Abstract
The coexistence of coronavirus disease 2019 (COVID-19) and pulmonary embolism (PE), two life-threatening illnesses, in the same patient presents a unique challenge. Guidelines have delineated how best to diagnose and manage patients with PE. However, the unique aspects of COVID-19 confound both the diagnosis and treatment of PE, and therefore require modification of established algorithms. Important considerations include adjustment of diagnostic modalities, incorporation of the prothrombotic contribution of COVID-19, management of two critical cardiorespiratory illnesses in the same patient, and protecting patients and health-care workers while providing optimal care. The benefits of a team-based approach for decision-making and coordination of care, such as that offered by pulmonary embolism response teams (PERTs), have become more evident in this crisis. The importance of careful follow-up care also is underscored for patients with these two diseases with long-term effects. This position paper from the PERT Consortium specifically addresses issues related to the diagnosis and management of PE in patients with COVID-19.
Key Words: catheter-directed thrombolysis, COVID-19, follow-up, PERT, prevention, pulmonary embolism, pulmonary embolism response team, systemic thrombolysis
Abbreviations: COVID-19, coronavirus disease 2019; CTA, CT angiography; ECMO, extracorporeal membrane oxygenation; PE, pulmonary embolism; PERT, pulmonary embolism response team; PPE, personal protective equipment; RV, right ventricular
The National Pulmonary Embolism Response Team (PERT) Consortium, the largest organization in the world specifically dedicated to improving outcomes in pulmonary embolism (PE) and advancing the science around the disease, recently published consensus recommendations for the diagnosis, treatment, and follow-up for patients with acute PE.1 Although many of these recommendations apply to patients with PE and coronavirus disease 2019 (COVID-19), some unique factors associated with the pandemic necessitate addressing these patients separately.
The current COVID-19 pandemic has illustrated the importance of aggressive evaluation and management, when considering exposure to a highly communicable viral disease that has multisystem effects and a high mortality rate. As such, COVID-19 presents an unprecedented challenge to the health-care system, and specifically for individuals directly responsible for delivering care, and for others in the medical environment who could be exposed. Those who become infected are potentially vectors of viral transmittance in homes, community, and throughout the world. Every exposure can further exacerbate the pandemic. Therefore, the goal must be to deliver optimal care without compromise to the patients, while mitigating direct or indirect exposure and spread of the virus. There is emerging evidence that patients infected with COVID-19 are prone to thrombosis and PE.2, 3, 4, 5, 6 Diagnosis and management of PE, challenging under usual circumstances, is even more complex when occurring in association with COVID-19. Using a multidisciplinary approach, as offered by PERT, can greatly assist during this unique time. To meet this need, a writing group was established from the PERT Board of Directors to create a position paper. The writing group brainstormed and developed key clinical topics related to COVID-19 and PE. These topics were then divided into questions focused in this paper based on each member content expertise. Each group pragmatically reviewed the relevant published literature and incorporated this information into responses. Because there is a lack of high-quality evidence surrounding issues related to COVID-19 and PE, the committee carefully addressed and refined each recommendation until agreement was reached. Differences in opinion were dealt with through electronic and telephone communications. The final document reflects the consensus opinion of the entire writing group. Authors with financial conflicts were allowed to contribute given their expertise in the subject matter. The writing committee mandated full and transparent disclosure of any conflicts to inform the readership.
This current position statement addresses issues solely related to how a PERT can be instrumental in the diagnosis and care of patients with suspected or confirmed PE and COVID-19 (Table 1 ), and offers a safe and efficient way to diagnose and treat PE without compromising patient care. Please refer to the Centers for Disease Control and Prevention (www.cdc.gov) website for the most up-to-date information specific to COVID-19.
Table 1.
Questions Addressed in This Statement: Diagnosis, Treatment, and Follow-up of PE During the COVID-19 Pandemic
| PERT |
|
| Diagnosis and risk stratification |
|
| Treatment considerations |
|
| Transfer of care |
|
| Follow-up |
|
COVID-19 = coronavirus disease 2019; PE = pulmonary embolism; PERT = pulmonary embolism response team.
PERT
PERTs bring together a multidisciplinary group of specialists to treat patients with severe PE.7 The goal is to coordinate and expedite the diagnosis and treatment of PE with a team of physicians from different specialties. There is no defined or optimal structure of a PERT, and the makeup varies by institution.7 A team may include all or a combination of cardiac surgery, critical care, emergency medicine, hematology, interventional and noninterventional cardiology, interventional radiology pulmonary medicine, vascular medicine, vascular surgery, and pharmacy. Each member brings their expert opinion to the team.8 One of the main advantages of a PERT is that this multidisciplinary approach occurs in real time and allows for the rapid evaluation of risks, formulation of a treatment plan that suits each patient, and mobilization of appropriate resources to provide the highest quality of care to patients with PE.9 This approach, now more than ever, seems essential in the COVID-19 era.
Although the exact incidence of VTE associated with COVID-19 is currently unknown, reports range from as low as 1% in the general wards to as high as 31% in ICUs.2 , 4, 5, 6 , 10 The coagulopathic state associated with COVID-19 and the resultant increased thrombin generation can increase the risk of VTE, including PE. Moreover, hospitalized patients have a variety of usual factors that increase risk for VTE, including sepsis, immobilization, respiratory failure, mechanical ventilation, pharmacologic sedation and paralysis, vasopressor use, and central venous catheter use. Therefore, prevention and early diagnosis and treatment of VTE is essential during this crisis and PERTs can be instrumental in these areas. Importantly, PERTs can take on many forms, and the organization will depend on the local resources and needs of each hospital.11 , 12 During this crisis, even if organizations do not have a formal PERT, we encourage providers to formally or informally connect PE specialists together to help care for this vulnerable population. When possible, consultations should be performed virtually via telephone or telemedicine (eg, Zoom) to avoid unnecessary exposure of patients and providers.
Position Statement
-
•
During the COVID-19 pandemic, using a multidisciplinary approach to diagnose and treat patients with PE is encouraged.
Diagnosis and Risk Stratification
Understanding of the relationship between PE and COVID-19 is evolving. It is clear that a hypercoagulable state can be associated with COVID-19 infection. Furthermore, the symptoms of PE may mimick or overlap with those of COVID-19 infection, making it challenging to identify causality. Accordingly, physicians evaluating patients with COVID-19 in either an out- or inpatient venue should have a low threshold for considering concurrent PE. Historical elements, including onset, severity, and rate of progression of COVID-19 symptoms, along with elicudation of additional classic risk factors for PE, may guide the physician in diagnosis.
The presence of PE complicating COVID-19 infection (or in a person under investigation) should be considered when a patient exhibits hemodynamic instability or poor gas exchange that is not fully explained or is out of proportion to the stage, duration, and rate of progression of COVID-19 infection. For example, PE testing should be considered in a patient presenting with minimal pulmonary infiltrates and mild, short-term symptoms, but with syncope, shock, acute respiratory failure, or signs of acute right ventricular (RV) overload. For patients already hospitalized with established or presumed COVID-19 infection, vigilance and attentiveness for the development of signs of PE, superimposed on existing cardiorespiratory compromise, is important. In particular, PE should be considered if a patient develops acute onset of shortness of breath, worsening of oxygenation, hypotension, or tachycardia, especially if imaging or clinical findings are not consistent with worsening COVID-19 pneumonia. The occurrence of these changes should trigger the need for diagnostic testing. Notably, data from France suggest that the PE seen in patients with COVID-19 occurred at a median of 6 days.2
The use of biomarkers such as D-dimer to help identify patients with COVID-19 at risk of developing VTE has been explored in several case series and cohort studies.6 , 13, 14, 15, 16 In a single-center cohort study of 198 patients, an elevated D-dimer was associated with a 50% increased risk of developing VTE.17 Similarly, in a study of 400 hospitalized patients with COVID-19, an elevated D-dimer at admission of 1,001 to 2,500 ng/mL had an OR for thrombotic complications of 3.04 (95% CI, 1.26-7.31) and a D-dimer of > 2,500 ng/mL had an OR of 6.79 (95% CI, 2.39-19.30; P < .001).13 However, this study also found that elevations in D-dimer > 2,500 ng/mL at initial presentation were also predictive of bleeding complications during hospitalization. In the same way, data from China indicated that patients at the highest risk of developing a PE were the same patients at the highest risk of bleeding.18 At this point and in line with the recently published CHEST guidelines,19 there is insufficient evidence to recommend using an elevated D-dimer or any other laboratory data to guide clinical practice for VTE diagnosis. Therefore, we recommend that biomarkers should not be used in the diagnostic evaluation for suspected DVT or PE.
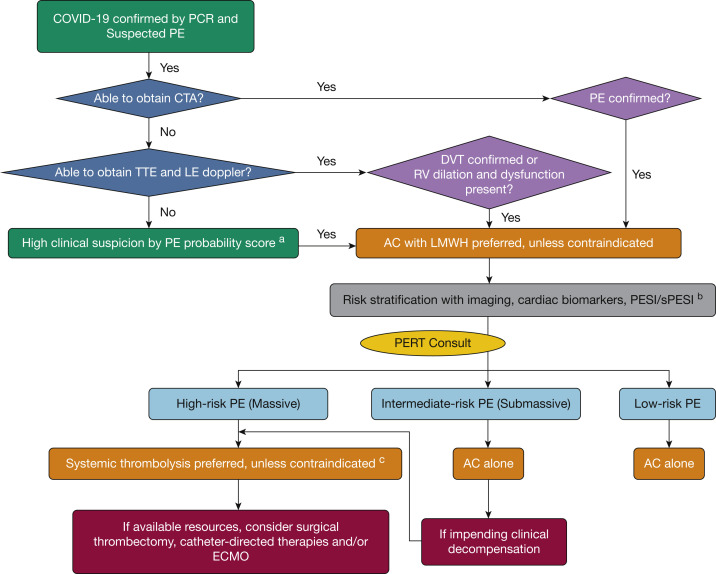
Given the limited data to support the use of biomarkers to diagnosis PE in patients with COVID-19, standard diagnostic testing should be considered (Fig 1 ). However, because of concerns about staff exposure, standard diagnostic tests, including CT angiography (CTA), may not be easily obtained. Alternatively, bedside echocardiogram and lower extremity ultrasonography can be important adjunct in establishing the diagnosis of PE. If evidence of acute, otherwise unexplained, RV dilatation or dysfunction, intracardiac thrombus, or clot-in-transit is noted, the patient should be presumed to have acute PE and treatment with full-dose anticoagulation should be initiated. No specific echocardiographic findings have been identified for COVID-19 infection. Evidence of right-sided pressure overload such as right-sided chamber dilatation, McConnell sign, or paradoxical septal movement, although nonspecific, remain diagnostic findings. Similarly, if DVT is identified, or is highly probable, the patient should be treated with full-dose anticoagulation. At times, a patient with COVID-19 may be unable to be evaluated by CTA, ultrasonography, or echocardiogram because of concerns of staff exposure to COVID-19 or cardiorespiratory instability. If the clinical presentation supports PE, the patient should be treated with full-dose anticoagulation, unless contraindicated, pending definitive diagnosis. A PERT consultation, given possible limitations in testing opportunities and the overlap of COVID-19 and PE symptoms, may help to clarify the diagnosis.
Figure 1.
PERT addendum algorithm for patients with COVID-19. aPE probability scores to consider include Wells criteria, Geneva score, and Pulmonary Embolism Rule-out Criteria. bPlease refer to the PERT Consortium1 consensus practice document for specific details on risk stratification. cBecause of the fluid nature of COVID-19 hotspots, the ability to handle patients with COVID-19 in catheterization laboratories and operating rooms with regard to transport, staff exposure/preparedness, and so forth has evolved since the start of the pandemic and will continue to evolve. This algorithm represents how to treat patients in high-volume COVID-19 institutions where resources may be limited. In low-volume areas, providers may be less likely to shunt a patient down a systemic tissue plasminogen activator pathway if the patient would benefit from an invasive therapy and there are no barriers or limited resources. AC = anticoagulation; COVID-19 = coronavirus disease 2019; CTA = CT angiography; ECMO = extracorporeal membrane oxygenation; LE = lower extremity; LMWH = low-molecular-weight heparin; PCR = polymerase chain reaction; PE = pulmonary embolism; PERT = pulmonary embolism response team; PESI = PE severity index; RV = right ventricular; sPESI = simplified PE severity index; TTE = transthoracic echocardiogram.
Once PE is diagnosed, standard risk stratification is recommended using a composite of clinical appearance, systolic BP, heart rate, respiratory rate, oxygen requirement, PE severity index or simplified PE severity index, imaging for RV dysfunction (CTA or echocardiogram), and/or biomarkers (troponin, brain natriuretic peptide, or NT-pro-brain natriuretic peptide). Troponin elevation may be seen in COVID-19 infection, therefore possibly confounding its clinical utility. Similarly, COVID-19-associated cardiomyopathy may be an alternative cause of RV dysfunction. However, the presence of cardiomyopathy does not exclude concurrent PE and may, indeed, be an independent risk factor for PE based on poor cardiac output. Risk stratification must take into account the relative contribution of COVID-19 lower respiratory tract infection, vs that of PE, as the cause of respiratory failure. The respiratory and hemodynamic compromise from both the viral pneumonia and the PE need to be investigated fully to determine which of these disorders is having the greatest impact and to determine optimal treatment. Consultation with the multidisciplinary PERT, if available, is recommended to assist with stratification and challenging diagnostic and therapeutic decision-making. Studies prior to COVID-19 have demonstrated that having a PERT decreases time to PE diagnosis, time to therapeutic anticoagulation, length of stay, and mortality.20, 21, 22
During this pandemic, a central challenge to a PERT consultation is the awareness of the communicable nature of COVID-19 for those exposed to patients in the ICU, catheterization laboratory, or operating room. If not already performed, recommendation of a COVID-19 test should be done simultaneously with determination of hemodynamic stability. If the patient requires emergent procedural intervention, all preventive measures must be taken for the patient and providers to, from, and in the catheterization laboratory or operating room. However, if the patient is stable enough to allow for testing to be completed, procedural intervention should be delayed pending results.
Although COVID-19 testing is pending, medical therapy with appropriate anticoagulation is carried out with recommendations by the PERT to the primary team. It is imperative for the PERT to remain aware of the patient’s status throughout this period. If the patient becomes unstable, intervention may be necessary before COVID-19 test results are available. An open line of communication with the treating ICU staff can keep the PERT up-to-date on the patient status and any changes that occur.
Once COVID-19 testing is complete, an interim patient status assessment should be performed. In general, patient stability supports continuation of ongoing treatment, regardless of COVID-19 status. Deterioration, on the other hand, supports intervention with care to protect providers from viral acquisition if the patient has tested COVID-19 positive. Additional details on specific interventional decision-making are subsequently discussed in the Interventions section.
Position Statement
-
•
Carefully assess the contribution of COVID-19 lower respiratory tract involvement to the presenting hemodynamic and gas-exchange abnormalities, and whether these abnormalities are out of proportion and require exploration for alternative explanation, such as PE.
-
•
Because patients with COVID-19 may exhibit a hypercoagulable state, the index of suspicion for concurrent PE should be high. In patients with clinical and imaging findings not entirely explained by COVID-19, evaluation for PE should be strongly considered.
-
•
Elevated D-dimer, in and of itself, should not be used to diagnose suspected PE.
-
•
PERT consultation should take into account, when clinically allowable, COVID-19 testing and results as a means of risk stratification and to protect allied health-care providers from the risk of viral transmission.
Treatment
Hospital Admissions
Pathways for hospital admission are predominantly the ED, physician’s office, or hospital transfers. To decrease exposure, patients with stable low-risk PE, absence of comorbidities, low COVID-19 risk factors or suspicion for infection, and mild clinical presentation may not require hospitalization. The decision to treat a patient in the in- or outpatient setting should be made on a case-by-case basis and according to standard recommendations and assessments; PERTs can be helpful in making these decisions. If the decision is to monitor at home, the safety and availability of direct oral anticoagulants or low-molecular-weight heparin should enable carefully selected patients to be treated as outpatients. Numerous clinical trials have demonstrated the safety of outpatient treatment of patients with low-risk PE.23, 24, 25, 26, 27 Moreover, the European Society of Cardiology28 and American College of Chest Physicians29 guidelines recommend selected low-risk patients are safe for early discharge or outpatient therapy. These guidelines, however, are for PE in general, and it is unknown if PE associated with COVID-19 is different in terms of response to therapy and outcomes. Therefore, decisions surrounding whom to admit must be made after risk stratification and with appropriate consultation (eg, PERT). Importantly, robust follow-up, communication, and coordination of care with a PE clinic and the primary care physician are essential for outpatient treatment.
Position Statement
-
•
For patients with mild COVID-19 symptoms and low-risk PE, outpatient treatment or early discharge may be considered, with close follow-up.
Anticoagulation
There are numerous guidelines and position papers on the use of anticoagulation (type, dose, and duration) in patients with COVID-19 for both prevention and treatment, and we defer to those documents because that is not the focus of this paper.19 , 30, 31, 32, 33, 34, 35, 36
Interventions
Decisions about procedural intervention should be grounded in a discussion of risk-benefit ratio with a multidisciplinary PERT consultation. During this pandemic, the relative risks and benefits have shifted because of the risk of viral transmission with transportation of patients who are COVID-19 positive to invasive laboratories or operating rooms. Furthermore, patient circumstances (eg, proning) may make interventional procedures unsafe or impossible. The evidence base regarding optimal treatment for higher-risk PE is evolving and remains unclear as to which patients might benefit from interventional therapies.37 Given the absence of a well-defined benefit for invasive therapy and the potential for viral transmission, a conservative approach leaning toward medical therapy (eg, anticoagulation, IV peripheral fibrinolysis) should be considered in patients with COVID-19. In general, procedural means of treating PE should be applied to only severe cases in which medical therapy is unlikely to be successful or contraindicated. Presently, there are no data to support one intervention over another and it is a strength of a PERT, using a multidisciplinary discussion platform, to arrive at these decisions based on each patient’s unique clinical scenario (Fig 1).
Hemodynamically stable patients should be medically managed with anticoagulation alone. Careful monitoring is prudent to identify patients who become unstable and require emergency intervention. Urgent and emergent cases can be identified as those that, acutely or subacutely, develop hemodynamic instability characterized by increasing vasopressor requirements, rising biomarkers, or a trend of worsening RV function on serial echocardiography. Clinical assessment of stability is critical and should be based on objective measures including BP, heart rate, oxygen requirements, assessment of RV function over time, and overall clinical trajectory, so as to avoid unnecessary resource utilization and to minimize unnecessary exposures. Furthermore, systemic anticoagulation, in the context of worsening RV dysfunction, may be an acceptable lone therapy if either a patient declines an additional procedural intervention or contraindications are present for which a procedural intervention carries more risk than benefit.
When an intervention is deemed necessary, rapid mobilization of resources, as can often be performed by PERTs, is essential. Sequential patient assessments should be performed in patients with known or suspected PE, to identify early signs of deterioration. Precautions should be in place to balance the need for intervention with concerns for safety of the patient and health-care personnel. Given that rushing a patient to the invasive laboratory or operating room may result in viral containment, planning ahead to avoid such a scenario is important. The therapeutic benefit and risk of both thrombolytic therapy and extracorporeal membrane oxygenation (ECMO) should be considered in extreme circumstances of cardiopulmonary collapse. Thrombolytic therapy offers a potential expedited therapeutic benefit. Consideration of using ECMO should take into account its supportive role, inherent need for additional personnel, use of resources, and lack of extensive data regarding outcomes and survival. When possible, placement of cannulae and initiation of the ECMO circuit at the bedside is preferred, given the complexity of transport of patients with COVID-19, particularly in urgent situations.
For patients with COVID-19 and confirmed PE who have imminent or ongoing hemodynamic collapse, immediate administration of systemic thrombolytic therapy is recommended (with consideration of contraindications). The appropriate dose and agent should be selected according to institutional protocol and by consensus of the treating team. Not to be overlooked, thrombolytic therapy carries a bleeding risk that must be factored into clinical decision-making. Few studies have highlighted the risks of bleeding in patients with COVID-19 and when reported, the rates are quite varied. One French study of ICU patients found a 2.7% rate of bleeding complications, whereas another French ICU study reported a 21% overall rate of hemorrhagic events, with 84% of the events occurring in patients on therapeutic anticoagulation.38 , 39 Importantly, no studies have reported on bleeding rates in patients administered thrombolytics. Nevertheless, the limited available reports emphasize the need to carefully balance the risks of bleeding with the benefits of an intervention. When a catheter-directed or surgical procedure is indicated, but not feasible given constraints of the COVID-19 pandemic, thrombolytic therapy can be administered in its place when risk-benefit suggests it is appropriate. Thrombolytic therapy is generally still reserved for patients who are experiencing hemodynamic instability or certain individuals who are progressively deteriorating but have yet to develop hypotension. Recommendations on following coagulation parameters when using thrombolytic agents are delineated in the PERT consensus document.1
Autopsies have identified microvascular thrombi in patients infected with COVID-19.40 , 41 Unlike most DVT and PE events, the clinical onset of these microthrombi is unknown and lacks specific physical findings or imaging signatures. Furthermore, there are no data to support the use of therapeutic anticoagulation or thrombolytic therapy based on these findings alone even in the context of patients who develop refractory respiratory failure. The role and impact of microvascular thrombosis needs further investigation to be able to draw evidence-based conclusions to warrant additional pharmacologic or other treatments.
The decision and subtleties involved in using hemodynamic modes of support, thrombolytics, or the timing of interventional procedures highlight the central role of the PERT. Given the multidisciplinary membership, the PERT is positioned to provide insight that guides management in multifaceted situations, not to mention the additional complexities associated with concurrent COVID-19 infection.
Position Statement
-
•
Indications and contraindications for thrombolysis remain unchanged.
-
•
Consider systemic thrombolysis as a viable alternative in certain patients with COVID-19 who are appropriate for advanced therapy, but in whom an invasive approach may not be available because of limited resources or concerns about viral transmission.
-
•
The risk-benefit ratio of medical and interventional therapy may require adjustment in patients with concurrent COVID-19 and PE.
-
•
PERT consultation provides a mechanism for evaluation of complex interventional options by a multidisciplinary group of PE experts.
Transfer of Care
Given the potential of exposure to other patients and staff, interhospital transfer of patients with COVID-19 should only occur when the receiving hospital offers treatment that is imminently needed and beyond the capabilities of the sending hospital. Whenever possible, COVID-19 status should be identified prior to transfer.
Physician-to-physician communication should occur regarding the necessity of transfer, and the logistics and potential liabilities. Existing hospital transfer policies for patients with PE, regardless of COVID-19 status, should incorporate the following: there must be a well-defined clinical benefit or resources or services that are not available at the sending hospital, such as ICU care, endovascular intervention or surgical services, ECMO, and neurosurgical services. PERTs can be instrumental not only to help make the decision as to whether or not to transfer a patient, but also to mobilize the resources and carry out the treatment plans that are deemed necessary once the transfer occurs.
All hospital, state, and federal guidelines for avoiding viral transmission, including those found on the Centers for Disease Control (www.cdc.gov) website, should be followed in any case of patient transfer.
Position Statement
-
•
Transfer of care for a patient with PE and COVID-19 should be requested when needed services are not available at originating institution and are necessary for best care of the patient.
-
•
The potential benefit vs risk should be carefully considered on a case-by-case basis via direct communication between physicians from transferring and receiving institutions. PERTs can aid in this decision.
Follow-up
Adequate follow-up for all patients with PE is essential for recovery and for optimal prevention of subsequent thromboembolic events. Such follow-up is even more critically important in the successful recovery of patients with concomitant COVID-19 and PE. Using PERT follow-up clinics can help assure that this crucial piece of care occurs (Table 2 ). If PERT follow-up clinics are not available, dedicated hematology, pulmonary, or vascular medicine clinics, for example, may provide similar continuity. Prior to discharge, providers must assure appropriate hand off to the next level of care, including securing the anticoagulants prescribed and scheduling a follow-up visit. Follow-up appointments, when possible, should be performed virtually via telephone or telemedicine to avoid additional exposure of patients and providers. Follow-up appointments should address etiology of the PE, discussion of anticoagulation treatment, need for additional testing, and assessment of new symptoms.
Table 2.
Important Considerations During Follow-up Clinic
| Issues to Address |
|---|
Hand off
|
Location of visit
|
Hospital course
|
Etiology of PE
|
Anticoagulation
|
Adjunctive therapies
|
Abnormal findings
|
Complications: evaluation and management
|
COVID-19/PE registry
|
CTEPH = chronic thromboembolic pulmonary hypertension; ECHO = echocardiogram; IVC = inferior vena cava; PCP = primary care physician; PSA = prostate-specific antigen; PTSD = posttraumatic stress disorder. See Table 1 legend for expansion of other abbreviations.
The coagulopathic state associated with COVID-19 and the resultant increased thrombin generation is unequivocally one of the etiologic factors responsible for PE in these patients. However, other possible contributing factors must be addressed as well. During follow-up, the physician should inquire into other conditions that may have played a role in thrombosis. Compliance with age-appropriate cancer screening should be ensured. Thrombophilia evaluation should only be considered if the results are going to change medical management of the patient or impact family members. Discussion regarding anticoagulation medications is another important aspect of follow-up. During hospitalization, patients with COVID-19 and PE are typically treated with low-molecular-weight heparin and often discharged home on a direct oral anticoagulant. At follow-up, the type and specific choice of agent, recommended duration of therapy, and potential side effects should be reviewed. The importance of compliance should be emphasized. Follow-up is also an opportunity to address any abnormal results from tests performed during hospitalization. Specifically, many patients with COVID-19 may have had abnormal coagulation tests, and these should be reevaluted and potentially repeated. Furthermore, if a patient had an abnormal echocardiogram, evidence of RV dysfunction, elevated pulmonary artery pressures, or cardiomyopathy, the follow-up appointment will ensure this can be repeated to assess for recovery. For patients who underwent placement of an inferior vena cava filter, device removal should be planned during the follow-up appointment. It should also be appreciated that PE can be associated with profound emotional distress; follow-up provides an opportunity to evaluate and manage the psychological well-being of these patients.
Finally, and perhaps most importantly, follow-up visits enable providers to assess patients for any new symptoms. In the months to years after PE, there is a spectrum of morbidity that may occur, ranging from persistent fatigue, dyspnea, and reduction in exercise tolerance, which are characteristic of post-PE syndrome, to chronic thromboembolic pulmonary hypertension. The follow-up appointment allows providers to assess for these conditions in each patient and, if present, to promptly evaluate and, if available, treat. The intermediate and long-term impact of COVID-19 is unknown, particularly in patients who have concurrent PE. Therefore, monitoring and collecting data during follow-up regarding the prevalence of chronic thromboembolic pulmonary hypertension and other post-PE syndromes, and perhaps other sequelae yet to be defined, will be essential for the medical community to understand the full impact of COVID-19. To address this need, the PERT Consortium recently created a COVID-19/PE registry with a goal to identify the clinical characteristics, diagnostic strategies, treatment approach, and short- and long-term outcomes of all patients diagnosed with both COVID-19 and PE. The consortium currently manages a mature database that is the largest prospective US registry of patients with PE. PERT will leverage the infrastructure of this existing registry not only to collect the necessary information for the COVID-19/PE registry, but also to quickly scale it up to meet the needs of this rapidly growing disease. Importantly, this registry is open to any facility taking care of patients with COVID-19 and PE. Institutions need not be members of the PERT Consortium or even have a PERT because the intent of this COVID-19/PE registry is to provide expedient public reporting of aggregate data on the clinical characteristics and outcomes of patients with COVID-19 and PE to increase our knowledge base and provide data that will inform decision-making and enhance outcomes.
Position Statement
-
•
Follow-up assessment of patients with COVID-19 and PE is critical to address issues surrounding anticoagulation, follow-up testing, recovery and persistent symptoms, and psychological well-being.
-
•
When possible, follow-up visits should be virtual. If persistent symptoms or concern for RV failure exist, long-term follow-up visits may be best evaluated in person.
-
•
Monitoring and collection of data obtained during follow-up visits will help the medical community understand the unique impact of concurrent COVID-19 and PE. Facilities caring for patients with COVID-19 and PE are encouraged to join the complimentary PERT Consortium COVID-19/PE registry, which will provide physicians with important data on presentation, assessment, and management of patients with COVID-19 and PE and help shape real-time decision-making and patient-level care as this pandemic unfolds.
Decision-making in Patients With PE and COVID-19
Evaluation of patients with PE and confirmed or suspected COVID-19 infection should be thorough, while acknowledging that certain testing may not be available because of concerns about viral transmission or in extremely ill patients who cannot be moved. Assimilation of all available data is of paramount importance. The uniquely complex nature of concurrent COVID-19 and PE underscores the benefits of a multidisciplinary team-based approach such as a PERT to facilitate prompt decision-making. Whenever available, use of an approved electronic platform enables real-time visualization of all available data (eg, physical examination, images, laboratory values) with all members of the care team, without necessitating patient contact, which reduces not only exposure but also the use of personal protective equipment (PPE). The benefits of using these tools include the ability to exchange information, to display images and pertinent laboratory values, and to discuss management options in real time. Furthermore, electronically mediated connection of members of the PERT and/or others involved enables the rapid response and prompt decision-making that is often required for these acutely ill patients. When deciding on recommendations, the input of experts from different disciplines can be critical for these complex patients; however, only those consultants involved in actually providing intervention should see and examine the patient in person, thereby reducing exposure and the use of PPE. Interventional or surgical consultants should evaluate the patient with the minimum personal contact that is necessary to achieve a thorough understanding of the case; however, patient care should never suffer from avoidance of first-hand patient evaluation. Ongoing open lines of communication to share patient data that is inherent in PERTs reduce the need for repeated personal patient evaluation. Adherence to these principles requires careful case-by-case assessment of each patient with COVID-19 and PE.
Position Statement
-
•
In all care of patients infected with COVID-19, exposure should be limited, without compromising the medical information necessary to make critical evidence-based management decisions.
-
•
Digital platforms for information exchange enable reduced exposure and facilitate real-time decision-making by a multidisciplinary team such as a PERT.
Destination: Catheterization Laboratory or Operating Room
When an intervention is necessary for patients with PE and COVID-19, invasive laboratory and operating room personnel should be kept to the minimum that is required for safe conduct of the procedure, and should don and doff specified and approved PPE. Careful planning should be exercised and, when possible, the team should anticipate and lay out the resources (medications, instruments, and devices) that will potentially be needed so as to expedite the procedure and minimize supply runs. Every precaution should be taken to prevent aerosolization of patient respiratory secretions throughout the procedure. After the procedure, the room should be terminally cleaned, in accordance with hospital policy regarding COVID-19 infection prevention.
Position Statement
-
•
Careful and thoughtful advanced planning for interventional or operative procedures, particularly regarding required personnel and equipment, will expedite procedures and minimize staff exposure.
Conclusions
The current COVID-19 era has complicated the diagnosis, risk stratification, and treatment of patients with PE. The PERT approach can significantly aid in the care of these vulnerable and complicated patients. Through a multidisciplinary clinical discussion, PERT evaluations assess the hemodynamic status, provide cardiopulmonary evaluation, weigh the impact of comorbid conditions, and define the best anticoagulation or interventional management. Vigilance and special measures are also required for management of such patients to optimize outcome while protecting others in the environment. Modifications of a previously defined algorithm for the diagnosis and management of PE must be considered. As more is learned about COVID-19, ongoing refinements will be necessary to address this vulnerable population. The PERT Consortium COVID-19 and PE registry will be a reservoir for such information.
PERTinent Pearls
Visit the PERT website (www.pertconsortium.org) for archived and upcoming webinars, literature updates, podcasts, and other resources regarding PE. Information about membership in the PERT Consortium and the upcoming 2020 PERT Consortium Annual Scientific Meeting, “Pulmonary Embolism - What is known and what we need to know,” is also available.
Acknowledgments
Author contributions: R. P. R. and C. G. wrote the first draft of the manuscript and contributed to the concept, design, creation of tables, critical revision, and final approval. R. C., G. A. D., J. S. G., J. H., C. K., R. L., G. M., T. A. M., B. R.-L., T. M. T., V. T., A. S. W., and K. R. contributed to the concept, critical revision, and final approval of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. S. G. discloses being on the advisory boards for Inari Medical & Astra Zeneca. C. K. discloses grants to his institution from Diagnostica Stago, Siemens Healthcare Diagnostics, and Janssen; and being a consultant for Boston Scientific and EKOS Corp. R. L. discloses being a consultant and on the medical advisory board for Boston Scientific and Medtronic. K. R. declares being a Consultant/on Scientific Advisory Board for Access Vascular, Angiodynamics, Philips, Boston Scierntific, Surmodics, Janssen, Magneto, Mayo Clinic, BMS-Pfizer, Summa Therapeutics, Thrombolex; and obtains Grants from NIH, Boston Scientific, Gettinge, Intact Vascular; and has Equity in Accolade, Access Vascular, Capture Vascular, Contego, Cruzar Systems, Embolitech, Endospan, Eximo, JanaCare, Magneto, Micell, Orchestra, PQ Bypass, Primacea, Silk Road, Shockwave Medical, Summa Therapeutics, Thrombolex, Valcare. R. P. R. discloses grants to her institution from BMS and Janssen; and is a consultant/on the advisory board for BMS, Dova, Janssen, and Portola. T. M. T. is a consultant for Medtronic and GE Healthcare. None declared (C. G., R. C., G. A. D., J. H., G. M., T. A. M., B. R.-L., V. T., A. S. W.).
Footnotes
Drs Rosovsky and Grodzin contributed equally to this manuscript.
References
- 1.Rivera-Lebron B., McDaniel M., Ahrar K., et al. Diagnosis, treatment and follow up of acute pulmonary embolism: consensus practice from the PERT Consortium. Clin Appl Thromb Hemost. 2019;25 doi: 10.1177/1076029619853037. 1076029619853037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 3.Klok F.A., Kruip M., van der Meer N.J.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goyal P., Choi J.J., Pinheiro L.C., et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosovsky R., Zhao K., Sista A., Rivera-Lebron B., Kabrhel C. Pulmonary embolism response teams: purpose, evidence for efficacy, and future research directions. Res Pract Thromb Haemost. 2019;3(3):315–330. doi: 10.1002/rth2.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosovsky R., Borges J., Kabrhel C., Rosenfield K. Pulmonary embolism response team: Inpatient structure, outpatient follow-up, and is it the current standard of care? Clin Chest Med. 2018;39(3):621–630. doi: 10.1016/j.ccm.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Rosovsky R., Chang Y., Rosenfield K., et al. Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10-year analysis. J Thromb Thrombolysis. 2019;47(1):31–40. doi: 10.1007/s11239-018-1737-8. [DOI] [PubMed] [Google Scholar]
- 10.Klok F.A., Kruip M., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes G.D., Kabrhel C., Courtney D.M., et al. Diversity in the pulmonary embolism response team model: an organizational survey of the National PERT Consortium Members. Chest. 2016;150(6):1414–1417. doi: 10.1016/j.chest.2016.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Barnes G., Giri J., Courtney D.M., et al. Nuts and bolts of running a pulmonary embolism response team: results from an organizational survey of the National PERT Consortium members. Hosp Pract (1995) 2017;45(3):76–80. doi: 10.1080/21548331.2017.1309954. [DOI] [PubMed] [Google Scholar]
- 13.Al-Samkari H., Karp Leaf R.S., Dzik W.H., et al. COVID and coagulation: bleeding and thrombotic manifestations of SARS-CoV2 infection. Blood. 2020;136(4):489–500. doi: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T., Chen R., Liu C., et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020;7(5):e362–e363. doi: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moores L.K., Tritschler T., Brosnahan S., et al. Prevention, diagnosis and treatment of venous thromboembolism in patients with COVID-19: CHEST Guideline and Expert Panel Report. Chest. 2020;158(3):1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhury P., Gadre S.K., Schneider E., et al. Impact of multidisciplinary pulmonary embolism response team availability on management and outcomes. Am J Cardiol. 2019;124(9):1465–1469. doi: 10.1016/j.amjcard.2019.07.043. [DOI] [PubMed] [Google Scholar]
- 21.Wright C., Elbadawi A., Chen Y.L., et al. The impact of a pulmonary embolism response team on the efficiency of patient care in the emergency department. J Thromb Thrombolysis. 2019;48(2):331–335. doi: 10.1007/s11239-019-01875-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xenos E.S., Davis G.A., He Q., Green A., Smyth S.S. The implementation of a pulmonary embolism response team in the management of intermediate- or high-risk pulmonary embolism. J Vasc Surg Venous Lymphat Disord. 2019;7(4):493–500. doi: 10.1016/j.jvsv.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Aujesky D., Roy P.M., Verschuren F., et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011;378(9785):41–48. doi: 10.1016/S0140-6736(11)60824-6. [DOI] [PubMed] [Google Scholar]
- 24.Frank Peacock W., Coleman C.I., Diercks D.B., et al. Emergency department discharge of pulmonary embolus patients. Acad Emerg Med. 2018;25(9):995–1003. doi: 10.1111/acem.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabrhel C., Rosovsky R., Baugh C., et al. The creation and implementation of an outpatient pulmonary embolism treatment protocol. Hosp Pract (1995) 2017;45(3):123–129. doi: 10.1080/21548331.2017.1318651. [DOI] [PubMed] [Google Scholar]
- 26.Wells P.S., Buller H.R. Outpatient treatment of patients with pulmonary embolism. Semin Vasc Med. 2001;1(2):229–234. doi: 10.1055/s-2001-18492. [DOI] [PubMed] [Google Scholar]
- 27.White B., Rosovsky R., Parry B.A., Kabrhel C. The outpatient treatment of venous thromboembolism: operational impact and the role of novel anticoagulants. Semin Thromb Hemost. 2016;42(8):846–856. doi: 10.1055/s-0036-1593542. [DOI] [PubMed] [Google Scholar]
- 28.Konstantinides S.V., Meyer G., Becattini C., et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41(4):543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 29.Kearon C., Akl E.A., Ornelas J., et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 30.Bikdeli B., Madhavan M.V., Jimenez D., et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt B., Retter A., McClintock C. Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. https://b-s-h.org.uk/media/18171/th-and-covid-25-march-2020-final.pdf
- 32.British Thoracic Society BTS guidance on venous thromboembolic disease in patients with COVID-19. https://www.brit-thoracic.org.uk/about-us/covid-19-information-for-the-respiratory-community/
- 33.Thachil J., Tang N., Gando S., et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Society of Hematology COVID-19 and VTE/anticoagulation: frequently asked questions; version 2.1. https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation
- 35.Barnes G.D., Burnett A., Allen A., et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50(1):72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhai Z., Li C., Chen Y., et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020;120(6):937–948. doi: 10.1055/s-0040-1710019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todoran T.M., Giri J., Barnes G.D., et al. Treatment of submassive and massive pulmonary embolism: a clinical practice survey from the second annual meeting of the Pulmonary Embolism Response Team Consortium. J Thromb Thrombolysis. 2018;46(1):39–49. doi: 10.1007/s11239-018-1659-5. [DOI] [PubMed] [Google Scholar]
- 38.Fraisse M., Logre E., Pajot O., Mentec H., Plantefeve G., Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24(1):275. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and cardiac pathology in African American patients with COVID-19: the first autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]