Abstract
Plant cell wall–associated polygalacturonase-inhibiting proteins (PGIPs) are widely distributed in the plant kingdom. They play a crucial role in plant defense against phytopathogens by inhibiting microbial polygalacturonases (PGs). PGs hydrolyze the cell wall polysaccharide pectin and are among the first enzymes to be secreted during plant infection. Recent studies demonstrated that herbivorous insects express their own PG multi-gene families, raising the question whether PGIPs also inhibit insect PGs and protect plants from herbivores. Preliminary evidence suggested that PGIPs may negatively influence larval growth of the leaf beetle Phaedon cochleariae (Coleoptera: Chrysomelidae) and identified BrPGIP3 from Chinese cabbage (Brassica rapa ssp. pekinensis) as a candidate. PGIPs are predominantly studied in planta because their heterologous expression in microbial systems is problematic and instability and aggregation of recombinant PGIPs has complicated in vitro inhibition assays. To minimize aggregate formation, we heterologously expressed BrPGIP3 fused to a glycosylphosphatidylinositol (GPI) membrane anchor, immobilizing it on the extracellular surface of insect cells. We demonstrated that BrPGIP3_GPI inhibited several P. cochleariae PGs in vitro, providing the first direct evidence of an interaction between a plant PGIP and an animal PG. Thus, plant PGIPs not only confer resistance against phytopathogens, but may also aid in defense against herbivorous beetles.
Keywords: LRR protein, aggregation, polygalacturonase, GH28, polygalacturonase-inhibiting protein, plant-insect interaction, herbivorous insect, plant protection, plant, plant cell wall, plant defense, insect, enzyme inhibitor, enzyme, protein aggregation
Plant cells are encased by cell walls of crosslinked polysaccharides that provide protection and structural integrity and contribute to cell–cell adhesion and signal transduction (1, 2). Pectin is a complex mixture of galacturonic acid–rich polysaccharides, which form a matrix embedding cellulose, hemicelluloses, and proteins, and account for ∼35% of primary cells walls in dicots and nongraminaceous monocots (1, 3). Polygalacturonases (PGs) (EC 3.2.1.15) of the glycoside hydrolase family 28 (GH28) depolymerize homogalacturonan, the main component of pectin (1, 3, 4). PGs are among the first enzymes to be secreted by phytopathogenic microorganisms during colonization (5–7) and have been shown to be important pathogenicity factors in fungi (8–11) and bacteria (12, 13). Plants defend themselves by secreting PG-inhibiting proteins (PGIPs) into their cell wall to counteract microbial PGs (14–16). Besides restricting the pectin degradation, inhibition of PGs by PGIPs favors the formation of oligogalacturonides (17, 18). Both, oligogalacturonides as well as the PG itself (independent from its enzymatic activity), elicit plant defense responses (19–21). Overexpression of PGIPs reduces infection symptoms and contributes to plant resistance against phytopathogens (22–24), whereas plants with reduced PGIP levels are more susceptible to pathogen infestation (25, 26), making PGIPs interesting candidates in plant protection.
Recently, animal-encoded PGs have also been discovered in the transcriptomes of phytophagous nematodes (27) and herbivorous insects (28–32). In piercing-sucking mirid bugs, PGs excreted from the salivary glands are considered a major cause of plant damage (33, 34). In chewing herbivores, their ecological impact remains elusive. The mustard leaf beetle (Phaedon cochleariae) possesses nine PG family members, which are specifically expressed in the insect gut (35). Of these, three were characterized as endo-PGs (PCO_GH28-1, -5, and -9), which catalyze the random hydrolysis of polygalacturonic acid (PGA), and one was described as an oligogalacturonase (PCO_GH28-4), cleaving trigalacturonic acid into dimers and monomers (30). In a proteomic analysis of P. cochleariae gut extracts, BrPGIP3 from the beetle's food plant Chinese cabbage (Brassica rapa ssp. pekinensis) was detected and hypothesized to interact with PG family members (35). In contrast to phytopathogens, comparably few studies are available about insect PG–PGIP interactions. PG activity from protein extracts of several mirid bugs (Hemiptera) was reduced by PGIPs in vitro (33, 36). Semi-purified PGIPs inhibited a PG from Diaprepes abbreviatus (Coleoptera: Curculionidae) (37). Here, we investigate whether beetle PGs directly interact with and are inhibited by a plant PGIP in vitro. Thereby, we aim to elucidate if the PGIP plant defense system not only targets microbial PGs but also influences the pectin digestion of an herbivorous beetle.
In general, using extracts from native or PGIP-overexpressing plants is the commonly preferred way to study PG inhibition by PGIPs (15, 16). There are well-established methods to express PGIPs in model plants such as Arabidopsis thaliana (23, 38, 39) or Nicotiana benthamiana (33, 40, 41). Several studies report the expression of various PGIPs in nonmodel plants as well (41–45). Although plant-based systems offer high success rates for PGIP expression, there are also some disadvantages: Even for plants with established transformation methods, the creation of stable lines is laborious and it takes several months until plants are available for experiments (46). Furthermore, PGIPs are ubiquitously distributed among the plant kingdom (15); reports range from 2 in the frequently used model plant A. thaliana (23) to 16 in Brassica napus (47). Screening the genome for homologues of B. napus PGIPs indicates that B. rapa ssp. pekinensis possesses at least 9 putative PGIPs (48). This ever-present background of PGIPs of mostly unknown or uncharacterized inhibitory properties harbors the potential of unwanted and unpredictable influences of these proteins. Thus, heterologous expression in and purification from a PGIP-free organism would be desirable to study the effect of single PGIPs on individual PGs.
Microbial expression systems are PGIP-free and fast-growing, can be scaled to the respective needs, and are usually high-yielding. However, studies on heterologous expression of PGIPs, apart from in planta, are scarce and limited to few candidate proteins. Some PGIPs were successfully expressed in Escherichia coli (39, 42, 44, 49), whereas other studies report on their accumulation into inclusion bodies (14, 50). When studying novel PGIPs, assessment of whether renaturation restores the native function is difficult, because the target PG is unknown. Yeast expression circumvents inclusion bodies and takes posttranslational modifications of the in vivo glycosylated PGIPs (14, 22, 51) into account. Nevertheless, it is used even more rarely for PGIP production. Bashi et al. (38) expressed one PGIP from B. napus, but reported difficulties for several others. In 2001, De Lorenzo et al. (14) even went so far as to rate yeast or other fungal expression systems as “unsuitable for PGIP expression,” as no protein could be detected despite high levels of transcripts. It took 10 years until they finally found a way to express enough protein to solve the X-ray structure of PvPGIP2 from the bean Phaseolus vulgaris in a complex with the fungal FpPG from Fusarium phyllophilum (52). The difficulty of expressing other PGIPs has resulted in PvPGIP2 being a very well-studied protein (53–56) that is often used in PGIP assays (33, 36, 57–59). However, which properties enable the expression and increased stability compared with other PGIPs in vitro is unknown.
Similar to other PGIPs, BrPGIP3 from B. rapa ssp. pekinensis turned out to be very challenging to express in various cell culture systems in our hands. In this study, we ascribed these difficulties to protein aggregation. Nonetheless, to investigate this promising candidate for beetle PG inhibition, we developed a method to circumvent BrPGIP3 aggregation. Fusion of BrPGIP3 to a glycosylphosphatidylinositol (GPI) membrane anchor, and thereby immobilization on the extracellular surface of the expressing cells, reduced protein–protein interactions and minimized the probability to form aggregates. Our new expression method considerably enhanced the stability of BrPGIP3_GPI compared with the soluble BrPGIP3 and enabled us to characterize this otherwise unstable protein. In in vitro assays, BrPGIP3_GPI directly interacted with and inhibited several PGs from the beetle P. cochleariae. We provide the first example of a direct interaction between an animal PG and a plant PGIP. Our findings indicate that plant PGIPs do not only confer resistance against phytopathogenic microorganisms, but also may play a role in plant defense against herbivorous beetles.
Results
GPI anchorage enhances BrPGIP3 stability
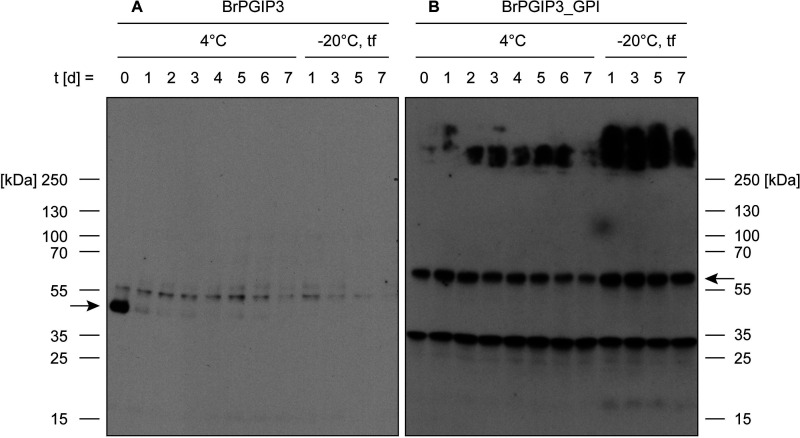
BrPGIP3 is unstable as a soluble protein (Fig. 1A). Expressed in yeast, we detected BrPGIP3 on the day of harvest in the culture medium, but the signal on the Western blotting drastically decreased after 1 day at 4°C and was undetectable after thawing the sample once from −20°C storage (Fig. 2A). Comparing signal intensities of corresponding samples between Western and dot blot demonstrated that BrPGIP3 was not degraded during storage. Instead, this indicated that BrPGIP3 aggregated in the culture medium as well as after purification despite the addition of stabilizing agents (Fig. S1). Because of its instability, BrPGIP3 could not be used in subsequent activity assays.
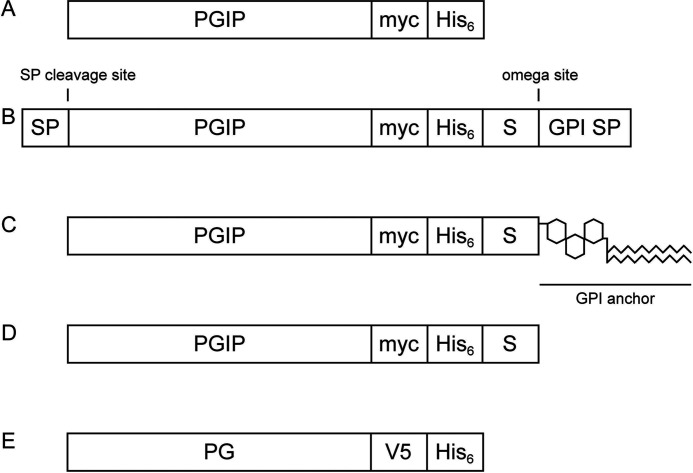
Figure 1.
Simplified schematic representation of heterologously expressed PGIPs and PGs. A, mature, soluble PGIP expressed in the yeast Kluyveromyces lactis. B, immature, pre-processed PGIP_GPI expressed in Sf9 insect cells. C, mature, membrane-anchored PGIP_GPI expressed on the extracellular surface of Sf9 insect cells. D, soluble PGIP_GPI after release from the membrane by PI-PLC treatment. E, mature, soluble PG expressed in the yeast Pichia pastoris. For detailed sequences see supporting information 2. SP, signal peptide for extracellular localization; myc, His6 and V5, protein tags for immunoblot detection or purification; GPI SP, GPI attachment signal peptide; S, 15-amino-acid spacer; omega site, GPI attachment site.
Figure 2.
Stability of membrane-anchored BrPGIP3_GPI is considerably enhanced compared with soluble BrPGIP3. A and B, culture medium from (A) BrPGIP3-expressing yeast and (B) membrane preparations of Sf9 insect cells with membrane-anchored BrPGIP3_GPI were stored for 7 days at 4°C and −20°C. The −20°C samples were repeatedly thawed and frozen on days 1, 3, 5, and 7 (tf). Equal volumes were applied onto a Western blot and the proteins of interest were detected with (A) an anti-His6 and (B) an anti-myc antibody. Arrows indicate the expected size of BrPGIP3 and BrPGIP3_GPI, respectively.
To prevent aggregation by minimizing protein–protein contacts through spatial separation, we immobilized BrPGIP3 as a GPI anchor fusion protein in the plasma membrane of Sf9 insect cells (Fig. 1, B and C). We detected BrPGIP3_GPI in membrane preparations for at least 7 days at 4°C and after multiple freezing and thawing cycles (Fig. 2B). The bands below 35 kDa result from unspecific binding of the anti-myc antibody to Sf9 cell membrane proteins (Fig. 2B and Fig. S2). The detection of bands of higher molecular weight for BrPGIP3_GPI but not BrPGIP3 indicated that aggregation was reduced to the point that protein precipitates were small enough to run in the gel matrix of the SDS-PAGE (Fig. 2). Thus, compared with the soluble BrPGIP3, membrane anchorage greatly enhanced the stability of BrPGIP3_GPI, which allowed for the use of BrPGIP3_GPI in follow-up assays.
PvPGIP2_GPI inhibits fungal PGs
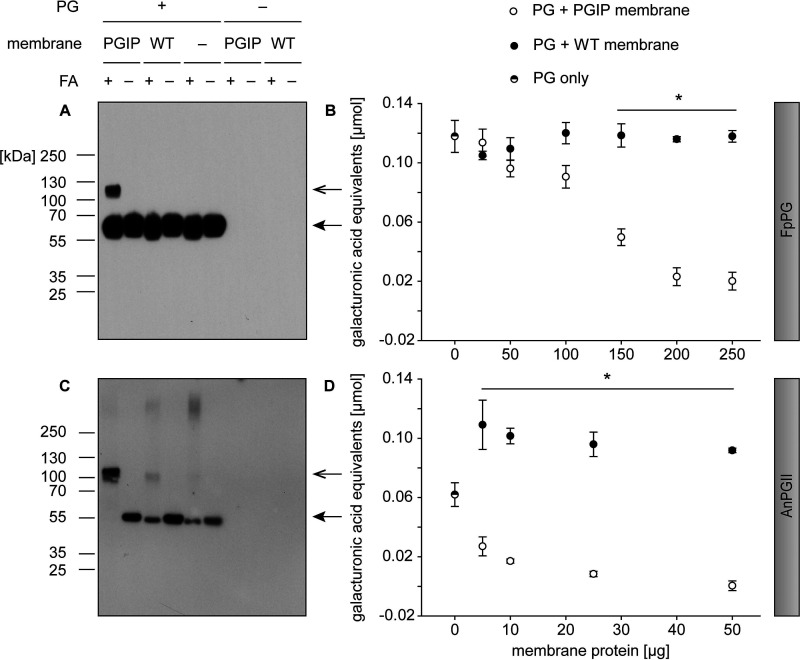
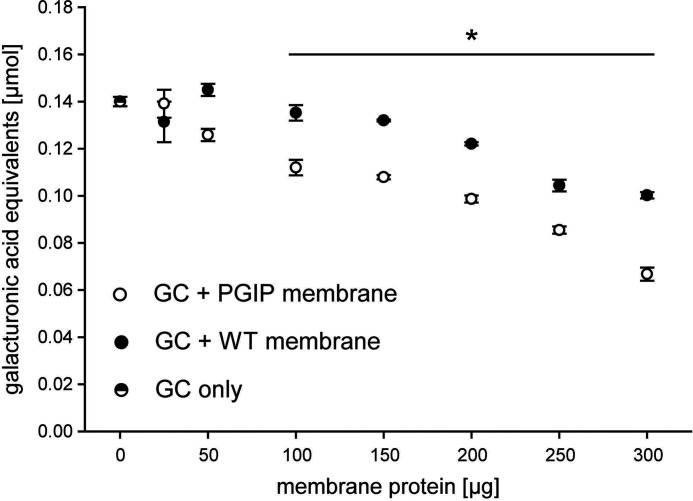
To exclude an influence of the GPI anchor or the membrane immobilization itself on PGIP activity, we used the well-studied interaction of PvPGIP2 with FpPG for proof of concept experiments. We enzymatically released PvPGIP2_GPI from the plasma membrane using a phosphatidylinositol-specific phospholipase C (PI-PLC) for interaction assays with FpPG to investigate whether the additional amino acid residues of PvPGIP2_GPI (Fig. 1D) resulting from the GPI anchor signal peptide fusion negatively influenced the binding to FpPG (Fig. 1E). As expected, a band the size of the combined molecular weight of FpPG and PvPGIP2_GPI appeared on a Western blot, when the proteins were crosslinked with formaldehyde (Fig. 3A). In contrast, such bands were not detected when formaldehyde was omitted from the assay or in control samples such as membrane preparations of WT cells as well as single protein incubations. We verified the PGIP-PG complex with separate antibodies labeling the PGIP and the PG (Fig. S3), further supporting a specific binding interaction of PvPGIP2_GPI with FpPG. This showed that the GPI anchor fusion of PvPGIP2_GPI did not interfere with the ability to bind FpPG. To test whether the immobilization to the plasma membrane itself interfered with PGIP activity, PG inhibition assays were performed directly with membrane preparations without prior release of PvPGIP2_GPI from the membrane (Fig. 1C). PvPGIP2_GPI–containing membrane proteins inhibited FpPG in a concentration-dependent manner, significantly reducing PG activity up to 83% compared with WT membrane proteins (Fig. 3B and Table S1). Hence, membrane anchorage did not interfere with the inhibitory activity of PvPGIP2_GPI.
Figure 3.
Both fungal PGs, FpPG and AnPGII, specifically bound to and inhibited by PvPGIP2_GPI in a concentration-dependent manner. A and C, membrane proteins from PvPGIP2_GPI-expressing as well as WT Sf9 cells were incubated with (A) FpPG as well as (C) AnPGII and crosslinked with formaldehyde (FA). The PGs were detected in a Western blotting with an anti-V5 antibody. Arrows indicate the expected size of PG (closed arrowhead) and PG-PGIP complex (open arrowhead), respectively. B and D, for inhibition assays, membrane preparations were incubated with (B) FpPG and (D) AnPGII and the activity was quantified by DNS assay. Statistical differences were analyzed between corresponding samples of equal amounts of total membrane proteins (n = 3, for detailed values see Table S1). Error bars represent S.D. Note that panels A and C are also presented in Fig. S3 to allow for a direct comparison with a Western blot using the anti-myc antibody.
To validate our method and assess its more general applicability, we applied it to another fungal PG (AnPGII from Aspergillus niger) (Fig. 1E), for which a binding interaction as well as an enzyme inhibition has been reported previously (36, 40). With our interaction assay, we detected the AnPGII-PvPGIP2_GPI complex with separate antibodies against PG and PGIP, indicating a direct and specific binding in vitro (Fig. 3C and Fig. S3). Furthermore, AnPGII enzyme activity was completely inhibited by membrane-anchored PvPGIP2_GPI in a concentration-dependent manner (Fig. 3D).
These proof of concept experiments not only demonstrated that the fusion to a GPI anchor did not interfere with the inhibitory activity of PvPGIP2_GPI but also indicated that GPI-anchored PGIPs could be used to study novel PG-PGIP interactions in general.
BrPGIP3_GPI inhibits P. cochleariae PGs and beetle gut PG activity
Applying our method to the comparatively unstudied field of beetle PG inhibition, we investigated the impact of BrPGIP3_GPI on all PG family members expressed by P. cochleariae (Fig. 1E). Binding interaction assays revealed bands of the combined molecular weight of soluble BrPGIP3_GPI (Fig. 1D) and PCO_GH28-1, -4, or -9 with antibodies binding the PG (Fig. 4, A and E, and Fig. S4) or separate antibodies labeling PG and PGIP (Fig. S4), demonstrating specific binding of BrPGIP3_GPI to these beetle PGs. In contrast, we found no such binding of BrPGIP3_GPI to the other tested PG family members, because bands of the combined molecular weight were either not visible (PCO_GH28-2, -3, and -8) or obscured by strong background signals (PCO_GH28-5 and -6) (Fig. 4C and Fig. S4).
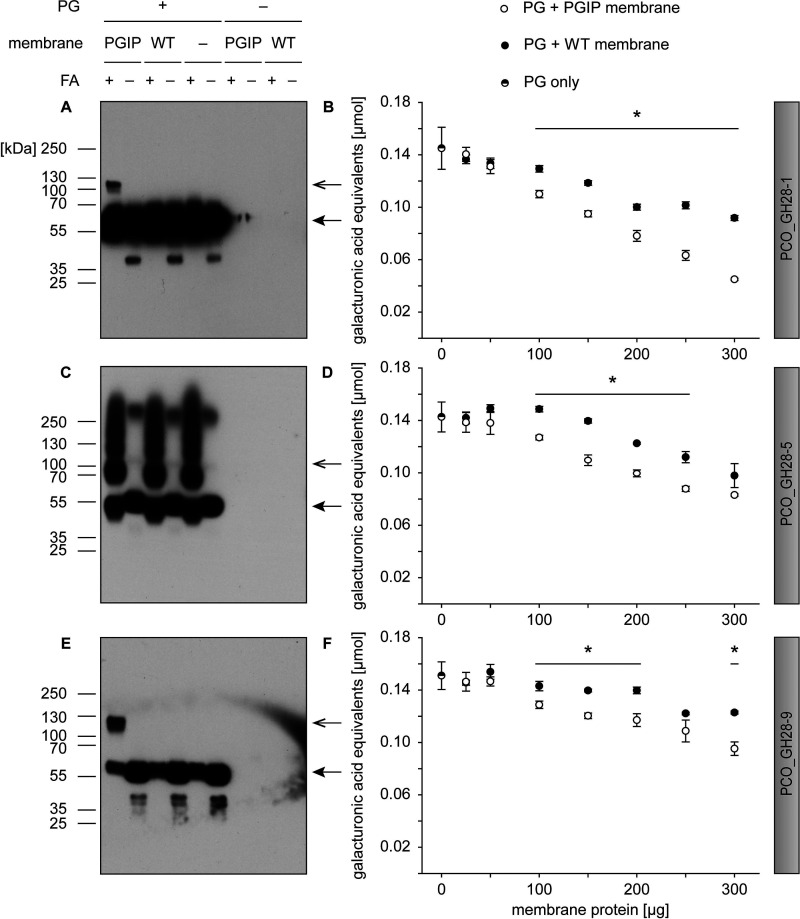
Figure 4.
Binding and inhibition assays of P. cochleariae PGs PCO_GH28-1, -5, and -9 with BrPGIP3_GPI. A, C, and E, membrane proteins from WT as well as BrPGIP3_GPI-expressing Sf9 cells were incubated with (A) PCO_GH28-1, (C) -5, and (E) -9 and crosslinked with formaldehyde (FA). The PGs were detected in a Western blot with an anti-V5 antibody. Arrows indicate the expected size of PG (closed arrowhead) and PG-PGIP complex (open arrowhead), respectively. B, D, and F, membrane preparations were incubated with (B) PCO_GH28-1, (D) -5, and (F) -9 and the activity was quantified by DNS assay. Statistical differences were analyzed between corresponding samples of equal amounts of total membrane proteins (n = 3) (for detailed values see Table S1). Error bars represent S.D. Note that panels A, C, and E are also presented in Fig. S4 to allow for a direct comparison with a Western blot using the anti-myc antibody.
We tested the inhibitory activity of BrPGIP3_GPI (Fig. 1C) against all P. cochleariae endo-PGs, PCO_GH28-1, -5, and -9. We were unable to reliably quantify the activity of the oligogalacturonase PCO_GH28-4 and therefore could not measure its potential inhibition by BrPGIP3_GPI. The membrane preparation from WT Sf9 cells inhibited galacturonic acid release in a concentration-dependent manner, and even greater specific inhibition was seen on some of the PGs by preparations with BrPGIP3_GPI. PCO_GH28-1 was specifically inhibited up to 51% (Fig. 4B and Table S1), PCO_GH28-9 up to 22% (Fig. 4F and Table S1), and, surprisingly, the activity of PCO_GH28-5 was specifically reduced up to 18% even though we could not clearly show an interaction of the latter with BrPGIP3_GPI (Fig. 4, C and D, and Table S1). Interestingly, the additional specific inhibition of PCO_GH28-5 and -9 by BrPGIP3_GPI did not correlate with the amount of BrPGIP3_GPI added.
Overall, BrPGIP3_GPI exhibited binding interactions with PCO_GH28-1, -4, and -9 and reduced the activity of all P. cochleariae endo-PGs. This is the first study showing that a plant PGIP directly interacts with and inhibits PGs of animal origin.
P. cochleariae PGs are specifically expressed in the gut and are responsible for the beetle gut PG activity (35, 60). We used beetle gut protein extracts to test if a single PGIP not only inhibited the individual endo-PGs, as we have shown above, but also could have a negative effect on the pectin digestion in the beetle gut in general. BrPGIP3_GPI–containing membrane proteins significantly inhibited the gut PG activity up to 33% compared with WT membrane proteins (Fig. 5 and Table S1). This study not only provides evidence that BrPGIP3_GPI is a versatile inhibitor of P. cochleariae PGs, but also that a single plant PGIP may be sufficient to significantly decrease PG activity in the gut of a leaf beetle.
Figure 5.
BrPGIP3_GPI inhibited PG activity in the gut of P. cochleariae. Membrane preparations of WT as well as BrPGIP3_GPI-expressing Sf9 cells were incubated with P. cochleariae gut content and the activity was quantified by DNS assay. Statistical differences were analyzed between the corresponding samples of equal membrane proteins (n = 3) (for detailed values see Table S1). Error bars represent S.D.
Discussion
Overcoming challenges in leucine-rich repeat (LRR) protein expression
Because PGIPs, like other LRR proteins, are challenging to express, we developed a novel method to enhance protein stability. With this, we could show for the first time that an animal PG directly interacted with and was inhibited by a plant PGIP.
Recombinant BrPGIP3 aggregated quickly and could not even be used from frozen aliquots. Several of the difficulties we encountered when expressing BrPGIP3 are mirrored in the literature. Interestingly, studies about not only the expression of PGIPs but also various other LRR proteins are studded with reports of “difficulties in producing recombinant LRR proteins in yields sufficient for biochemical analysis” (61). The proteins were generally not expressed (14, 38), accumulated into inclusion bodies (14, 62), occurred in very low yields (38), or were simply unstable (63–65). Challenges have been partially overcome for individual proteins with alternative solutions. The extracellular domains of a human (Homo sapiens) LRR kinase or a zebrafish (Danio rerio) Toll-like receptor (TLR) could be kept in solution, when supplemented with certain detergents (65) or the natural ligand (64), respectively. The fusion of truncated fragments of the proteins of interest with stable flanking modules from another LRR protein stabilized several TLR LRR domains of various animal origins (64, 66, 67).
Expression systems that secrete recombinant proteins into the culture medium are suitable for naturally soluble, extracellular proteins. PGIPs, however, are bound to the plant cell wall upon secretion into the apoplast (55, 68) and are not free but imbedded in the insoluble polysaccharide matrix (69, 70). Our approach to immobilize BrPGIP3_GPI above the plasma membrane not only mimics the natural cell wall association more accurately, but also minimizes protein–protein contacts and thereby the probability to form aggregates. Indeed, compared with soluble BrPGIP3, membrane-anchored BrPGIP3_GPI showed a considerably increased stability, allowing us to study an otherwise aggregating LRR protein. Interestingly, in contrast to PGIPs, plant-encoded inhibitors of fungal cell wall–modifying pectin methylesterases are naturally GPI anchored (71). Furthermore, by choosing a lipid anchor over other possibilities of membrane immobilization (e.g. transmembrane domain), only a few amino acids are added to the protein of interest, reducing the risk of unpredictable influences on protein folding and activity (72–74). As an additional advantage, membrane fractions containing recombinant proteins can be pelleted by centrifugation, allowing for rapid pulldown of protein complexes as well as an easy buffer exchange and protein concentration adjustment. In case the membrane itself interferes with the assay conditions, the GPI anchor can be cleaved with commercially available enzymes, allowing for a convenient release of the protein directly into the assay buffer.
Proof of concept validates inhibitory activity of a GPI-anchored PGIP
As a proof of concept as well as to investigate a potential interference of the GPI anchorage with binding and inhibition, we tested our method on the well-studied interaction of PvPGIP2 with FpPG. Soluble as well as membrane-anchored, PvPGIP2_GPI specifically interacted with and inhibited FpPG, respectively. This is in agreement with the literature (36, 52) and demonstrates that neither the additional amino acids nor the immobilization in the membrane impairs the formation of the PvPGIP2_GPI-FpPG complex. To demonstrate that our method is applicable for more than this “gold standard” of PG-PGIP interaction, we tested PvPGIP2_GPI with another fungal PG, AnPGII. PvPGIP2_GPI bound to and completely inhibited AnPGII, which is in agreement with previous findings (36, 40). For both fungal PGs, FpPG and AnPGII, PvPGIP2_GPI retained its binding and PG-inhibiting activity despite the fusion to the membrane anchor. This makes GPI-anchored PGIPs a suitable and convenient system to identify novel PG-PGIP interactions in vitro.
Plant PGIPs may play a role in the defense against herbivorous beetles
Although PG inhibition is well-studied for phytopathogenic microorganisms, comparably few studies address the impact of PGIPs on herbivores. Mechanistically, these systems differ considerably. Bacteria and fungi secrete PGs directly into the apoplast to initiate cell wall maceration. Thus, it has been demonstrated multiple times that incorporation of PGIPs in the cell wall confers resistance to phytopathogens (14–16). Piercing-sucking herbivores excrete PGs from their salivary glands and penetrate the plant tissue with their mouth parts (27, 33, 34). PG activity of several crude protein extracts from piercing-sucking mirid bugs was inhibited by PvPGIP3 and -4 in vitro (33, 36). In A. thaliana, AtPGIP1 has been shown to attenuate root infections by the herbivorous nematode Heterodera schachtii in vivo (75). In chewing herbivores, however, PGs encounter the plant material when the food bolus passes through the digestive tract. Accordingly, to be effective against their pectinolytic enzymes, plant PGIPs need to withstand degradation in the gut environment and indeed, in proteomic analyses, PGIPs have been detected in beetle gut contents (35). Thus, it is not surprising that preliminary evidence suggests that these robust inhibitory proteins may be involved in defense against chewing insects as well. A PG from D. abbreviatus (Coleoptera, Curculionidae) was inhibited by a semi-purified PGIP from orange peels (37). In mung bean (Vigna radiata), resistance to seed beetles (Callosobruchus spp.) was associated with genes encoding putative PGIPs (76, 77). Feeding assays on A. thaliana revealed the first ecological relevance of PGIPs for herbivorous beetles, as the fitness of P. cochleariae correlated with the PGIP composition of the diet (78).
Previously, the P. cochleariae PGs PCO_GH28-1 and -9 have been found to co-elute with BrPGIP3 in a fractionated separation of the beetle's gut contents (35). With our method, we directly confirm in vitro, that this co-elution is indeed the consequence of an interaction in vivo. We also detected an additional interaction of the oligogalacturonase PCO_GH28-4 with BrPGIP3_GPI. Furthermore, BrPGIP3_GPI significantly inhibited all P. cochleariae endo-PGs (PCO_GH28-1, -5, and -9). PCO_GH28-5 was inhibited, even though detection of a possible complex with BrPGIP3_GPI was obscured by high background signals in the assay. Instead of the PGIP binding to the pectolytic enzyme, inhibition could be explained by PGIP binding to the substrate, shielding it from degradation without direct contact with the PG (55, 68). For example, VvPGIP1 from grapevine (Vitis vinifera) limited fungal infection symptoms without evidence for an in vitro interaction (79). Our results with tagged recombinant proteins from PGIP-free expression systems clearly show for the first time that a plant PGIP directly interacts with and inhibits several PGs of animal origin.
In our inhibition assays, PCO_GH28-1 was clearly more affected by BrPGIP3_GPI than PCO_GH28-5 and -9. This differential inhibition of beetle PGs matches previous reports on phytopathogens that one PGIP may not be sufficient to effectively inhibit all PGs of a single organism. For example, VvPGIP1 inhibited some, but not all fungal PGs from Botrytis cinerea or A. niger (79, 80). Like PGs, PGIPs belong to large multi-gene families, which are believed to have been shaped by an evolutionary arms race (81). Specificity of PG-PGIP interaction is maintained by a few positively selected hot spots (16, 81). The exchange of a single amino acid in either the PGIP or PG can suffice to enable a novel recognition or evade an existing interaction (40, 53). An expansion of PGs may facilitate not only a more effective pectin digestion, but also a strategy to evade inhibition. PGIPs, on the other hand, possess different specificities toward PGs. This is most pronounced in P. vulgaris, where not only individual inhibitory activities of PvPGIPs toward various fungal PGs were demonstrated (33, 36), but also a subfunctionalization against fungi and insects. PG activity of several mirid bugs was reduced by PvPGIP3 and -4, but was unaffected by PvPGIP1 and -2 (33, 36).
Including our study, only two of nine BrPGIPs from B. rapa ssp. pekinensis have been investigated so far. Overexpression of BrPGIP2 increased plant resistance against Pectobacterium carotovorum ssp. carotovorum and crude plant extracts inhibited a PG from this phytopathogenic bacterium (41). Here, we demonstrated that BrPGIP3_GPI is a versatile inhibitor of several beetle PGs. The inhibition spectrum is mirrored in the regulation of gene expression, as BrPGIP2, but not BrPGIP3, was induced by bacterial infection of B. rapa ssp. pekinensis (41). BrPGIP2 and -3 show 99% sequence similarity with their orthologues BnPGIP2 and -3 in the closely related B. napus. Similarly, BnPGIP2 was up-regulated upon fungal infection, whereas BnPGIP3 was unresponsive to this treatment (47). This suggests that not only in P. vulgaris but also in these Brassicaceous plants, a subfunctionalization of PGIPs against phytopathogenes and insects has evolved. In future studies, we aim to characterize the specificities and inhibitory activities of the remaining BrPGIPs, especially toward P. cochleariae PGs. Because PCO_GH28-5 and -9 were less susceptible to BrPGIP3_GPI than was PCO_GH28-1, we will investigate whether any of the other BrPGIPs specifically target these PGs, or whether their expression enables the beetle to counteract inhibition by PGIPs.
The total PG activity and hence overall pectin digestion of the beetle gut was significantly reduced by BrPGIP3_GPI in vitro. Our study demonstrates that a single PGIP is sufficient to negatively affect pectin digestion in an herbivorous beetle. Previous feeding assays on A. thaliana also revealed an impact of plant PGIPs on P. cochleariae. PG activity as well as larval weight gain was reduced if two instead of only one AtPGIP were present in the food plant (78). Thus, PGIPs are not only important in defending plants against phytopathogenic bacteria and fungi, but may also play a role in the protection against herbivorous beetles.
In general, beetle performance depends on the food plant species and quality (82, 83). Because PGIP profiles differ between plants and each PG-PGIP interaction is unique, predicting the impact of certain PGIPs in vivo is difficult. Whereas a single AtPGIP influenced P. cochleariae performance on the model plant A. thaliana (78), the beetle pectinolytic system has been shown to be robust when feeding on B. rapa ssp. pekinensis. Even a major reduction of PG activity in the gut did not significantly impair beetle fitness (60). Despite the impact of PGIPs that our study and Kirsch et al. (78) demonstrated, the gut content of P. cochleariae feeding on B. rapa ssp. pekinensis as well as A. thaliana still retains some residual PG activity (35, 60, 78). This suggests that P. cochleariae has adapted to the PG-inhibiting defenses of its food plants in vivo. Adaptation of the beetle to the PGIP composition of its diet may occur through the aforementioned expansion and subfunctionalization, which may have led to the evolution of PGs escaping or being less sensitive to inhibition by PGIPs. Furthermore, catalytically inactive PG family members (pseudoenzymes) may help to maintain total beetle PG activity. RNAi-mediated knockdown in P. cochleariae demonstrated that they play an important role in the pectin digestion pathway (60). They have been hypothesized to bind PGIPs as “decoys,” thereby protecting the active enzymes from inhibition (35). Here, we did not observe an interaction of BrPGIP3_GPI with those pseudoenzymes, but many more PGIPs remain to be studied in B. rapa ssp. pekinensis. Our system now offers the possibility to test all these various combinations of inactive and active PG family members with PGIPs in vitro to elucidate the role these pseudoenzymes play in the beetle's pectin digestion pathway. Moreover, testing these interactions systematically will provide an exhaustive picture of how plant PGIPs interfere with pectin degradation in the beetle and how this has affected both enzymes and inhibitors in the course of evolution.
In conclusion, we have developed a novel method to stably express a PG-inhibiting protein from a plant by reducing its tendency to aggregate, and demonstrated for the first time that a plant PGIP directly interacts with and is a versatile inhibitor of beetle PGs. Our study provides evidence that PGIPs not only confer resistance against phytopathogenic microorganisms but may also be involved in plant defense against herbivorous beetles. In future studies, we intend to elucidate the impact of other plant PGIPs on P. cochleariae and how pectin digestion in herbivorous insects and the defensive plant PGIP system have co-evolved.
Experimental Procedures
Expression of PGs in P. pastoris and Sf9 insect cells
The ORFs of FpPG (plasmid kindly provided by Felice Cervone, Sapienza University of Rome, Italy) and AnPGII were cloned into vectors pIB/V5-His-TOPO® TA or pMIB/V5-His B, respectively, to attach a C-terminal His6 and V5 epitope. During the subsequent cloning into the yeast expression vector pPICZα A, the native signal peptides were replaced by the vector's secretion signal peptide (identification of signal peptides by SignalP 4.1) (84). All vectors were from Thermo Fischer Scientific. Constructs were verified by sequencing. The fungal PGs (including the His6 and V5 epitope) were expressed in the yeast P. pastoris according to the manufacturer's instructions of the EasySelectTM Pichia Expression Kit (Thermo Fischer Scientific). Differing from this protocol, the BMGY pre-culture was inoculated from a dense 5 ml BMGY culture instead of a single colony to be able to accurately calculate the growth, because a precise A600 was essential for expression success. The expression was induced approximately every 12 h with 1% methanol. The P. cochleariae PG family members PCO_GH28-1, -2, -3, -4, -5, -6, -8, and -9 were transiently expressed in Sf9 insect cells in 6-well plates (2 wells per construct) and dialyzed against H2O as described previously (30). Aliquots of culture medium were used for interaction assays and dialyzed samples for inhibition assays. Protein expression was verified by Western blots using antibodies against the His6 or V5 tags, and PG activity was detected by an agarose diffusion assay as described previously (35), using demethylated PGA (1% w/v in H2O, P-PGACT, Megazyme Ltd., Ireland).
Purification of PGs
Purification of PGs from yeast culture medium was performed according to the manufacturer's instructions for batch purification using HisPurTM Cobalt Resin (Thermo Fischer Scientific). The elution fractions were passed over the resin multiple times to enhance the yield per fraction and subsequently concentrated and desalted with Amicon® Ultra Centrifugal Filters (Merck KGaA). Protein concentrations were determined by Quick StartTM Bradford Protein Assay (Bio-Rad Laboratories) and PG activity by agarose diffusion assay (see above).
Expression of BrPGIP3 in Kluyveromyces lactis
The ORF of BrPGIP3 (Bra005919 in EnsemblPlants (48), for full sequence see supporting information 2) was cloned into pPICZα A (Thermo Fischer Scientific) to attach a C-terminal His6 and myc epitope. There and during the subsequent cloning into the yeast expression vector pKLAC2 (New England Biolabs), the native signal peptide was replaced by the vectors' secretion signal peptides (identification of signal peptides by SignalP 4.1 (84)). Constructs were verified by sequencing. BrPGIP3 (including the His6 and myc epitope) was expressed in the yeast K. lactis according to the manufacturer's instructions of the K. lactis Protein Expression Kit (New England Biolabs). Expression was verified by Western blotting using antibodies against the His6 or myc tag.
Construction of pMIB/V5_GPI plasmid
For the expression of membrane-anchored proteins in Sf9 insect cells, the vector pMIB/V5-His A (Thermo Fischer Scientific) was modified to attach the genes of interest (GOIs) to a GPI anchor. PredGPI (85) was used for GPI anchor prediction of an insect aminopeptidase N (AY358034.1) (86). The GPI anchor transmembrane domain, omega site and 15 upstream amino acids (spacer) were amplified from Helicoverpa armigera cDNA. RNA isolation and cDNA synthesis was performed as described previously (87). The PCR product was inserted in the BamHI/NotI restriction site of the vector's multiple cloning site. The restriction sites upstream of BamHI were retained, allowing for insertion of GOIs (seamless, if using the BamHI restriction site). Constructs were verified by sequencing. For a full sequence of pMIB/V5_GPI see supporting information 3.
Expression of PvPGIP2_GPI and BrPGIP3_GPI in stable Sf9 insect cell lines
The ORFs of PvPGIP2 (plasmid kindly provided by Felice Cervone, Sapienza University of Rome, Italy) and BrPGIP3 were cloned into pPICZα A as described above and the GOIs (including the His6 and myc epitope) were fused to the GPI anchor signal peptide sequence in pMIB/V5_GPI. During protein biosynthesis, the GPI signal peptide is removed and the nascent protein of interest is transferred to the C-terminally attached GPI membrane anchor (88). For full amino acid sequences for BrPGIP3 and BrPGIP3_GPI pre- and post-processing, see supporting information 2. Constructs were verified by sequencing. Stable monoclonal Sf9 cell lines expressing BrPGIP3_GPI were created as described previously using the cloning cylinder method and 50 µg/ml blasticidin S (Thermo Fischer Scientific) for selection (87). Stable polyclonal Sf9 cell lines expressing PvPGIP2_GPI were selected with 80 µg/ml blasticidin S as described previously (30). Both cell lines were maintained in Sf-900 II SFM (Thermo Fischer Scientific) with 10 µg/ml blasticidin S and protein expression was confirmed by Western blotting using antibodies against the His6 or myc tags.
Sf9 cell membrane preparation
The Sf9 cell membrane fractions were isolated by differential centrifugation. Strict handling of the samples on ice was of importance here, because the GPI-anchored proteins can be released from the membrane at elevated temperatures (89). After harvesting, the cells were washed with 1× PBS twice (500 × g, 4°C) and subsequently lysed with a Dounce homogenizer in hypotonic buffer (20 mm Tris-HCl pH 7.5, 5 mm EDTA, 1 mm DTT). Sucrose buffer was added (hypotonic buffer with 0.5 m sucrose) in a 1:1 ratio and nuclei and intact cells were pelleted by centrifugation (1200 × g, 4°C). Centrifugation of the supernatant (10,000 × g, 4°C) pellets the membrane fraction, which was washed with 40 mm citrate phosphate buffer, pH 5.0, to remove soluble proteins. Aliquots in 40 mm citrate phosphate buffer, pH 5.0, were stored at −20°C until further use. The expression of proteins of interest and their localization in the membrane fraction was verified by Western blotting. Protein concentrations were determined by Quick StartTM Bradford Protein Assay (Bio-Rad).
Stability and aggregation test
To test the stability of expressed proteins, aliquots of the same protein solution were stored at 4°C for up to 7 days. Daily, samples were frozen in SDS-PAGE buffer. Additionally, aliquots were stored at −20°C for up to 7 days and cumulatively thawed and refrozen on days 1, 3, 5, and 7, freezing it in SDS-PAGE buffer on the last day. Hence, this resulted in one for the day 1 and a total of four times thawing and freezing for the day 7 sample. Soluble protein abundance was monitored by Western blotting. To test for protein aggregation, equal volumes of the same protein sample were applied to produce both a Western blot and a dot blot. Equal treatment of both membranes and detection of the luminescence signal on a single film allowed for a comparison of signal intensities. A signal is absent from both blots if the proteins are degraded. If the proteins form aggregates that are too large to be separated by SDS-PAGE but that can be blotted onto the membrane, they will be visible in the dot blot but not the Western blot. As a reference, a stable soluble protein (PCO_GH28-1) was applied onto both the Western and the dot blots.
Western blot and dot blot
For the Western blot, protein samples in SDS-PAGE buffer (1× XT Sample Buffer, 1× XT Reducing Agent, 1% SDS) were boiled for 5 min and separated on CriterionTM XT Bis-Tris Precast Gels (125 V for 1.5 h in XT MES Running Buffer) (all from Bio-Rad). The PageRuler Plus Prestained Protein Ladder (Thermo Fischer Scientific) was used as a size standard. Proteins were blotted onto an Immun-Blot PVDF Membrane (Bio-Rad) at 100 V for 30 min. For dot blots, equal volumes of untreated protein samples were spotted onto activated Immun-Blot PVDF Membrane in 5 µl steps. After evaporation of the liquid, the membranes were treated analogous to the Western blotting membrane. Both membranes were blocked with 5% milk powder in TBST at room temperature (RT) for 1 h and incubated with the respective antibody at 4°C overnight. A 1:5000 dilution of anti-His(C-term)-HRP antibody (no. R931-25), 1:20,000 anti-V5-HRP antibody (no. R961-25, both Invitrogen and Thermo Fischer Scientific) or 1:1000 and 1:300,000 anti-myc-HRP antibody (sc-40 HRP, Santa Cruz Biotechnology, Inc. and A190-104P, Bethyl Laboratories, Inc., Montgomery, TX, USA) was used in 5% milk powder in TBST, respectively. Chemiluminescence of the SuperSignalTM West Dura Extended Duration Substrate Kit (Thermo Fischer Scientific) was documented with Amersham Biosciences Hyperfilm DCL chemiluminescence films (GE Healthcare Life Sciences), GBX Developer and Replenisher, and GBX Fixer and Replenisher (both Kodak).
Interaction assay PG-PGIP
The GPI-anchored PGIPs (BrPGIP3_GPI and PvPGIP2_GPI) were released from the membrane by cleavage of the GPI anchor by a PI-PLC (P6466, Thermo Fischer Scientific). 0.2 units PI-PLC were added to 226 µg total membrane proteins and incubated at RT (21°C) for 1 h. Centrifugation (10,000 × g, 30 min, 4°C) separated soluble proteins from the membrane fraction and the supernatant was used for further assays. The interaction of PGIPs with PGs was tested in a crosslinking assay modified from Benedetti et al. (52). In a 40 µl assay, 56.5 µg of membrane proteins containing BrPGIP3_GPI or PvPGIP2_GPI were co-incubated with PGs (2.5 ng FpPG, 100 ng AnPGII, or 10 µl PCO_GH28 Sf9 insect cell culture medium) for 1 h at 4°C in 40 mm citrate phosphate buffer, pH 5.0. Subsequently, they were supplemented with a final concentration of 1% formaldehyde (in 1× PBS) or 1× PBS and incubated overnight at 16°C. Equal volumes of sample were used for Western blotting without boiling of the sample, to avoid reversal of formaldehyde crosslinking by heat (90). PGs and PGIPs can be distinguished in the Western blotting by their different tags.
Inhibition assay PG-PGIP
To test the inhibitory activity of the GPI-anchored PGIPs, the Sf9 membrane preparations were incubated directly with PGs or gut contents without prior release of the proteins from the membrane. The gut contents of 35 P. cochleariae third instar larval guts were pooled in 500 µl 50 mm citrate phosphate buffer, pH 5.0, and subsequently dialyzed against H2O. For 60 µl assays, up to 300 µg Sf9 cell membrane proteins were transferred into a total of 26 µl H2O and pre-incubated with 102 ng FpPG, 33 ng AnPGII, or 10 µl PGs or gut content (in H2O) and 8 µl 0.2 m citrate phosphate buffer, pH 5.0, for 30 min (AnPGII at 4°C, gut content, FpPG, PCO_GH28-1, -5, and -9 at RT). Of six replicates per treatment, three were boiled after pre-incubation at 99°C for 5–10 min as background controls. Subsequently, 12 µl 1% demethylated PGA (w/v in H2O) and 4 µl 0.2 m citrate phosphate buffer, pH 5.0, were added and the mixture was incubated at 40°C for 30–90 min. The amounts and incubation times of individual PGs or beetle gut contents were chosen to ensure that PG activity was well above the detection limit and in the linear range below saturation. Mixing of the samples every 15 min during pre-incubation and incubation ensured contact of the soluble PGs with the PGIPs bound to sedimenting membranes. Afterward, quantification of released galacturonic acid residues was performed by the colorimetric 3,5-dinitrosalicylic acid (DNS) method as described previously (31). Upon hydrolysis of PGA, the increase of free reducing groups resulted in the reduction and thus color change of DNS from yellow to brown, which was quantified at 575 nm (91). The average of the respective background controls was subtracted from the samples. Statistical analysis was performed with SigmaPlot (Systat Software, San Jose, CA, USA) comparing samples with WT and PGIP-containing membrane proteins with a Student's t test (for normally distributed samples with equal variances) or a rank sum test (Mann-Whitney, when samples were not normally distributed or had unequal variances).
Data availability
All data presented in this paper are contained within the article.
Supplementary Material
Acknowledgments
We thank Felice Cervone and Daniela Pontiggia (both Sapienza University of Rome, Italy) for kindly providing FpPG and PvPGIP2 plasmids. Furthermore, we thank Bianca Wurlitzer and Domenica Schnabelrauch for technical assistance as well as Theresa Sporer for fruitful discussions.
This article contains supporting information.
Author contributions—W. H., D. G. H., Y. P., and R. K. conceptualization; W. H. and J. H. data curation; W. H., J. H., Y. P., and R. K. formal analysis; W. H., J. H., Y. P., and R. K. validation; W. H. investigation; W. H. writing-original draft; W. H., D. G. H., Y. P., and R. K. writing-review and editing; D. G. H. resources; D. G. H., Y. P., and R. K. supervision; D. G. H. and R. K. funding acquisition.
Funding and additional information—This work was supported by Max Planck Society and German Science Foundation DFG Grants KI1917/1-1 and KI1917/1-2.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- PG
- polygalacturonases
- PGIP
- PG-inhibiting proteins
- GPI
- glycosylphosphatidylinositol
- GH28
- glycoside hydrolase family 28
- PGA
- polygalacturonic acid
- PI-PLC
- phosphatidylinositol-specific phospholipase C
- LRR
- leucine-rich repeat
- TLR
- Toll-like receptor
- GOI
- genes of interest
- RT
- room temperature
- DNS
- dinitrosalicylic acid.
References
- 1. Caffall K. H., and Mohnen D. (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 344, 1879–1900 10.1016/j.carres.2009.05.021 [DOI] [PubMed] [Google Scholar]
- 2. Brett C., and Waldron K. (1990) Physiology and Biochemistry of Plant Cell Walls, 1st Ed., Springer Netherlands, Dordrecht, Netherlands [Google Scholar]
- 3. Mohnen D. (2008) Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 11, 266–277 10.1016/j.pbi.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 4. Gilbert H. J. (2010) The biochemistry and structural biology of plant cell wall deconstruction. Plant Physiol. 153, 444–455 10.1104/pp.110.156646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Leone G., and van den Heuvel J. (1987) Regulation by carbohydrates of the sequential in vitro production of pectic enzymes by Botrytis cinerea. Can. J. Bot. 65, 2133–2141 10.1139/b87-294 [DOI] [Google Scholar]
- 6. Ramos A. M., Gally M., Szapiro G., Itzcovich T., Carabajal M., and Levin L. (2016) In vitro growth and cell wall degrading enzyme production by Argentinean isolates of Macrophomina phaseolina, the causative agent of charcoal rot in corn. Rev. Argent. Microbiol. 48, 267–273 10.1016/j.ram.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 7. Kikot G. E., Hours R. A., and Alconada T. M. (2010) Extracellular enzymes of Fusarium graminearum isolates. Braz. Arch. Biol. Technol. 53, 779–783 10.1590/S1516-89132010000400005 [DOI] [Google Scholar]
- 8. Oeser B., Heidrich P. M., Müller U., Tudzynski P., and Tenberge K. B. (2002) Polygalacturonase is a pathogenicity factor in the Claviceps purpurea/rye interaction. Fungal Genet. Biol. 36, 176–186 10.1016/S1087-1845(02)00020-8 [DOI] [PubMed] [Google Scholar]
- 9. Isshiki A., Akimitsu K., Yamamoto M., and Yamamoto H. (2001) Endopolygalacturonase is essential for citrus black rot caused by Alternaria citri but not brown spot caused by Alternaria alternata. Mol. Plant Microbe Interact. 14, 749–757 10.1094/MPMI.2001.14.6.749 [DOI] [PubMed] [Google Scholar]
- 10. Bravo Ruiz G., Di Pietro A., and Roncero M. I. G. (2016) Combined action of the major secreted exo- and endopolygalacturonases is required for full virulence of Fusarium oxysporum. Mol. Plant Pathol. 17, 339–353 10.1111/mpp.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. ten Have A., Mulder W., Visser J., and van Kan J. A. L. (1998) The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol. Plant Microbe Interact. 11, 1009–1016 10.1094/MPMI.1998.11.10.1009 [DOI] [PubMed] [Google Scholar]
- 12. Huang Q., and Allen C. (2000) Polygalacturonases are required for rapid colonization and full virulence of Ralstonia solanacearum on tomato plants. Physiol. Mol. Plant Path. 57, 77–83 10.1006/pmpp.2000.0283 [DOI] [Google Scholar]
- 13. Rodriguez-Palenzuela P., Burr T. J., and Collmer A. (1991) Polygalacturonase is a virulence factor in Agrobacterium tumefaciens Biovar 3. J. Bacteriol. 173, 6547–6552 10.1128/jb.173.20.6547-6552.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Lorenzo G., D'Ovidio R., and Cervone F. (2001) The role of polygalacturonase-inhibiting proteins (PGIPs) in defense against pathogenic fungi. Annu. Rev. Phytopathol. 39, 313–335 10.1146/annurev.phyto.39.1.313 [DOI] [PubMed] [Google Scholar]
- 15. Kalunke R. M., Tundo S., Benedetti M., Cervone F., De Lorenzo G., and D'Ovidio R. (2015) An update on polygalacturonase-inhibiting protein (PGIP), a leucine-rich repeat protein that protects crop plants against pathogens. Front. Plant Sci. 6, 146 10.3389/fpls.2015.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rathinam M., Rao U., and Sreevathsa R. (2020) Novel biotechnological strategies to combat biotic stresses: Polygalacturonase inhibitor (PGIP) proteins as a promising comprehensive option. Appl. Microbiol. Biotechnol. 104, 2333–2342 10.1007/s00253-020-10396-3 [DOI] [PubMed] [Google Scholar]
- 17. De Lorenzo G., and Ferrari S. (2002) Polygalacturonase-inhibiting proteins in defense against phytopathogenic fungi. Curr. Opin. Plant Biol. 5, 295–299 10.1016/S1369-5266(02)00271-6 [DOI] [PubMed] [Google Scholar]
- 18. De Lorenzo G., Cervone F., Bellincampi D., Caprari C., Clark A. J., Desiderio A., Devoto A., Forrest R., Leckie F., Nuss L., and Salvi G. (1994) Polygalacturonase, PGIP and oligogalacturonides in cell-cell communication. Biochem. Soc. Trans. 22, 394–397 10.1042/bst0220394 [DOI] [PubMed] [Google Scholar]
- 19. Cervone F., De Lorenzo G., Caprari C., Clark A. J., Desiderio A., Devoto A., Leckie F., Nuss L., Salvi G., and Toubart P. (1993) The interaction between fungal endopolygalacturonase and plant cell wall PGIP (polygalacturonase-inhibiting protein). in Mechanisms of Plant Defense Responses (Fritig B., and Legrand M., eds.) 1st Ed., pp. 64–67, Springer Netherlands, Dordrecht, Netherlands [Google Scholar]
- 20. Ferrari S., Savatin D. V., Sicilia F., Gramegna G., Cervone F., and De Lorenzo G. (2013) Oligogalacturonides: Plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 4, 49 10.3389/fpls.2013.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poinssot B., Vandelle E., Bentéjac M., Adrian M., Levis C., Brygoo Y., Garin J., Sicilia F., Coutos-Thévenot P., and Pugin A. (2003) The endopolygalacturonase 1 from Botrytis cinerea activates grapevine defense reactions unrelated to its enzymatic activity. Mol. Plant Microbe Interact. 16, 553–564 10.1094/MPMI.2003.16.6.553 [DOI] [PubMed] [Google Scholar]
- 22. Powell A. L. T., van Kan J., ten Have A., Visser J., Greve L. C., Bennett A. B., and Labavitch J. M. (2000) Transgenic expression of pear PGIP in tomato limits fungal colonization. Mol. Plant Microbe Interact. 13, 942–950 10.1094/MPMI.2000.13.9.942 [DOI] [PubMed] [Google Scholar]
- 23. Ferrari S., Vairo D., Ausubel F. M., Cervone F., and De Lorenzo G. (2003) Tandemly duplicated Arabidopsis genes that encode polygalacturonase-inhibiting proteins are regulated coordinately by different signal transduction pathways in response to fungal infection. Plant Cell 15, 93–106 10.1105/tpc.005165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Manfredini C., Sicilia F., Ferrari S., Pontiggia D., Salvi G., Caprari C., Lorito M., and De Lorenzo G. (2005) Polygalacturonase-inhibiting protein 2 of Phaseolus vulgaris inhibits BcPG1, a polygalacturonase of Botrytis cinerea important for pathogenicity, and protects transgenic plants from infection. Physiol. Mol. Plant Path. 67, 108–115 10.1016/j.pmpp.2005.10.002 [DOI] [Google Scholar]
- 25. Ferrari S., Galletti R., Vairo D., Cervone F., and De Lorenzo G. (2006) Antisense expression of the Arabidopsis thaliana AtPGIP1 gene reduces polygalacturonase-inhibiting protein accumulation and enhances susceptibility to Botrytis cinerea. Mol. Plant Microbe Interact. 19, 931–936 10.1094/MPMI-19-0931 [DOI] [PubMed] [Google Scholar]
- 26. Hou W., Mu J., Li A., Wang H., and Kong L. (2015) Identification of a wheat polygalacturonase-inhibiting protein involved in Fusarium head blight resistance. Eur. J. Plant Pathol. 141, 731–745 10.1007/s10658-014-0574-7 [DOI] [Google Scholar]
- 27. Jaubert S., Laffaire J.-B., Abad P., and Rosso M.-N. (2002) A polygalacturonase of animal origin isolated from the root-knot nematode Meloidogyne incognita. FEBS Lett. 522, 109–112 10.1016/S0014-5793(02)02906-X [DOI] [PubMed] [Google Scholar]
- 28. Girard C., and Jouanin L. (1999) Molecular cloning of cDNAs encoding a range of digestive enzymes from a phytophagous beetle, Phaedon cochleariae. Insect Biochem. Mol. Biol. 29, 1129–1142 10.1016/S0965-1748(99)00104-6 [DOI] [PubMed] [Google Scholar]
- 29. Pauchet Y., Wilkinson P., Chauhan R., and Ffrench-Constant R. H. (2010) Diversity of beetle genes encoding novel plant cell wall degrading enzymes. PLoS One 5, e15635 10.1371/journal.pone.0015635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kirsch R., Gramzow L., Theißen G., Siegfried B. D., Ffrench-Constant R. H., Heckel D. G., and Pauchet Y. (2014) Horizontal gene transfer and functional diversification of plant cell wall degrading polygalacturonases: Key events in the evolution of herbivory in beetles. Insect Biochem. Mol. Biol. 52, 33–50 10.1016/j.ibmb.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 31. Kirsch R., Heckel D. G., and Pauchet Y. (2016) How the rice weevil breaks down the pectin network: Enzymatic synergism and sub-functionalization. Insect Biochem. Mol. Biol. 71, 72–82 10.1016/j.ibmb.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 32. Shelomi M., Jasper W. C., Atallah J., Kimsey L. S., and Johnson B. R. (2014) Differential expression of endogenous plant cell wall degrading enzyme genes in the stick insect (Phasmatodea) midgut. BMC Genomics 15, 917 10.1186/1471-2164-15-917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frati F., Galletti R., De Lorenzo G., Salerno G., and Conti E. (2006) Activity of endo-polygalacturonases in mirid bugs (Heteroptera: Miridae) and their inhibition by plant cell wall proteins (PGIPs). Eur. J. Entomol. 103, 515–522 10.14411/eje.2006.067 [DOI] [Google Scholar]
- 34. Shackel K. A., de la Paz Celorio-Mancera M., Ahmadi H., Greve L. C., Teuber L. R., Backus E. A., and Labavitch J. M. (2005) Micro-injection of lygus salivary gland proteins to simulate feeding damage in alfalfa and cotton flowers. Arch. Insect Biochem. Physiol. 58, 69–83 10.1002/arch.20033 [DOI] [PubMed] [Google Scholar]
- 35. Kirsch R., Wielsch N., Vogel H., Svatoš A., Heckel D. G., and Pauchet Y. (2012) Combining proteomics and transcriptome sequencing to identify active plant-cell-wall-degrading enzymes in a leaf beetle. BMC Genomics 13, 587 10.1186/1471-2164-13-587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. D'Ovidio R., Raiola A., Capodicasa C., Devoto A., Pontiggia D., Roberti S., Galletti R., Conti E., O'Sullivan D., and De Lorenzo G. (2004) Characterization of the complex locus of bean encoding polygalacturonase-inhibiting proteins reveals subfunctionalization for defense against fungi and insects. Plant Physiol. 135, 2424–2435 10.1104/pp.104.044644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doostdar H., McCollum T. G., and Mayer R. T. (1997) Purification and characterization of an endo-polygalacturonase from the gut of West Indies sugarcane rootstalk borer weevil (Diaprepes abbreviatus L.) larvae. Comp. Biochem. Physiol. 118, 861–867 10.1016/S0305-0491(97)00285-X [DOI] [Google Scholar]
- 38. Bashi Z. D., Rimmer S. R., Khachatourians G. G., and Hegedus D. D. (2013) Brassica napus polygalacturonase inhibitor proteins inhibit Sclerotinia sclerotiorum polygalacturonase enzymatic and necrotizing activities and delay symptoms in transgenic plants. Can. J. Microbiol. 59, 79–86 10.1139/cjm-2012-0352 [DOI] [PubMed] [Google Scholar]
- 39. Liu N., Zhang X., Sun Y., Wang P., Li X., Pei Y., Li F., and Hou Y. (2017) Molecular evidence for the involvement of a polygalacturonase-inhibiting protein, GhPGIP1, in enhanced resistance to Verticillium and Fusarium wilts in cotton. Sci. Rep. 7, 39840 10.1038/srep39840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leckie F., Mattei B., Capodicasa C., Hemmings A., Nuss L., Aracri B., De Lorenzo G., and Cervone F. (1999) The specificity of polygalacturonase-inhibiting protein (PGIP): A single amino acid substitution in the solvent-exposed β-strand/β-turn region of the leucine-rich repeats (LRRs) confers a new recognition capability. EMBO J. 18, 2352–2363 10.1093/emboj/18.9.2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hwang B. H., Bae H., Lim H.-S., Kim K. B., Kim S. J., Im M.-H., Park B.-S., Kim D. S., and Kim J. (2010) Overexpression of polygalacturonase-inhibiting protein 2 (PGIP2) of Chinese cabbage (Brassica rapa ssp. pekinensis) increased resistance to the bacterial pathogen Pectobacterium carotovorum ssp. carotovorum. Plant Cell Tissue Org. Cult. 103, 293–305 10.1007/s11240-010-9779-4 [DOI] [Google Scholar]
- 42. HuangFu H., Guan C., Jin F., and Yin C. (2014) Prokaryotic expression and protein function of Brassica napus PGIP2 and its genetic transformation. Plant Biotechnol. Rep. 8, 171–181 10.1007/s11816-013-0307-y [DOI] [Google Scholar]
- 43. Wang Z., Wan L., Xin Q., Chen Y., Zhang X., Dong F., Hong D., and Yang G. (2018) Overexpression of OsPGIP2 confers Sclerotinia sclerotiorum resistance in Brassica napus through increased activation of defense mechanisms. J. Exp. Bot. 69, 3141–3155 10.1093/jxb/ery138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chen X., Chen Y., Zhang L., He Z., Huang B., Chen C., Zhang Q., and Zuo S. (2019) Amino acid substitutions in a polygalacturonase inhibiting protein (OsPGIP2) increases sheath blight resistance in rice. Rice 12, 56 10.1186/s12284-019-0318-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Agüero C. B., Uratsu S. L., Greve C., Powell A. L. T., Labavitch J. M., Meredith C. P., and Dandekar A. M. (2005) Evaluation of tolerance to Pierce's disease and Botrytis in transgenic plants of Vitis vinifera L. expressing the pear PGIP gene. Mol. Plant Pathol. 6, 43–51 10.1111/j.1364-3703.2004.00262.x [DOI] [PubMed] [Google Scholar]
- 46. Bernhardt K., Vigelius S. K., Wiese J., Linka N., and Weber A. P. M. (2012) Agrobacterium-mediated Arabidopsis thaliana transformation: An overview of T-DNA binary vectors, floral dip and screening for homozygous lines. J. Endocyt. Cell Res. 22, 19–28 [Google Scholar]
- 47. Hegedus D. D., Li R., Buchwaldt L., Parkin I., Whitwill S., Coutu C., Bekkaoui D., and Rimmer S. R. (2008) Brassica napus possesses an expanded set of polygalacturonase inhibitor protein genes that are differentially regulated in response to Sclerotinia sclerotiorum infection, wounding and defense hormone treatment. Planta 228, 241–253 10.1007/s00425-008-0733-1 [DOI] [PubMed] [Google Scholar]
- 48. Howe K. L., Contreras-Moreira B., De Silva N., Maslen G., Akanni W., Allen J., Alvarez-Jarreta J., Barba M., Bolser D. M., Cambell L., Carbajo M., Chakiachvili M., Christensen M., Cummins C., Cuzick A., et al. (2020) Ensembl Genomes 2020-enabling non-vertebrate genomic research. Nucleic Acids Res. 48, D689–D695 10.1093/nar/gkz890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang R., Lu L., Pan X., Hu Z., Ling F., Yan Y., Liu Y., and Lin Y. (2015) Functional analysis of OsPGIP1 in rice sheath blight resistance. Plant Mol. Biol. 87, 181–191 10.1007/s11103-014-0269-7 [DOI] [PubMed] [Google Scholar]
- 50. Zhang C., Feng C., Wang J., Kong F., Sun W., and Wang F. (2016) Cloning, expression analysis and recombinant expression of a gene encoding a polygalacturonase-inhibiting protein from tobacco, Nicotiana tabacum. Heliyon 2, e00110 10.1016/j.heliyon.2016.e00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bergmann C. W., Cook B., Darvill A. G., Albersheim P., Bellincampi D., and Caprari C. (1996) The effect of glycosylation of endopolygalacturonases and polygalacturonase inhibiting proteins on the production of oligogalacturonides. in Progress in Biotechnology (Visser J., and Voragen A. G. J., eds.), 1st Ed., pp. 275–282 Elsevier, Amsterdam, Netherlands [Google Scholar]
- 52. Benedetti M., Leggio C., Federici L., De Lorenzo G., Pavel N. V., and Cervone F. (2011) Structural resolution of the complex between a fungal polygalacturonase and a plant polygalacturonase-inhibiting protein by small-angle X-ray scattering. Plant Physiol. 157, 599–607 10.1104/pp.111.181057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Benedetti M., Andreani F., Leggio C., Galantini L., Di Matteo A., Pavel N. V., De Lorenzo G., Cervone F., Federici L., and Sicilia F. (2013) A single amino-acid substitution allows endo-polygalacturonase of Fusarium verticillioides to acquire recognition by PGIP2 from Phaseolus vulgaris. PLoS One 8, e80610 10.1371/journal.pone.0080610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Benedetti M., Bastianelli E., Salvi G., De Lorenzo G., and Caprari C. (2011) Artificial evolution corrects a repulsive amino acid in polygalacturonase inhibiting proteins (PGIPs). J. Plant Pathol. 93, 89–95 10.4454/jpp.v93i1.277 [DOI] [Google Scholar]
- 55. Spadoni S., Zabotina O., Di Matteo A., Mikkelsen J. D., Cervone F., De Lorenzo G., Mattei B., and Bellincampi D. (2006) Polygalacturonase-inhibiting protein interacts with pectin through a binding site formed by four clustered residues of arginine and lysine. Plant Physiol. 141, 557–564 10.1104/pp.106.076950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Benedetti M., Pontiggia D., Raggi S., Cheng Z., Scaloni F., Ferrari S., Ausubel F. M., Cervone F., and De Lorenzo G. (2015) Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc. Natl. Acad. Sci. U.S.A. 112, 5533–5538 10.1073/pnas.1504154112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. D'Ovidio R., Roberti S., Di Giovanni M., Capodicasa C., Melaragni M., Sella L., Tosi P., and Favaron F. (2006) The characterization of the soybean polygalacturonase-inhibiting proteins (PGIP) gene family reveals that a single member is responsible for the activity detected in soybean tissues. Planta 224, 633–645 10.1007/s00425-006-0235-y [DOI] [PubMed] [Google Scholar]
- 58. Janni M., Bozzini T., Moscetti I., Volpi C., and D'Ovidio R. (2013) Functional characterisation of wheat Pgip genes reveals their involvement in the local response to wounding. Plant Biol. 15, 1019–1024 10.1111/plb.12002 [DOI] [PubMed] [Google Scholar]
- 59. Habrylo O., Evangelista D. E., Castilho P. V., Pelloux J., and Henrique-Silva F. (2018) The pectinases from Sphenophorus levis: Potential for biotechnological applications. Int. J. Biol. Macromol. 112, 499–508 10.1016/j.ijbiomac.2018.01.172 [DOI] [PubMed] [Google Scholar]
- 60. Kirsch R., Kunert G., Vogel H., and Pauchet Y. (2019) Pectin digestion in herbivorous beetles: Impact of pseudoenzymes exceeds that of their active counterparts. Front. Physiol. 10, 685 10.3389/fphys.2019.00685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kolade O. O., Bamford V. A., Ancillo Anton G., Jones J. D., Vera P., and Hemmings A. M. (2006) In vitro characterization of the cysteine-rich capping domains in a plant leucine rich repeat protein. Biochim. Biophys. Acta 1764, 1043–1053 10.1016/j.bbapap.2006.03.014 [DOI] [PubMed] [Google Scholar]
- 62. Smallwood M., Worrall D., Byass L., Elias L., Ashford D., Doucet C. J., Holt C., Telford J., Lillford P., and Bowles D. J. (1999) Isolation and characterization of a novel antifreeze protein from carrot (Daucus carota). Biochem. J. 340, 385–391 10.1042/bj3400385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hohmann U., and Hothorn M. (2019) Crystal structure of the leucine-rich repeat ectodomain of the plant immune receptor kinase SOBIR1. Acta Crystallogr. D Struct. Biol. 75, 488–497 10.1107/S2059798319005291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hong M., Yoon S.-I., and Wilson I. A. (2012) Recombinant expression of TLR5 proteins by ligand supplementation and a leucine-rich repeat hybrid technique. Biochem. Biophys. Res. Commun. 427, 119–124 10.1016/j.bbrc.2012.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vancraenenbroeck R., Lobbestael E., Weeks S. D., Strelkov S. V., Baekelandt V., Taymans J. M., and De Maeyer M. (2012) Expression, purification and preliminary biochemical and structural characterization of the leucine rich repeat namesake domain of leucine rich repeat kinase 2. Biochim. Biophys. Acta 1824, 450–460 10.1016/j.bbapap.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 66. Kim H. M., Park B. S., Kim J. I., Kim S. E., Lee J., Oh S. C., Enkhbayar P., Matsushima N., Lee H., Yoo O. J., and Lee J.-O. (2007) Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist eritoran. Cell 130, 906–917 10.1016/j.cell.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 67. Jin M. S., Kim S. E., Heo J. Y., Lee M. E., Kim H. M., Paik S. G., Lee H., and Lee J.-O. (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082 10.1016/j.cell.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 68. Protsenko M. A., Buza N. L., Krinitsyna A. A., Bulantseva E. A., and Korableva N. P. (2008) Polygalacturonase-inhibiting protein is a structural component of plant cell wall. Biochemistry (Mosc.) 73, 1053–1062 10.1134/s0006297908100015 [DOI] [PubMed] [Google Scholar]
- 69. Jamet E., Canut H., Boudart G., and Pont-Lezica R. F. (2006) Cell wall proteins: A new insight through proteomics. Trends Plant Sci. 11, 33–39 10.1016/j.tplants.2005.11.006 [DOI] [PubMed] [Google Scholar]
- 70. Komatsu S., and Yanagawa Y. (2013) Cell wall proteomics of crops. Front. Plant Sci. 4, 17 10.3389/fpls.2013.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. De Caroli M., Lenucci M. S., Di Sansebastiano G.-P., Dalessandro G., De Lorenzo G., and Piro G. (2011) Protein trafficking to the cell wall occurs through mechanisms distinguishable from default sorting in tobacco. Plant J. 65, 295–308 10.1111/j.1365-313X.2010.04421.x [DOI] [PubMed] [Google Scholar]
- 72. Arnau J., Lauritzen C., Petersen G. E., and Pedersen J. (2006) Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr. Purif. 48, 1–13 10.1016/j.pep.2005.12.002 [DOI] [PubMed] [Google Scholar]
- 73. Vogl T., Ahmad M., Krainer F. W., Schwab H., and Glieder A. (2015) Restriction site free cloning (RSFC) plasmid family for seamless, sequence independent cloning in Pichia pastoris. Microb. Cell Fact. 14, 103 10.1186/s12934-015-0293-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ishikawa N., Chiba T., Chen L. T., Shimizu A., Ikeguchi M., and Sugai S. (1998) Remarkable destabilization of recombinant α-lactalbumin by an extraneous N-terminal methionyl residue. Protein Eng. 11, 333–335 10.1093/protein/11.5.333 [DOI] [PubMed] [Google Scholar]
- 75. Shah S. J., Anjam M. S., Mendy B., Anwer M. A., Habash S. S., Lozano-Torres J. L., Grundler F. M. W., and Siddique S. (2017) Damage-associated responses of the host contribute to defence against cyst nematodes but not root-knot nematodes. J. Exp. Bot. 68, 5949–5960 10.1093/jxb/erx374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chotechung S., Somta P., Chen J., Yimram T., Chen X., and Srinives P. (2016) A gene encoding a polygalacturonase-inhibiting protein (PGIP) is a candidate gene for bruchid (Coleoptera: bruchidae) resistance in mungbean (Vigna radiata). Theor. Appl. Genet. 129, 1673–1683 10.1007/s00122-016-2731-1 [DOI] [PubMed] [Google Scholar]
- 77. Kaewwongwal A., Liu C., Somta P., Chen J., Tian J., Yuan X., and Chen X. (2020) A second VrPGIP1 allele is associated with bruchid resistance (Callosobruchus spp.) in wild mungbean (Vigna radiata var. sublobata) accession ACC41. Mol. Genet. Genomics 295, 275–286 10.1007/s00438-019-01619-y [DOI] [PubMed] [Google Scholar]
- 78. Kirsch R., Vurmaz E., Schaefer C., Eberl F., Sporer T., Haeger W., and Pauchet Y. (2020) Plants use identical inhibitors to protect their cell wall pectin against microbes and insects. Ecol. Evol. 10, 3814–3824 10.1002/ece3.6180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Joubert D. A., Kars I., Wagemakers L., Bergmann C., Kemp G., Vivier M. A., and van Kan J. A. L. (2007) A polygalacturonase-inhibiting protein from grapevine reduces the symptoms of the endopolygalacturonase BcPG2 from Botrytis cinerea in Nicotiana benthamiana leaves without any evidence for in vitro interaction. Mol. Plant Microbe Interact. 20, 392–402 10.1094/MPMI-20-4-0392 [DOI] [PubMed] [Google Scholar]
- 80. Joubert D. A., Slaughter A. R., Kemp G., Becker J. V. W., Krooshof G. H., Bergmann C., Benen J., Pretorius I. S., and Vivier M. A. (2006) The grapevine polygalacturonase-inhibiting protein (VvPGIP1) reduces Botrytis cinerea susceptibility in transgenic tobacco and differentially inhibits fungal polygalacturonases. Transgenic Res. 15, 687–702 10.1007/s11248-006-9019-1 [DOI] [PubMed] [Google Scholar]
- 81. Casasoli M., Federici L., Spinelli F., Di Matteo A., Vella N., Scaloni F., Fernandez-Recio J., Cervone F., and De Lorenzo G. (2009) Integration of evolutionary and desolvation energy analysis identifies functional sites in a plant immunity protein. Proc. Natl. Acad. Sci. U.S.A. 106, 7666–7671 10.1073/pnas.0812625106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Müller T., and Müller C. (2016) Adult beetles compensate for poor larval food conditions. J. Insect Physiol. 88, 24–32 10.1016/j.jinsphys.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 83. Müller T., and Müller C. (2017) Phenotype of a leaf beetle larva depends on host plant quality and previous test experience. Behav. Processes 142, 40–45 10.1016/j.beproc.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 84. Petersen T. N., Brunak S., von Heijne G., and Nielsen H. (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods 8, 785–786 10.1038/nmeth.1701 [DOI] [PubMed] [Google Scholar]
- 85. Pierleoni A., Martelli P. L., and Casadio R. (2008) PredGPI: A GPI-anchor predictor. BMC Bioinform. 9, 392 10.1186/1471-2105-9-392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Knight P. J. K., Crickmore N., and Ellar D. J. (1994) The receptor for Bacillus thuringiensis CrylA(c) δ-endotoxin in the brush border membrane of the lepidopteran Manduca sexta is aminopeptidase N. Mol. Microbiol. 11, 429–436 10.1111/j.1365-2958.1994.tb00324.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Bretschneider A., Heckel D. G., and Pauchet Y. (2016) Three toxins, two receptors, one mechanism: Mode of action of Cry1A toxins from Bacillus thuringiensis in Heliothis virescens. Insect Biochem. Mol. Biol. 76, 109–117 10.1016/j.ibmb.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 88. Ferguson M. A. J., Hart G. W., and Kinoshita T. (2017) Glycosylphosphatidylinositol anchors. in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., Schnaar R. L., and Seeberger P. H., eds.) 3rd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA [Google Scholar]
- 89. Henning J. (2019) Interaction of Beetle Polygalacturonases with Putative Plant Inhibitory Proteins. M.Sc. thesis, Friedrich Schiller University, Jena, Germany [Google Scholar]
- 90. Klockenbusch C., and Kast J. (2010) Optimization of formaldehyde cross-linking for protein interaction analysis of non-tagged integrin β1. J. Biomed. Biotechnol. 2010, 927585 10.1155/2010/927585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Miller G. L. (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428 10.1021/ac60147a030 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this paper are contained within the article.