Abstract
Objectives
To retrospectively investigate the incidence of acute adrenal infarction (AAI) in patients who underwent chest CT for severe SARS-CoV-2 infection and to correlate findings with prognosis.
Methods
The local ethics committee approved this retrospective study and waived the need of informed consent. From March 9 to April 10, 2020, all patients referred to our institution for a clinical suspicion of COVID-19 with moderate to severe symptoms underwent a chest CT for triage. Patients with a/parenchymal lesion characteristics of COVID-19 involving at least 50% of lung parenchyma and b/positive RT-PCR for SARS-CoV-2 were retrospectively included. Adrenal glands were reviewed by two independent readers to look for AAI. Additional demographics and potential biological markers of adrenal insufficiency were obtained. Correlations with ICU stay and mortality were sought.
Results
Out of the 219 patients with critical (n = 52) and severe lung (n = 167) parenchyma lesions, 51 (23%) had CT scan signs of AAI, which was bilateral in 45 patients (88%). Four patients had an acute biological adrenal gland insufficiency (8%). Univariate analysis in AAI+ patients demonstrated a higher rate of ICU stay (67% vs. 45%, p < 0.05) and a longer stay (more than 15 days for 31% for AAI+ vs. 19%, p < 0.05) compared with AAI− patients. Mortality rate was similar (27%, p = 0.92).
Conclusions
Acute adrenal infarction on initial chest evaluation of severe COVID-19 is frequent (51/219, 23%) and might be a sign of poorer prognosis.
Key Points
• Acute adrenal infarction on initial chest CT evaluation of severe COVID-19 is frequent (51/219).
• AAI might be a factor of poorer prognosis, with increased rate of ICU hospitalization and length of stay.
Keywords: Adrenal insufficiency, Coronavirus, Diagnostic imaging, Multidetector computed tomography
Introduction
The SARS-CoV-2 viral infection has a variable prognosis, with up to 5% of cases being dramatically severe. The initial immune response to viral load is followed in certain cases by an uncontrolled “cytokine storm” with hyperinflammation and immunosuppression [1]. In critically ill patients, uncontrolled viral propagation induces cytotoxicity and hyperactivation of immune cells, leading to increased vascular inflammation, clotting, and thromboembolism complications [2]. Reports of hypothalamic-pituitary-adrenal (HPA) axis dysfunction have emerged in the literature but the detailed pathophysiology of immune response and the potential damages affecting especially the adrenal gland are still unclear [3].
Acute adrenal infarction (AAI) is a rare but serious complication of hypercoagulable conditions such as primary antiphospholipid antibody syndrome or pregnancy [4, 5]. CT diagnosis of AAI is based on an enlargement and a low attenuation of the infarcted adrenal gland, sometimes associated with a high attenuation surrounding rim with fat stranding and “capsular sign” [6–8]. The incidence and potential consequences of this condition have never been evaluated in SARS-CoV-2 infection, where thromboembolic complications are frequently reported [9].
Consequently, the purpose of this study is to retrospectively investigate the incidence of AAI in patients with severe SARS-CoV-2 infection and to correlate findings to prognosis data.
Material and methods
Patient population
The local ethics committee of Strasbourg University Hospital approved this retrospective study and waived the need of informed consent.
From March 9 to April 10, 2020, all consecutive patients who underwent chest CT for clinical suspicion of SARS-CoV-2-infection at one of our 2 hospital sites (Nouvel Hôpital Civil, Hôpital de Hautepierre) were retrospectively screened for inclusion. Chest CT was used as a systematic triage tool in all patients with a clinical suspicion of COVID-19 and moderate to severe symptoms.
Inclusion criteria were:
Severe or critical lung parenchyma lesion characteristics of COVID-19, i.e., involving at least 50% of the total lung parenchyma [10];
Presence of both entire adrenal glands in the inferior part of the volume of acquisition;
Positive RT-PCR for SARS-CoV-2 at the time of chest CT.
There was no exclusion criterion.
For all patients, the additional following data at the time of CT examination were retrieved from their Electronic Health Record: age, sex, body mass index (BMI), time from the onset of symptoms, hospitalization status (ICU, medicine ward, Emergency Department outpatient, patient discharged), and vital status.
Chest CT acquisition
Chest CT were acquired on 64 row or greater scanners without injection of iodine contrast medium. Images were reconstructed with a slice thickness of 1 mm in mediastinal and parenchymal windows. Tube voltage was set at 100 to 135 kV depending on patient’s morphotype, and automatic tube-current modulation was used, with a maximum mAs varying between scanners but always set below 350 mAs. When possible, patients were instructed to hold their breath and raise their arms above their head to minimize artifacts. Images were transmitted to post-processing workstations for multiplanar reconstructions and maximum intensity projection reconstructions.
Lung parenchyma analysis
Lung parenchyma of all chest CT were reviewed by the same radiologist (P.L.) and the global percentage of abnormal lung parenchyma was visually estimated following the ESR and ESTI guidelines [10]. CT typically shows bilateral ground glass opacities, with a predominantly peripheral and subpleural location [11]. In patients evaluated several days after the beginning of symptoms, linear and extensive consolidation in the most severe presentation could also be present [12]. Severe disease was defined as lesion characteristics of COVID-19 involving 50 to 75% of the total lung parenchyma, while critical disease was defined as involving 76 to 100% of the total lung parenchyma.
Adrenal gland analysis
Two independent readers (M.O. and C.R., radiologists with respectively 10 and 30 years of experience), blinded to the clinical and laboratory data, reviewed all included CT using the soft tissue reconstruction.
Both adrenal glands were analyzed. The specific imaging criteria used to diagnose AAI was the enlargement of one or both adrenal glands with peripheral fat stranding in the suprarenal space. When AAI was present, a two-level quantification was performed (grade 1 = adrenal gland enlargement with moderate peripheral fat stranding; and grade 2 = adrenal gland enlargement with severe peripheral fat stranding). Discrepancies between readers were resolved by consensus.
Laboratory analyses
All patients were screened with RT-PCR technique for SARS-CoV-2, with all initial samples obtained using a nasopharyngeal swab.
Sodium and potassium blood levels and glycemia were recorded for all patients who had CT signs of AAI. Biological adrenal gland insufficiency was defined by a combination of hyperkalemia (> 5 mmol/L), hyponatremia (< 130 mmol/L), and hypoglycemia (< 3.9 mmol/L).
Statistical analyses
Statistical analyses were performed using R 3.4.3 Software (R Foundation for Statistical Computing) at a significance level of 0.05.
Continuous data were tested for Gaussian distribution using the Shapiro-Wilk test and quantile-quantile plot.
Student test or Mann-Whitney-Wilcoxon tests were used to compare quantitative parameters between both groups.
Statistical correlations tests used Pearson and Spearman coefficient.
Kappa statistics were used for overall inter-rater agreement.
Results
Patients and flowchart
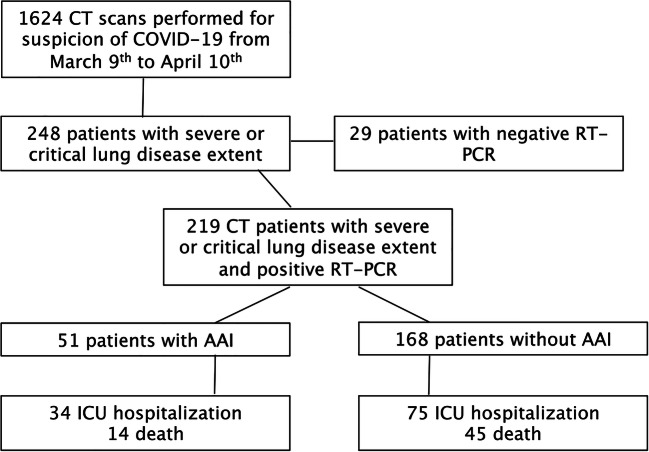
From March 9 to April 10, 2020, 1624 CT scans were performed for a clinical suspicion of COVID-19 with moderate to severe symptoms. Among these 1614 examinations, 219 chest CT corresponding to 219 patients were ultimately included, following the flowchart detailed in Fig. 1.
Fig. 1.
Flowchart of the study
At the time of data gathering (April 21, 2020) and after a median follow-up of 24 days (range 11; 43):
59/219 patients (27%) were dead (14 in the AAI+ group),
29 were still in ICU (11 in the AAI+ group),
115 patients were still hospitalized in medicine ward (24 in the AAI+ group),
6 patients were discharged from ICU (1 in the AAI+ group), and
10 patients were discharged home (1 in the AAI+ group).
Lung parenchyma analysis
One hundred sixty-seven cases (76%) involved 50 to 75% of the total lung parenchyma and 52 cases (24%) involved more than 75%.
Adrenal gland analysis
COVID-19 patients with AAI
A total of 51/219 patients (23%) had CT signs of AAI on their initial chest CT.
Thirty-six were men (sex ratio 3:1), their mean age was 67 years old ± 11 (range 42; 88), and their mean BMI was 28 kg m−2 ± 6 (range 16; 46). The median time interval between developing symptoms and chest CT was 7 days (range 1; 21). Thirty-four patients were hospitalized in ICU during the follow-up. Length of stay was greater than 15 days in 16 patients (31%) (Table 1).
Table 1.
Clinical and biological data for patients undergoing chest CT and classified as COVID-19 infection (RT-PCR+)
| Clinical and CT features | Adrenal gland infarction present (n = 51) | Adrenal gland infarction absent (n = 168) | p (Student t test) |
|---|---|---|---|
| Male | 36 (71%) | 123 (73%) | 0.07 |
| Age—mean ± SD (year old) | 67 ± 11 | 67 ± 15 | 0.73 |
| BMI—mean ± SD (kg/m2) | 28 ± 6 | 30 ± 5 | 0.62 |
| ICU hospitalization | 34 (67%) | 75 (45%) | < 0.05 |
| Length of stay above 15 days | 16 (31%) | 32 (19%) | < 0.05 |
| Death | 14 (27%) | 45 (27%) | 0.92 |
| Patients discharged | 2 (4%) | 14 (8%) | 0.23 |
| Acute adrenal insufficiency | 4 (8%) | 3 (2%) | 0.09 |
| Interval between symptoms and CT—median IQR (days) | 7 ± 5 | 7 ± 6 | 0.06 |
| Lung parenchyma lesions 50–75% | 25 (49%) | 27 (16%) | < 0.05 |
| Lung parenchyma lesions 76–100% | 26 (51%) | 141 (84%) | < 0.05 |
| Bilateral adrenal gland infarction | 45 (88%) | – | – |
| Unilateral gland infarction | 6 (12%) | – | – |
The adrenal gland infarct was bilateral in 45 cases (88%). Among them, 4 patients had an acute biological adrenal gland insufficiency (8%)—all had bilateral AAI.
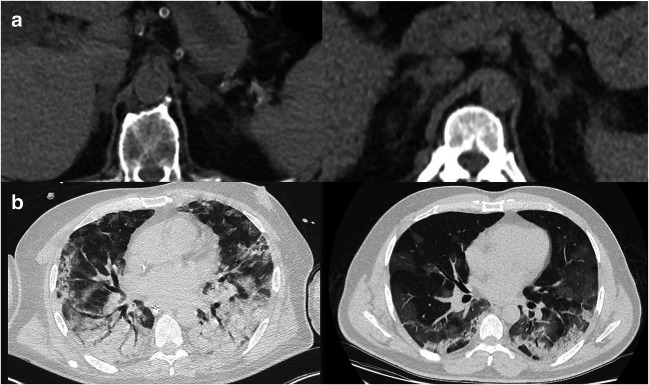
Based on qualitative visual assessment, 33 (65%) moderate AAI and 18 (35%) severe AAI were found. Inter-reader agreement on this two-level classification was high (κ = 0.86, p < 0.05). An illustration of these CT findings is presented in Fig. 2.
Fig. 2.
Initial chest CT of two different patients with RT-PCR confirmed COVID-19 infection. a Upper abdominal view focusing on the adrenal glands and showing enlargement with moderate (left) and severe (right) fat stranding in the suprarenal space. b Corresponding lung parenchyma lesions
Subgroup analyses, presented in Table 2, revealed a trend of increasing mortality when increasing the severity of adrenal damages (p = 0.77). Yet, biological adrenal insufficiency as well as ICU hospitalization were not correlated with the severity of adrenal damages (p = 0.92 and 0.91, respectively).
Table 2.
Subgroup severity analyses for patients with AAI
| AAI severity | N | Death | ICU hospitalization | Acute adrenal insufficiency |
|---|---|---|---|---|
| Moderate (grade 1) | 33 (65%) | 8 (24%) | 22 (67%) | 2 (6%) |
| Severe (grade 2) | 18 (35%) | 6 (33%) | 12 (67%) | 1 (6%) |
| p (Student t test) | 0.88 | 0.77 | 0.91 | 0.92 |
COVID-19 without AAI
A total of 168/219 patients (77%) had no sign of AAI on their initial chest CT scan.
One hundred twenty-three were men (sex ratio 4:1), their mean age was 67 years old ± 15 (range 22; 96), and their mean BMI was 30 kg m−2 ± 5 (range 19; 50). The median time interval between developing symptoms and chest CT was 7 days (range 1; 27). Seventy-five patients were hospitalized in ICU during the follow-up. Length of stay was more than 15 days in 32 patients (19%).
Comparison of AAI+ and AAI− patients
ICU admission was significantly higher in the AAI group than in the internal control group (67% vs. 45%, p < 0.05) and the length of stay significantly longer (more than 15 days in 31% of patients in the AAI group vs. 19%, p < 0.05). Mortality rate was similar (27%, p = 0.92).
Details are given in Table 1.
Discussion
Our study is the first to report the prevalence of AAI in initial chest CT performed for clinical suspicion of COVID-19, demonstrating on 219 severe patients that 51 (23%) had imaging features of AAI. Presence of AAI on initial chest CT was associated with a higher ICU admission rate and a longer stay.
The phenomena of AAI due to “viral sepsis” doesn’t seem to be explained by hypovolemic conditions as one could see in other causes of septic shock [1]. These would favor a systemic disease spread related to a high activation of blood coagulation, as seen in severe COVID-19 patients secondary to a systemic inflammatory response syndrome [13]. It is hypothesized that SARS-CoV-2 is able to directly attack vascular endothelial cells expressing high levels of ACE2 and then lead to abnormal coagulation [14, 15]. All these systemic responses could explain the pro-thrombotic conditions that directly conduce to AAI.
Since the outbreak of SARS in 2003 with the first reported coronavirus (SARS-CoV), there has been a growing body of evidence to consider this pathology as a systemic disease and not only an acute respiratory insufficiency [16]. This is supported by the wide expression of ACE2 in various human tissues, which are used by SARS-CoV-2 as a target receptor to invade human cells [17]. Systemic vasculitis with edema, localized fibrinoid necrosis, and infiltration of monocytes, lymphocytes, and plasma cells into vessel walls was described in the heart, liver, kidney, and adrenal gland [18], and could explain the pathophysiology behind our findings.
In this context of viral induced adrenal insufficiency, it will be interesting to consider potential therapeutics implications. Based on prior experience in patients with SARS, the absence of benefit on overall survival has discouraged the use of glucosteroids [19]. Yet, the recent press release of the Recovery UK trial [20] highlights a potential benefit for dexamethasone in severe COVID-19. One could therefore hypothesize targeting the glucosteroids usage to patients with imaging signs of AAI, which would however require much more evidence-based approaches [3, 21].
Our work has several limitations.
First, the median follow-up was limited and a significant portion of patients was still in ICU at the end of the inclusion—notably in the AAI group (11 patients), which could partly explain the absence of mortality rate difference at the time of writing. Additionally, it must be noted that a low proportion of critically ill patients had to be transferred to other tertiary center, due to the sanitary conditions, and were therefore lost for follow-up. Other longitudinal observational studies will be needed to evaluate the potential long-term effects of AAI.
Second, our cohort represents the initial phase of the epidemic, when clinicians where not familiar with the potential systemic complications of the disease. Consequently, comprehensive biological explorations for adrenal insufficiency and hypothalamic-pituitary-adrenal tract are lacking in this preliminary study. Indeed, basic laboratory data collected at the day of chest CT examination could not reflect the potential delayed evolution of AAI. We hypothesize that a larger proportion of patients with AAI at initial imaging could have later clinical and biological repercussions that were not explored in the present work. Nonetheless, as we could see in other pathological conditions of AAI (i.e., antiphospholipid antibody syndrome), the relationship between the severity of lesions and the presence of an insufficiency is not clearly demonstrated. In addition, it is not possible to derive any “pathological” diagnosis based on unenhanced CT scans. Edematous changes due to inflammation, reactive increase in blood flow and congestion, and small hemorrhagic foci can also result in such morphological changes. More specific imaging features of adrenal infarct were described in the literature (e.g., “capsular sign”) [8] in CT exploration realized at portal venous phase or with DWI-MRI [22]. Our retrospective study included only non-enhanced chest CT performed at Emergency Department arrival to expedite patient’s triage in this pandemic context. Conversely, these more specific imaging signs could not be assessed in our preliminary findings.
Thirdly, the high proportion of severe extent of lung disease in the AAI group could explain a possible inclusion bias in global prognosis estimation.
Finally, our work was done in one tertiary university center in France, with a local population of patients, and results might not be generalizable to other countries.
To conclude, our work demonstrated a high incidence of acute adrenal infarction on initial chest CT of severe COVID-19 (51/219, 23%), which might be a sign of a poorer prognosis. We recommend radiologists to be aware of this finding, and to mention it in their report when present. Future works will evaluate the biological and clinical consequences of these lesions, all the more since therapeutic implications could be considered.
Abbreviations
- AAI
Acute adrenal infarction
- ACE2
Angiotensin-converting enzyme 2
- ARDS
Acute respiratory distress syndrome
- CT
Computed tomography
- HPA
Hypothalamic-pituitary-adrenal
- ICU
Intensive care unit
- RT-PCR
Reverse transcriptase polymerase chain reaction
Funding information
The authors state that this work has not received any funding.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Catherine ROY, MD.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
The local ethics committee approved this retrospective study and waived the need of informed consent.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Case-control study
• Performed at one institution
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li H, Liu L, Zhang D et al (2020) SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed]
- 2.Jose RJ, Manuel A (2020) COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 10.1016/S2213-2600(20)30216-2 [DOI] [PMC free article] [PubMed]
- 3.Isidori AM, Pofi R, Hasenmajer V, Lenzi A, Pivonello R (2020) Use of glucocorticoids in patients with adrenal insufficiency and COVID-19 infection. Lancet Diabetes Endocrinol. 10.1016/S2213-8587(20)30149-2 [DOI] [PMC free article] [PubMed]
- 4.Riddell AM, Khalili K. Sequential adrenal infarction without MRI-detectable hemorrhage in primary antiphospholipid-antibody syndrome. AJR Am J Roentgenol. 2004;183:220–222. doi: 10.2214/ajr.183.1.1830220. [DOI] [PubMed] [Google Scholar]
- 5.Glomski SA, Guenette JP, Landman W, Tatli S. Acute nonhemorrhagic adrenal infarction in pregnancy: 10-year MRI incidence and patient outcomes at a single institution. AJR Am J Roentgenol. 2018;210:785–791. doi: 10.2214/AJR.17.18739. [DOI] [PubMed] [Google Scholar]
- 6.Ames DE, Asherson RA, Ayres B, Cassar J, Hughes GR (1992) Bilateral adrenal infarction, hypoadrenalism and splinter haemorrhages in the “primary” antiphospholipid syndrome. Br J Rheumatol 31:117–120. 10.1093/rheumatology/31.2.117 [DOI] [PubMed]
- 7.Khandelwal A, Krishna JS, Khandelwal K, Virmani V, Ryan J (2013) Bilateral adrenal infarction in Crohn’s disease. Indian J Endocrinol Metab 17:933–935. 10.4103/2230-8210.117227 [DOI] [PMC free article] [PubMed]
- 8.Moschetta M, Telegrafo M, Pignatelli A, Ianora AAS, Angelelli G (2015) Value of the CT “capsular sign” as a potential indicator of acute adrenal ischemia. Emerg Radiol 22:533–538. 10.1007/s10140-015-1327-4 [DOI] [PubMed]
- 9.Oudkerk M, Büller HR, Kuijpers D et al (2020) Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: report of the National Institute for Public Health of the Netherlands. Radiology 201629. 10.1148/radiol.2020201629 [DOI] [PMC free article] [PubMed]
- 10.Revel M-P, Parkar AP, Prosch H et al (2020) COVID-19 patients and the radiology department - advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI). Eur Radiol. 10.1007/s00330-020-06865-y [DOI] [PMC free article] [PubMed]
- 11.Ye Z, Zhang Y, Wang Y, Huang Z, Song B (2020) Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol:1–9. 10.1007/s00330-020-06801-0 [DOI] [PMC free article] [PubMed]
- 12.Zhao W, Zhong Z, Xie X, Yu Q, Liu J (2020) Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol 214:1072–1077. 10.2214/AJR.20.22976 [DOI] [PubMed]
- 13.Varga Z, Flammer AJ, Steiger P et al (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed]
- 14.Leonard-Lorant I, Delabranche X, Severac F et al (2020) Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology 201561. 10.1148/radiol.2020201561 [DOI] [PMC free article] [PubMed]
- 15.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203:631–637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed]
- 17.Li M-Y, Li L, Zhang Y, Wang X-S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson E. RECOVERY trial: the UK covid-19 study resetting expectations for clinical trials. BMJ. 2020;369:m1626. doi: 10.1136/bmj.m1626. [DOI] [PubMed] [Google Scholar]
- 21.Shang L, Zhao J, Hu Y, Du R, Cao B (2020) On the use of corticosteroids for 2019-nCoV pneumonia. Lancet 395:683–684. 10.1016/S0140-6736(20)30361-5 [DOI] [PMC free article] [PubMed]
- 22.Molière S, Gaudineau A, Koch A, Leroi T, Roedlich MN, Veillon F (2017) Usefulness of diffusion-weighted imaging for diagnosis of adrenal ischemia during pregnancy: a preliminary report. Emerg Radiol 24:705–708. 10.1007/s10140-017-1530-6 [DOI] [PubMed]