delay between blood and PET tissue measurements. Motion correction between PET frames was done by realigning the PET frames to a single reference frame with the most uptake on average. Whole-brain T1-weighted MRI images were acquired by a Philips 3 T Ingenuity PET/MR hybrid scanner. The individual T1-weighted images were co-registered to the sum of the realigned PET frames. Inverse normalization parameters obtained from normalizing the co-registered T1-weighted images to MNI space were used to fit the Hammersmith anatomical atlas to the PET frames (Hammers et. al 2003). Data preprocessing were performed using Statistical Parametric Mapping 12 (http://www.fil.ion.ucl.ac.uk/spm) and MATLAB R2014b (Mathworks Inc., Natick, MA).
London study
Continuous, 90-min PET scans were acquired on a PET/CT (Hi-Rez Biograph 6 CT44931; Siemens Medical Solutions, Kemnath, Germany) in 3D, after bolus injection of 314 ± 34.4 MBq of [11C]MePPEP, as previously described48–50. CT scans were acquired prior to each PET scan for correction of attenuation and scatter. Continuous arterial blood sampling took place for the first 15 min of the scan that was followed by discrete blood sampling at 2, 5, 10, 15, 20, 25, 35, 40, 50, 60, 70, 80, and 90 min after radioligand injection. Images were reconstructed with filtered back-projection, including corrections for attenuation and scatter. There were no significant group differences in injected mass, injected activity, or specific activity. To aid anatomical localization, each volunteer received a high-resolution structural 3D T1-weighted magnetic resonance scan acquired on a General Electric MR750 3 T scanner (MR750; GE Healthcare, Chicago, IL).
Data preprocessing was performed using a combination of Statistical Parametric Mapping 12 (http://www.fil.ion.ucl.ac.uk/spm) and FSL (http://www.fsl.fmrib.ox.ac.uk/fsl) functions, as implemented in MIAKAT (http://miakat.org). Motion correction was applied to non-attenuation-corrected images51. Non-attenuated-corrected frames were realigned to a single “reference” frame (corresponding to that with the highest number of counts) by employing a mutual information algorithm. The transformation parameters were then applied to the corresponding attenuated-corrected dynamic images, creating a movement-corrected dynamic image that was used for subsequent analysis. Realigned frames were then summated to create single-subject motion-corrected maps that were then used for MRI and PET co-registration, prior to PET data quantification. T1-weighted structural images were co-registered to the PET image using rigid body transformation. Normalization parameters were obtained by warping the co-registered structural MRI to MNI space (International Consortium for Brain Mapping ICBM/MNI) using bias-corrected segmentation. The inverse of these parameters was used to fit a neuroanatomical atlas to each individual PET scan using the Hammersmith atlas52. Whole-blood time–activity curves (TACs) were fitted using a multi-exponential function as derived by Feng’s model53. For each scan, a time delay was fitted and applied to the input functions (both parent and whole-blood TACs) to account for any temporal delay between blood sample measurement and target tissue data. Regions of interest (ROI) were harmonized with the Turku PET dataset.
In both Turku and London studies, CB1R availability was indexed using the volume of distribution (VT ml/cm3) of the respective PET tracer, as calculated using the Logan graphical method with a metabolite-corrected arterial plasma input function. The resulting model was validated as described previously33,54. The temporal lobe, frontal lobe, anterior cingulate cortex, pcc, striatum, parietal lobe, occipital lobe, putamen, nucleus caudatus, insula, amygdala, and whole brain were chosen as ROI for PET. Hippocampal, amygdala, superior temporal gyral, superior frontal, middle frontal, inferior frontal, and orbitofrontal subregions were included to explore regional differences, within relevant ROI. Due to the use of different PET tracers used in both sites, the CB1R availability data were scaled to unit variance and zero mean within each study, when analyzed together.
Analysis of serum endocannabinoids
The following peripheral endocannabinoids and related structures were measured using a targeted LC–QqQMS method: THC-COOH, arachidonoyl ethanolamide (AEA), N-arachidonoyl dopamine (NADA), 2-AG, 1-AG, OEA, PEA, 2-arachidonoyl glycerol ether, AA, and stearoyl ethanolamide from the arterial serum collected during the PET imaging. The serum collection was unified at both sites, in order to allow for direct comparison of results. Serum samples were stored at −80 °C for 12.1 months (±8.72 months) in Turku study site and 24.9 months (±8.66 months) at KCL. The analytical method was developed for serum samples and all solvents were LC-grade (Sigma-Aldrich Inc., St. Louis, MO). Initially, the internal standards (10 µL, 100 ng/mL, of THC-COOH-d9, 2-AG-d5, NADA-d8, and AEA-d8 in EtOH) were added to 200 µL of serum in silylated 2 mL glass chromatography vials. The proteins were then precipitated using acetonitrile (400 µL) containing 0.1% v/v formic acid (LC-grade, Sigma-Aldrich). This was done in metal and glass syringes to ensure no contamination from plastic. The samples were then briefly vortexed and placed at −20 °C for 30 min. The samples were then centrifuged (10,000 × g, 4 °C) for 10 min. The supernatant was then removed and placed in fresh silylated vials and stored for no more than 24 h before solid-phase extraction (SPE) of the endocannabinoids.
The SPE (reverse-phase HLB, 30 mg sorbent) was performed in 96-well plates (Waters Inc., Milford, MA) and performed on ice to reduce plastic contamination. Initially, 1 mL of ultrapure H2O was added to the top of each well. The supernatant was then transferred into H2O via a glass Pasteur pipette, and gently mixed by pipetting up and down. The vacuum pump (−3 mbar) was then started and the wells allowed to dry. The wells were then washed twice with 15% ACN in ultrapure H2O (500 µL, 0.1% v/v formic acid). The pressure was then reduced to −15 mbar and the wells dried for 15 min. The waste container under the 96-well sorbet plate was then replaced with 750 µL silylated glass insert vials in a deep 96-well plate. The endocannabinoids were eluted by allowing acetone (350 µL, 0.1% v/v formic acid) to pass through the sorbent bed twice. The samples were then evaporated to dryness under a stream of N2 prior to resuspension in a 70% solution of ACN (100 µL, 30% ultrapure water, 1% v/v acetic acid). The samples were then vortexed briefly and stored at −20 °C prior to the LC–QqQMS experiment.
The endocannabinoids were separated using ultra-high-performance liquid chromatography. The two eluent solvents were (i) H2O (1% v/v acetic acid) and (ii) ACN. The gradient was set up as follows: the initial conditions were 70% B for 4.5 min increasing to 80% B by 7 min. By 8 min, the gradient was increased to 95% B and held there for 10 min. The gradient was reduced to the starting conditions by 10.5 min and the column flushed with a minimum of ten column volumes. The flow rate was set at 0.5 mL/min throughout the run and the column temperature set to 60 °C. The column used for the separation was a Waters ACQUITY BEH reverse-phase C18 column (130 Å, 1.7 µm, 2.1 mm × 50 mm). The injection volume was 10 µL. The needle was washed three times both before and after the injection with two solvents: (i) IPA:ACN (1:1) and (ii) H2O:MeOH (1:1). The mass spectrometer 5500 QTRAP (AB Sciex Inc., Framingham, MA) was set up in scheduled multiple reaction monitoring (MRM) mode that allows for the setup of detection windows around the peak of interest to improve sensitivity. The details of the MRM transitions can be found in Supplementary Table 1. The analytes were quantified using a six-point standard curve and, where possible, with the matched labeled standard. Where no labeled standard was available, we used the closest related molecule as the internal standard.
Statistical analysis
The nonparametric Mann–Whitney U test was used for comparing the levels of circulating endocannabinoids using the log-transformed data, and performed using GraphPad Prism v. 7.04 (GraphPad Software Inc., San Diego, CA). In order to compare the association between circulating endocannabinoids with brain CB1R availability, two methods were utilized: (i) PLS-R modeling was used in order to combine multiple brain regions and circulating endocannabinoid measures. This analysis was performed using PLS-Toolbox v. 8.6 (Eigenvector Research Inc., Manson, WA) and MATLAB 2017b (Mathworks Inc., Natick, MA). Due to the small number of samples, a leave-one-out cross-validation, using the number of iterations of the smallest group size, was utilized to ensure model validity. (ii) Spearman correlation coefficients were calculated using the statistical toolbox in MATLAB 2017b and p-values < 0.05 (two-tailed) were considered significant for the correlations. The individual spearman correlation coefficients (R) were illustrated as a heatmap using the “corrplot” package for the R statistical programming language55. The individual correlations between the serum endocannabinoids and CB1R availability were plotted, and analyzed using GraphPad Prism.
Generation of the statistical association brain maps
In order to visualize the associations of CB1R availability across different brain regions, the R2 values from the individual brain regions arising from the PLS-R modeling were mapped onto the same brain template as the PET image analysis, using MATLAB 2017b. The resultant image was then visualized using CARIMAS v. 2.9 (Turku PET Centre, Turku, Finland). The color scale for each brain region was defined by the HC in each cohort and then applied to the FEP patients.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
This project has received funding from the European Union’s Seventh Framework Programme for project METSY—Neuroimaging platform for characterization of metabolic comorbidities in psychotic disorders (grant no. 602478). We thank to Aidan McGlinchey for assistance with editing the manuscript. We would like to thank the Turku Metabolomics Centre and Biocenter Finland for their support in the metabolomics analysis. We also wish to acknowledge the following METSY project investigators: Raimo K. R. Salokangas, Tuula Ilonen, Päivi Jalo, Akseli Mäkelä, Tiina From, Janina Paju, Anna Toivonen, Reetta-Liina Armio, Mirka Kolkka, Maija Walta, Max Karukivi, Juha Mäkelä, Maria Tikka, Olof Solin, Merja Haaparanta-Solin, Aidan McGlinchey, Juha Pajula, Mark van Gils, Juha M. Kortelainen, Carmen Moreno, Joost Janssen, Javier Santonja, Covadonga M. Diaz-Caneja, Miriam Ayora Rodriguez, Celso Arango, Alberto Rodriguez-Quiroga, Fabian Hernández-Álvarez, Jose L. Ayuso-Mateos, Roberto Rodriguez-Jimenez, Angela Ibañez, Jaana Suvisaari, Maija Lindgren, Teemu Mäntylä, Tuula Kieseppä, Outi Mantere, Eva Rikandi, Tuukka T. Raij, Dieter Maier, Elisabeth Frank, and Markus Butz-Ostendorf. Open access funding provided by Örebro University.
Author contributions
M.O., J.H., and O.H. initiated, designed, and supervised the study. A.M.D., T.R., T.L., and T.H. acquired serum endocannabinoid data by mass spectrometry. F.B., H.L., T.M., and M.V. acquired neuroimage data. A.M.D., F.B., H.L., and M.O. analyzed the data. A.M.D., S.L., and M.O. wrote the first draft of the manuscript. All authors approved the final version.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
The MATLAB scripts used for statistical analysis in this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alex M. Dickens, Faith Borgan, Heikki Laurikainen.
Contributor Information
Alex M. Dickens, Email: alex.dickens@utu.fi
Matej Orešič, Email: matej.oresic@utu.fi.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41537-020-00110-7.
References
- 1.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch. Gen. Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 2.Ringen PA, Engh JA, Birkenaes AB, Dieset I, Andreassen OA. Increased mortality in schizophrenia due to cardiovascular disease−a non-systematic review of epidemiology, possible causes, and interventions. Front. Psychiatry. 2014;5:137. doi: 10.3389/fpsyt.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherjee S, Schnur DB, Reddy R. Family history of type 2 diabetes in schizophrenic patients. Lancet. 1989;1:495. doi: 10.1016/s0140-6736(89)91392-5. [DOI] [PubMed] [Google Scholar]
- 4.Arango C, Bobes J, Kirkpatrick B, Garcia-Garcia M, Rejas J. Psychopathology, coronary heart disease and metabolic syndrome in schizophrenia spectrum patients with deficit versus non-deficit schizophrenia: findings from the CLAMORS study. Eur. Neuropsychopharmacol. 2011;21:867–875. doi: 10.1016/j.euroneuro.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Pillinger T, et al. Impaired glucose homeostasis in first-episode schizophrenia: a systematic review and meta-analysis. JAMA Psychiatry. 2017;74:261–269. doi: 10.1001/jamapsychiatry.2016.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillinger T, Beck K, Stubbs B, Howes OD. Cholesterol and triglyceride levels in first-episode psychosis: systematic review and meta-analysis. Br. J. Psychiatry. 2017;211:339–349. doi: 10.1192/bjp.bp.117.200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covell NH, Weissman EM, Essock SM. Weight gain with clozapine compared to first generation antipsychotic medications. Schizophr. Bull. 2004;30:229–240. doi: 10.1093/oxfordjournals.schbul.a007074. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z, et al. Metformin for treatment of clozapine-induced weight gain in adult patients with schizophrenia: a meta-analysis. Shanghai Arch. Psychiatry. 2015;27:331–340. doi: 10.11919/j.issn.1002-0829.215071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suvitaival T, et al. Serum metabolite profile associates with the development of metabolic co-morbidities in first-episode psychosis. Transl. Psychiatry. 2016;6:e951. doi: 10.1038/tp.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newell KA, Deng C, Huang XF. Increased cannabinoid receptor density in the posterior cingulate cortex in schizophrenia. Exp. Brain Res. 2006;172:556–560. doi: 10.1007/s00221-006-0503-x. [DOI] [PubMed] [Google Scholar]
- 11.Koethe D, et al. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br. J. Psychiatry. 2009;194:371–372. doi: 10.1192/bjp.bp.108.053843. [DOI] [PubMed] [Google Scholar]
- 12.De Marchi N, et al. Endocannabinoid signalling in the blood of patients with schizophrenia. Lipids Health Dis. 2003;2:5. doi: 10.1186/1476-511X-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parolaro D, Realini N, Vigano D, Guidali C, Rubino T. The endocannabinoid system and psychiatric disorders. Exp. Neurol. 2010;224:3–14. doi: 10.1016/j.expneurol.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Leweke FM, et al. Anandamide levels in cerebrospinal fluid of first-episode schizophrenic patients: impact of cannabis use. Schizophr. Res. 2007;94:29–36. doi: 10.1016/j.schres.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 15.Bioque M, et al. Peripheral endocannabinoid system dysregulation in first-episode psychosis. Neuropsychopharmacology. 2013;38:2568–2577. doi: 10.1038/npp.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 1999;10:1665–1669. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- 17.Giuffrida A, et al. Cerebrospinal anandamide levels are elevated in acute schizophrenia and are inversely correlated with psychotic symptoms. Neuropsychopharmacology. 2004;29:2108–2114. doi: 10.1038/sj.npp.1300558. [DOI] [PubMed] [Google Scholar]
- 18.Iannotti FA, Di Marzo V, Petrosino S. Endocannabinoids and endocannabinoid-related mediators: targets, metabolism and role in neurological disorders. Prog. Lipid Res. 2016;62:107–128. doi: 10.1016/j.plipres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Matias I, Di Marzo V. Endocannabinoids and the control of energy balance. Trends Endocrinol. Metab. 2007;18:27–37. doi: 10.1016/j.tem.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Di Marzo V, Piscitelli F, Mechoulam R. Cannabinoids and endocannabinoids in metabolic disorders with focus on diabetes. Handb. Exp. Pharmacol. 2011;203:75–104. doi: 10.1007/978-3-642-17214-4_4. [DOI] [PubMed] [Google Scholar]
- 21.Lo Verme J, et al. Regulation of food intake by oleoylethanolamide. Cell Mol. Life Sci. 2005;62:708–716. doi: 10.1007/s00018-004-4494-0. [DOI] [PubMed] [Google Scholar]
- 22.Fu J, Oveisi F, Gaetani S, Lin E, Piomelli D. Oleoylethanolamide, an endogenous PPAR-alpha agonist, lowers body weight and hyperlipidemia in obese rats. Neuropharmacology. 2005;48:1147–1153. doi: 10.1016/j.neuropharm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Fu J, et al. Food intake regulates oleoylethanolamide formation and degradation in the proximal small intestine. J. Biol. Chem. 2007;282:1518–1528. doi: 10.1074/jbc.M607809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desfossés J, Stip E, Bentaleb LA, Potvin S. Endocannabinoids and schizophrenia. Pharmaceuticals. 2010;3:3101–3126. [Google Scholar]
- 25.Silvestri C, Di Marzo V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013;17:475–490. doi: 10.1016/j.cmet.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Pertwee RG. The pharmacology of cannabinoid receptors and their ligands: an overview. Int. J. Obes. 2006;30:S13–S18. doi: 10.1038/sj.ijo.0803272. [DOI] [PubMed] [Google Scholar]
- 27.Frank E, et al. Platform for systems medicine research and diagnostic applications in psychotic disorders-The METSY project. Eur. Psychiatry. 2018;50:40–46. doi: 10.1016/j.eurpsy.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Borgan F, et al. The effects of cannabinoid 1 receptor compounds on memory: a meta-analysis and systematic review across species. Psychopharmacology (Berl.) 2019;236:3257–3270. doi: 10.1007/s00213-019-05283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hungund BL, et al. Upregulation of CB1 receptors and agonist-stimulated [35S]GTPgammaS binding in the prefrontal cortex of depressed suicide victims. Mol. Psychiatry. 2004;9:184–190. doi: 10.1038/sj.mp.4001376. [DOI] [PubMed] [Google Scholar]
- 30.Neumeister A, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol. Psychiatry. 2013;18:1034–1040. doi: 10.1038/mp.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hietala J. 42.4 The endocannabinoid system in first-episode psychosis. Schizophr. Bull. 2018;44:S69–S69. [Google Scholar]
- 32.Ranganathan M, et al. Reduced brain cannabinoid receptor availability in schizophrenia. Biol. Psychiatry. 2016;79:997–1005. doi: 10.1016/j.biopsych.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borgan F, et al. In vivo availability of cannabinoid 1 receptor levels in patients with first-episode psychosis. JAMA Psychiatry. 2019;76:1074–1084. doi: 10.1001/jamapsychiatry.2019.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minichino A, et al. Measuring disturbance of the endocannabinoid system in psychosis: a systematic review and meta-analysis. JAMA Psychiatry. 2019;76:914–923. doi: 10.1001/jamapsychiatry.2019.0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaddurah-Daouk R, et al. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol. Psychiatry. 2007;12:934–945. doi: 10.1038/sj.mp.4002000. [DOI] [PubMed] [Google Scholar]
- 36.Oresic M, et al. Metabolome in schizophrenia and other psychotic disorders: a general population-based study. Genome Med. 2011;3:19. doi: 10.1186/gm233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holmes E, et al. Metabolic profiling of CSF: evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS Med. 2006;3:e327. doi: 10.1371/journal.pmed.0030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quinones MP, Kaddurah-Daouk R. Metabolomics tools for identifying biomarkers for neuropsychiatric diseases. Neurobiol. Dis. 2009;35:165–176. doi: 10.1016/j.nbd.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 39.Oresic M, et al. Metabolome in progression to Alzheimer’s disease. Transl. Psychiatry. 2011;1:e57. doi: 10.1038/tp.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bogdanov M, et al. Metabolomic profiling to develop blood biomarkers for Parkinson’s disease. Brain. 2008;131:389–396. doi: 10.1093/brain/awm304. [DOI] [PubMed] [Google Scholar]
- 41.Foley DL, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch. Gen. Psychiatry. 2011;68:609–616. doi: 10.1001/archgenpsychiatry.2011.2. [DOI] [PubMed] [Google Scholar]
- 42.Henderson DC, Vincenzi B, Andrea NV, Ulloa M, Copeland PM. Pathophysiological mechanisms of increased cardiometabolic risk in people with schizophrenia and other severe mental illnesses. Lancet Psychiatry. 2015;2:452–464. doi: 10.1016/S2215-0366(15)00115-7. [DOI] [PubMed] [Google Scholar]
- 43.Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. doi: 10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- 44.Jourdan T, Godlewski G, Kunos G. Endocannabinoid regulation of beta-cell functions: implications for glycaemic control and diabetes. Diabetes Obes. Metab. 2016;18:549–557. doi: 10.1111/dom.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leweke FM, et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry. 2012;2:e94–e94. doi: 10.1038/tp.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fanelli F, et al. Estimation of reference intervals of five endocannabinoids and endocannabinoid related compounds in human plasma by two dimensional-LC/MS/MS. J. Lipid Res. 2012;53:481–493. doi: 10.1194/jlr.M021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mechoulam R, Fride E, Di Marzo V. Endocannabinoids. Eur. J. Pharm. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 48.Cravatt BF, et al. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 49.Dinh TP, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. PNAS. 2002;99:10819. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rask-Andersen M, Olszewski PK, Levine AS, Schiöth HB. Molecular mechanisms underlying anorexia nervosa: focus on human gene association studies and systems controlling food intake. Brain Res. Rev. 2010;62:147–164. doi: 10.1016/j.brainresrev.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 51.Engeli S, et al. Activation of the peripheral endocannabinoid system in human obesity. Diabetes. 2005;54:2838–2843. doi: 10.2337/diabetes.54.10.2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J. Neurosci. 2014;34:932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J. Neurosci. 2012;32:215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rikandi E, et al. Connectivity of the precuneus-posterior cingulate cortex with the anterior cingulate cortex-medial prefrontal cortex differs consistently between control subjects and first-episode psychosis patients during a movie stimulus. Schizophr. Res. 2018;199:235–242. doi: 10.1016/j.schres.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 55.DiPatrizio NV, Piomelli D. The thrifty lipids: endocannabinoids and the neural control of energy conservation. Trends Neurosci. 2012;35:403–411. doi: 10.1016/j.tins.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boorman E, Zajkowska Z, Ahmed R, Pariante CM, Zunszain PA. Crosstalk between endocannabinoid and immune systems: a potential dysregulation in depression? Psychopharmacology (Berl.) 2016;233:1591–1604. doi: 10.1007/s00213-015-4105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narayan S, Head SR, Gilmartin TJ, Dean B, Thomas EA. Evidence for disruption of sphingolipid metabolism in schizophrenia. J. Neurosci. Res. 2009;87:278–288. doi: 10.1002/jnr.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bioque M, et al. Peripheral endocannabinoid system dysregulation in first-episode psychosis. Neuropsychopharmacology. 2013;38:2568–2577. doi: 10.1038/npp.2013.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Centonze D, Battistini L, Maccarrone M. The endocannabinoid system in peripheral lymphocytes as a mirror of neuroinflammatory diseases. Curr. Pharm. Des. 2008;14:2370–2342.. doi: 10.2174/138161208785740018. [DOI] [PubMed] [Google Scholar]
- 60.Westerbacka J, et al. Splanchnic balance of free fatty acids, endocannabinoids, and lipids in subjects with nonalcoholic fatty liver disease. Gastroenterology. 2010;139:1961–1971 e1961. doi: 10.1053/j.gastro.2010.06.064. [DOI] [PubMed] [Google Scholar]
- 61.Oresic M, et al. Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia. 2013;56:2266–2274. doi: 10.1007/s00125-013-2981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osei-Hyiaman D, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodhams SG, Sagar DR, Burston JJ, Chapman V. The role of the endocannabinoid system in pain. Handb. Exp. Pharm. 2015;227:119–143. doi: 10.1007/978-3-662-46450-2_7. [DOI] [PubMed] [Google Scholar]
- 64.Guzmán M, et al. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α) J. Biol. Chem. 2004;279:27849–27854. doi: 10.1074/jbc.M404087200. [DOI] [PubMed] [Google Scholar]
- 65.Fu J, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- 66.Provensi G, et al. Satiety factor oleoylethanolamide recruits the brain histaminergic system to inhibit food intake. Proc. Natl Acad. Sci. USA. 2014;111:11527–11532. doi: 10.1073/pnas.1322016111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tam J, et al. The therapeutic potential of targeting the peripheral endocannabinoid/CB1 receptor system. Eur. J. Intern. Med. 2018;49:23–29. doi: 10.1016/j.ejim.2018.01.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The MATLAB scripts used for statistical analysis in this study are available from the corresponding author upon reasonable request.