Abstract
Background:
Cancer patients often experience decreased quality of life during chemotherapy. This study aimed to determine the preliminary efficacy and safety of Reishi & Privet Formula (RPF) for maintaining quality of life among patients with non–small cell lung cancer (NSCLC) undergoing chemotherapy.
Methods:
We conducted a phase II randomized, double-blind, placebo-controlled clinical trial in China. Adults with NSCLC scheduled to receive chemotherapy were randomly assigned (3:1 ratio) to receive oral RPF (3.36 g/day) or placebo daily for 6 weeks. The main outcome was the Functional Assessment of Cancer Therapy–Lung (FACT-L). We evaluated RPF’s safety profile using the Common Terminology Criteria for Adverse Events and assessed changes in outcome measures from baseline to weeks 3 and 6 using a linear mixed effects model.
Results:
We enrolled 82 participants across 8 cancer centers in China. The median age was 59 years, 56 (68%) had advanced cancer. Compared with the placebo group, the RPF group had nonstatistically significant higher quality of life as measured by the FACT-L total score (P = .086) over 2 cycles of chemotherapy. The RPF group was associated with a nonsignificant better general health (P = .050) and emotional well-being (P = .090) than the placebo group. Adverse events rates did not differ between groups.
Conclusions:
This study demonstrated preliminary safety and suggests a promising trend in RPF’s effect on maintaining quality of life and emotional well-being among NSCLC patients undergoing chemotherapy. Future adequately powered randomized-controlled trials are needed to verify the efficacy and safety of RPF in cancer patients undergoing chemotherapy.
Keywords: Reishi & Privet formula, traditional Chinese medicine, herbs, quality of life, non–small cell lung cancer, chemotherapy, randomized placebo-controlled trial
Background
Lung cancer is the most frequently diagnosed cancer and the leading cause of cancer deaths worldwide.1 Non–small cell lung cancer (NSCLC) accounts for approximately 85% of all new lung cancer cases. The average 5-year survival rate of NSCLC is no more than 23%.2 Patients often present at an advanced stage with a dismal prognosis (6% 5-year survival rate at distant metastatic stage)3 and suffer from a high burden of symptoms and poor quality of life (QoL). Improving QoL is one of the most important goals for NSCLC patients.4 Chemotherapy remains an essential component of NSCLC treatment even in the era of precision medicine.5 Unfortunately, its toxic side-effects have a detrimental effect on patients’ QoL6-8 and lead to premature treatment discontinuation.9,10 Furthermore, lower QoL and depressed mood are associated with shorter survival among patients.11-14 Therefore, effective interventions to prevent the decline of QoL in patients undergoing chemotherapy are critically needed.
The fruit body of Ganoderma lucidum (Leyss ex Fr) Karst, also known as Reishi mushroom (Lingzhi, Ganoderma), is one of the most popular herbal dietary supplements used by cancer patients around the world.15 Preclinical findings reveal that Reishi mushroom (Lingzhi) has chemopreventive, tumoricidal, and immunostimulating abilities,16-24 increases the overall survival of tumor bearing mice,25 alleviates chemotherapy-induced side effects,26-28 and has a possible synergistic effect with cisplatin.21 A number of clinical studies have suggested that G lucidum is well tolerated among cancer patients, has potential effects on immune modulation, and improves QoL and survival outcomes.29-33 However, these trials often include various cancer types, with heterogeneous cancer treatment, and have small sample sizes. More clinical research is needed to determine its safety and efficacy in lung cancer patients undergoing chemotherapy.
The Reishi & Privet Formula (RPF) consists of the dried sporederm-broken spores of the artificially cultivated G lucidum Karst and ethanol extracts and water extracts from the dried mature fruit (glossy Privet fruit, NuZhenzi, Ligustri Lucidi Fructus) of Ligustrum lucidum Ait. Glossy Privet fruit (NuZhenzi) is also widely used by cancer patients in China as an adjuvant because of its immunomodulatory,34,35 antitumor,36-38 anti-inflammatory, and hepatoprotective properties39,40 as demonstrated in in vivo and in vitro studies. This specific formula aims to achieve better synergetic efficacy and safety than each individual component.
RPF was developed from clinical practice in China based on traditional Chinese medicine theory, which underlies Jun-Chen-Zuo-Shi medicinal compatibility strategy. Reishi mushroom (Lingzhi) spores, the principal component (Jun herb) of RPF, perform the primary action of treating qi deficiency. Glossy Privet fruit (NuZhenzi), the adjuvant component (Chen herb) in treating yin deficiency, synergizes with Jun to strengthen its therapeutic effects and reduce or eliminate possible adverse effects. NSCLC patients undergoing chemotherapy usually demonstrate a cluster of symptoms41,42 (eg, fatigue, drowsiness, loss of appetite, shortness of breath, cough, anxiety, insomnia, and dry mouth), which may be diagnosed as a qi-yin deficiency symptom pattern.43 The RPF formula is designed to tonify energy, reinforce deficiency, and relieve symptoms in a patient-centered manner for those with qi-yin deficiency symptom pattern.
Based on previous clinical experience, preclinical and clinical research data, RPF may play a role of improving cancer patients’ QoL in multiple ways. However, clinical studies of RPF are lacking. We proposed to investigate the preliminary efficacy and safety of RPF for NSCLC patients undergoing chemotherapy to inform the development and design of future adequately powered randomized controlled trials (RCTs) to test the specific efficacy and safety of this product.
Patients and Methods
Study Design
This was a phase II randomized, double-blind, placebo-controlled clinical trial conducted at 8 cancer research centers in China (Appendix 1; available online). The independent ethics committees at each center approved the study (Appendix 1).
Participants
Eligible patients were 18 to 75 years of age with confirmed NSCLC and greater than 3 months of life expectancy who were scheduled to receive at least 2 cycles of chemotherapy with either paclitaxel plus cisplatin (TP, paclitaxel 135 mg/m2 on day 1, cisplatin 75 mg/m2 on day 2, every 3 weeks) or paclitaxel plus carboplatin (TC, paclitaxel 200 mg/m2 on day 1, carboplatin AUC [area under the curve] 6 on day 1, every 3 weeks).
Exclusion criteria were as follows: (1) Karnofsky performance status < 70; (2) laboratory values obtained prior to randomization: white blood cell <3 × 109/L, absolute neutrophil count <1.5 × 109/L, platelet <75 × 1012/L, hemoglobin <8 g/dL, creatinine >1.5 × upper normal limit (UNL), bilirubin >2 × UNL, aspartate aminotransferase, alanine aminotransferase, or alkaline phosphatase >2.5 × UNL (>5 × UNL in presence of liver metastases); (3) severe/uncontrolled systemic diseases, including gastrointestinal dysfunction, bleeding, cardiac dysfunction, endocrine dysfunction, or infection; (4) significant cognitive impairment or psychiatric disturbance; (5) allergy to RPF or active ingredients; (6) use of other investigational drug within 30 days before study entry; and (7) any relevant condition potentially interfering with study evaluation. We recorded concomitant medications taken during the study.
We recruited subjects via media and print advertisements. Patients provided written informed consent before participating in the study. A trained study coordinator made initial contact with the subjects; all study-related procedures were performed at 8 cancer research centers in China.
Randomization and Blinding
Randomization was done via a computer-generated list and stratified by center. An independent biostatistician with no clinical involvement in the trial prepared the randomization sequence and allocation concealment. Sealed envelopes containing allocation assignment and packaged medication/placebo were sent to each of the 8 centers. Investigators enrolled patients at each center. The blinded pharmacist at each center provided the study team with ready-to-use blinded medication/placebo according to enrollment order.
All investigators, staff, participants, and sponsor personnel or delegate(s) who were involved in the treatment administration or clinical evaluation of the subjects were blinded to the group assignments. The chemotherapy agents were open-label. Researchers were required to document the chemotherapy regimen patients received prior to randomization. Study medications were supplied in a blinded manner as RPF/placebo capsules and were packaged identically. Each patient received 2 boxes of RPF or placebo, one for the first treatment period (day 1 to day 21) and another for the second treatment period (day 22 to day 42).
Intervention
Patients scheduled to receive either TP or TC were randomly assigned (3:1 ratio) to receive oral RPF (3.36 g/day) or placebo daily for 6 weeks concurrent with their chemotherapy. Patients were instructed to take 4 RPF/placebo capsules orally 3 times a day (in the morning, at noon, and in the afternoon) for a total of 3.36 g/day. Each RPF capsule (Nanjing Zhongke Pharmaceutical Co, Ltd, batch number B20160401) weighed 0.28 g and contained 0.15 g of sporederm-broken Reishi mushroom (Lingzhi) spore, 0.11 g of glossy Privet fruit (NuZhenzi) extract, 5% crospovidone, and 2% silica. Each capsule was standardized to contain >1% polysaccharide and >2.0 mg of oleanolic acid (C30H48O3). It was manufactured in accordance with good manufacturing practices (Certificate of GMP No. JS20160640) and tested for batch-to-batch consistency according to the State Pharmacopoeia of the People’s Republic of China (2000).44 Reishi mushroom (Lingzhi) spore is the dried spore of G lucidium Karst, which is collected and dried when the artificially cultivated plants grow mature and release spores. Glossy Privet fruit (NuZhenzi) is the dried mature fruit of Ligustrum lucidum Ait, which is harvested in winter when the fruits are mature. Glossy Privet fruit (NuZhenzi) was extracted using 70% ethanol and the residual was further extracted using water. The extracts were then combined and mixed evenly with the shell-broken Reishi mushroom (Lingzhi) spore. After drying, an appropriate amount of microcrystalline cellulose was added, and the mixture was granulated and dried. Crospovidone (5%) and silica (2%) were added to make the capsule. The placebo capsule (Nanjing Zhongke Pharmaceutical Co, Ltd) was filled with 52.8% caramel color, 40.7% rice powder, and 6.5% brown iron oxide pigment. The placebo pill also weighed 0.28 g and was packed in identical capsules as the RPF. The study medication and placebo were stored in a locked cabinet at room temperature at each research center.
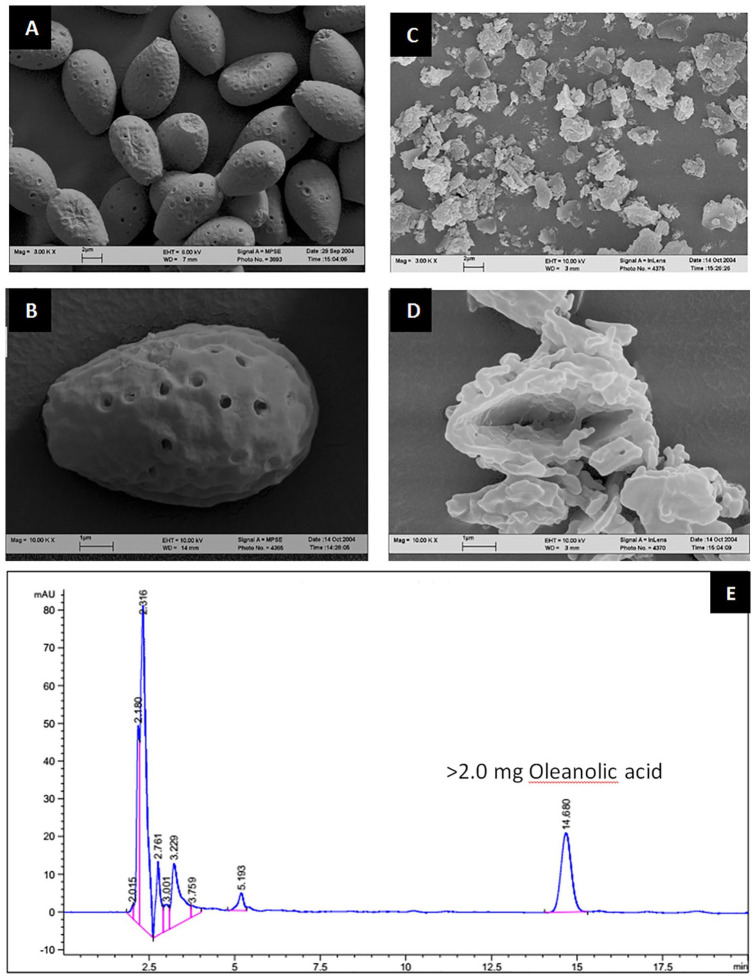
The sporederm-broken Ganoderma spore micrographs produced by electron microscopy are shown in Figure 1a. The RPF was subjected to high-performance liquid chromatography (HPLC); a HPLC fingerprint of the extract is shown in Figure 1b.
Figure 1.
(a) Reishi & Privet Formula (RPF) micrographs. (A) Raw Ganoderma spore. (B) Raw Ganoderma spore. (C) Sporederm-broken Ganoderma spore. (D) Sporederm-broken Ganoderma spore. (b) HPLC fingerprint of the Reishi & Privet formula. HPLC, high-performance liquid chromatography.
Outcomes
The main outcome was the 36-item self-report Functional Assessment of Cancer Therapy–Lung (FACT-L 4.0,45,46 Chinese version), a disease-specific questionnaire including the 27-item FACT-G (core instrument), and the 9-item Lung Cancer Subscale (LCS). The total FACT-G score is calculated by summing the subscale scores of physical well-being, social/family well-being, emotional well-being, and functional well-being. A total FACT-L score is obtained by summing the FACT-G score with the LCS (2 of the 9 items are not used in the scoring), thereby augmenting the FACT-G with lung cancer-specific QoL information. We also calculated a 21-item Trial Outcome Index (TOI) by summing physical well-being, functional well-being, and the LCS. All questions are rated on 5-point Likert-type scales ranging from 0 (not at all) to 4 (very much). A higher score indicates better QoL or fewer symptoms. We collected all patient-reported outcome measures at baseline (before starting treatment), and at 3 and 6 weeks.
Investigators evaluated adverse events each week and graded them according to the Common Terminology Criteria for Adverse Events version 4.0.47 Symptomatic adverse events evaluated during the treatment included fatigue, insomnia, decreased appetite, constipation, diarrhea, bloating, and nausea. We evaluated white blood cell, neutrophil, hemoglobin, and platelets in the peripheral blood at baseline and every week after the start of chemotherapy. We evaluated creatinine, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase in the peripheral blood at baseline, 3 weeks, and 6 weeks.
Statistical Analysis
This study was designed to estimate the initial effect size and safety. It is important to note that statistical significance is not the primary goal in determining the success of a phase II trial. We estimated that with 84 patients, the study would have 80% power to detect a significant between-group difference in the change in total FACT-L score from baseline to 6 weeks, with a large effect size of 0.7 SD.48 The primary outcome of the pilot study was change from baseline to 6 weeks in the FACT-L total score.
Our analyses were guided by intention-to-treat principles. We used the χ2 test to compare baseline variables between groups. Because our primary and secondary outcome measures were repeated measures over time, we assessed differences in changes from baseline to week 3 and week 6 using mixed effect models. We treated time and treatment as categorical variables and included a random intercept term in the mixed effect model. Tests of intention-to-treat differences between intervention arms with regard to the change were based on time-intervention interactions in the mixed effect models. We have presented results as between-group differences with 95% confidence intervals (CIs). All statistical tests were 2-sided.
Statistical significance was set at the <.05 level. All statistical analysis was conducted using STATA (version 15.0; STATA Corporation) and SAS (version 9.4; SAS Institute).
Results
Baseline Characteristics of the Participants
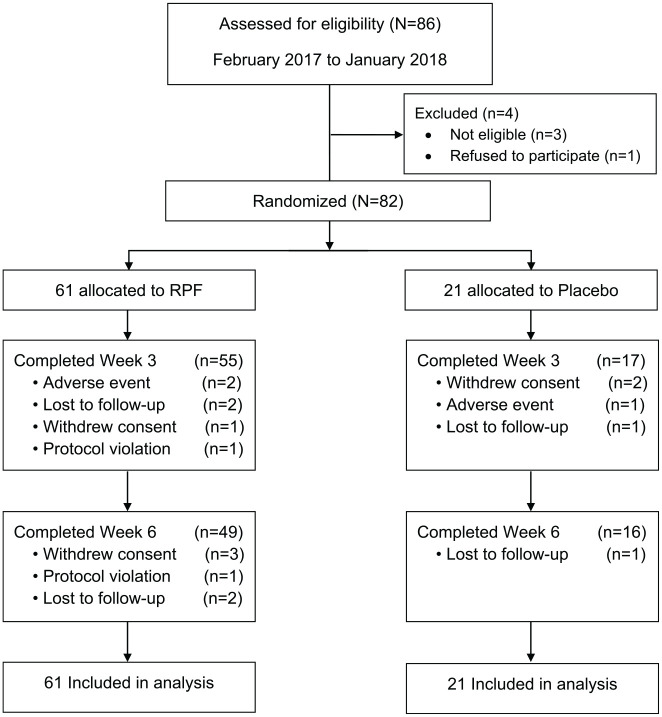
Between February 2017 and January 2018, we enrolled 82 participants across 8 cancer centers in China. In the RFT group, 80% (N = 49) completed all study-related activities; 76% (N = 16) in the placebo group completed all study-related activities (Figure 2). In the RFT group, 2 out of 61 (3.3%) discontinued therapy due to adverse events compared with 1 out of 21 (4.8%) in the placebo group. In addition, 4 (6.6%) patients in the RPF group and 2 (9.5%) patients in the placebo group withdrew consent; 4 (6.6%) patients in RPF and 2 (9.5%) in placebo were lost to follow-up; and the study PI withdrew 2 (3.3%) patients from the RPF group due to protocol violation (Figure 2).
Figure 2.
CONSORT diagram.
Patient demographic and clinical characteristics were well balanced between the 2 treatment groups and are summarized in Table 1. Of the 82 randomized patients, the median age was 59 years (range = 29-75 years), 58 (71%) were males, and the median Karnofsky performance score was 90 (range = 70-100). Among the participants, 49 (60%) had a diagnosis of non-squamous cell lung cancer. The majority (n = 56, 68%) had advanced (stages IIIB and IV) lung cancer, 48 (59%) had no prior history of chemotherapy before enrollment into the study, 48 (59%) received TP, and 34 (41%) received TC as a chemotherapy regimen. The mean FACT-L total score was 89.1 and the mean FACT-G score was 70.5.
Table 1.
Baseline Patient Characteristics.
| Total (N = 82) | RPF (N = 61) | Placebo (N = 21) | |
|---|---|---|---|
| Age in years, median (range) | 59 (29-75) | 59 (29-75) | 55 (39-70) |
| <65, n (%) | 66 (80) | 48 (79) | 18 (86) |
| ≥65, n (%) | 16 (20) | 13 (21) | 3 (14) |
| Gender, n (%) | |||
| Male | 58 (71) | 45 (74) | 13 (62) |
| Female | 24 (29) | 16 (26) | 8 (38) |
| Cancer stage, n (%) | |||
| Stage I | 5 (6) | 4 (7) | 1 (5) |
| Stage II | 15 (18) | 11 (18) | 4 (19) |
| Stage IIIA | 6 (7) | 4 (7) | 2 (9) |
| Stage IIIB | 14 (17) | 9 (14) | 5 (24) |
| Stage IV | 42 (51) | 33 (54) | 9 (43) |
| Histology, n (%) | |||
| Squamous | 32 (40) | 24 (40) | 8 (38) |
| Non-squamous | 49 (60) | 36 (60) | 13 (62) |
| Chemotherapy scheme, n (%) | |||
| Paclitaxel plus cisplatin (TP) | 48 (59) | 35 (57) | 13 (62) |
| Paclitaxel plus carboplatin (TC) | 34 (41) | 26 (43) | 8 (38) |
| Past chemotherapya, n (%) | |||
| Yes | 34 (41) | 25 (41) | 9 (43) |
| No | 48 (59) | 36 (59) | 12 (57) |
| KPS score, median (range) | 90 (70-100) | 90 (70-90) | 90 (70-100) |
| FACT-L, mean (SD) | |||
| FACT-L total | 89.1 (17.1) | 88.1 (16.8) | 91.9 (18.2) |
| FACT-G total | 70.5 (14.4) | 69.7 (14.4) | 72.7 (14.3) |
| Trial Outcome indexb | 53.6 (11.3) | 52.8 (10.5) | 56.1 (13.3) |
| Lung cancer subscale | 18.6 (4.2) | 18.3 (4.0) | 19.2 (4.8) |
| Physical well-being | 20.8 (4.7) | 20.6 (4.7) | 21.6 (4.7) |
| Social/family well-being | 20.0 (4.9) | 19.9 (4.5) | 20.0 (5.9) |
| Emotional well-being | 15.5 (4.9) | 15.4 (4.9) | 15.8 (4.9) |
| Functional well-being | 14.3 (2.5) | 13.9 (4.9) | 15.2 (6.0) |
Abbreviations: RPF, Reishi & Privet formula; KPS, Karnofsky performance score; FACT-L, Functional Assessment of Cancer Therapy–Lung; FACT-G, FACT-General.
Past chemotherapy means whether patients had received any chemotherapy before this study.
Trial Outcome Index = physical well-being + functional well-being + lung cancer subscale.
Quality of Life
We have presented our results in Table 2. Overall, the RPF group showed a better QoL measured by FACT-L total score (higher score indicates better QoL) compared with the placebo group from baseline to 6 weeks of treatment, although not statistically significant (P = .086 for treatment and time interaction term). At week 3 after the first cycle of chemotherapy, the RPF group reported a 7.99 (95% CI = 0.9 to 15.0) point higher in the FACT-L total score than the placebo group. At week 6 after 2 cycles of chemotherapy, the between group difference in the FACT-L total change score was 4.63 (95% CI = −2.5 to 11.7), favoring the RPF group (see Figure 3). We observed a similar trend in the FACT-G score and the emotional well-being domain score change. The RPF group showed a greater increase in the FACT-G score from baseline than the placebo group after 6 weeks with a P value of .05 approaching statistical significance. At week 3, the between group difference in the FACT-G score was 7.46 (95% CI = 1.5 to 13.4) favoring the RPF group. At week 6, the RPF group’s change from baseline in FACT-G score was still greater than the placebo group with a between group difference of 3.88 (95% CI = −2.0 to 9.8). In the emotional well-being domain, at week 3, the RPF group showed a 1.97 (95% CI = 0.2 to 3.7) point greater increase than the placebo group. At week 6, this trend was maintained with a between group difference of 0.8 (95% CI = −1.0 to 2.6) favoring the RPF group with an overall P value of .090 for the treatment and time interaction term.
Table 2.
Mean Change in FACT-L Scores From Baseline to 6 Weeks.
| Outcomes | Visit | Change from baseline, mean (95% CI) | Between group differences, mean (95% CI) | P a | |
|---|---|---|---|---|---|
| RPF (N = 52) | Placebo (N = 17) | RPF versus placebo | |||
| FACT-Lb | .086 | ||||
| Week 3 | 2.39 (−1.10 to 5.88) | −5.60 (−11.7 to 0.5) | 7.99 (0.9 to 15.0) | ||
| Week 6 | −0.05 (−3.62 to 3.52) | −4.68 (−10.8 to 1.5) | 4.63 (2.5 to 11.7) | ||
| Physical well-being | .32 | ||||
| Week 3 | 0.23 (−0.75 to 1.21) | −0.84 (−2.6 to 0.9) | 1.08 (−0.9 to 3.1) | ||
| Week 6 | −0.83 (−1.83 to 0.17) | 0.38 (−2.1 to 1.4) | −0.45 (−2.5 to 1.6) | ||
| Social/family well-being | .41 | ||||
| Week 3 | 0.16 (−1.25 to 1.57) | −1.5 (−4.0 to 1.0) | 1.66 (−1.2 to 4.5) | ||
| Week 6 | 0.29 (−1.16 to 1.74) | −1.4 (−3.9 to 1.1) | 1.69 (−1.2 to 4.6) | ||
| Emotional well-being | .090 | ||||
| Week 3 | 1.02 (0.14 to 1.90) | −0.95 (−2.5 to 0.6) | 1.97 (0.2 to 3.7) | ||
| Week 6 | 0.68 (−0.22 to 1.58) | −0.13 (−1.7 to 1.4) | 0.8 (−1.0 to 2.6) | ||
| Functional well-being | .10 | ||||
| Week 3 | 0.52 (−0.79 to 1.83) | −2.38 (−4.7 to −0.1) | 2.90 (0.2 to 5.6) | ||
| Week 6 | −0.64 (−1.99 to 0.71) | −2.43 (−4.7 to −0.1) | 1.79 (−0.9 to 4.5) | ||
| Lung cancer subscale | .80 | ||||
| Week 3 | 0.52 (−0.58 to 1.62) | −0.03 (−2.0 to 1.9) | 0.55 (−1.7 to 2.8) | ||
| Week 6 | 0.44 (−0.68 to 1.56) | −0.28 (−2.2 to 1.6) | 0.73 (−1.5 to 2.9) | ||
| Trial outcome indexc | .18 | ||||
| Week 3 | 1.21 (−1.10 to 3.52) | −3.21 (−7.3 to 0.8) | 4.42 (−0.2 to 9.1) | ||
| Week 6 | −1.09 (−3.46 to 1.28) | −3.17 (−7.2 to 0.9) | 2.08 (−2.6 to 6.8) | ||
| FACT-Gd | .050 | ||||
| Week 3 | 1.90 (−1.02 to 4.82) | −5.56 (−10.7 to −0.4) | 7.46 (1.5 to 13.4) | ||
| Week 6 | −0.48 (−3.46 to 2.50) | −4.36 (−9.5 to 0.8) | 3.88 (−2.0 to 9.8) | ||
Abbreviations: FACT-L, Functional Assessment of Cancer Therapy–Lung, CI, confidence interval; RPF, Reishi & Privet formula; FACT-G, FACT-General.
Mixed effect P-value from interaction term between visit and treatment.
FACT-L = FACT-G + LCS.
Trial Outcome Index = physical well-being + functional well-being + lung cancer subscale.
FACT-G = physical well-being + social/family well-being + emotional well-being + functional well-being.
Figure 3.
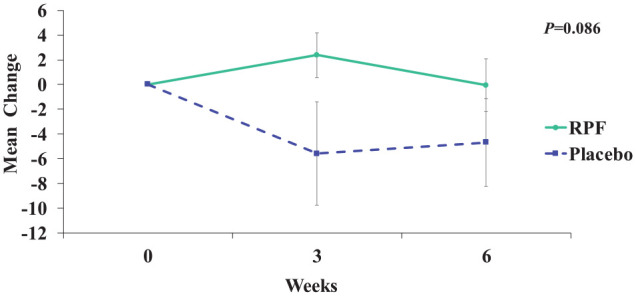
Mean change in FACT-L (Functional Assessment of Cancer Therapy–Lung) total scores from baseline to 6 weeks.
RPF, Reishi & Privet formula.
Safety
Adverse events were generally mild and moderate in severity and were similar between treatment groups. The most frequently reported chemotherapy-related adverse events were gastrointestinal, neutropenia, anemia, fatigue, and decreased appetite. Two adverse events (abdominal bloating and insomnia) were classified as possibly related to the RPF study drug. Among patients who reported fatigue after the start of chemotherapy in the RPF group, fatigue either improved or remained unchanged in 13 out of 15 (87%) patients. However, in the placebo group only 1 in 3 (33%) patients who experienced fatigue at the start of the chemotherapy reported improved or unchanged fatigue. No group differences were observed in the frequency and severity of other adverse events such as neutropenia, anemia, thrombocytopenia, and hepatobiliary and renal disorders.
Discussion
In this phase II, randomized, double-blind, placebo-controlled clinical trial, we evaluated the preliminary efficacy and safety of RPF for maintaining QoL among patients with NSCLC undergoing chemotherapy. Participants who received RPF had nonsignificant higher QoL scores than those who were randomized to the placebo group. We observed a greater increase in the RPF group’s week 3 and week 6 FACT-L, FACT-G, and emotional well-being scores compared with the placebo group. More patients in the RPF group who experienced fatigue at the beginning of chemotherapy were able to either maintain or reduce their fatigue during chemotherapy than those in the placebo group. The herbal formula was also well tolerated with few related adverse events. To our knowledge, this is the first clinical study of this Reishi mushroom-based herbal formula targeting QoL during chemotherapy in people with NSCLC and assessed by a well-established patient-reported outcome (FACT-L 4.0).
It is important to place our findings in the context of prior research involving Reishi mushroom products. Gao et al33 found the polysaccharide fractions extracted from G lucidum enhanced immune responses (cytokines, T-cell subsets, mitotic response to phytohemagglutinin, and natural killer activity) in advanced cancer patients. A pilot study suggested that spore powder of G lucidum may have beneficial effects on cancer-related fatigue and QoL, but the study population was breast cancer patients undergoing endocrine therapy.31 Two systematic reviews indicated G lucidum might have potential benefits on overall survival, tumor response, immunity stimulation, and Karnofsky performance status.29,30 We contributed to the literature by suggesting the potential effect of this Reishi mushroom-based formula in maintaining QoL and emotional wellbeing for NSCLC patients undergoing chemotherapy.
Considering that the majority of the study population was advanced NSCLC patients undergoing chemotherapy, deterioration in QoL was expected. The results of the Eastern Cooperative Oncology Group trial showed that chemotherapy regimens such as TP demonstrated significant decreases in QoL scores over 6 months. Their study6 used the TOI to measure the change in QoL (TOI is a subscale of the FACT-L and an indicator of the physical component of QoL). Of patients treated with TP, 36% reported worsened TOI of ≥5 units at week 6. TOI and FACT-L total scores decreased 8.3 and −9.9 points, respectively, at 6 months after receiving the first course of chemotherapy.
Although our study did not reach statistical significance, the magnitude of difference in QoL has potential clinical implications. The patients in the RPF group maintained FACT-L scores from baseline to 6 weeks (mean change = −0.05, 95% CI = −3.62 to 3.52), while patients in the placebo group had decreased QoL (mean change = −4.68, 95% CI = −10.8 to 1.5). In the RPF group, 38 (62%) patients showed improvement in the FACT-L at week 6, while only 9 (43%) patients in the placebo group showed improvement in FACT-L score. Meanwhile, the potential benefit over the placebo group in the FACT-G total score (P = .050) and emotional well-being (P = .090) was also consistent as seen in the FACT-L total scores. Compared with the results of the Eastern Cooperative Oncology Group trial,6,49 our study demonstrated that with RPF treatment, the FACT-L and FACT-G total scores did not decrease, and in fact showed some increase over time. This suggests that RPF might have the potential to improve QoL of lung cancer patients going through active treatments, a promising clinical benefit that requires further investigation.
Our study provided preliminary safety and tolerability data for the concurrent use of RPF with chemotherapy. RPF-related adverse events were rare and mild. A meta-analysis of clinical results showed similar evidence that Reishi mushroom (Lingzhi) was generally well tolerated with minimal side effects including nausea and insomnia, and no reports of significant hematological or hepatological toxicity.30 Although we did not observe any sign of RPF preventing objective adverse events such as neutropenia, anemia, or thrombocytopenia, we did observe that a greater proportion of patients in the RPF group who experienced fatigue at the start of chemotherapy were able to either maintain or reduce their fatigue during the rest of treatment. This might indicate that RPF has the potential to reduce chemotherapy-induced fatigue. One pilot RCT of 48 breast cancer patients undergoing endocrine therapy in a short-term (4 week) intervention study31 also suggests that the spore powder of G lucidum may have beneficial effects on the physical well-being domain and fatigue subscale as assessed by FACT-F and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Version 3.0 questionnaires. Future larger trials are needed to provide more definitive data of the safety of RPF and its impact on chemotherapy-related QoL measures.
Limitations
Our study has several limitations. This trial was designed as an unregistered phase II trial with a small sample size, which prevented us from providing definitive evidence on the safety and efficacy of RPF for QoL. Based on the knowledge gained from this trial, future prospectively registered and adequately powered trials are needed to verify the preliminary effects identified in this study. QoL and symptom burden typically gets worse within 1 week of chemotherapy infusion and gradually improve over the next few weeks; therefore, our infrequent patient-reported outcomes assessment may not have fully captured the impact of RPF on QoL. Finally, our study was conducted among a Chinese population; studies in other populations are needed to increase its generalizability.
Conclusions
Rigorous clinical research is essential in building the evidence base for the safe and effective use of herbal medicine for cancer patients.50 Our double-blinded RCT found preliminary efficacy and safety of RPF, a Reishi mushroom herbal product, in improving QoL for patients with NSCLC undergoing chemotherapy. These preliminary findings need to be verified in adequately powered, well-designed clinical trials to establish RPF’s definitive safety and efficacy.
Supplemental Material
Supplemental material, 4_Reishi_appendix_1_April_24_2020_Clean for Preliminary Efficacy and Safety of Reishi & Privet Formula on Quality of Life Among Non–Small Cell Lung Cancer Patients Undergoing Chemotherapy: A Randomized Placebo-Controlled Trial by Jie Liu, Jun J. Mao, Susan Qing Li and Hongsheng Lin in Integrative Cancer Therapies
Acknowledgments
We thank Nanjing Zhongke Pharmaceutical Co, Ltd (Nanjing, China) for providing the standardized RRF and placebo for the study. We would like to thank all study staff and participants. We thank Christina Seluzicki for her editorial assistance in the preparation of this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors report grant funding from Nanjing Zhongke Pharmaceutical Co, Ltd. Dr. Mao has also received grant funding unrelated to this study from Tibet Cheezheng Tibetan Medicine Co, Ltd.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Nanjing Zhongke Pharmaceutical Co, Ltd. Dr. Liu and Dr. Lin are supported by International Cooperative Research in Chinese Medicine of the National Administration of Traditional Chinese Medicine of the People’s Republic of China (Grant Number GZYYGJ2017008). Dr. Mao is supported by the Memorial Sloan Kettering Cancer Center’s Translational and Integrative Medicine Research Fund and a National Institutes of Health/National Cancer Institute Cancer Center grant (Grant Number P30 CA008748). The funding agencies had no role in the design, conduct, or analysis of the study, or the decision to submit the manuscript for publication.
ORCID iD: Jie Liu  https://orcid.org/0000-0002-6112-2947
https://orcid.org/0000-0002-6112-2947
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 2. American Society of Clinical Oncology. Non-small cell lung cancer survival rates, by stage. https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html.
- 3. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014. National Cancer Institute; 2017. [Google Scholar]
- 4. Gralla RJ, Hollen PJ, Msaouel P, Davis BV, Petersen J. An evidence-based determination of issues affecting quality of life and patient-reported outcomes in lung cancer: results of a survey of 660 patients. J Thorac Oncol. 2014;9:1243-1248. [DOI] [PubMed] [Google Scholar]
- 5. Hellmann MD, Li BT, Chaft JE, Kris MG. Chemotherapy remains an essential element of personalized care for persons with lung cancers. Ann Oncol. 2016;27:1829-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonomi P, Kim K, Fairclough D, et al. Comparison of survival and quality of life in advanced non-small-cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: results of an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2000;18:623-631. [DOI] [PubMed] [Google Scholar]
- 7. Langer CJ, Manola J, Bernardo P, et al. Cisplatin-based therapy for elderly patients with advanced non-small-cell lung cancer: implications of Eastern Cooperative Oncology Group 5592, a randomized trial. J Natl Cancer Inst. 2002;94:173-181. [DOI] [PubMed] [Google Scholar]
- 8. Sandler AB, Nemunaitis J, Denham C, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2000;18:122-130. [DOI] [PubMed] [Google Scholar]
- 9. Felip E, Rosell R, Maestre JA, et al. ; Spanish Lung Cancer Group. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol. 2010;28:3138-3145. [DOI] [PubMed] [Google Scholar]
- 10. Nadpara P. Determinants of early discontinuation of first-line chemotherapy, and associated outcomes among elderly with advanced non-small cell lung cancer. J Clin Oncol. 2019;37(15 suppl):9102-9102. [Google Scholar]
- 11. Maione P, Perrone F, Gallo C, et al. Pretreatment quality of life and functional status assessment significantly predict survival of elderly patients with advanced non-small-cell lung cancer receiving chemotherapy: a prognostic analysis of the multicenter Italian lung cancer in the elderly study. J Clin Oncol. 2005;23:6865-6872. [DOI] [PubMed] [Google Scholar]
- 12. Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol. 2009;27:5816-5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pirl WF, Temel JS, Billings A, et al. Depression after diagnosis of advanced non-small cell lung cancer and survival: a pilot study. Psychosomatics. 2008;49:218-224. [DOI] [PubMed] [Google Scholar]
- 14. Cherif HBS, Habibech S, Racil H, et al. Chemotherapy toxicity in advanced non-small cell lung cancer and its impact on survival. Eur Respir J. 2016;48(60):PA4841. [Google Scholar]
- 15. Hou YN, Deng G, Mao JJ. Practical application of “About Herbs” website: herbs and dietary supplement use in oncology settings. Cancer J. 2019;25:357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weng CJ, Yen GC. The in vitro and in vivo experimental evidences disclose the chemopreventive effects of Ganoderma lucidum on cancer invasion and metastasis. Clin Exp Metastasis. 2010;27:361-369. [DOI] [PubMed] [Google Scholar]
- 17. Min BS, Gao JJ, Nakamura N, Hattori M. Triterpenes from the spores of Ganoderma lucidum and their cytotoxicity against meth-A and LLC tumor cells. Chem Pharm Bull. 2000;48:1026-1033. [DOI] [PubMed] [Google Scholar]
- 18. Tang W, Liu JW, Zhao WM, Wei DZ, Zhong JJ. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006;80:205-211. [DOI] [PubMed] [Google Scholar]
- 19. Cao QZ, Lin ZB. Ganoderma lucidum polysaccharides peptide inhibits the growth of vascular endothelial cell and the induction of VEGF in human lung cancer cell. Life Sci. 2006;78:1457-1463. [DOI] [PubMed] [Google Scholar]
- 20. Yun TK. Update from Asia. Asian studies on cancer chemoprevention. Ann N Y Acad Sci. 1999;889:157-192. [DOI] [PubMed] [Google Scholar]
- 21. Furusawa E, Chou SC, Furusawa S, Hirazumi A, Dang A. Antitumour activity of Ganoderma lucidum, an edible mushroom, on intraperitoneally implanted Lewis lung carcinoma in synergenic mice. Phytother Res. 1992;6:300-304. [Google Scholar]
- 22. Wang SY, Hsu ML, Hsu HC, et al. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer. 1997;70:699-705. [DOI] [PubMed] [Google Scholar]
- 23. Zhu XL, Lin ZB. Modulation of cytokines production, granzyme B and perforin in murine CIK cells by Ganoderma lucidum polysaccharides. Carbohydr Polym. 2006;63:188-197. [Google Scholar]
- 24. Kimura Y, Taniguchi M, Baba K. Antitumor and antimetastatic effects on liver of triterpenoid fractions of Ganoderma lucidum: mechanism of action and isolation of an active substance. Anticancer Res. 2002;22(6A):3309-3318. [PubMed] [Google Scholar]
- 25. Lee SS, Wei YH, Chen CF, Wang SY, Chen KY. Antitumor effects of Ganoderma lucidum. J Chin Med. 1995;6:1-12. [Google Scholar]
- 26. Wang CZ, Basila D, Aung HH, et al. Effects of Ganoderma lucidum extract on chemotherapy-induced nausea and vomiting in a rat model. Am J Chin Med. 2005;33:807-815. [DOI] [PubMed] [Google Scholar]
- 27. Ouyang MZ, Lin LZ, Lv WJ, et al. Effects of the polysaccharides extracted from Ganoderma lucidum on chemotherapy-related fatigue in mice. Int J Biol Macromol. 2016;91:905-910. [DOI] [PubMed] [Google Scholar]
- 28. Pillai TG, John M, Sara Thomas G. Prevention of cisplatin induced nephrotoxicity by terpenes isolated from Ganoderma lucidum occurring in southern parts of India. Exp Toxicol Pathol. 2011;63:157-160. [DOI] [PubMed] [Google Scholar]
- 29. Zhong L, Yan P, Lam WC, Yao L, Bian Z. Coriolus versicolor and Ganoderma lucidum related natural products as an adjunct therapy for cancers: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. 2019;10:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jin X, Ruiz Beguerie J, Sze DM, Chan GC. Ganoderma lucidum (Reishi mushroom) for cancer treatment. Cochrane Database Syst Rev. 2016;(4):CD007731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao H, Zhang Q, Zhao L, Huang X, Wang J, Kang X. Spore powder of Ganoderma lucidum improves cancer-related fatigue in breast cancer patients undergoing endocrine therapy: a pilot clinical trial. Evid Based Complement Alternat Med. 2012;2012:809614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bao PP, Lu W, Cui Y, et al. Ginseng and Ganoderma lucidum use after breast cancer diagnosis and quality of life: a report from the Shanghai Breast Cancer Survival Study. PLoS One. 2012;7:e39343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gao Y, Zhou S, Jiang W, Huang M, Dai X. Effects of ganopoly (a Ganoderma lucidum polysaccharide extract) on the immune functions in advanced-stage cancer patients. Immunol Invest. 2003;32:201-215. [DOI] [PubMed] [Google Scholar]
- 34. Sun Y, Hersh EM, Talpaz M, et al. Immune restoration and/or augmentation of local graft versus host reaction by traditional Chinese medicinal herbs. Cancer. 1983;52:70-73. [DOI] [PubMed] [Google Scholar]
- 35. Wang J, Shan A, Liu T, Zhang C, Zhang Z. In vitro immunomodulatory effects of an oleanolic acid-enriched extract of Ligustrum lucidum fruit (Ligustrum lucidum supercritical CO2 extract) on piglet immunocytes. Int Immunopharmacol. 2012;14:758-763. [DOI] [PubMed] [Google Scholar]
- 36. Rittenhouse JR, Lui PD, Lau BH. Chinese medicinal herbs reverse macrophage suppression induced by urological tumors. J Urol. 1991;146:486-490. [DOI] [PubMed] [Google Scholar]
- 37. Lau BH, Ruckle HC, Botolazzo T, Lui PD. Chinese medicinal herbs inhibit growth of murine renal cell carcinoma. Cancer Biother. 1994;9:153-161. [DOI] [PubMed] [Google Scholar]
- 38. Hsieh TC, Lu X, Guo J, et al. Effects of herbal preparation Equiguard on hormone-responsive and hormone-refractory prostate carcinoma cells: mechanistic studies. Int J Oncol. 2002;20:681-689. [PubMed] [Google Scholar]
- 39. Lau KM, He ZD, Dong H, Fung KP, But PP. Anti-oxidative, anti-inflammatory and hepato-protective effects of Ligustrum robustum. J Ethnopharmacol. 2002;83:63-71. [DOI] [PubMed] [Google Scholar]
- 40. Yim TK, Wu WK, Pak WF, Ko KM. Hepatoprotective action of an oleanolic acid-enriched extract of Ligustrum lucidum fruits is mediated through an enhancement on hepatic glutathione regeneration capacity in mice. Phytother Res. 2001;15:589-592. [DOI] [PubMed] [Google Scholar]
- 41. Cleeland CS, Zhao F, Chang VT, et al. The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group Symptom Outcomes and Practice Patterns study. Cancer. 2013;119:4333-4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mendoza TR, Wang XS, Lu C, et al. Measuring the symptom burden of lung cancer: the validity and utility of the lung cancer module of the M.D. Anderson Symptom Inventory. Oncologist. 2011;16:217-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin H. Clinical Practice Guidelines of Chinese Medicine in Oncology. People’s Medical Publishing House; 2016. [Google Scholar]
- 44. Committee of the Ministry of Health of the Republic. State Pharmacopoeia Commission of the People’s Republic of China. Chemical Industry Press; 2000. [Google Scholar]
- 45. Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199-220. [DOI] [PubMed] [Google Scholar]
- 46. Cella D. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) measurement system, Version 4. Center on Outcomes, Research and Education (CORE), Evanston Northwestern Healthcare and Northwestern University; 1997. [Google Scholar]
- 47. US Department of Health and Human Services; National Institute of Health; National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 4.0. Published May 28, 2009. Accessed July 8, 2020 https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 48. Cella D. The Functional Assessment of Cancer Therapy-Lung and Lung Cancer Subscale assess quality of life and meaningful symptom improvement in lung cancer. Semin Oncol. 2004;31(3 suppl 9):11-15. [DOI] [PubMed] [Google Scholar]
- 49. Cella D, Eton DT, Fairclough DL, et al. What is a clinically meaningful change on the Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire? Results from Eastern Cooperative Oncology Group (ECOG) Study 5592. J Clin Epidemiol. 2002;55:285-295. [DOI] [PubMed] [Google Scholar]
- 50. Liu J, Mao JJ, Wang XS, Lin H. Evaluation of traditional Chinese medicine herbs in oncology clinical trials. Cancer J. 2019;25:367-371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, 4_Reishi_appendix_1_April_24_2020_Clean for Preliminary Efficacy and Safety of Reishi & Privet Formula on Quality of Life Among Non–Small Cell Lung Cancer Patients Undergoing Chemotherapy: A Randomized Placebo-Controlled Trial by Jie Liu, Jun J. Mao, Susan Qing Li and Hongsheng Lin in Integrative Cancer Therapies