Abstract
There is a striking age-related disparity in the prevalence and severity of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced coronavirus disease 2019 infections, which might be explained by age-dependent immunological mechanisms. These include age-related physiological differences in immunological responses, cross-neutralizing antibodies, and differences in levels and binding affinity of angiotensin-converting enzyme 2, the SARS-CoV-2 target receptor; antibody-dependent enhancement in adults manifesting with an overexuberant systemic inflammation in response to infection; and the increased likelihood of comorbidities in adults and the elderly. Emerging immunological phenomena such as Pediatric Multi-System Inflammatory Disorder Temporally associated with SARS-CoV-2 or Multisystem Inflammatory Syndrome in Children are now being observed, though the underlying mechanisms are still unclear. Understanding the mechanisms through which pediatric patients are protected from severe novel coronaviruses infections will provide critical clues to the pathophysiology of coronavirus disease 2019 infection and inform future therapeutic and prophylactic interventions. Asymptomatic carriage in children may have major public health implications, which will have an impact on social and health care policies on screening and isolation practices, school reopening, and safe distancing requirements in the community.
Key words: COVID-19, Pediatric Multi-System Inflammatory Disorder, SARS-CoV-2
Abbreviations used: ACE, Angiotensin-converting enzyme; ACE-2, Angiotensin-converting enzyme 2; Ang, Angiotensin; ADE, Antibody-dependent enhancement; CoV, Coronavirus; COVID-19, Coronavirus disease 2019; HCoV, Human coronavirus; KD, Kawasaki disease; MIS-C, Multisystem Inflammatory Syndrome in Children; PMIS-TS, Pediatric Multi-System Inflammatory Disorder Temporally associated with SARS-CoV-2; S protein, Spike protein; SARS, Severe acute respiratory syndrome; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
Background
Severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), is the newest human coronavirus (HCoV) that first emerged in December 2019 and has now spread to more than 215 countries, with more than 13.2 million people infected and approximately 575,663 deaths.1 The spectrum of infection ranges from asymptomatic or mild upper respiratory tract symptoms to severe pneumonia and acute respiratory distress syndrome.2 Marked disparities in disease prevalence and severity have been observed between pediatric and adult populations. In this review, we summarize the age-dependent differences in COVID-19 phenotypes, and postulate immunological mechanisms that may explain these observations.
Clinical Presentation of COVID-19 in Pediatric and Elderly Populations
Although insufficient data exist on the incidence of SARS-CoV-2 infection in children versus adults, particularly in asymptomatic individuals, COVID-19 rates are clearly different between these groups. Most patients with COVID-19 are aged 30 to 79 years (87%), and the highest fatality rate (14.8%) has been reported in those older than 80 years. A systematic review of all COVID-19 literature published between January 1, 2020, and March 18, 2020, found that children accounted for just 1% to 5% of all COVID-19 cases.3
A clinically mild disease phenotype has been a consistent finding in pediatric COVID-19 infection. In the largest study of pediatric patients, the prevalence of severe pediatric cases (as defined by the presence of hypoxemia <92%) was 5.9%, a third of that in adults (18.5%).4 Case reports indicate that infected pediatric patients may demonstrate minimal symptoms and the prevalence of asymptomatic infections may be up to 15.8%.5 Few pediatric patients with COVID-19 have required intensive care or mechanical ventilation.5 In contrast, the elderly have a much higher risk of severe disease, intensive care and mechanical ventilation requirements, and fatality.6 Case-fatality rates in Italy and China show an increasing trend with advancing age—from 3.5% to 3.6% (age 60-69 years), 8.0% to 12.8% (age 70-79 years) to 14.8% to 20.2% (age 80 years and above).7
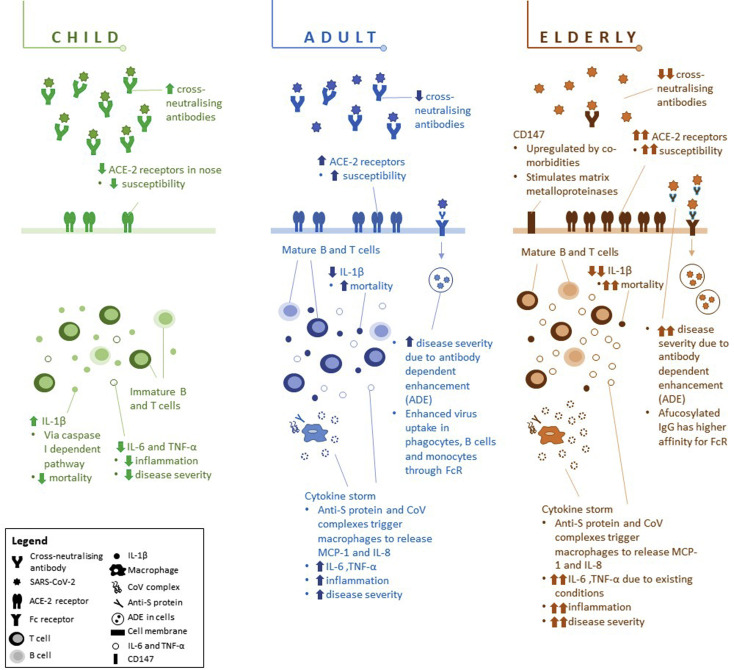
Several explanations for the relatively low rate and severity of disease in children have been postulated. Low community exposure alone would not explain this because children are commonly exposed to large community gatherings such as school and childcare. Inherent biological differences in immune responses between age groups that influence susceptibility to infection and/or progression to disease and clinical manifestations and the higher prevalence of comorbidities in older adults may potentially better explain the discrepant clinical observations. Figure 1 illustrates possible mechanisms contributing to the differences in infection rates and disease severity between children, adults, and the elderly.
Figure 1.
Differences in physiological responses of children, adults, and elderly to SARS-CoV-2. Children generally experience infrequent, mild, and self-limiting infections, which may be due to (a) higher levels of cross-neutralizing antibodies, (b) lower levels of ACE-2 receptors in nasal epithelium, which lowers susceptibility to infection, (c) immature B and T cells and higher regulatory T-cell response, and (d) lower IL-6 and TNF-α production, limiting inflammatory response. Moreover, adults may experience ADE where the S protein enhances entry into cells via Fc receptors, resulting in cytokine storms, which cause severe lung injury. Elderly may be even more susceptible to ADE because they have more afucosylated IgG, which has a higher affinity with Fc receptors. Existing comorbidities in elderly also result in upregulation of CD147, increasing viral entry as well as exacerbation of proinflammatory responses, which increase mortality risk.
Postulated Mechanisms for the Age-Dependent Differences in Immunological Responses to COVID-19
Cross-protective neutralizing anticoronavirus antibodies
An intriguing possibility for the reduced susceptibility of children may be cross-protection from previous exposure to endemic coronaviruses (CoVs) implicated in the common cold. It is hypothesized that seroconversion to HCoV-NL63 and HCoV-OC43 (non–SARS-HCoVs) may produce antibodies to spike protein (S protein) of CoVs that have some degree of neutralizing and cross-protective activity against infection to another HCoV from the same group.8 Thus, it is possible that high and sustained seroconversion toward the common non–SARS-HCoVs, which has been demonstrated in the pediatric population, may confer protection against SARS-CoV-2 as well.
Seropositivity to HCoVs also increases gradually with age until early adulthood,9 but subsequently wanes with increasing age.10 Other studies have demonstrated high seroprevalence to NL63 and 229E in young children. Dijkman et al11 found that 75% and 65% of the children aged 2.5 to 3.5 years were HCoV-NL63 and HCoV-229E seropositive, respectively. A study of seasonal CoV infection in healthy children in the community found that OC43 and NL63 were the most frequently implicated HCoVs.8 Hovi et al12 also showed that antibody titers to HCoV-OC43 increased rapidly up to age 14 years before tapering off and decreasing after age 60 years. Because this protective cross-neutralizing effect may be attenuated with the waning of neutralizing antibody titers with age, this may possibly manifest as higher infection rates and more severe disease presentation in adulthood.
Antibody-dependent enhancement in adults
An effective humoral response to infection is influenced by the quality and magnitude of the antibody response, with high-affinity antibodies specific to the virus important in achieving viral neutralization.13 , 14 SARS-CoV-2 infection is initiated by the binding of surface S proteins on viruses to angiotensin-converting enzyme 2 (ACE-2) receptors present on various host cell types.15 , 16 This results in the production of SARS-CoV-2–specific IgM and IgG antibodies.
However, cross-reactive antibodies from other similar virus serotypes may have varying neutralizing abilities on SARS-CoV-2, depending on antibody titers, isotypes, and specificity/affinity for the ACE-2 receptor.
Antibody-dependent enhancement (ADE) occurs when antibodies produced to one virus serotype interacts with a second serotype (cross-reactive) without neutralizing it fully, resulting in enhanced virulence and downstream inflammatory responses.17 The Fc receptor on monocytes/macrophages and granulocytes has been implicated as the primary receptor to which virus-antibody complexes bind.18 One of the postulated mechanisms behind ADE in CoV infections involves a circulating neutralizing antibody from a previous infection with a closely related virus binding to a CoV's virion spike, forming an antibody-Fc receptor complex that mimics a viral receptor, triggering a conformational change in the spike, which enhances viral entry into various cells, and an ensuing exuberant inflammatory response.19
Antibody concentrations appear to modulate the immune response to SARS-CoV infection. In vitro assays for SARS in human cell lines showed that exposure to antisera diluted 10- to 100-fold demonstrated greater viral neutralization, whereas antisera diluted 1000- to 2000-fold facilitated infection and increased cell apoptosis.20 This is similar to reports from dengue infections where viral neutralization was observed in the presence of high antibody concentrations, which saturated available virion-binding sites, contrasting with low antibody concentrations, which resulted in partial receptor binding facilitating viral enhancement effects, mediated by interactions between the virion-bound antibody and target cells' Fc receptors.21 These anti–S-IgG and FcR interactions then trigger robust inflammatory responses akin to a cytokine storm, manifesting with acute respiratory distress syndrome and respiratory failure.16 Higher titers of cross-neutralizing antibodies were linked to lower odds of reinfection, whereas lower subneutralizing levels were linked to ADE.22 It is thus possible that higher cross-reactive antibody titers in children may be protective, whereas low antibody titers in adults may facilitate ADE.
Differences in antibody isotype, specificity, and affinity also influence the host response toward neutralization or ADE.13 In murine studies of viral vector vaccines encoding the SARS-CoV S protein and nucleocapsid protein, respectively, nucleocapsid protein–immunized mice showed significant upregulation of proinflammatory processes and lung disease.23 In contrast, antibodies to different epitopes of the S protein demonstrated differential immune responses, such as protective responses generated by antibodies to the receptor-binding domain or the HR2 domain, and ADE by antibodies specific to other S epitopes.24
In addition, older adults and elderly may exhibit greater afucosylation of IgG.25 Patients with dengue hemorrhagic fever or dengue shock syndrome caused by ADE were found to produce greater quantities of afucosylated IgG with stronger affinity to Fc receptors.26 A preprint by Larsen et al27 also highlighted that patients with severe COVID-19 expressed heightened afucosylated IgG responses. The higher likelihood of increased afucosylated IgG in adults and elderly may account for the higher incidence of ADE and aggravated symptoms.
Finally, ADE may also arise de novo as a pathogenic process unrelated to cross-reactive antibodies. The “multiple hit” model of neutralization proposes that viral neutralization corresponds to the number of antibodies coating the virion, which is influenced by the affinity and concentration of antibodies.28
Physiological differences in immunological responses in children versus adults and elderly
Immature immune system in children compared with adults
Children generally develop milder forms of viral disease, which may be due to their relatively immature immune systems.29 Children exhibit predominantly TH2, TH17, and low TH1, IFN type 1 immune responses,30 as well as lower levels of memory T and B cells due to reduced lifetime exposure to foreign antigens.30 , 31 Regulatory T cells, more abundant in children, exert stronger immune regulatory effects than those in adults, which may protect them against severe disease manifestations.32
Children also appear to exhibit limited proinflammatory responses. A study in 8 pediatric patients with SARS showed that selective activation of the caspase I–dependent pathway in infected macrophages resulted in a large increase in circulating IL-1β levels but only marginal increases in other proinflammatory cytokines such as IL-6 and IL-12.33 In clinical studies, it has been observed that most children with COVID-19 also do not have increased inflammatory markers such as procalcitonin, C-reactive protein, and IL-6.34
In contrast, adults manifest with heightened TH1-inflammatory responses are associated with increased disease severity. SARS-infected adults demonstrated elevated IL-1β, IL-6, and IL-12 levels with activation of the nuclear factor kappa B pathway.35 , 36 The overproduction of proinflammatory cytokines (cytokine storm) has been shown to result in severe inflammatory lung damage in adult SARS fatalities.37
Although definitive immunophenotyping of pediatric and adult immune responses to SARS-CoV-2 is yet to be published, the SARS experience suggests that similar mechanisms may be applicable in SARS-CoV-2 infections due to their high viral sequence homology. A preprint by Rodriguez et al38 on longitudinal immune profiling of adult patients with COVID-19 showed that proinflammatory IL-6 and IFN-γ levels correlated with severity of COVID-19.
The role of immunosenescence and inflammaging
Aging is associated with 2 profound biological changes in the immune system: Immunosenescence is a gradual decline in the host ability to mount robust immune responses to pathogens, while inflammaging is a chronic increase in low-grade inflammation arising from an overactive yet ineffective alert system.39 , 40 Immunosenescence has been shown to impair innate and humoral immunity, whereby immune cells exhibit functional impairment such as reduced migratory, phagocytic, and proliferative capacity resulting in poorer responses and antibody generation along with ineffective clearance of the foreign antigen.41 , 42 Toll-like receptors (TLRs), T-cell receptor expression and diversity, as well as downstream cytokine responses have also been shown to decline with age.43 , 44 These changes may thus increase disease susceptibility and hamper the mounting of an effective immune response against SARS-CoV-2 in the elderly. The interplay between immunosenescence and inflammaging has been hypothesized to be responsible for the phenomenon of COVID-19 “cytokine storm” in the elderly.45 In the aged immune system, an initial ineffective innate immune response leads to poor viral clearance and greater viral replication.46 , 47 High levels of infected cells drive increased inflammatory cytokine signaling,48 resulting in sustained dysregulated immune activation, which triggers the cytokine storm.
Balance in renin-angiotensin system pathways
The renin-angiotensin system plays a crucial role in regulating host cardiovascular and renal physiology.49 Two of the key enzymes in the renin-angiotensin system are the angiotensin-converting enzyme (ACE) and ACE-2. ACE converts angiotensin (Ang) I to Ang II, and also binds to angiotensin receptor subtype 1a AT1aR, which is responsible for driving lung injury through production of proinflammatory cytokines.50 , 51
However, ACE-2 counteracts ACE activity by converting Ang II to Ang (1-7).52 Consequently, ACE-2 is protective against severe acute lung injury, as depicted in murine models.53 ACE-2 is also shown to reduce hyperoxic lung injury in mice by inhibiting the proinflammatory nuclear factor kappa B pathway and promoting the Nrf2 pathway, which increases production of antioxidants HO-1 and NQO1.54
In COVID-19, binding of SARS-CoV and SARS-CoV-2 virus to cell surface ACE-2 receptors allows viral entry and downregulates ACE-2 expression.55 This results in reduced protection against lung injury and upregulation of the proinflammatory Ang II pathway, manifesting as increased disease severity.56 Likewise, increased serum Ang II levels correlating to viral load and lung injury have been observed in individuals with COVID-19.55 Children (<10 years) were found to have lower ACE-2 expression (2.4 mean log2, counts per million) in the nasal epithelium, one of the main points of entry of SARS-CoV-2. A marked increase with mean log2 counts per million of 2.77 and 3.02 were seen in older children and young adults, respectively, which may explain the lower incidence of COVID-19 in the younger age group.57 Evidence from murine models also showed increased expression of ACE-2 in olfactory epithelium with age58; hence, the elderly may be more susceptible to infection.
Impact of comorbidities on immunological responses to COVID-19
Epidemiological studies show that the presence of comorbidities is a risk factor for COVID-19 infection and severe disease. A meta-analysis of 6 studies found that 17.1% of patients with COVID-19 were hypertensive, 16.4% had cardiac/cerebrovascular disease, and 9.7% were diabetic.59 In this study, patients requiring intensive care were also 2 to 3 times more likely to be hypertensive (28.8% vs 14.1% in non–intensive care unit cases; relative risk, 2.03; 95% CI, 1.54-2.68), have cardio/cerebrovascular disease (16.7% vs 6.2%; relative risk, 3.30; 95% CI, 2.03-5.36), or have diabetes (11.7% vs 4.0%; relative risk, 2.21; 95% CI, 0.88-5.57).
Cardiovascular disease
Several mechanisms have been proposed for the increased cardiac morbidity in COVID-19: (1) direct viral-induced myocardial damage, (2) indirect myocardial injury through viral-mediated cytokine storm,60 and (3) upregulation of ACE-2 receptors by drugs.
Direct viral-induced myocardial damage
SARS-CoV–infected mice demonstrated an ACE-2–dependent myocardial infection, with downregulated ACE-2 protein expression, which mediates increased pulmonary vascular permeability resulting in pulmonary edema and respiratory failure.61 Autopsy samples from deceased patients with SARS also showed detectable viral RNA, marked macrophage infiltration, and myocardial damage in myocardial samples,61 demonstrating the ability of SARS-CoV to mediate myocardial inflammation and damage (myocarditis), which is likely responsible for the high cardiovascular morbidity, particularly in patients with preexisting cardiovascular disease.
Indirect cardiac injury due to cytokine storm
Severe pneumonia or acute respiratory distress syndrome induces a significant inflammatory response termed a cytokine storm, producing high levels of proinflammatory cytokines, which in turn induce myocyte damage and impairment of myocardial function,62 as well as exacerbate systemic hypoperfusion, myocardial and multiorgan ischemia, and ventilation-perfusion mismatch. Autopsy specimens from SARS-infected patients demonstrated high levels of MCP-1TGF-β1, TNF-α, IL-1β, and IL-6, along with dense infiltration of T cells, monocytes/macrophages, and lymphocytes in pulmonary interstitial tissues, and significant apoptosis of pneumocytes, demonstrating the viral cytopathic effect and immunologically mediated cell damage, which may be due to a combination of cytokine storm and ADE effects.63
Upregulation of ACE-2 receptors by drugs
ACE-2 expression can be upregulated by drugs such as ACE inhibitors or Ang II type I receptor blockers.64 ACE inhibitors block ACE receptors from converting Ang I to Ang II, whereas ACE inhibitors or Ang II type I receptor blockers prevent Ang II from binding to Ang II type 1 receptors.65 Human intestinal cells expressed higher levels of ACE-2 after treatment with ACE inhibitors in vitro.66
Diabetes mellitus
Murine diabetes mellitus models have demonstrated increased ACE-2 expression in the lung, kidney, heart, and pancreas.67 Novel genetic epidemiology tools such as phenome-wide Mendelian randomization study have enabled investigation of association between genetic variants and disease phenotypes.68 Phenome-wide Mendelian randomization study demonstrated a tentative causal association between diabetes-related traits and ACE-2 expression in the lung.69 Type 2 diabetes mellitus is characterized by increased proinflammatory TH1 and TH17 cells and decrease in anti-inflammatory regulatory T cells,70 accentuating the systemic inflammatory responses in the COVID-19–associated cytokine storm, which leads to more severe end-organ damage and increased morbidity in patients with diabetes mellitus.71
In addition to ACE-2, CD147 has been identified as a second receptor for SARS-CoV-2.72 CD147 and matrix metalloproteinase expression levels are often upregulated in inflammatory diseases, suggesting that the increased mortality in patients with other comorbidities may be due to the high expression of CD147 and matrix metalloproteinase, hence increasing susceptibility to infection.73 , 74
Pediatric multisystem inflammatory disorder
Recent reports of a new postinfectious pediatric multisystem inflammatory disorder have emerged from regions recovering from severe COVID-19 outbreaks. This entity has been described as PMIS-TS (Pediatric Multi-System Inflammatory Disorder Temporally associated with SARS-CoV-2) or MIS-C (Multisystem Inflammatory Syndrome in Children).71 , 75, 76, 77 The first published series of cases from London, United Kingdom, described 8 children with a hyperinflammatory syndrome akin to Kawasaki disease (KD) shock syndrome. These children presented with fever, rash, conjunctivitis, peripheral edema, and evidence of coronary artery inflammation similar to that of KD, a well-known pediatric autoinflammatory systemic vasculitis thought to be viral-triggered. A second case series from Italy compared clinical, laboratory, and immunological characteristics of 10 children with PIMS-TS to a retrospective cohort of 19 patients with KD. In contrast to the retrospective cohort, patients were generally older (mean age, 7.5 years vs 3.0 years), more likely to present with incomplete KD, and had prominent gastrointestinal and meningeal symptoms, with significant lymphopenia and thrombocytopenia. There was a higher incidence of severe disease manifesting with hypotension and hypoperfusion, abnormal echocardiography, elevated cardiac enzymes, and increased adjunctive steroid requirements.78 The 2 largest series of MIS-C to date reported clinical data on 91 cases from New York, and 186 cases from 26 other states.79 , 80 Although features of KD were found in one-third of the cohort, only 60% fulfilled criteria for typical or atypical KD, with younger children (below 12 years old) being more likely to present with KD. The course of illness was severe, with 80% requiring intensive care unit admissions, 50% with hypotension requiring inotropic support, and a mortality rate of 0.1% to 2%. Cardiac dysfunction, coagulopathy, gastrointestinal symptoms, and significantly raised inflammatory markers were prominent features consistent with a systemic hyperinflammatory state. Most of the patients received immunomodulatory treatment with intravenous immunoglobulin, and glucocorticoids, IL-6 inhibitors (toculizumab and siltuximab), and IL-1Ra inhibitor (anakinra) were used in a subset of patients.
The pathogenesis and immune mechanisms underlying PMIS-TS/MIS-C are still poorly understood. The prevalent hypothesis involves an abnormal immune response to SARS-CoV-2 in genetically susceptible populations. There is a strikingly higher incidence of typical KD in East Asian populations compared with Western populations (239.6 vs 20.8 and 14.7 per 100,000 children <5 years in Japan, the United States, and Italy, respectively),81 , 82 suggesting that genetic predisposition is an important factor in the pathogenesis of KD. The case series from the United States and the United Kingdom found a higher proportion of PMIS-TS/MIS-C in black and Hispanic children than in the general population, although this may also reflect the higher incidence of COVID-19 in these communities.79 , 80 Certain endemic HCoVs (in particular HCoV-229E) have previously been implicated as triggers of KD in Japanese children.83 A novel CoV, New Haven HCoV, was also speculated to be a trigger for KS in a cohort of children from the United States.84 This association was not found in a larger restrospective study from Japan, which found an RNA sequence of HCoV-NH in 5% of controls, but none in children with KD.85
Interestingly, no confirmed cases of PMIS-TS have been reported in Asia so far, although many of these countries have experienced similar large COVID-19 outbreaks, and earlier in the pandemic due to proximity to China. This could be due to under recognition, an increased genetic susceptibility to PMIS-TS/MIS-C in non-Asian populations, and/or differing viral strains in different countries, with a predominant viral strain in Europe and the United States responsible for this geographically limited immune phenomenon.
Further study is urgently required to delineate the clinical, laboratory, and immunological features of PMIS and long-term sequelae, establish causative links to COVID-19 infection, and investigate the genetic, epigenetic, and immunological mechanisms underlying PMIS-TS. Despite valid concerns about the emergence of post-infectious PMIS as a cause of significant morbidity in a small group of children, overall morbidity from COVID-19 in children remains markedly low in comparison to the adult population.3
Conclusions and Social Implications for Policymakers
The pediatric population appears to be spared the brunt of COVID-19 morbidity. The disparity between the pediatric and adult populations might be explained by inherent biological differences in immunological responses to CoV infections and the presence of comorbidities in older adults. Understanding the immunological differences between the young and the old could potentially lay the groundwork for future therapeutics such as convalescent plasma infusions,86 mAbs,87 or vaccine development.88
In addition, the unique features of pediatric COVID-19 may have an impact on clinical decision making and health care policy frameworks addressing the growing threat of COVID-19. Most pediatric COVID-19 cases are detected only in family cluster screening, displaying very mild or no symptoms at all89 and demonstrating prolonged viral shedding in their nasopharynx and stools for up to 2 to 4 weeks after infection.90 In addition, the detection of a high prevalence of asymptomatic/presymptomatic carriage in children would strongly support screening and quarantine of whole family clusters upon a positive diagnosis in an adult to reduce the risk of perpetuating community spread.
The evidence on seroprevalence and changes in antibodies with age suggest that natural immunity to SARS-CoV-2 may be relatively short lived. Antibody titers in survivors of SARS, the most closely related virus to SARS-CoV-2, have been shown to decline after a few years.91 , 92 It is also possible that subsequent SARS-CoV-2 infections could be more severe than the initial infection. Moreover, because vaccine-induced immunity is typically less potent and less enduring than naturally acquired immunity, it might be postulated that vaccinated individuals may be susceptible to more severe COVID-19 sooner than those who acquire immunity through natural infection. Although this is beyond the scope of the current review, it has potentially far-reaching implications for vaccine development, which is currently touted as the solution to the current global crisis.
Large-scale seroepidemiological studies will be required to definitively characterize trends of transmissibility, infection, asymptomatic carriage, and longevity of immunity across separate age groups. Future research should also focus on identifying predictors of clinical phenotypes, prognosis, and outcomes across the different age groups and aim to elucidate protective immunological mechanisms in patients with mild clinical phenotypes, to guide therapeutics and vaccine development. Health policies governing containment efforts such as social distancing, school and workplace closures as well as plans for economic restoration should also be made in careful consideration of the biological differences in clinical manifestations, viral carriage, and transmissibility in pediatric versus adult populations.
Footnotes
E. H. Tham is supported by the National Medical Research Council Transition Award grant (MOH-TA18nov-0003).
Disclosure of potential conflict of interest: P. A. Tambyah reports grants from Roche, Sanofi Pasteur, Johnson & Johnson and GlaxoSmithKine outside the submitted work. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Worldometer . 2020. COVID-19 Coronavirus pandemic.https://www.worldometers.info/coronavirus/ Available from: [Google Scholar]
- 2.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. J Emerg Med. 2020;58:712–713. [Google Scholar]
- 5.Lu X., Zhang L., Du H., Zhang J., Li Y.Y., Qu J. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 8.Dijkman R., Jebbink M.F., Gaunt E., Rossen J.W.A., Templeton K.E., Kuijpers T.W. The dominance of human coronavirus OC43 and NL63 infections in infants. J Clin Virol. 2012;53:135–139. doi: 10.1016/j.jcv.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao X., Guo X., Esper F., Weibel C., Kahn J.S. Seroepidemiology of group I human coronaviruses in children. J Clin virol. 2007;40:207–213. doi: 10.1016/j.jcv.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan C.M., Tse H., Wong S.S., Woo P.C., Lau S.K., Chen L. Examination of seroprevalence of coronavirus HKU1 infection with S protein-based ELISA and neutralization assay against viral spike pseudotyped virus. J Clin Virol. 2009;45:54–60. doi: 10.1016/j.jcv.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijkman R., Jebbink M.F., El Idrissi N.B., Pyrc K., Müller M.A., Kuijpers T.W. Human coronavirus NL63 and 229E seroconversion in children. J Clin Microbiol. 2008;46:2368–2373. doi: 10.1128/JCM.00533-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovi T., Kainulainen H., Ziola B., Salmi A. OC43 strain-related coronavirus antibodies in different age groups. J Med Virol. 1979;3:313–320. doi: 10.1002/jmv.1890030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwasaki A., Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. 2020;20:339–341. doi: 10.1038/s41577-020-0321-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pierson T.C., Fremont D.H., Kuhn R.J., Diamond M.S. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe. 2008;4:229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu Y., Cheng Y., Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tirado S.M.C., Yoon K.-J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003;16:69–86. doi: 10.1089/088282403763635465. [DOI] [PubMed] [Google Scholar]
- 18.Takada A., Kawaoka Y. Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev Med Virol. 2003;13:387–398. doi: 10.1002/rmv.405. [DOI] [PubMed] [Google Scholar]
- 19.Wan Y., Shang J., Sun S., Tai W., Chen J., Geng Q. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol. 2020;94:e02015–e02019. doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S.-F., Tseng S.-P., Yen C.-H., Yang J.-Y., Tsao C.-H., Shen C.-W. Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins. Biochem Biophys Res Commun. 2014;451:208–214. doi: 10.1016/j.bbrc.2014.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burton D.R., Williamson R.A., Parren P.W. Antibody and virus: binding and neutralization. Virology. 2000;270:1–3. doi: 10.1006/viro.2000.0239. [DOI] [PubMed] [Google Scholar]
- 22.Negro F. Is antibody-dependent enhancement playing a role in COVID-19 pathogenesis? Swiss Med Wkly. 2020;150:w20249. doi: 10.4414/smw.2020.20249. [DOI] [PubMed] [Google Scholar]
- 23.Yasui F., Kai C., Kitabatake M., Inoue S., Yoneda M., Yokochi S. Prior immunization with severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) nucleocapsid protein causes severe pneumonia in mice infected with SARS-CoV. J Immunol. 2008;181:6337. doi: 10.4049/jimmunol.181.9.6337. [DOI] [PubMed] [Google Scholar]
- 24.Wang Q., Zhang L., Kuwahara K., Li L., Liu Z., Li T. Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2016;2:361–376. doi: 10.1021/acsinfecdis.6b00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Haan N., Reiding K.R., Driessen G., van der Burg M., Wuhrer M. Changes in healthy human IgG Fc-glycosylation after birth and during early childhood. J Proteome Res. 2016;15:1853–1861. doi: 10.1021/acs.jproteome.6b00038. [DOI] [PubMed] [Google Scholar]
- 26.Wang T.T., Sewatanon J., Memoli M.J., Wrammert J., Bournazos S., Bhaumik S.K. IgG antibodies to dengue enhanced for FcγRIIIA binding determine disease severity. Science. 2017;355:395–398. doi: 10.1126/science.aai8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen M.D., de Graaf E.L., Sonneveld M.E., Plomp H.R., Linty F., Visser R. Afucosylated immunoglobulin G responses are a hallmark of enveloped virus infections and show an exacerbated phenotype in COVID-19. https://www.biorxiv.org/content/10.1101/2020.05.18.099507v1 Available from: Accessed July 5, 2020.
- 28.Klasse P.J. Neutralization of virus infectivity by antibodies: old problems in new perspectives. Adv Biol. 2014;2014:157895. doi: 10.1155/2014/157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prendergast A.J., Klenerman P., Goulder P.J. The impact of differential antiviral immunity in children and adults. Nat Rev Immunol. 2012;12:636–648. doi: 10.1038/nri3277. [DOI] [PubMed] [Google Scholar]
- 30.Dowling D.J., Levy O. Ontogeny of early life immunity. Trends Immunol. 2014;35:299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon A.K., Hollander G.A., McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Royal Soc B Biol Sci. 2015;282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thome J.J., Bickham K.L., Ohmura Y., Kubota M., Matsuoka N., Gordon C. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med. 2016;22:72. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng P.C., Lam C.W., Li A.M., Wong C.K., Cheng F.W., Leung T.F. Inflammatory cytokine profile in children with severe acute respiratory syndrome. Pediatrics. 2004;113:e7–e14. doi: 10.1542/peds.113.1.e7. [DOI] [PubMed] [Google Scholar]
- 34.Henry B.M., Lippi G., Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020;58:1135–1138. doi: 10.1515/cclm-2020-0272. [DOI] [PubMed] [Google Scholar]
- 35.Wong C., Lam C., Wu A., Ip W., Lee N., Chan I. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang C.-W., Lee Y.-Z., Hsu H.-Y., Shih C., Chao Y.-S., Chang H.-Y. Targeting coronaviral replication and cellular JAK2 mediated dominant NF-κB activation for comprehensive and ultimate inhibition of coronaviral activity. Sci Rep. 2017;7:1–13. doi: 10.1038/s41598-017-04203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshikawa T., Hill T., Li K., Peters C.J., Tseng C.-T.K. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez L., Pekkarinen P., Tadepally L.K., Tan Z., Rosat Consiglio C., Pou C. Systems-level immunomonitoring from acute to recovery phase of severe COVID-19. Cell Rep Med. 2020;1:100078. doi: 10.1016/j.xcrm.2020.100078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franceschi C., Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69:S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 40.Franceschi C., Bonafè M., Valensin S., Olivieri F., De Luca M., Ottaviani E. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 41.Oh S.J., Lee J.K., Shin O.S. Aging and the immune system: the impact of immunosenescence on viral infection, immunity and vaccine immunogenicity. Immune Netw. 2019;19:e37. doi: 10.4110/in.2019.19.e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poland G.A., Ovsyannikova I.G., Kennedy R.B., Lambert N.D., Kirkland J.L. A systems biology approach to the effect of aging, immunosenescence and vaccine response. Curr Opin Immunol. 2014;29:62–68. doi: 10.1016/j.coi.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panda A., Qian F., Mohanty S., van Duin D., Newman F.K., Zhang L. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol. 2010;184:2518–2527. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bektas A., Schurman S.H., Sen R., Ferrucci L. Human T cell immunosenescence and inflammation in aging. J Leukocyte Biol. 2017;102:977–988. doi: 10.1189/jlb.3RI0716-335R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging. 2020;12:9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shaw A.C., Joshi S., Greenwood H., Panda A., Lord J.M. Aging of the innate immune system. Curr Opin Immunol. 2010;22:507–513. doi: 10.1016/j.coi.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovacs E.J., Boe D.M., Boule L.A., Curtis B.J. Inflammaging and the lung. Clin Geriatr Med. 2017;33:459–471. doi: 10.1016/j.cger.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26:842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 49.Imai Y., Kuba K., Ohto-Nakanishi T., Penninger J.M. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circulation J. 2010;74:405–410. doi: 10.1253/circj.cj-10-0045. [DOI] [PubMed] [Google Scholar]
- 50.Nicholls J., Peiris M. Good ACE, bad ACE do battle in lung injury, SARS. Nat Med. 2005;11 doi: 10.1038/nm0805-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jerng J.-S., Hsu Y.-C., Wu H.-D., Pan H.-Z., Wang H.-C., Shun C.-T. Role of the renin-angiotensin system in ventilator-induced lung injury: an in vivo study in a rat model. Thorax. 2007;62:527–535. doi: 10.1136/thx.2006.061945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calò L.A., Rigato M., Bertoldi G. ACE2/Angiotensin 1-7 protective antiinflammatory and antioxidant role in hyperoxic lung injury: support from studies in Bartter’s and Gitelman’s syndromes. QJM. 2020;113:440–441. doi: 10.1093/qjmed/hcz319. [DOI] [PubMed] [Google Scholar]
- 53.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fang Y., Gao F., Liu Z. Angiotensin-converting enzyme 2 attenuates inflammatory response and oxidative stress in hyperoxic lung injury by regulating NF-κB and Nrf2 pathways. QJM. 2019;112:914–924. doi: 10.1093/qjmed/hcz206. [DOI] [PubMed] [Google Scholar]
- 55.Arnold R.H. COVID-19—does this disease kill due to imbalance of the renin angiotensin system (RAS) caused by genetic and gender differences in the response to viral ACE 2 attack? Heart Lung Circ. 2020;29:964–972. doi: 10.1016/j.hlc.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.AlGhatrif M., Cingolani O., Lakatta E.G. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: insights from cardiovascular aging science. JAMA Cardiol. 2020;5:747–748. doi: 10.1001/jamacardio.2020.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bunyavanich S., Do A., Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bilinska K., Jakubowska P., Von Bartheld C.S., Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 61.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Investig. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He L., Ding Y., Zhang Q., Che X., He Y., Shen H. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saleem T.M., Bharani K., Gauthaman K. ACE inhibitors–angiotensin II receptor antagonists: a useful combination therapy for ischemic heart disease. Open Access Emerg Med. 2010;2:51. doi: 10.2147/oaem.s10507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vuille-dit-Bille R.N., Camargo S.M., Emmenegger L., Sasse T., Kummer E., Jando J. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693–705. doi: 10.1007/s00726-014-1889-6. [DOI] [PubMed] [Google Scholar]
- 67.Wysocki J., Ye M., Soler M.J., Gurley S.B., Xiao H.D., Bernstein K.E. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55:2132–2139. doi: 10.2337/db06-0033. [DOI] [PubMed] [Google Scholar]
- 68.Bush W.S., Oetjens M.T., Crawford D.C. Unravelling the human genome-phenome relationship using phenome-wide association studies. Nat Rev Genet. 2016;17:129–145. doi: 10.1038/nrg.2015.36. [DOI] [PubMed] [Google Scholar]
- 69.Rao S., Lau A., So H.-C. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of 2019-nCov: a Mendelian randomization analysis highlights tentative relevance of diabetes-related traits. https://www.medrxiv.org/content/10.1101/2020.03.04.20031237v2 Available from: Accessed July 9, 2020. [DOI] [PubMed]
- 70.Zeng C., Shi X., Zhang B., Liu H., Zhang L., Ding W. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J Mol Med (Berl) 2012;90:175–186. doi: 10.1007/s00109-011-0816-5. [DOI] [PubMed] [Google Scholar]
- 71.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes/Metab Res Rev. 2020:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang K., Chen W., Zhou Y.-S., Lian J.-Q., Zhang Z., Du P. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. https://www.biorxiv.org/content/10.1101/2020.03.14.988345v1 Available from: Accessed May 10, 2020.
- 73.Kong L.-M., Liao C.-G., Zhang Y., Xu J., Li Y., Huang W. A regulatory loop involving miR-22, Sp1, and c-Myc modulates CD147 expression in breast cancer invasion and metastasis. Cancer Res. 2014;74:3764–3778. doi: 10.1158/0008-5472.CAN-13-3555. [DOI] [PubMed] [Google Scholar]
- 74.Wang S., Liu C., Liu X., He Y., Shen D., Luo Q. Effects of matrix metalloproteinase inhibitor doxycycline and CD147 antagonist peptide-9 on gallbladder carcinoma cell lines. Tumor Biol. 2017;39 doi: 10.1177/1010428317718192. 1010428317718192. [DOI] [PubMed] [Google Scholar]
- 75.World Health Organization . 2020. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19 [Scientific Brief]https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 Available from: Accessed May 28, 2020. [Google Scholar]
- 76.Royal College of Paediatrics and Child Health . 2020. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19.https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf Available from: Accessed May 12, 2020. [Google Scholar]
- 77.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Holman R.C., Belay E.D., Christensen K.Y., Folkema A.M., Steiner C.A., Schonberger L.B. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29:483–488. doi: 10.1097/INF.0b013e3181cf8705. [DOI] [PubMed] [Google Scholar]
- 82.Marchesi A., Tarissi de Jacobis I., Rigante D., Rimini A., Malorni W., Corsello G. Kawasaki disease: guidelines of the Italian Society of Pediatrics, part I—definition, epidemiology, etiopathogenesis, clinical expression and management of the acute phase. Ital J Pediatr. 2018;44:102. doi: 10.1186/s13052-018-0536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shirato K., Imada Y., Kawase M., Nakagaki K., Matsuyama S., Taguchi F. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J Med Virol. 2014;86:2146–2153. doi: 10.1002/jmv.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esper F., Shapiro E.D., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ebihara T., Endo R., Ma X., Ishiguro N., Kikuta H. Lack of association between New Haven coronavirus and Kawasaki disease. J Infect Dis. 2005;192:351–352. doi: 10.1086/430797. author reply 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salazar G., Zhang N., Fu T.-M., An Z. Antibody therapies for the prevention and treatment of viral infections. NPJ Vaccines. 2017;2:19. doi: 10.1038/s41541-017-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Young B.E., Chen M. Influenza in temperate and tropical Asia: a review of epidemiology and vaccinology. Hum Vaccin Immunother. 2020;16:1659–1667. doi: 10.1080/21645515.2019.1703455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hu Z., Song C., Xu C., Jin G., Chen Y., Xu X. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kam K.Q., Yung C.F., Cui L., Lin Tzer Pin R., Mak T.M., Maiwald M. A well infant with coronavirus disease 2019 (COVID-19) with high viral load. Clin Infect Dis. 2020;71:847–849. doi: 10.1093/cid/ciaa201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cao W.C., Liu W., Zhang P.H., Zhang F., Richardus J.H. Disappearance of antibodies to SARS-associated coronavirus after recovery. N Engl J Med. 2007;357:1162–1163. doi: 10.1056/NEJMc070348. [DOI] [PubMed] [Google Scholar]
- 92.Mo H., Zeng G., Ren X., Li H., Ke C., Tan Y. Longitudinal profile of antibodies against SARS-coronavirus in SARS patients and their clinical significance. Respirol. 2006;11:49–53. doi: 10.1111/j.1440-1843.2006.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]