Myeloid cells, by producing inflammatory mediators, play an important role in regulating ILC2‐driven allergic inflammation.
Keywords: allergic inflammation, group 2 innate lymphoid cells, myeloid cell
Summary
Group 2 innate lymphoid cells (ILC2s) are an important component of the innate immune system that execute important effector functions at barrier surfaces, such as lung and skin. Like T helper type 2 cells, ILC2s are able to release high amounts of type 2 cytokines that are essential in inducing allergic inflammation and eliminating helminth infections. The past few years have contributed to our better understanding of the interactions between ILC2s and other cells of the immune system via soluble factors or in a cell–cell contact manner. Myeloid cells, including mononuclear leukocytes and polymorphonuclear leukocytes, are excellent sensors of tissue damage and infection and can influence ILC2 responses in the process of allergic inflammation. In this review, we summarize recent insights on how myeloid cell subsets regulate ILC2 activation with focus on soluble factors in the context of allergic inflammation.
Abbreviations
- DCs
dendritic cells
- GATA3
GATA binding protein 3
- G‐CSF
granulocyte colony‐stimulating factor
- IFN
interferon
- IL
interleukin
- ILC2s
group 2 innate lymphoid cells
- mDCs
myeloid DCs
- MDSCs
myeloid‐derived suppressor cells
- M‐MDSCs
monocytic MDSCs
- MIF
migration inhibitory factor; MΦ: macrophage
- pDCs
plasmacytoid DCs
- PGD2
group V phospholipase A2
- Pla2g5
group V phospholipase A2
- PMN‐MDSCs
polymorphonuclear MDSCs
- Th2
T helper type 2
- TSLP
thymic stromal lymphopoietin
Introduction
Allergic diseases such as asthma and atopic dermatitis are common inflammatory disorders characterized by the dysregulated type 2 immune responses to environmental antigens. It is known that type 2 cytokines including interleukin‐4 (IL‐4), IL‐5 and IL‐13 from activated T helper type 2 (Th2) cells are crucial for the emergence of allergic outcomes, such as release of IgE, mucus production, smooth muscle cell contraction and recruitment of eosinophils, basophils and mast cells. 1 Recently, evidence is accumulating that group 2 innate lymphoid cells (ILC2s) are a major source of type 2 cytokines and have become one of the key effector immune cells in driving allergic inflammation. 2 , 3 , 4
The ILC2s, which resemble Th2 cells, rely on the expression of transcription factor GATA binding protein 3 (GATA3) for their development and function. However, ILC2s lack antigen‐specific receptor but respond quickly to epithelium‐derived cytokines including IL‐33, IL‐25 and thymic stromal lymphopoietin (TSLP) during allergic inflammation. 5 Upon activation, ILC2s produce large amounts of type 2 cytokines IL‐5, IL‐13 and IL‐9, and thereby contribute to allergic responses and clearance of helminth infections. 6 On the other hand, ILC2s secrete amphiregulin, which promotes the restoration of damaged tissues. 7 Of note, ILC2s are recognized as tissue‐resident cells and act as ‘early sentinel’ cells that elicit type 2 immune responses in lung, intestine, skin and adipose tissue. 8 Recently, numerous studies have demonstrated that ILC2 responses during allergic inflammation can be modulated by a variety of factors including cytokines, hormones, lipid mediators, neuropeptides, nutrients and cell surface molecules, as excellently reviewed elsewhere. 6 , 9 , 10 Meanwhile, emerging data have indicated that ILC2s communicate with cells of the innate and adaptive immune systems and contribute to inflammatory processes in the context of allergic diseases. 11 , 12 Although activated ILC2s are known to modulate the recruitment and function of myeloid cells, the regulation of ILC2 responses by myeloid cells is of great interest in enhancing our understanding of how mediators from myeloid cells influence type 2 immune responses during allergic inflammation.
In mammals, myeloid cell development arises in a stepwise fashion that begins in the yolk sac and ends in the bone marrow. 13 During myelopoiesis, myeloid progenitors branch up into monocytic and granulocytic lineages generating mononuclear leukocytes including monocytes, macrophages (MΦ) and dendritic cells (DCs), as well as polymorphonuclear leukocytes including neutrophils, mast cells, basophils and eosinophils. They circulate through the blood and lymphatic system and rapidly migrate to sites of tissue damage and infection through a variety of chemokine receptors. 13 Then, they use various pattern recognition receptors to recognize pathogen‐associated molecular patterns in tissues, and thereby display innate immunity such as phagocytosis and production of effector molecules (i.e. cytokines or lipid mediators). On the other hand, like ILC2s, some myeloid cells are tissue‐resident cells and respond quickly to microbial and other tissue‐derived signals. 14 , 15 Although ILC2‐derived cytokines are critical in modulating the recruitment and activation of myeloid cells, 11 acute and chronic tissue inflammation is often accompanied by activation of local myeloid cells followed by crosstalk with ILC2s. 8 , 12 , 16 This highlights the great importance of myeloid cells in regulating ILC2‐driven allergic inflammation.
Understanding the complex social networking of immune cells with ILC2s in local tissues during health and disease would definitely contribute to finding critical checkpoints that can be used for developing therapeutic strategies. To this end, we highlight how various myeloid cells regulate ILC2‐mediated type 2 immune responses, focusing on their roles in allergic diseases such as asthma and atopic dermatitis.
Mononuclear leukocytes and ILC2s
Monocytes/ MΦ
Monocytes develop from progenitors in the bone marrow and migrate into peripheral tissues such as lung via the bloodstream under homeostatic and inflammatory conditions. 17 Recruitment of monocytes is important for effective control and clearance of viral, bacterial, fungal and protozoal infections, but they are also involved in the pathogenesis of ILC2‐mediated lung inflammation. One study showed that Ly6c‐positive monocytes recruited to the lung can produce IL‐33, a key ILC2 activator, which may contribute to the pathogenesis of house‐dust‐mite‐induced airway inflammation. 18
MΦ, which are phagocytes that can originate from monocytes, are critical effectors and regulators of inflammation. Currently, three major classes of lung MΦ have been recognized based on their ontogeny, mode of maintenance and location within the tissue. 19 Two of these, ‘primitive’ interstitial MΦ and alveolar MΦ, originate from hematopoietic progenitors arising from the yolk sac and fetal liver, respectively. The third major population of ‘definitive’ interstitial MΦ comes from circulating monocytes and becomes the ‘primitive’ interstitial MΦ over time. Of note, MΦ also play an essential role in lung inflammation by regulating ILC2 responses. Alveolar MΦ can activate ILC2s by producing IL‐33 in a model of influenza A virus‐induced airway hyperreactivity, 20 whereas one study showed that resident alveolar MΦ suppressed allergic lung inflammation in house dust mite and ovalbumin murine models. 21 This discrepancy may be the result of the use of different airway inflammation murine models. Further, in a mouse model of hookworm‐mediated lung injury, Nieves et al. demonstrated that defective AMP‐activated protein kinase activity in alveolar MΦ and conventional DCs impairs ILC2 responses through increased IL‐12/23p40 production. 22 Another study, in an Alternaria‐induced pulmonary inflammation, found that macrophage‐associated group V phospholipase A2 (Pla2g5) enhances lung ILC2 activation through the regulation of IL‐33 induction and free fatty acid production. 16 Still, one recent study showed that MΦ migration inhibitory factor (MIF) is required for ILC2 responses and MΦ polarization into M2 phenotype which is essential for the clearance of intestinal helminth parasites. 23 As MΦ can produce migration inhibitory factor, it may regulate ILC2 activation in allergic lung inflammation. Moreover, IL‐5 and IL‐13 produced by activated ILC2s can also promote the MΦ activation. 24 Hence, concerted actions of monocyte/MΦ and ILC2s may contribute to lung inflammation upon an allergen challenge.
Dendritic cells
Dendritic cells, the most potent professional antigen‐presenting cells, act as key mediators at the interface of the innate and adaptive immune systems. Human DCs are a heterogeneous population of cells that can be divided into myeloid DCs (mDCs) and plasmacytoid DCs (pDCs). 25 The mDCs are thought to promote allergic inflammation by eliciting type 2 immunity to inhaled allergens; 26 however, pDCs, the major source of type I interferon (IFN), exert a negative regulation in airway inflammation by dampening the Th2 responses. 27 Similar to MΦ, mouse bone marrow‐derived DCs are found to produce IL‐33 via Toll‐like receptor/nuclear factor‐κB signaling pathways, and CD11c+ DCs in ocular mucosal surface and the draining cervical lymph nodes can produce IL‐33, 28 implying that DC‐derived IL‐33 may enhance ILC2 responses in allergic inflammation. One recent study showed that mDCs from blood in patients with allergic rhinitis promoted ILC2 proliferation and ILC2s secreting IL‐13 and IL‐9 through the IL‐33/ST2 pathway, whereas activation of pDCs suppressed ILC2 activation via IL‐6. 29 However, whether mDCs can affect ILC2 responses in vivo needs further investigation. Interestingly, studies have found that both type I and type II IFNs suppress ILC2 function, suggesting that IFN‐secreting cells, including DCs, serve as important negative regulators of ILC2 responses. 30 , 31 Indeed, several studies demonstrated that pDC‐derived IFN negatively regulates ILC2 cells in murine asthma models. 32 , 33 , 34 Furthermore, DCs are critical to activate Th2 responses during ongoing airway inflammation. 35 In a model of papain‐induced lung inflammation, one study demonstrated that ILC2‐derived IL‐13 activates CD11b+ CD103− lung DCs to produce the chemokine CCL17, promoting the recruitment of CCR4+ memory Th2 cells to the lung. 36 Taken together, DCs are key players in regulating ILC2s and Th2‐driven allergic airway inflammation, and modulating DC activity may have great clinical implications in asthma treatment.
Polymorphonuclear leukocytes and ILC2s
Neutrophils
Neutrophils, a type of polymorphonuclear leukocyte, originate from bone marrow myeloid precursors. Notably, neutrophils are the first leukocytes that migrate to an inflammatory site, where they contribute to eliminating pathogens by multiple means, such as phagocytosis, degranulation and neutrophil extracellular traps. 37 , 38 Although neutrophils are undoubtedly key players of acute infection, several lines of evidence show that they are also major effectors of allergic airway inflammation. Neutrophilic asthma is a typically non‐Th2/type 2 asthma that is prevalent among individuals with steroid‐resistant asthma. 39 Interestingly, Patel et al. recently demonstrated that the regulatory role of neutrophils on ILC2s may rationally account for the failure of neutrophil‐targeting therapies for people with asthma. 40 In a mouse model of house‐dust‐mite‐mediated allergic airway inflammation, they found that depletion of neutrophils resulted in a dramatic increase in systemic granulocyte colony‐stimulating factor (G‐CSF) concentrations, which are ordinarily negatively controlled in the periphery by transmigrated lung neutrophils. G‐CSF then functioned to augment allergen sensitization either by activating ILC2s or acting on bone marrow progenitors to drive monocytosis and finally caused the exacerbated Th2 inflammation, epithelial remodeling and airway resistance. 40 Intriguingly, a subpopulation of Lin– GATA3+ ST2+ ILC2s, in the presence of IL‐33 and leukotrienes, was found to produce IL‐17 in vitro and in a mouse of model of IL‐33 or papain‐induced lung inflammation, 41 which suggests that some ILC2s may promote the migration of neutrophils to the lung by producing IL‐17. Moreover, a recent study showed that short‐chain fatty acids derived from fermentation of dietary fibers by the gut microbiota modulated pulmonary ILC2s to secrete IL‐17A, which is linked to enhanced neutrophil recruitment to the lung. 42 It is noteworthy that IL‐17 is typically not a signature cytokine for ILC2s, the source and role of IL‐17‐producing ILC2s during allergic inflammation requires further exploration. Additionally, type 2 immune‐associated neutrophil infiltration also requires the mouse RNase A homologue, eosinophil‐associated ribonuclease 11, which is secreted by alternatively activated macrophages downstream of IL‐25‐stimulated ILC2s. 43 ILC2‐derived IL‐5 can also directly initiate CXCR2+ neutrophils to produce IL‐5 during traumatic injury. 44 Altogether, these studies highlight the complex regulatory role of neutrophils in asthma, at least in part by affecting ILC2 function or being regulated upon ILC2 activation.
Polymorphonuclear myeloid‐derived suppressor cells
Myeloid‐derived suppressor cells (MDSCs) are a heterogeneous population of immature myeloid cells with a potent ability to suppress T‐cell responses under various pathological conditions, including cancer, inflammation, trauma and infection. 45 Based on morphology and specific cell‐surface molecules, MDSCs can be classified into two subpopulations: polymorphonuclear MDSCs (PMN‐MDSCs) and monocytic MDSCs (M‐MDSCs). 46 Two studies have shown that ILC2‐derived IL‐13 can activate M‐MDSCs in cancers, 47 , 48 suggesting the importance of the ILC2–MDSC axis in pathological conditions. Meanwhile, given their remarkable immunosuppressive ability towards Th2 cells, the negative regulation of MDSCs in airway inflammation has been well demonstrated. 49 , 50 Interestingly, Cao et al. recently found that PMN‐MDSCs, but not M‐MDSCs, effectively inhibited ILC2 function both in vitro and in vivo, which attenuated allergic airway inflammation. 51 They further showed that cyclo‐oxygenase‐1, which is required for PMN‐MDSCs to inhibit Th2 responses, 49 may mediate the suppressive effects of PMN‐MDSCs on ILC2 activation. Therefore, enhancing PMN‐MDSCs may be beneficial for treating ILC2‐driven allergic asthma.
Mast cell
Mast cells, rich in cytoplasmic granules, originate from bone‐marrow‐derived hematopoietic progenitors that can traffic into all vascularized tissues where they complete their development. They, especially IgE‐primed mast cells, regulate both the innate and the adaptive immune responses in inflammatory disorders including allergic inflammation. 52 In particular, mast cells contribute to the outcome of lung inflammation through the secretion of mediators that act on other cell types, including ILC2s. For instance, mast cells can express IL‐33 upon IgE stimulation. 53 Using models of skin anaphylaxis, one study showed that mast‐cell‐derived IL‐33 can initiate neutrophilic inflammation by communicating with basophils. 54 Moreover, IL‐33 can also potentiate IgE‐mediated human mast cell responses by increasing both mast cell degranulation frequency and degranulation magnitude. 55 Interestingly, IL‐33 produced by mast cells is crucial for the induction of IL‐13‐producing ILC2s and the clearance of helminth infections. 56 However, Morita et al. found that IL‐33‐stimulated mast‐cell‐derived IL‐2 enhances expansion of numbers of regulatory T cells, thereby suppressing papain‐induced allergic inflammation, 57 suggesting that mast cells may impair type 2 immune responses in the acute phase of allergic inflammation. Another study found that IL‐9 increases IL‐2 production by mast cells, which leads to expansion of CD25+ ILC2s and subsequent activation of Th9 cells, which promotes lung inflammation in cystic fibrosis. 58 Notably, IgE‐primed mast‐cell‐derived prostaglandin D2 (PGD2) is an important and potent activator of human ILC2s, 59 whereas dermal ILC2‐derived IL‐13 may play a role in suppressing mast cell activation. 60 Taken together, the mast cell–ILC2 axis may display distinct actions in early and chronic allergic inflammation, and a better understanding of the role of mast cells in modulating human ILC2‐driven allergic airway inflammation requires further investigation.
Basophil
Basophils are the least common type of granulocyte that mature in the bone marrow from myeloid stem cells and then enter the circulation. Similar to mast cells, basophils express high‐affinity IgE receptors (FcεRI), and secrete histamine and Th2 cytokines after activation. 61 , 62 However, basophils and mast cells are distinct cell lineages and basophils display important and non‐redundant roles in protective immunity against parasitic infections, and in allergic or autoimmune pathologies. 61 , 62 , 63 In particular, basophils are closely associated with allergic inflammation by participating in Th2 skewing by producing IL‐4, IL‐6 and IL‐13. 64 Basophils derived from individuals with asthma, which express ST2 (IL‐33 receptor α chain), can produce IL‐4 and IL‐13 upon IL‐33 stimulation. 65 Interestingly, Motomura et al. reported that basophil‐derived IL‐4 can enhance the expression of the chemokine CCL11, as well as IL‐5, IL‐9 and IL‐13 in ILC2s, resulting in eosinophilic lung inflammation induced by protease allergens in mice. 66 In humans, the number of activated basophils is enhanced in the sputum of people with asthma and correlated with airway and blood eosinophils. 67 Two studies also showed that IL‐33 or TSLP can induce basophils to produce IL‐4, which enhanced ILC2 proliferation and their production of IL‐5 and IL‐13, and exacerbated the atopic dermatitis‐like inflammation. 68 , 69 These results highlight the importance of basophils in modulating ILC2 function during allergic inflammation. Apart from IL‐4, basophils secrete a variety of effector molecules such as pro‐inflammatory eicosanoids that contribute to allergic diseases. 61 As studies have demonstrated that both murine and human ILC2s directly respond to leukotrienes, 70 , 71 , 72 it is possible that other mediators produced by basophils may act together with IL‐4 to regulate ILC2 function. Further, TSLP‐elicited and IL‐3‐elicited basophils display distinct responsiveness and functional potential in response to IL‐3, IL‐18, or IL‐33, 73 whereas how the distinct regulation of basophils impacts on ILC2 biology needs to be addressed.
Eosinophils
Eosinophils develop in the bone marrow and migrate to inflammatory foci driven by pro‐inflammatory chemokines, such as eotaxin. It is well established that eosinophilia is one of the hallmarks in allergic diseases including asthma. 74 Lung ILC2‐derived IL‐5 plays a key role in the activation and recruitment of eosinophils to the airways. 75 In response to inflammatory stimuli, eosinophils degranulate and release active mediators, such as major basic protein or eosinophil peroxidase, which are critical for eliminating parasites. 76 Additionally, they also produce type 2 cytokines (such as IL‐4 and IL‐13), 77 , 78 suggesting a role in promoting allergic inflammation.
Although the emergence of numerous eosinophils has become an important parameter of ILC2 activation, recent data showed that eosinophils can also influence ILC2 responses. Depletion of eosinophils in IL‐33 or ovalbumin or house dust mite allergen‐induced lung inflammation caused a significant reduction of total and activated pulmonary ILC2s, 79 suggesting a critical role for eosinophils in the maintenance of ILC2s. A recent study found that eosinophil extracellular traps can induce the lung epithelium to produce IL‐33 and TSLP, and thereby activated ILC2 responses and increased airway hyperresponsiveness in mice, 80 suggesting a critical role of eosinophil extracellular traps in reinforcing the type 2 immune responses in severe asthma. In humans, the number of eosinophils with extracellular traps is positively correlated with ILC2s in severe asthma. 80 Additionally, as eosinophils are a major source of IL‐4, a activator for human ILC2, it is possible that IL‐4‐producing eosinophils can act on human ILC2s in eosinophil‐associated inflammation. 81 Together, these studies have shown that eosinophils and ILC2s engage in reciprocal regulation, and their crosstalk over inflammation remains to be analyzed.
Concluding remarks
The ILC2s are a prominent source of type 2 cytokines in both lymphoid and non‐lymphoid organs, and play an essential role in the onset and/or maintenance of allergic inflammation, as well as in eliminating parasites. As ILC2s are tissue‐resident cells, it is becoming increasingly evident that regulation of ILC2 responses during allergic inflammation involves a variety of soluble factors, immune cells and non‐immune cells. Myeloid cells, with their properties of rapid activation and recruitment, are critical in tuning ILC2 proliferation and function by releasing soluble mediators or possibly by a cell–cell contact manner in the process of allergic inflammation (Fig. 1). Meanwhile, emerging data also show that ILC2‐derived cytokines can act on the migration and activation of myeloid cells, and thereby reinforce the type 2 immune responses in allergic diseases. 11 , 12 Defining the complex interactions between myeloid cells and ILC2s during acute and chronic inflammation will greatly advance our understanding of the contributions of the myeloid cell–ILC2 networks in the pathogenesis of allergic diseases. Furthermore, ILC2s can be divided into conventional ILC2s and inflammatory ILC2s induced by IL‐33 and IL‐25, respectively. 82 , 83 Recent studies have also found IL‐10‐producing ILC2s, 84 and IL‐17‐producing ILC2s 41 , 85 in allergic airway inflammation. However, whether myeloid cells can affect ILC2 plasticity in health and disease remains to be investigated. Finally, although mouse models are very useful in addressing the relationships between myeloid cells and ILC2s, future investigations on the human myeloid cell–ILC2 regulatory axis may shed new light on critical checkpoints that can be manipulated for treating ILC2‐driven allergic inflammation, as well as for improving immunity against helminths.
Figure 1.
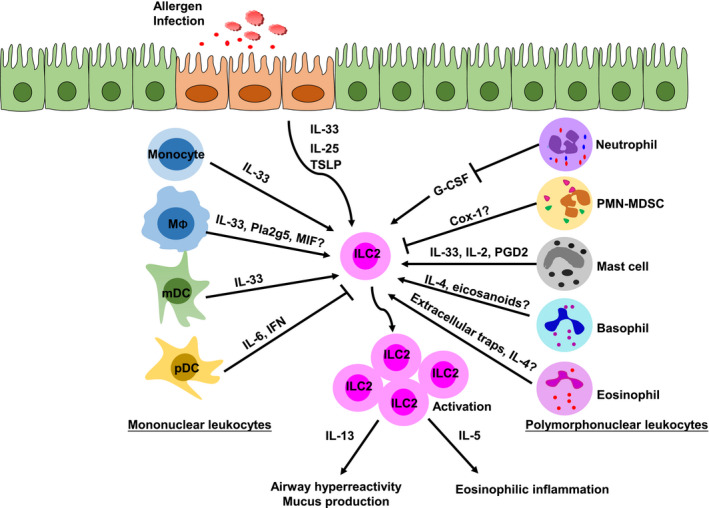
The effect of myeloid cells on the group 2 innate lymphoid cell (ILC2) responses. The role of mononuclear leukocytes (left) and polymorphonuclear leukocytes (right) in the regulation of ILC2s are shown, along with key molecules involved in each. MΦ, macrophage. ‘?’ denotes that the regulation remains to be investigated.
Funding information
AL is supported by the National Natural Science Foundation of China (No. 81800031), the Natural Science Foundation of Guangdong Province (No. 2018A030313648) and the Research Foundation of Education Bureau of Human Province, China (No. 18C0458). RL is supported by the Natural Science Foundation of Hunan Province, China (No. 2020JJ4521) and the Research Foundation of Education Bureau of Hunan Province, China (No. 19B496). YH is supported by the National Natural Science Foundation of Guangdong (No. 2019A1515011435) and the Science and Technology Program of Guangzhou (No. 201904010073). QY is supported by the Natural Science Foundation of Guangdong (No. 2016A030310182). This work was also supported by the Foundation of Hunan Provincial Key Laboratory for Special Pathogens Prevention and Control (No. 2014‐5) and the Hunan Province Cooperative Innovation Center for Molecular Target New Drug Study (2015‐351).
Disclosures
The authors have no conflict of interest to report.
Contributor Information
Aihua Lei, Email: leiaihua18@usc.edu.cn.
Ranhui Li, Email: ranhui81@163.com.
References
- 1. Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity 2019; 50:975–91. [DOI] [PubMed] [Google Scholar]
- 2. McKenzie AN. Type‐2 innate lymphoid cells in asthma and allergy. Ann Am Thorac Soc 2014; 11(Suppl 5):S263–S270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stier MT, Peebles RS Jr. Innate lymphoid cells and allergic disease. Ann Allergy Asthma Immunol 2017; 119:480–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lei AH, Xiao Q, Liu GY, Shi K, Yang Q, Li X et al ICAM‐1 controls development and function of ILC2. J Exp Med 2018; 215:2157–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 2016; 17:765–74. [DOI] [PubMed] [Google Scholar]
- 6. Kabata H, Moro K, Koyasu S. The group 2 innate lymphoid cell (ILC 2) regulatory network and its underlying mechanisms. Immunol Rev 2018; 286:37–52. [DOI] [PubMed] [Google Scholar]
- 7. Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA et al Innate lymphoid cells promote lung‐tissue homeostasis after infection with influenza virus. Nat Immunol 2011; 12:1045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Messing M, Jan‐Abu SC, McNagny K. Group 2 innate lymphoid cells: central players in a recurring theme of repair and regeneration. Int J Mol Sci 2020; 21:1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lei A, Zhou J. Cell‐surface molecule‐mediated cell–cell interactions in the regulation of ILC2‐driven allergic inflammation. Cell Mol Life Sci 2019; 76:4503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hurrell BP, Jahani PS, Akbari O. Social networking of group two innate lymphoid cells in allergy and asthma. Front Immunol 2018; 9:2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Symowski C, Voehringer D. Interactions between innate lymphoid cells and cells of the innate and adaptive immune system. Front Immunol 2017; 8:1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mortha A, Burrows K. Cytokine networks between innate lymphoid cells and myeloid cells. Front Immunol 2018; 9:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Kleer I, Willems F, Lambrecht B, Goriely S. Ontogeny of myeloid cells. Front Immunol 2014; 5:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guilliams M, Scott CL. Does niche competition determine the origin of tissue‐resident macrophages? Nat Rev Immunol 2017; 17:451–60. [DOI] [PubMed] [Google Scholar]
- 15. Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol 2016; 17:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamaguchi M, Samuchiwal SK, Quehenberger O, Boyce JA, Balestrieri B. Macrophages regulate lung ILC2 activation via Pla2g5‐dependent mechanisms. Mucosal Immunol 2018; 11:615–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011; 11:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tashiro H, Takahashi K, Hayashi S, Kato G, Kurata K, Kimura S et al Interleukin‐33 from monocytes recruited to the lung contributes to house dust mite‐induced airway inflammation in a mouse model. PLoS One 2016; 11:e0157571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan SY, Krasnow MA. Developmental origin of lung macrophage diversity. Development 2016; 143:1318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE et al Innate lymphoid cells mediate influenza‐induced airway hyper‐reactivity independently of adaptive immunity. Nat Immunol 2011; 12:631–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaslona Z, Przybranowski S, Wilke C, van Rooijen N, Teitz‐Tennenbaum S, Osterholzer JJ et al Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma. J Immunol 2014; 193:4245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nieves W, Hung LY, Oniskey TK, Boon L, Foretz M, Viollet B et al Myeloid‐restricted AMPKα1 promotes host immunity and protects against IL‐12/23p40‐dependent lung injury during hookworm infection. J Immunol 2016; 196:4632–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Filbey KJ, Varyani F, Harcus Y, Hewitson JP, Smyth DJ, McSorley HJ et al Macrophage migration inhibitory factor (MIF) Is essential for type 2 effector cell immunity to an intestinal helminth parasite. Front Immunol 2019; 10:2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A et al Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 2013; 210:535–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kadowaki N. The divergence and interplay between pDC and mDC in humans. Front Biosci (Landmark Ed) 2009; 14:808–17. [DOI] [PubMed] [Google Scholar]
- 26. Mo JH, Chung YJ, Hayashi T, Lee J, Raz E. The role of plasmacytoid and myeloid dendritic cells in induction of asthma in a mouse model and the effect of a TLR9 agonist on dendritic cells. Allergy Asthma Immunol Res 2011; 3:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA et al Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 2004; 200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Su Z, Lin J, Lu F, Zhang X, Zhang L, Gandhi NB et al Potential autocrine regulation of interleukin‐33/ST2 signaling of dendritic cells in allergic inflammation. Mucosal Immunol 2013; 6:921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng YQ, Qin ZL, Fang SB, Xu ZB, Zhang HY, Chen D et al Effects of myeloid and plasmacytoid dendritic cells on ILC2s in patients with allergic rhinitis. J Allergy Clin Immunol 2020; 145:855–67. e8. [DOI] [PubMed] [Google Scholar]
- 30. Duerr CU, McCarthy CD, Mindt BC, Rubio M, Meli AP, Pothlichet J et al Type I interferon restricts type 2 immunopathology through the regulation of group 2 innate lymphoid cells. Nat Immunol 2016; 17:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moro K, Kabata H, Tanabe M, Koga S, Takeno N, Mochizuki M et al Interferon and IL‐27 antagonize the function of group 2 innate lymphoid cells and type 2 innate immune responses. Nat Immunol 2016; 17:76–86. [DOI] [PubMed] [Google Scholar]
- 32. Maazi H, Banie H, Aleman Muench GR, Patel N, Wang B, Sankaranarayanan I et al Activated plasmacytoid dendritic cells regulate type 2 innate lymphoid cell‐mediated airway hyperreactivity. J Allergy Clin Immunol 2018; 141:893–905. e6. [DOI] [PubMed] [Google Scholar]
- 33. Thio CL, Lai AC, Chi PY, Webster G, Chang YJ. Toll‐like receptor 9‐dependent interferon production prevents group 2 innate lymphoid cell‐driven airway hyperreactivity. J Allergy Clin Immunol 2019; 144:682–97. e9. [DOI] [PubMed] [Google Scholar]
- 34. Wu M, Gao L, He M, Liu H, Jiang H, Shi K et al Plasmacytoid dendritic cell deficiency in neonates enhances allergic airway inflammation via reduced production of IFN‐α . Cell Mol Immunol 2020; 17:519–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C et al In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 2005; 201:981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Halim TY, Hwang YY, Scanlon ST, Zaghouani H, Garbi N, Fallon PG et al Group 2 innate lymphoid cells license dendritic cells to potentiate memory TH2 cell responses. Nat Immunol 2016; 17:57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13:159–75. [DOI] [PubMed] [Google Scholar]
- 38. Zhou J, Nefedova Y, Lei A, Gabrilovich D. Neutrophils and PMN‐MDSC: Their biological role and interaction with stromal cells. Semin Immunol 2018; 35:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nabe T. Steroid‐resistant asthma and neutrophils. Biol Pharm Bull 2020; 43:31–35. [DOI] [PubMed] [Google Scholar]
- 40. Patel DF, Peiro T, Bruno N, Vuononvirta J, Akthar S, Puttur F et al Neutrophils restrain allergic airway inflammation by limiting ILC2 function and monocyte‐dendritic cell antigen presentation. Sci Immunol 2019; 4:eaax7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cai T, Qiu J, Ji Y, Li W, Ding Z, Suo C et al IL‐17‐producing ST2+ group 2 innate lymphoid cells play a pathogenic role in lung inflammation. J Allergy Clin Immunol 2019; 143:229–44. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lewis G, Wang B, Shafiei Jahani P, Hurrell BP, Banie H, Aleman Muench GR et al Dietary fiber‐induced microbial short chain fatty acids suppress ILC2‐dependent airway inflammation. Front Immunol 2019; 10:2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Panova V, Gogoi M, Rodriguez‐Rodriguez N, Sivasubramaniam M, Jolin HE, Heycock MWD et al Group‐2 innate lymphoid cell‐dependent regulation of tissue neutrophil migration by alternatively activated macrophage‐secreted Ear11. Mucosal Immunol 2020. 10.1038/s41385-020-0298-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu J, Guardado J, Hoffman R, Xu H, Namas R, Vodovotz Y et al IL33‐mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: a reverse translation study from a human cohort to a mouse trauma model. PLoS Medicine 2017; 14:e1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gabrilovich DI, Nagaraj S. Myeloid‐derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid‐derived suppressor cells in tumor‐bearing mice. J Immunol 2008; 181:5791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trabanelli S, Chevalier MF, Martinez‐Usatorre A, Gomez‐Cadena A, Salome B, Lecciso M et al Tumour‐derived PGD2 and NKp30‐B7H6 engagement drives an immunosuppressive ILC2‐MDSC axis. Nat Commun 2017; 8:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chevalier MF, Trabanelli S, Racle J, Salome B, Cesson V, Gharbi D et al ILC2‐modulated T cell‐to‐MDSC balance is associated with bladder cancer recurrence. J Clin Invest 2017; 127:2916–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shi M, Shi G, Tang J, Kong D, Bao Y, Xiao B et al Myeloid‐derived suppressor cell function is diminished in aspirin‐triggered allergic airway hyperresponsiveness in mice. J Allergy Clin Immunol 2014; 134:1163–74. e16. [DOI] [PubMed] [Google Scholar]
- 50. Fan HZ, Yu HP, Yu R, Zhang Y, Deng HJ, Chen X. Passive transfer of lipopolysaccharide‐derived myeloid‐derived suppressor cells inhibits asthma‐related airway inflammation. Eur Rev Med Pharmacol Sci 2015; 19:4171–81. [PubMed] [Google Scholar]
- 51. Cao Y, He Y, Wang X, Liu Y, Shi K, Zheng Z et al Polymorphonuclear myeloid‐derived suppressor cells attenuate allergic airway inflammation by negatively regulating group 2 innate lymphoid cells. Immunology 2019; 156:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y et al Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol 2012; 188:1809–18. [DOI] [PubMed] [Google Scholar]
- 53. Hsu CL, Neilsen CV, Bryce PJ. IL‐33 is produced by mast cells and regulates IgE‐dependent inflammation. PLoS One 2010; 5:e11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hsu CL, Chhiba KD, Krier‐Burris R, Hosakoppal S, Berdnikovs S, Miller ML et al Allergic inflammation is initiated by IL‐33‐dependent crosstalk between mast cells and basophils. PLoS One 2020; 15:e0226701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Joulia R, L'Faqihi FE, Valitutti S, Espinosa E. IL‐33 fine tunes mast cell degranulation and chemokine production at the single‐cell level. J Allergy Clin Immunol 2017; 140:497–509. e10. [DOI] [PubMed] [Google Scholar]
- 56. Shimokawa C, Kanaya T, Hachisuka M, Ishiwata K, Hisaeda H, Kurashima Y et al Mast cells are crucial for induction of group 2 innate lymphoid cells and clearance of helminth infections. Immunity 2017; 46:863–74. e4. [DOI] [PubMed] [Google Scholar]
- 57. Morita H, Arae K, Unno H, Miyauchi K, Toyama S, Nambu A et al An interleukin‐33–mast cell–interleukin‐2 axis suppresses papain‐induced allergic inflammation by promoting regulatory T cell numbers. Immunity 2015; 43:175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Moretti S, Renga G, Oikonomou V, Galosi C, Pariano M, Iannitti RG et al A mast cell–ILC2–Th9 pathway promotes lung inflammation in cystic fibrosis. Nat Commun 2017; 8:14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xue L, Salimi M, Panse I, Mjosberg JM, McKenzie AN, Spits H et al Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor‐homologous molecule expressed on TH2 cells. J Allergy Clin Immunol 2014; 133:1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS et al Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol 2013; 14:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Karasuyama H, Miyake K, Yoshikawa S, Yamanishi Y. Multifaceted roles of basophils in health and disease. J Allergy Clin Immunol 2018; 142:370–80. [DOI] [PubMed] [Google Scholar]
- 62. Voehringer D. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol 2013; 13:362–75. [DOI] [PubMed] [Google Scholar]
- 63. Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 2010; 33:364–74. [DOI] [PubMed] [Google Scholar]
- 64. Nakashima C, Otsuka A, Kabashima K. Recent advancement in the mechanism of basophil activation. J Dermatol Sci 2018; 91:3–8. [DOI] [PubMed] [Google Scholar]
- 65. Salter BM, Oliveria JP, Nusca G, Smith SG, Tworek D, Mitchell PD et al IL‐25 and IL‐33 induce Type 2 inflammation in basophils from subjects with allergic asthma. Respir Res 2016; 17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA et al Basophil‐derived interleukin‐4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity 2014; 40:758–71. [DOI] [PubMed] [Google Scholar]
- 67. Suzuki Y, Wakahara K, Nishio T, Ito S, Hasegawa Y. Airway basophils are increased and activated in eosinophilic asthma. Allergy 2017; 72:1532–9. [DOI] [PubMed] [Google Scholar]
- 68. Imai Y, Yasuda K, Nagai M, Kusakabe M, Kubo M, Nakanishi K et al IL‐33‐induced atopic dermatitis‐like inflammation in mice is mediated by group 2 innate lymphoid cells in concert with basophils. J Invest Dermatol 2019; 139:2185–94. e3. [DOI] [PubMed] [Google Scholar]
- 69. Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA et al Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol 2014; 193:3717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol 2013; 132:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S et al Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med 2013; 5:174ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lund SJ, Portillo A, Cavagnero K, Baum RE, Naji LH, Badrani JH et al Leukotriene C4 potentiates IL‐33‐induced group 2 innate lymphoid cell activation and lung inflammation. J Immunol 2017; 199:1096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA et al TSLP promotes interleukin‐3‐independent basophil haematopoiesis and type 2 inflammation. Nature 2011; 477:229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Trivedi SG, Lloyd CM. Eosinophils in the pathogenesis of allergic airways disease. Cell Mol Life Sci 2007; 64:1269–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB et al Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013; 502:245–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Specht S, Saeftel M, Arndt M, Endl E, Dubben B, Lee NA et al Lack of eosinophil peroxidase or major basic protein impairs defense against murine filarial infection. Infect Immun 2006; 74:5236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schmid‐Grendelmeier P, Altznauer F, Fischer B, Bizer C, Straumann A, Menz G et al Eosinophils express functional IL‐13 in eosinophilic inflammatory diseases. J Immunol 2002; 169:1021–7. [DOI] [PubMed] [Google Scholar]
- 78. Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N et al Eosinophils secrete IL‐4 to facilitate liver regeneration. Proc Natl Acad Sci USA 2013; 110:9914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Jacobsen EA, LeSuer WE, Nazaroff CD, Ochkur SI, Doyle AD, Wright BL et al Eosinophils induce recruitment and activation of ILC2s. J Aller Clin Immunol 2019; 143:AB289. [Google Scholar]
- 80. Choi Y, Kim YM, Lee HR, Mun J, Sim S, Lee DH et al Eosinophil extracellular traps activate type 2 innate lymphoid cells through stimulating airway epithelium in severe asthma. Allergy 2020; 75:95–103. [DOI] [PubMed] [Google Scholar]
- 81. Bal SM, Bernink JH, Nagasawa M, Groot J, Shikhagaie MM, Golebski K et al IL‐1β, IL‐4 and IL‐12 control the fate of group 2 innate lymphoid cells in human airway inflammation in the lungs. Nat Immunol 2016; 17:636–45. [DOI] [PubMed] [Google Scholar]
- 82. Huang Y, Guo L, Qiu J, Chen X, Hu‐Li J, Siebenlist U et al IL‐25‐responsive, lineage‐negative KLRG1hi cells are multipotential 'inflammatory' type 2 innate lymphoid cells. Nat Immunol 2015; 16:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang K, Xu X, Pasha MA, Siebel CW, Costello A, Haczku A et al Cutting edge: notch signaling promotes the plasticity of group‐2 innate lymphoid cells. J Immunol 2017; 198:1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Seehus CR, Kadavallore A, Torre B, Yeckes AR, Wang Y, Tang J et al Alternative activation generates IL‐10 producing type 2 innate lymphoid cells. Nat Commun 2017; 8:1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bernink JH, Ohne Y, Teunissen MBM, Wang J, Wu J, Krabbendam L et al c‐Kit‐positive ILC2s exhibit an ILC3‐like signature that may contribute to IL‐17‐mediated pathologies. Nat Immunol 2019; 20:992–1003. [DOI] [PubMed] [Google Scholar]


