ABSTRACT
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay on anal swabs was recently reported to be persistently positive even after throat testing was negative during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. However, data about the consistent performance of RT-PCR assay on throat and anal swabs remain limited in paediatric patients. Here, we retrospectively reviewed RT-PCR-testing results of 212 paediatric patients with suspected SARS-CoV-2 infection at Wuhan Children’s Hospital. The diagnostic potential of these two types of specimens showed significant difference (positive rate: 78.2% on throat swabs vs. 52.6% on anal swabs, McNemar Test P = 0.0091) and exhibited a weak positive consistency (Kappa value was 0.311, P < 0.0001) in paediatric patients. Furthermore, viral loads detected on both throat and anal swabs also showed no significant difference (P = 0.9511) and correlation (Pearson r = 0.0434, P = 0.8406), and exhibited an inconsistent kinetic change through the course of SARS-CoV-2 infection. Besides, viral loads in the throat and anal swabs were correlated with different types of immune states, immune-reactive phase, and the resolution phase/immunologic tolerance, respectively. These findings revealed that RT-PCR-testing on throat and anal swabs showed significant difference for monitoring SARS-CoV-2 infection and correlated with different immune state in paediatric patients.
KEYWORDS: SARS-CoV-2, paediatric patients, RT-PCR, diagnostic potential, viral load
Introduction
There had been 414,179 confirmed cases and 18,440 deaths caused by the ongoing global outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection as of March 26, 2020 [1]. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) assay has been widely used for clinical diagnosis and SARS-CoV-2 has been detected in specimens from multiple sites, including bronchoalveolar lavage fluid, sputum, nasal, anal, and throat swabs of patients with COVID-19 [2]. Although lower respiratory tract samples most often testing positive (93%) for the virus, testing on nasopharyngeal or throat swabs (63–72%) were typically used to confirm the diagnosis.
According to the data collected by Chinese Center for Disease Control and Prevention, 1–2% of Chinese confirmed cases were paediatric patients [3], and more than 90% of paediatric patients were mild or moderate cases [4,5]. The epidemiological and clinical features of children infected with SARS-CoV-2 infection has been reported in our previous study, and about 15.8% laboratory throat swab-testing confirmed cases did not have any symptoms of infection or radiologic features of pneumonia and which were admitted to hospital due to infected family members [6]. Furthermore, several studies have recently reported that anal swabs-testing was persistently positive even after nasopharyngeal testing was negative and maybe more useful in judging the effectiveness of treatment and determining the timing of termination of quarantine [7,8]. Thus, we evaluated the consistency of RT-PCR assay on different types of swabs in paediatric patients through the course of SARS-CoV-2 infection.
Methods
Study design and participants
We did a retrospective review of RT-PCR-testing results of 2138 paediatric patients with suspected SARS-CoV-2 infection in Wuhan Children’s Hospital, Huazhong University of Science and Technology (Wuhan, China) from Jan. 1 to Mar. 18, 2020. Wuhan Children’s Hospital is responsible for the treatments of paediatric SARS-CoV-2 infection assigned by the government. Diagnosis of COVID-19 was based on the New Coronavirus Pneumonia Prevention and Control Program (5th edition) published by the National Health Commission of China [9]. All cases were performed RT-PCR assay on throat swabs, and 212 cases were simultaneously tested on anal swabs. 217 cases were tested positive for SARS-CoV-2 either on throat or anal swabs (Fig. S1). Kinetic changes of viral load in both throat and anal swabs were monitored in 13 patients.
This study was reviewed and approved by the Medical Ethical Committee of Wuhan Children’s Hospital, Huazhong University of Science and Technology (approval number IEC-2020R003-E01), and written informed consent was waived because of the retrospective nature of the study. Anonymous clinical data were used for analysis.
RT-PCR assay
Throat/anal swab samples were collected and tested for SARS-CoV-2 with the Chinese Center for Disease Control and Prevention (CDC) recommended Kit. All samples were processed at the Department of Laboratory Medicine of Wuhan Children’s Hospital. Total RNA was extracted within 2 h using the Nucleic Acid Isolation kit (DAAN Gene, Wuhan, China). The real-time RT-PCR assay was performed using a SARS-CoV-2 nucleic acid detection kit according to the manufacturer’s protocol (BGI Biotechnology, Wuhan, China). Target gene open reading frame 1ab (ORF1ab) was amplified and tested during the real-time RT-PCR assay. A cycle threshold value (Ct-value) in FAM channel ≤38 was defined as a positive test result and a Ct-value of >40 or no amplification curve was defined as a negative test.
Statistical analysis
We presented continuous variables as median (IQR) or median SD, and categorical variables as number (%). Means for continuous variables were compared using unpaired or paired t-tests when the data were normally distributed; otherwise, the Mann–Whitney U test was used. Proportions for categorical variables were compared using the χ2 test. All statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 22.0 software (SPSS Inc). A 2-sided α of less than 0.05 was considered statistically significant.
Results
Diagnostic difference of RT-PCR assay on throat and anal swabs
RT-PCR assay on throat and anal swabs were simultaneously tested in 212 of 2138 paediatric patients with suspected SARS-CoV-2 infection in Wuhan Children’s Hospital. 78 of 212 patients were confirmed with SARS-CoV-2 infection according to the positive results obtained from either throat or anal swabs (Table 1). Of the 78 patients, 17 were positive on anal swabs and 37 were positive on throat swabs, as well as 24 were double positive. The diagnostic potential of these two types of specimens showed significant difference (positive rate: 78.2% on throat swabs vs. 52.6% on anal swabs, McNemar Test P = 0.0091) and exhibited a weak positive consistency with Kappa value was 0.311 (P < 0.0001).
Table 1. Diagnostic difference of RT-PCR assay on throat and anal swabs.
| Throat swabs | |||
|---|---|---|---|
| + | − | ||
| Anal swabs | + | 24 | 17 |
| − | 37 | 134 | |
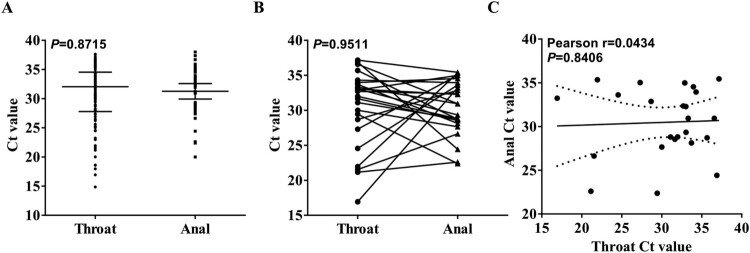
One thousand nine hundreds twenty-six or 1926 of 2138 cases were only performed RT-PCR assay on throat swabs, and finally 217 cases were tested positive for SARS-CoV-2 either on throat or anal swabs. The viral loads (inversely related to Ct value) between throat swabs (32.05, IQR: 27.80–34.53) and anal swabs (32.28, IQR: 28.63–34.54) showed no difference (Figure 1(A), P = 0.8715). In addition, viral loads detected on both throat and anal swabs of 24 patients also showed no significant difference (Figure 1(B), P = 0.9511) and correlation (Figure 1(C), Pearson r = 0.0434, P = 0.8406).
Figure 1.
The difference and correlation of Ct value between throat and anal swabs-testing. (A) The difference between Ct value obtained by RT-PCR-testing on throat swabs (200 cases) and anal swabs (41 cases). (B) The difference between Ct value obtained by RT-PCR-testing on paired throat swabs and anal swabs in 24 cases. The data were normally distributed and a paired t-test was used to compare statistical differences. (C) The pearson correlation between Ct value obtained by RT-PCR-testing on paired throat swabs and anal swabs in 24 cases.
Correlation of viral loads to indexes of SARS-CoV-2 infection
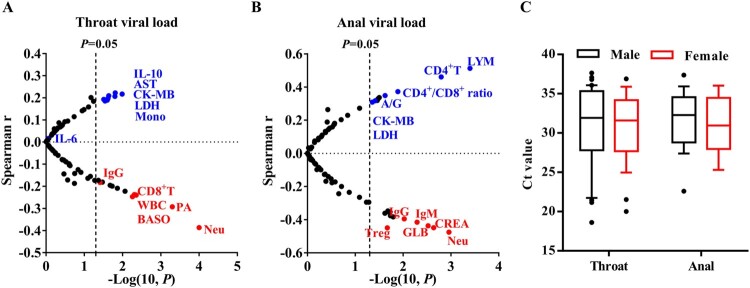
The correlations between viral loads with indexes of SARS-CoV-2 infection, including sex, age, blood routine, blood chemistry, infection-related biomarkers (CRP, PCT, and FERR), immunoglobulins, complements, B cells, NK cells, and subsets of T cells were further evaluated. Viral loads in both throat and anal swabs were positively correlated with myocardial zymogram, CK-MB and LDH in paediatric patients, while negatively correlated with neutrophils (Neu) (Figure 2 (A,B)). IL-10 was positively correlated with throat viral loads, whereas CD8+ T cells and white blood cells (WBC) showed a negative correlation (Figure 2(A)). Counts of CD4+ T cells were positively correlated with anal loads, whereas Tregs, IgG, and IgM were negatively correlated with anal loads (Figure 2(B)). Inflammatory IL-6 levels showed no significant correlation with both throat and anal viral loads in paediatric patients. Also, viral loads in both throat and anal swabs showed no difference between males and females (Figure 2(C)).
Figure 2.
Correlation of viral loads to indexes of SARS-CoV-2 infection. (A) The correlation of viral loads to indexes of SARS-CoV-2 infection. Ct values were detected in throat swabs obtained from 200 cases and viral loads were inversely correlated to Ct value. (B) The correlation of viral loads in anal swabs of 41 cases to indexes of SARS-CoV-2 infection. (C) The difference of Ct value detected in throat and anal swabs between males and females.
Kinetic changes of viral loads through the course of SARS-CoV-2 infection
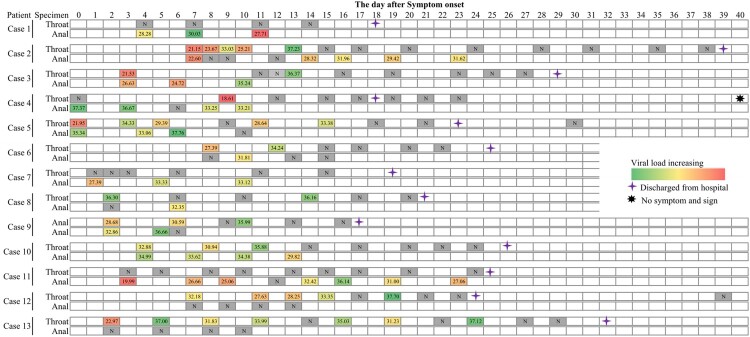
RT-PCR-testing on specimens obtained for respiratory tract was recommended for judging the time of hospital discharge or discontinuation of quarantine. Thus, dynamic changes of viral load were only detected in both throat and anal swabs of 13 patients concerning the day of confirmation (Figure 3). In our results, we noticed that throat swab-testing was persistently negative in Case 1, Case 7, and Case 11, and anal swab-testing remain relatively higher levels 1–4 days before discharging from hospital. RT-PCR-testing results of Case 12 and Case 13 were persistently negative on anal swabs. The difference of clinical features between with anal single positive result and throat single positive were further investigated, and the results revealed that clinical features, including signs and symptoms, CT scan, underlying diseases, comorbid conditions, hospital stay, and time from positive to negative for PCR assay showed no significant difference (Table 2).
Figure 3.
Timeline of RT-PCR-testing results from throat and anal swabs through the course of SARS-CoV-2 infection for 13 cases, January to March 2020.
Table 2. Difference of clinical features between patients with anal single positive result and throat single positive.
| Anal single positive (n = 17) | Throat single positive (n = 37) | χ2 | P-value | |
|---|---|---|---|---|
| Sex (n. %) | 0.17 | 0.4089 | ||
| Male | 11 (64.7) | 26 (70.3) | ||
| Female | 6 (35.3) | 11 (29.7) | ||
| Age (m, IQR) | 74 (32–100) | 90 (39–140) | 0.4055 | |
| Onset of symptom to Hospital admission (d, IQR) | 10 (7–12) | 5 (3–10) | 0.0184 | |
| Signs and symptoms (n. %) | 3.36 | 0.0668 | ||
| None | 10 (58.8) | 12 (32.4) | ||
| Yes | 7 (41.2) | 25 (67.6) | ||
| Gastrointestinal symptoms (n. %) | 0.3035 | |||
| None | 11 (64.7) | 30 (81.1) | ||
| Yes | 6 (35.3) | 7 (18.9) | ||
| Cough (n. %) | 1.19 | 0.1635 | ||
| None | 13 (76.5) | 21 (56.8) | ||
| Yes | 4 (23.5) | 16 (43.2) | ||
| Fever (n. %) | 2.85 | 0.0917 | ||
| None | 12 (70.6) | 17 (45.9) | ||
| Yes | 5 (29.4) | 20 (54.1) | ||
| CT evidence of pneumonia (n. %) | 1.19 | 0.5507 | ||
| None | 7 (41.2) | 12 (32.4) | ||
| Unilateral | 8 (47.1) | 16 (43.3) | ||
| Bilateral | 2 (11.7) | 9 (24.3) | ||
| Underlying diseases (n. %) | 0.7027 | |||
| None | 15 (88.2) | 30 (81.1) | ||
| Yes | 2 (11.8) | 7 (18.9) | ||
| Comorbid conditions (n. %) | 0.3653 | |||
| None | 14 (82.4) | 34 (91.9) | ||
| Yes | 3 (17.6) | 3 (8.1) | ||
| Hospital stay (d, IQR) | 13 (11–15) | 16 (10–20) | 0.4573 | |
| Time from positive to negative for PCR assay (d, IQR) | 6 (4–10) | 7 (5–14) | 0.2354 |
Discussion
As the only centre assigned by the central government for treating infected children under 16 years of age in Wuhan, we presented the inconsistent viral presentation of RT-PCR assay on throat and anal swabs for monitoring paediatric SARS-CoV-2 infection in this study. This report, to our knowledge, is the largest case series to date for evaluating the monitoring performance of paediatric patients with SARS-CoV-2 infection.
According to the study conducted by Gong et al in 10 paediatric patients, they reported that testing on anal swabs remained detectable well even after throat testing was negative and suggested the possibility of faecal-oral transmission besides respiratory transmission [8]. Cell entry of SARS-CoV-2 virus depends on the binding of the viral spike (S) proteins to cellular receptor ACE2 and S protein priming by host cell protease TMPRSS2 [10]. Lung type II pneumocytes, nasal goblet secretory cells, and ileal absorptive enterocytes are highly susceptive to SARS-CoV2 infection due to simultaneously expression of ACE2 and TMPRSS2 [11,12]. In contrary to the report that infectious virus could not be isolated from stool samples conducted by Wendter et al. [13], Li et al recently isolated live SARS-CoV2 virus from faecal samples in 3 of 11 adult patients, and the potential reason may be the diverse mutations in S proteins of the virus isolated from an individual patient [14]. In our study, we found that viral loads showed no difference and correlation between throat and anal swabs. Furthermore, the clinical features between anal single positive result and throat single positive showed no significant difference. Thus, similar to SARS-CoV and MERS coronavirus [15], faecal-oral transmission is also one route for SARS-CoV-2 infection.
CD4+ T helps cells limit exhaustion of memory CD8+ cytotoxic T lymphocyte (CTL) during influenza A virus infection [16], and Tregs were most important for calming inflammation during the resolution phase of viral infection [17]. Huang et al reported that faecal samples from 33 (45%) of 74 patients were negative for SARS CoV-2 RNA whereas their respiratory swabs were positive from first symptom onset, and positive results obtained from faecal samples were delayed from throat swabs through the course of 41 patients with SARS-CoV-2 infection [7]. Although we did not conduct strictly paired time-course analysis, we noticed that some cases were persistently negative in throat swabs and others were persistently negative in anal swabs. Thus, we suspect that increased viral loads in throat swabs may represent an acute infection (immune-overreactive) phase of SARS-CoV-2 infection and predict the exhaustion of memory CTL, which frequently occurred in patients associated with acute respiratory distress syndrome [18]. No matter what the transmission potential of patients with anal or faecal positive results, decreased viral loads in anal swabs may indicate the resolution phase of SARS-CoV-2 infection and has the potential to be used for monitoring the effectiveness of therapeutic responses as which negatively correlated with Tregs, IgM, and IgG.
In conclusion, RT-PCR-testing on throat and anal swabs showed significant difference for monitoring SARS-CoV-2 infection and correlated with different immune states in paediatric patients. Although testing on anal swabs or faecal samples was not included in guidelines for New Coronavirus Pneumonia Prevention and Control Program [19], it has caused attention to the decision on when to discontinue precautions to prevent transmission [20]. Transmission potential of anal swabs-testing positive patients, especially children, which are more like to be asymptomatic carriers, need be urgently evaluated for the control of outbreak SARS-CoV-2 infection.
Funding Statement
This work was supported by grants from the Top Medical Young Talents of Hubei Province.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Coronavirus disease (COVID-2019) situation report 101 (WHO, 2020) . Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- 2.Wang WL, Xu YL, Gao RQ, et al. Detection of SARS-CoV-2 in different types of clinical specimens. Jama. 2020: e203786. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu ZY, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. Jama. 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 4.Dong YY, Mo X, Hu YB, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020: e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 5.Qiu HY, Wu JH, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu XX, Zhang LQ, Du H, et al. SARS-CoV-2 infection in children. N Engl J M. 2020. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu YJ, Guo C, Tang LT, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5(5):434–435. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Y, Li XF, Zhu B, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26(4):502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Commission of China . New coronavirus pneumonia prevention and control program (5th Edition). 2020 Feb 4 [cited 2020 Feb 4]. Available from: http://www.gov.cn/zhengce/zhengceku/2022/05/5474791/files/de44557832ad4be1929091dcbcfca891.pdf; in Chinese).
- 10.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
- 14.Yao HP, Lu XY, Chen Q, et al. Patient-derived mutations impact pathogenicity of SARS-CoV-2. medRxiv. 2020. doi: 10.1101/2020.04.14.20060160. [DOI] [Google Scholar]
- 15.Yeo C, Kaushal S, Yeo D.. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol. 2020;5(4):335–337. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cullen JG, McQuilten HA, Quinn KM, et al. CD4+ t help promotes influenza virus-specific CD8+ T cell memory by limiting metabolic dysfunction. Proc Natl Acad Sci U S A. 2019;116(10):4481–4488. doi: 10.1073/pnas.1808849116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laidlaw BJ, Cui WG, Amezquita RA, et al. Production of IL-10 by CD4+ regulatory T cells during the resolution of infection promotes the maturation of memory CD8+ T cells. Nat Immunol. 2015;16(8):871–879. doi: 10.1038/ni.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Z, Shi L, Wang YJ, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S221600(20)30076-X doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Health Commission of China . New coronavirus pneumonia prevention and control program (7th edition). 2020 Mar 3 [cited 2020 Mar 3]. Available from: http://www.nhc.gov.cn/xcs/zhengcwj/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf; in Chinese).
- 20.Munster VJ, Koopmans M, Doremalen NV D, et al. A novel coronavirus emerging in China – key questions for impact assessment. N Engl J Med. 2020;382(8):692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]