Key Points
Question
What are the characteristics and trends of clinical trials registered in ClinicalTrials.gov over time, and how do they differ by sponsor type?
Findings
In this cross-sectional study of ClinicalTrials.gov registration data on 245 999 interventional studies started between 2000 and 2019 that were sponsored by the National Institutes of Health or other US government agencies, industry, or other sources (foundations, universities, hospitals, clinics, and others), most trials were small, single-site studies that did not have US Food and Drug Administration–defined phases and were sponsored by other sources. Median sample sizes and years to trial completion decreased over time.
Meaning
The findings suggest that the composition and design of trials changed between 2000 and 2019 and differed substantially by sponsor type; increased funding toward larger randomized clinical trials may be warranted to inform clinical decision-making and guide future research.
Abstract
Importance
ClinicalTrials.gov is a valuable resource that can be used to trace the state and nature of trials. Since its launch in 2000, more than 345 000 trials have been registered. Little is known about the characteristics and trends in clinical trials over time and how they differ by sponsor type.
Objective
To assess trends in clinical trials registered in ClinicalTrials.gov over time and by sponsor type.
Design, Setting, and Participants
This cross-sectional study included clinical trials (interventional studies) registered in ClinicalTrials.gov from January 1, 2000, through December 31, 2019. The trials were grouped by lead sponsor: National Institutes of Health (NIH) and other US government agencies, industry, and other sources (foundations, universities, hospitals, clinics, and others). A static version of the Clinical Trials Transformation Initiative Aggregate Analysis of ClinicalTrials.gov database was downloaded on January 1, 2020, for analysis.
Main Outcomes and Measures
ClinicalTrials.gov registration fields, including overall status, phase, intervention, number of sites, use of masking and randomization, sample size, and time to study completion by start year and lead sponsor (organization that provided funding or support for a clinical study).
Results
A total of 245 999 clinical trials (interventional studies) were started between 2000 and 2019, of which 135 144 (54.9%) were completed. Among completed trials, 5113 (3.8%) were sponsored by the NIH or a US government agency, 48 668 (36.0%) by industry, and 81 363 (60.2%) by other sources. Most trials were single center (61.3%), randomized (65.6%), and phase 1 to 2 (35.5%) or did not have a US Food and Drug Administration–defined phase (38.4%), with fewer drug trials being conducted over time. Sample sizes were small (median, 60; interquartile range [IQR], 30-160) and diminished over time. Trial median completion times varied by lead sponsor: 3.4 years (IQR, 1.9-5.0 years) for NIH- and US government–sponsored trials, 1.2 years (IQR, 0.5-2.4 years) for industry trials, and 2.1 years (IQR, 1.1-3.7) for trials sponsored by other sources.
Conclusions and Relevance
The findings suggest that the composition and design of trials changed from 2000 to 2019 and differed substantially by sponsor type. Increased funding toward larger randomized clinical trials may be warranted to inform clinical decision-making and guide future research.
This cross-sectional study assesses characteristics and trends in clinical trials registered in ClinicalTrials.gov from 2000 to 2019 and how they differed by sponsor type.
Introduction
Since ClinicalTrials.gov was launched in 2000, more than 345 000 interventional and observational studies have been registered.1,2,3 ClinicalTrials.gov is managed by the National Library of Medicine and is an online resource for health care professionals, researchers, patients, and the general public. It is an important resource that can be used to view and access clinical trials registration data. Analyzing clinical trials metadata can illuminate important trends over time, such as the composition, size, design, and types of trials being funded.
There have been updates to the clinical trials registration and reporting requirements since implementation of the US Food and Drug Administration (FDA) Modernization Act of 1997, which mandated clinical trials registration and led to the establishment of ClinicalTrials.gov.4,5 In 2005, the International Committee of Medical Journal Editors (ICMJE) required registration of clinical trials as a prerequisite for publication.6 Subsequently, the FDA Act (FDAAA 801) of 2007 expanded requirements to the types of trials being registered, key data elements being entered, and basic results being reported.7 The Final Rule became effective in January 2017, further clarifying and expanding on the registration and requirements of FDAAA 801.8 Some changes include the types of trials subject to the requirements, the information that must be submitted and data elements that are required to be entered on registration, and additional results information reporting requirements for trials.8 Simultaneously, a policy was issued by the National Institutes of Health (NIH) to require registration and results reporting for all trials funded by the NIH regardless of whether the trials are covered by the FDAAA 801 requirements of the Final Rule.8
Availability of the Clinical Trials Transformation Initiative Aggregate Analysis of ClinicalTrials.gov (CTTI AACT) database has facilitated and improved the ability to analyze ClinicalTrials.gov registration data.9 In 2017, the CTTI AACT database was upgraded to a cloud-based platform that allows for open access to the complete set of trials registered in ClinicalTrials.gov for download and analysis. Its restructured and relational format facilitates analysis and provides access to additional fields that are not readily available in direct exports from ClinicalTrials.gov.
Previous reports of ClinicalTrials.gov registration data have focused analyses on specific funders, such as the NIH; on a single condition; or on a particular registration element within ClinicalTrials.gov.10,11,12 To our knowledge, no studies have characterized trials by sponsor type during this 20-year time span. Thus, our objective was to assess the characteristics and trends of clinical trials started from January 1, 2000, through December 31, 2019, and to compare trends by sponsor type.
Methods
Study Design and Setting
This cross-sectional study included clinical trials (interventional studies) with start dates between January 1, 2000, and December 31, 2019, that were registered in ClinicalTrials.gov and accessed using the CTTI AACT database.13 Observational studies and studies with expanded access were excluded from the analysis. CTTI AACT is a relational cloud-based database that includes aggregated and restructured data from ClinicalTrials.gov. Content is updated daily and can be publicly accessed using pgAdmin (pgAdmin Development Team), R (R Foundation for Statistical Computing), SAS (SAS Institute Inc), or pSQL (PostgreSQL Global Development Group). Characterization of the CTTI AACT content and navigation through the CTTI AACT database followed definitions from the publicly available CTTI AACT comprehensive data dictionary14 and definitions available in ClinicalTrials.gov. A static version of the CTTI AACT database was downloaded for analysis on January 1, 2020. This is an analysis of publicly available aggregate trial data; thus, institutional review board approval was not required. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Variables of Interest
ClinicalTrials.gov registration fields, as coded in the CTTI AACT database, included the following: trial start and completion dates; study type (interventional or observational); overall status (completed, withdrawn, terminated, suspended, open to enrollment, recruiting, not yet recruiting, or status unknown); enrollment number; study phase (early phase 1, phase 1, phase 1 to 2, phase 2, phase 3, phase 4, and trials that do not have an FDA-defined phase [phase not applicable (NA)]); treatment assignment (randomized or not randomized); masking (open label or masked); facilities (single center or multicenter); posted results; and lead sponsor (NIH or other US government agency, industry, and all other sponsors). Lead sponsor is defined in ClinicalTrials.gov as the “organization or person who initiates the study and who has authority and control over the study.”1 This variable is not the same as funder type, which is derived from multiple data elements in ClinicalTrials.gov and is not available as a discrete field in the database download. Additional calculated variables included time to completion (calculated as the difference between actual completion date and start date for completed trials) and times to posted results. Anticipated and actual enrollment counts were also assessed by comparing target sample size provided at trial registration with the sample size provided on trial completion. A description of each variable as defined in ClinicalTrials.gov and used for the purpose of this article is available in eTable 1 in the Supplement.
Statistical Analysis
Results were grouped by lead sponsor and start date in 5-year periods: 2000 to 2004, 2005 to 2009, 2010 to 2014, and 2015 to 2019. These year groupings align with changes in the registration and reporting regulations, including the launch of ClinicalTrials.gov, the ICMJE edict, and implementation of FDAAA 801. Trial start dates were used to classify periods because registration dates can be entered retrospectively and thus are more likely to be inaccurate or lead to time misclassification.
Multivariable regression models were fitted to evaluate the association between sample size and sponsor type and were adjusted for start year and other trial design characteristics. An interaction term between start year and lead sponsor was included in the model in which a significant result would indicate an interactive effect. Anticipated and actual sample sizes were compared across sponsor types for trials started and completed between 2010 and 2019 using the available CTTI AACT archived databases for each year. Median times to completion were calculated from start date to actual completion date for completed trials. A 2-sided P < .05 was considered to be statistically significant. All tabulations and analyses were duplicated (A.G.G. and J.L.M.) using postgreSQL and SAS. The postgreSQL codes used to generate tables are available in the eAppendix in the Supplement. Additional analyses were performed using Stata, version 15 (StataCorp LLC).
Results
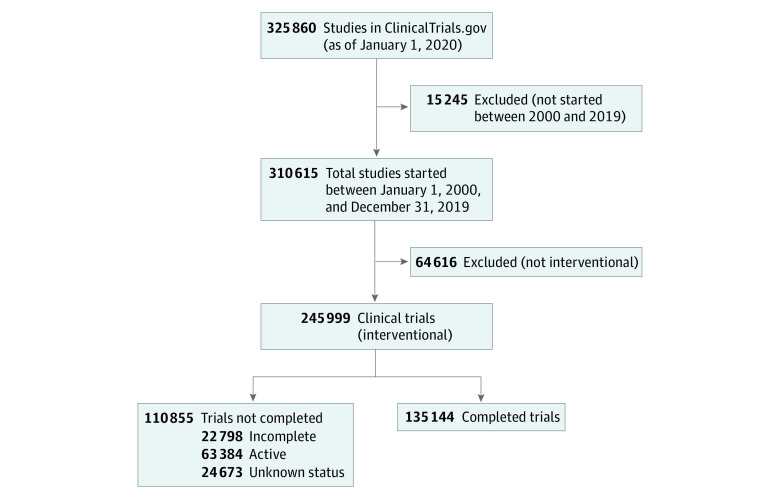
There were 325 860 registrations on ClinicalTrials.gov as of January 1, 2020, of which 245 999 were clinical trials (interventional studies) started between 2000 and 2019; 135 144 trials (54.9%) were completed (Figure). Overall, there were 8023 NIH- or US government–sponsored trials (3.3%), 70 329 industry-sponsored trials (28.5%), and 167 647 trials sponsored by other funding sources (68.1%). Among the NIH- and US government–sponsored trials, 63.7% were completed, 11.4% were incomplete, 20.2% were active, and 4.6% had unknown status (Table 1). Industry-sponsored trials had the highest percentage of completed trials (69.2%) and the lowest percentage of active trials (16.8%), whereas trials sponsored by other sources had the lowest completion rates (48.5%) and the highest percentage of active trials (29.8%), including trials that were not yet recruiting, were recruiting, were enrolling by invitation, or were active and not recruiting. The number of NIH- and US government–sponsored trials started each year decreased over time, in contrast to the number of trials started per year that were sponsored by industry and other funding sources, which increased over time (eTable 2 in the Supplement).
Figure. Flow Diagram.
Incomplete indicates terminated, suspended, or withdrawn, and active indicates recruiting, not yet recruiting, active, or enrolling by invitation.
Table 1. Status of the 245 999 Trials Published From 2000 Through 2019 by Lead Sponsor.
| Start year, lead sponsor | Trials, No. (%) | ||||
|---|---|---|---|---|---|
| Total | Completed | Incomplete | Active | Unknown | |
| 2000-2004 | |||||
| NIH or US government | 2077 | 1784 (85.9) | 172 (8.3) | 22 (1.0) | 99 (4.8) |
| Industry | 6772 | 6130 (90.5) | 507 (7.5) | 6 (0.1) | 129 (1.9) |
| Other | 9954 | 7884 (79.2) | 995 (10.0) | 214 (2.1) | 861 (8.6) |
| Total | 18 803 | 15 798 (84.0) | 1674 (8.9) | 242 (1.3) | 1089 (5.8) |
| 2005-2009 | |||||
| NIH or US government | 2246 | 1740 (77.5) | 290 (12.9) | 87 (3.9) | 129 (5.7) |
| Industry | 19 088 | 16 057 (84.1) | 2426 (12.7) | 131 (0.7) | 474 (2.5) |
| Other | 30 690 | 21 423 (69.8) | 4243 (13.8) | 925 (3.0) | 4099 (13.4) |
| Total | 52 024 | 39 220 (75.4) | 6959 (13.4) | 1143 (2.2) | 4702 (9.0) |
| 2010-2014 | |||||
| NIH or US government | 1895 | 1171 (61.8) | 318 (16.8) | 311 (16.4) | 95 (5.0) |
| Industry | 21 648 | 16 905 (78.1) | 2675 (12.4) | 1043 (4.8) | 1025 (4.7) |
| Other | 51 761 | 31 702 (61.2) | 5491 (10.6) | 5220 (10.1) | 9348 (18.1) |
| Total | 75 304 | 49 778 (66.1) | 8484 (11.3) | 6574 (8.7) | 10 468 (13.9) |
| 2015-2019 | |||||
| NIH or US government | 1805 | 418 (23.2) | 134 (7.4) | 1204 (66.7) | 49 (2.7) |
| Industry | 22 821 | 9576 (42.0) | 1762 (7.7) | 10 615 (46.5) | 868 (3.8) |
| Other | 75 242 | 20 354 (27.1) | 3785 (5.0) | 43 606 (58.0) | 7497 (10.0) |
| Total | 99 868 | 30 348 (30.4) | 5681 (5.7) | 55 425 (55.5) | 8414 (8.4) |
| 2000-2019 | |||||
| NIH or US government | 8023 | 5113 (63.7) | 914 (11.4) | 1624 (20.2) | 372 (4.6) |
| Industry | 70 329 | 48 668 (69.2) | 7370 (10.5) | 11 795 (16.8) | 2496 (3.5) |
| Other | 167 647 | 81 363 (48.5) | 14 514 (8.7) | 49 965 (29.8) | 21 805 (13.0) |
| Total | 245 999 | 135 144 (54.9) | 22 798 (9.3) | 63 384 (25.8) | 24 673 (10.0) |
Abbreviation: NIH, National Institutes of Health.
Design characteristics of completed trials ordered by lead sponsors and start year are given in Table 2. Most trials were single center (61.3%), randomized (65.6%), open label (55.7%), phase 1 to 2 (35.5%), or lacking an FDA-defined phase (38.4%). Percentages of completed trials that were double-masked, multisite, and randomized were 31.4% for industry-sponsored trials, 12.3% for NIH- and US government–sponsored trials, and 11.0% for other trials and remained stable over time. The overall percentage of drug trials completed decreased from 2000 to 2019 (70.5% in 2000-2004, 61.8% in 2005-2009, 48.9% in 2010-2014, and 40.0% in 2015-2019). This finding is in contrast to a doubling of trials that involved nondrug interventions from 2000 through 2004 (29.6%) to 2015 through 2019 (60.0%). These trends are reflected in the decreasing number of phase 1 to 2 and phase 3 to 4 trials being completed and the increasing number of trials lacking an FDA-defined phase (phase NA). The NIH and US government agencies were the lead sponsors for a larger percentage of completed phase 1 to 2 trials, whereas industry was the lead sponsor for more phase 3 to 4 trials completed over time. Trials sponsored by other sources involved mostly trials lacking an FDA-defined phase. More industry-sponsored trials were multicenter (65.6%) compared with NIH- and US government–sponsored trials (34.2%) and trials sponsored by other sources (27.7%).
Table 2. Design Characteristics of the 135 144 Completed Trials by Lead Sponsor and Start Year.
| Start year, characteristic | Completed trials, No. (%) | |||
|---|---|---|---|---|
| NIH or US government | Industry | Other | Total | |
| 2000-2004 | ||||
| Facilities | ||||
| Multisite | 449 (25.2) | 4575 (74.6) | 2736 (34.7) | 7760 (49.1) |
| Single site | 1335 (74.8) | 1555 (25.4) | 5148 (65.3) | 8038 (50.9) |
| Allocation | ||||
| Randomized | 841 (47.1) | 4346 (70.9) | 4949 (62.8) | 10 136 (64.2) |
| Nonrandomized | 149 (8.4) | 1260 (20.5) | 1178 (14.9) | 2587 (16.4) |
| NA | 794 (44.5) | 524 (8.5) | 1757 (22.3) | 3075 (19.4) |
| Masking | ||||
| Masked | 469 (26.3) | 2756 (45.0) | 2847 (36.1) | 6072 (38.4) |
| Open label | 906 (50.8) | 3227 (52.6) | 4479 (56.8) | 8612 (54.5) |
| Missing | 409 (22.9) | 147 (2.4) | 558 (7.1) | 1114 (7.1) |
| Interventions | ||||
| Drug | 1095 (61.4) | 5369 (87.6) | 4666 (59.2) | 11 130 (70.5) |
| Other | 686 (38.6) | 761 (12.4) | 3218 (40.8) | 4668 (29.6) |
| Phasea | ||||
| 1-2 | 1156 (64.8) | 2549 (41.6) | 3127 (39.7) | 6832 (43.3) |
| 3-4 | 281 (15.8) | 3323 (54.2) | 2495 (31.6) | 6099 (38.6) |
| NA | 347 (19.5) | 258 (4.2) | 2262 (28.7) | 2867 (18.2) |
| Sample sizeb | ||||
| <100 | 1023 (63.3) | 2408 (41.4) | 4303 (59.2) | 7734 (52.6) |
| 100-1000 | 497 (30.8) | 2972 (51.1) | 2593 (35.7) | 6062 (41.2) |
| >1000 | 96 (5.9) | 440 (7.6) | 368 (5.1) | 904 (6.2) |
| Multisite, randomized, and masked | 135 (7.6) | 2172 (35.4) | 826 (10.5) | 3133 (19.8) |
| Total | 1784 (100) | 6130 (100) | 7884 (100) | 15 798 (100) |
| 2005-2009 | ||||
| Facilities | ||||
| Multisite | 603 (34.7) | 11 025 (68.7) | 5667 (26.5) | 17 295 (44.1) |
| Single site | 1137 (65.3) | 5032 (31.3) | 15 756 (73.5) | 21 925 (55.9) |
| Allocation | ||||
| Randomized | 1013 (58.2) | 11 036 (68.7) | 14 573 (68.0) | 26 622 (67.9) |
| Nonrandomized | 221 (12.7) | 2834 (17.7) | 2742 (12.8) | 5797 (14.8) |
| NA | 506 (29.1) | 2187 (13.6) | 4108 (19.2) | 6801 (17.3) |
| Masking | ||||
| Masked | 617 (35.5) | 7917 (49.3) | 9369 (43.7) | 17 903 (45.7) |
| Open label | 1013 (58.2) | 7972 (49.7) | 11 845 (55.3) | 20 830 (53.1) |
| Missing | 110 (6.3) | 168 (1.0) | 209 (1.0) | 487 (1.2) |
| Interventions | ||||
| Drug | 993 (57.1) | 12 832 (79.9) | 10 409 (48.6) | 24 234 (61.8) |
| Other | 747 (42.9) | 3225 (20.1) | 11 014 (51.4) | 14 986 (38.2) |
| Phasesa | ||||
| 1-2 | 1053 (60.5) | 8503 (53.0) | 6603 (30.8) | 16 159 (41.2) |
| 3-4 | 219 (12.6) | 6382 (39.7) | 5906 (27.6) | 12 507 (31.9) |
| NA | 468 (26.9) | 1172 (7.3) | 8914 (41.6) | 10 554 (26.9) |
| Sample sizeb | ||||
| <100 | 1150 (66.3) | 8243 (51.7) | 13 931 (65.6) | 23 324 (60.0) |
| 100-1000 | 509 (29.3) | 6908 (43.4) | 6483 (30.5) | 13 900 (35.7) |
| >1000 | 76 (4.4) | 781 (4.9) | 812 (3.8) | 1669 (4.3) |
| Multisite, randomized, and masked | 238 (13.7) | 5487 (34.2) | 2123 (9.9) | 7848 (20.0) |
| Total | 1740 (100) | 16 057 (100) | 21 423 (100) | 39 220 (100) |
| 2010-2014 | ||||
| Facilities | ||||
| Multisite | 412 (35.2) | 10 423 (61.7) | 8665 (27.3) | 19 500 (39.2) |
| Single site | 759 (64.8) | 6482 (38.3) | 23 037 (72.7) | 30 278 (60.8) |
| Allocation | ||||
| Randomized | 747 (63.8) | 11 228 (66.4) | 22 576 (71.2) | 34 551 (69.4) |
| Nonrandomized | 154 (13.1) | 1877 (11.1) | 2585 (8.2) | 4616 (9.3) |
| NA | 270 (23.1) | 3800 (22.5) | 6541 (20.6) | 10 611 (21.3) |
| Masking | ||||
| Masked | 487 (41.6) | 8098 (47.9) | 15 319 (48.3) | 23 904 (48.0) |
| Open label | 665 (56.8) | 8759 (51.8) | 16 257 (51.3) | 25 681 (51.6) |
| Not reported | 19 (1.6) | 48 (0.3) | 126 (0.4) | 193 (0.4) |
| Interventions | ||||
| Drug | 509 (43.5) | 12 422 (73.5) | 11 410 (36.0) | 24 341 (48.9) |
| Other | 662 (56.5) | 4483 (26.5) | 20 292 (64.0) | 25 437 (51.1) |
| Phasesa | ||||
| 1-2 | 632 (54.0) | 9488 (56.1) | 6829 (21.5) | 16 949 (34.1) |
| 3-4 | 95 (8.1) | 5122 (30.3) | 6581 (20.8) | 11 798 (23.7) |
| NA | 444 (37.9) | 2295 (13.6) | 18 292 (57.7) | 21 031 (42.2) |
| Sample sizeb | ||||
| <100 | 817 (69.8) | 9991 (59.2) | 21 173 (66.9) | 31 981 (64.3) |
| 100-1000 | 317 (27.1) | 6317 (37.4) | 9321 (29.5) | 15 955 (32.1) |
| >1000 | 36 (3.1) | 574 (3.4) | 5104 (25.1) | 1771 (3.6) |
| Multisite, randomized, and masked | 177 (15.1) | 5066 (30.0) | 3795 (12.0) | 9038 (18.2) |
| Total | 1171 (100) | 16 905 (100) | 31 702 (100) | 49 778 (100) |
| 2015-2019 | ||||
| Facilities | ||||
| Multisite | 285 (31.8) | 4752 (49.6) | 4729 (23.2) | 9686 (31.9) |
| Single site | 133 (68.2) | 4824 (50.4) | 15 625 (76.8) | 20 662 (68.1) |
| Allocation | ||||
| Randomized | 308 (73.7) | 6311 (65.9) | 14 797 (72.7) | 21 416 (70.6) |
| Nonrandomized | 37 (8.8) | 842 (8.8) | 1625 (8.0) | 2504 (8.3) |
| NA | 73 (17.5) | 2423 (25.3) | 3932 (19.3) | 6428 (21.2) |
| Masking | ||||
| Masked | 222 (53.1) | 4740 (49.5) | 10 260 (50.4) | 15 222 (50.2) |
| Open label | 196 (46.9) | 4822 (50.4) | 10 038 (49.3) | 15 056 (49.6) |
| Not reported | 0 (0.0) | 14 (0.2) | 56 (0.3) | 70 (0.2) |
| Interventions | ||||
| Drug | 127 (30.4) | 6680 (69.8) | 5322 (26.2) | 12 129 (40.0) |
| Other | 291 (69.6) | 2896 (30.2) | 15 032 (73.8) | 18 219 (60.0) |
| Phasea | ||||
| 1-2 | 186 (44.5) | 5316 (55.5) | 2532 (12.4) | 8034 (26.5) |
| 3-4 | 28 (6.7) | 2092 (21.9) | 2708 (13.3) | 4828 (15.9) |
| NA | 204 (48.8) | 2168 (22.6) | 15 114 (73.3) | 17 486 (57.6) |
| Sample sizeb | ||||
| <100 | 311 (74.4) | 6419 (67.1) | 14 653 (72.0) | 21 383 (70.5) |
| 100-1000 | 97 (23.2) | 2916 (30.5) | 5104 (25.1) | 8117 (26.8) |
| >1000 | 10 (2.4) | 230 (2.4) | 590 (2.9) | 830 (2.7) |
| Multisite, randomized, and masked | 77 (18.4) | 2549 (26.6) | 2212 (10.9) | 4838 (15.9) |
| Total | 418 (100) | 9576 (100) | 20 354 (100) | 30 348 (100) |
| 2000-2019 | ||||
| Facilities | ||||
| Multisite | 1597 (34.2) | 30 847 (65.6) | 21 797 (27.7) | 54 241 (38.7) |
| Single site | 3516 (67.8) | 17 821 (34.4) | 59 566 (72.3) | 80 903 (61.3) |
| Allocation | ||||
| Randomized | 2909 (56.8) | 32 921 (63.9) | 56 895 (66.7) | 92 725 (65.6) |
| Nonrandomized | 561 (12.0) | 6813 (14.2) | 8130 (10.1) | 15 504 (11.4) |
| NA | 1643 (31.3) | 8934 (21.9) | 16 338 (23.2) | 26 915 (23.1) |
| Masking | ||||
| Masked | 1795 (35.0) | 23 511 (45.7) | 37 795 (42.7) | 63 101 (43.3) |
| Open label | 2780 (57.7) | 24 780 (53.6) | 42 619 (56.4) | 70 179 (55.7) |
| Not reported | 538 (7.3) | 377 (0.7) | 949 (0.88) | 1864 (1.0) |
| Interventions | ||||
| Drug | 2724 (34.4) | 37 303 (76.7) | 31 807 (13.8) | 71 834 (53.2) |
| Other | 2389 (65.6) | 11 365 (23.3) | 49 556 (86.2) | 63 308 (46.9) |
| Phasea | ||||
| 1-2 | 3027 (59.2) | 25 856 (53.1) | 19 091 (23.5) | 47 974 (35.5) |
| 3-4 | 623 (12.2) | 16 919 (34.8) | 17 690 (21.7) | 35 232 (26.1) |
| NA | 1463 (28.6) | 5893 (12.1) | 44 582 (54.8) | 51 938 (38.4) |
| Sample sizeb | ||||
| <100 | 3301 (66.8) | 27 061 (56.1) | 54 060 (67.2) | 84 422 (63.2) |
| 100-1000 | 1420 (28.8) | 19 113 (39.7) | 23 501 (29.2) | 44 034 (32.9) |
| >1000 | 218 (4.4) | 2025 (4.2) | 2931 (3.6) | 5174 (3.9) |
| Multisite, randomized, and masked | 627 (12.3) | 15 274 (31.4) | 8956 (11.0) | 24 857 (18.4) |
| Total | 5113 (100) | 48 668 (100) | 81 363 (100) | 135 144 (100) |
Abbreviations: NA, not applicable; NIH, National Institutes of Health.
Phase 1 to 2 includes early phase 1, phase 1, phase 1 to 2, and phase 2 trials. Phase 3 to 4 includes phase 2 to 3, phase 3, and phase 4 trials. Phase NA is defined as trials without a US Food and Drug Administration–defined phase, including trials of devices or behavioral interventions.
Actual and anticipated sample sizes. Tabulations exclude missing enrollment data.
Median sample sizes for completed trials by sponsor and phase over time are given in Table 3. The overall median sample size for trials between 2000 and 2019 was 60 individuals (interquartile range [IQR], 30-160 individuals) and decreased between 2000 and 2019 for all sponsors. Sample sizes were largest for industry-sponsored trials, with a median of 75 individuals (IQR, 32-236 individuals) compared with NIH- and US government–sponsored trials (median, 55 individuals; IQR, 28-140 individuals) and trials funded by other sources (median, 58 individuals; IQR, 28-128 individuals) (Table 3). Trial sample sizes were less than 100 individuals in 56.1% of industry-sponsored trials compared with 66.8% of NIH- and US government–sponsored trials and 67.2% of trials sponsored by other sources overall.
Table 3. Sample Sizes for the 135 144 Completed Trials by Lead Sponsor and Phase Over Time.
| Start year, lead sponsor | Sample size, median (IQR) | |||
|---|---|---|---|---|
| Phase 1-2a | Phase 3-4b | Phase NAc | All trials | |
| 2000-2004 | ||||
| NIH/US government | 48 (29-85) | 223 (80-727) | 150 (50-400) | 60 (32-157) |
| Industry | 51 (30-120) | 300 (122-600) | 109 (36-341) | 140 (48-400) |
| Other | 42 (24-78) | 120 (50-338) | 99 (40-243) | 68 (32-195) |
| Total | 47 (27-92) | 210 (80-500) | 100 (40-277) | 86 (38-266) |
| 2005-2009 | ||||
| NIH/US government | 44 (24-83) | 150 (49-400) | 98 (30-257) | 55 (26-139) |
| Industry | 48 (26-108) | 258 (106-538) | 72 (35-173) | 91 (36-273) |
| Other | 38 (20-67) | 85 (40-206) | 70 (32-172) | 60 (29-140) |
| Total | 42 (24-87) | 150 (60-400) | 70 (32-178) | 66 (30-190) |
| 2010-2014 | ||||
| NIH/US government | 36 (20-69) | 149 (64-480) | 70 (33-206) | 48 (25-124) |
| Industry | 42 (24-90) | 251 (101-513) | 60 (30-140) | 66 (30-204) |
| Other | 33 (18-64) | 74 (40-162) | 62 (30-153) | 59 (28-130) |
| Total | 39 (20-77) | 120 (50-325) | 62 (30-152) | 60 (29-150) |
| 2015-2019 | ||||
| NIH/US government | 37 (20-72) | 111 (61-357) | 53 (28-123) | 47 (25-100) |
| Industry | 39 (22-74) | 247 (105-515) | 46 (23-100) | 53 (25-140) |
| Other | 30 (15-60) | 70 (36-134) | 52 (27-109) | 50 (25-104) |
| Total | 36 (19-70) | 110 (50-291) | 51 (26-108) | 51 (25-114) |
| 2000-2019d | ||||
| NIH/US government | 42 (24-80) | 180 (64-506) | 89 (34-253) | 55 (28-140) |
| Industry | 45 (24-95) | 261 (108-540) | 57 (28-131) | 75 (32-236) |
| Other | 36 (20-66) | 80 (40-198) | 60 (30-145) | 58 (28-128) |
| Total | 40 (22-80) | 140 (56-379) | 60 (30-146) | 60 (30-160) |
Abbreviations: IQR, interquartile range; NA, not applicable; NIH, National Institutes of Health.
Includes early phase 1, phase 1, phase 1 to 2, and phase 2 trials.
Includes phase 2 to 3, phase 3, phase 3 to 4, and phase 4 trials.
Phase NA is defined as trials without a US Food and Drug Administration–defined phase, including trials of devices or behavioral interventions.
Totals exclude missing data. Enrollment data are missing for 1514 records.
In multivariable regression of completed trials (eTable 3 in the Supplement), sample sizes decreased by 8.2 persons every 5 years. Phase 1 to 2 trials decreased by 2.2 persons (95% CI, 1.5-2.9 persons), phase 3 to 4 trials by 8.8 persons (95% CI, 5.3-12.3 persons), and trials lacking an FDA-defined phase by 4.2 persons (95% CI, 0.9-7.5 persons) every 5 years. Comparing sample sizes by sponsor, NIH- and US government–sponsored trials were smaller than industry-sponsored trials for phase 1 to 2 trials (−2.5; 95% CI, −4.0 to 1.0; P < .001), phase 3 to 4 trials (−82.7; 95% CI, −96.4 to −69.0; P < .001), and overall (−12.7; 95% CI, −14.9 to −10.6; P < .001). Interaction terms for start year and sponsor were statistically significant (eTable 3 in the Supplement). Planned sample sizes at the beginning of the trial were larger than actual sample sizes when trials were completed across all phases and sponsor types (eTable 4 and eFigure 3 in the Supplement).
Median times to trial completion by lead sponsor were 3.4 years (IQR, 1.9-5.0 years) for NIH- and US government–sponsored trials, 1.2 years (IQR, 0.5-2.4 years) for industry trials, and 2.1 years (IQR, 1.1-3.7 years) for trials sponsored by other sources between 2000 and 2019 (eFigure 1 in the Supplement). Table 4 shows median times to completion and IQRs for completed trials by sponsor type and start year, which decreased over time (eFigure 2 in the Supplement). For example, median years to completion for phase 3 to 4 trials sponsored by the NIH and other US government agencies were 5.4 (IQR, 3.7-7.7) in 2000, 3.8 (IQR, 2.3-5.9) in 2005, 3.7 (IQR, 2.9-4.9) in 2010, and 3.2 (IQR, 1.9-3.7) in 2015. Times to completion for industry-sponsored phase 3 to 4 trials remained relatively steady over time: 3.2 years (IQR, 1.9-5.2 years) in 2000, 1.7 years (IQR, 0.9-2.7 years) in 2005, 1.7 years (IQR, 0.9-3.1 years) in 2010, and 1.6 years (IQR, 0.9-2.5 years) Times to completion for phase 3 to 4 trials sponsored by other sources were 6.0 years (IQR, 3.9-9.1 years) in 2000, 3.1 years (IQR, 1.8-5.0 years) in 2005, 3.0 years (IQR, 1.6-4.4 years) in 2010, and 1.6 years (IQR, 0.9-2.5 years) in 2015.
Table 4. Time to Trial Completion for the 135 144 Completed Trials by Lead Sponsor Over Timea.
| Start year | Time to trial completion, median (IQR), y | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Phase 1-2 | Phase 3-4 | Phase NAb | |||||||
| NIH or US government | Industry | Other | NIH or US government | Industry | Other | NIH or US government | Industry | Other | |
| 2000 | 3.8 (1.9-5.7) | 2.3 (1.0-5.1) | 5.4 (3.5-7.6) | 5.4 (3.7-7.7) | 3.2 (1.9-5.2) | 6.0 (3.9-9.1) | 4.6 (3.0-5.9) | 3.7 (1.5-9.3) | 5.3 (3.6-7.7) |
| 2001 | 3.7 (2.2-5.9) | 1.8 (0.7-3.5) | 5.7 (3.4-8.2) | 4.9 (3.2-5.9) | 2.5 (1.6-4.2) | 5.1 (3.3-7.5) | 4.2 (2.9-5.4) | 4.9 (2.3-8.5) | 4.7 (2.9-7.1) |
| 2002 | 4.0 (2.7-6.1) | 1.2 (0.2-2.7) | 5.2 (3.0-7.5) | 4.6 (3.0-6.0) | 2.0 (1.1-3.3) | 4.4 (2.8-6.3) | 3.8 (2.7-5.3) | 1.0 (0.2-4.0) | 4.0 (2.7-5.8) |
| 2003 | 3.7 (2.4-5.9) | 1.5 (0.4-3.0) | 4.7 (2.9-6.9) | 4.3 (2.4-5.7) | 1.8 (1.1-3.1) | 4.0 (2.4-5.7) | 3.1 (2.2-4.9) | 2.5 (0.2-5.4) | 3.9 (2.4-5.8) |
| 2004 | 4.0 (2.5-6.4) | 1.4 (0.4-2.7) | 4.4 (2.7-6.2) | 3.0 (2.0-5.2) | 1.7 (1.0-2.8) | 3.3 (2.1-5.1) | 3.2 (2.1-4.5) | 1.0 (0.1-3.7) | 3.4 (1.9-5.2) |
| 2005 | 3.8 (2.0-5.5) | 1.4 (0.5-2.8) | 3.9 (2.2-6.0) | 3.8 (1.8-5.8) | 1.7 (0.9-2.7) | 3.1 (1.8-5.0) | 3.0 (2.0-4.3) | 1.7 (0.3-5.2) | 2.9 (1.6-4.9) |
| 2006 | 3.9 (2.1-5.9) | 1.4 (0.6-2.8) | 3.8 (2.3-5.9) | 3.2 (1.5-5.5) | 1.7 (1.0-2.7) | 2.9 (1.8-4.5) | 3.1 (1.8-4.7) | 2.2 (0.6-4.8) | 2.7 (1.4-4.5) |
| 2007 | 4.0 (2.3-6.1) | 1.3 (0.5-2.7) | 3.6 (1.9-5.6) | 3.7 (1.6-5.6) | 1.6 (0.9-2.7) | 2.7 (1.6-4.4) | 3.4 (1.9-4.8) | 1.8 (0.4-3.8) | 2.7 (1.3-4.2) |
| 2008 | 4.0 (2.4-5.8) | 1.0 (0.4-2.3) | 3.4 (1.9-5.2) | 3.5 (1.9-5.2) | 1.6 (0.8-2.7) | 2.6 (1.4-4.3) | 3.1 (1.5-4.4) | 1.2 (0.3-3.0) | 2.6 (1.4-4.5) |
| 2009 | 3.3 (1.3-5.6) | 1.0 (0.3-2.4) | 3.3 (1.8-5.2) | 3.9 (2.3-5.3) | 1.7 (0.9-3.1) | 2.5 (1.3-4.0) | 3.5 (2.2-5.3) | 1.2 (0.5-2.8) | 2.6 (1.3-4.2) |
| 2010 | 3.6 (1.9-5.0) | 0.9 (0.3-2.2) | 3.1 (1.7-4.8) | 3.7 (2.9-4.9) | 1.7 (0.9-2.9) | 2.4 (1.2-3.9) | 3.0 (1.6-4.4) | 1.3 (0.5-3.5) | 2.4 (1.2-3.9) |
| 2011 | 3.5 (2.0-5.0) | 1.0 (0.3-2.2) | 2.7 (1.5-4.3) | 3.5 (2.3-4.4) | 1.7 (1.0-2.9) | 2.3 (1.3-3.7) | 3.2 (1.8-4.4) | 1.3 (0.6-3.0) | 2.2 (1.1-3.7) |
| 2012 | 3.3 (1.7-4.6) | 1.1 (0.3-2.2) | 2.6 (1.4-4.1) | 3.2 (1.8-4.7) | 1.7 (1.0-2.8) | 2.2 (1.2-3.5) | 3.2 (1.9-4.6) | 1.2 (0.6-2.7) | 2.2 (1.1-3.7) |
| 2013 | 3.5 (2.0-4.9) | 1.0 (0.3-2.2) | 2.5 (1.3-3.8) | 3.7 (2.7-4.3) | 1.7 (0.9-2.8) | 2.1 (1.2-3.2) | 3.1 (1.9-4.2) | 1.3 (0.5-2.6) | 2.0 (1.1-3.3) |
| 2014 | 2.7 (1.7-3.9) | 0.9 (0.3-2.0) | 2.0 (1.1-3.2) | 2.5 (1.5-4.0) | 1.6 (0.9-2.6) | 1.8 (1.0-2.9) | 3.3 (1.6-4.2) | 1.3 (0.5-2.4) | 1.8 (1.0-2.4) |
| 2015 | 2.6 (1.6-3.6) | 0.9 (0.3-1.9) | 1.7 (0.9-2.8) | 3.2 (1.9-3.7) | 1.6 (0.9-2.4) | 1.6 (0.9-2.5) | 2.2 (1.1-3.3) | 1.3 (0.5-2.6) | 1.6 (0.9-2.5) |
| 2016 | 1.8 (1.1-2.4) | 0.8 (0.4-1.6) | 1.6 (0.9-2.3) | 1.6 (0.8-2.8) | 1.4 (0.9-2.2) | 1.4 (0.8-2.1) | 2.0 (1.0-2.4) | 1.3 (0.5-2.4) | 1.3 (0.7-2.0) |
| 2017 | 1.7 (1.1-2.1) | 0.7 (0.3-1.2) | 1.3 (0.8-1.8) | 1.2 (1.1-1.2) | 1.2 (0.7-1.7) | 1.1 (0.7-1.6) | 1.5 (0.7-1.9) | 1.0 (0.5-1.9) | 1.0 (0.6-1.5) |
| 2018 | 0.8 (0.6-1.3) | 0.5 (0.2-0.8) | 0.8 (0.4-1.1) | 1.4 (1.4-1.4) | 0.7 (0.5-1.0) | 0.7 (0.4-1.0) | 0.9 (0.6-1.1) | 0.7 (0.3-1.5) | 0.6 (0.4-1.0) |
| 2019 | 0.6 (0.6-0.6) | 0.2 (0.1-0.4) | 0.3 (0.1-0.5) | NR | 0.2 (0.1-0.5) | 0.4 (0.2-0.5) | 0.2 (0.1-0.5) | 0.6 (0.3-1.2) | 0.3 (0.2-0.5) |
| All | 3.5 (1.9-5.3) | 1.0 (0.3-2.2) | 2.9 (1.5-4.8) | 3.8 (2.3-5.4) | 1.7 (0.9-2.7) | 2.3 (1.3-3.9) | 3.0 (1.7-4.4) | 1.0 (0.3-2.2) | 1.7 (0.9-3.1) |
Abbreviations: IQR, interquartile range; NIH, National Institutes of Health; NR, not reported.
Trends in time to trial completion should be interpreted with caution within the past 3 to 5 years because fewer trials were completed and trials may not have had sufficient time to be completed. Data are presented for descriptive purposes.
Phase NA is defined as trials without a US Food and Drug Administration–defined phase, including trials of devices or behavioral interventions.
From 2007, when posting results became required, to 2019, the percentages of completed trials posting results by agency were 47.7% for NIH- and US government-sponsored trials, 37.8% for industry-sponsored trials, and 16.0% for trials sponsored by other sources. The median times to posting were 1.4 years (IQR, 0.9-3.0 years) for all trials, 1.3 years (IQR, 0.9-2.9 years) for industry-sponsored trials, 1.6 years (IQR, 1.0-3.3 years) for NIH- and US government-sponsored trials, and 1.6 years (IQR, 0.9-3.1 years) for trials sponsored by other sources.
Discussion
ClinicalTrials.gov is an important resource that can be used to characterize the state and nature of trials. We describe trends and characteristics of 245 999 trials that were registered in ClinicalTrials.gov and started between 2000 and 2019. We found that trials had smaller sample sizes and were being completed in less time and that most trials were sponsored by other sources (foundations, universities, hospitals, clinics, and others) from 2000 to 2019.
Observed Trends in Clinical Trial Characteristics Over Time
The number of trials started per year increased between 2000 and 2019, with the largest increase observed in the number of trials started each year by other sources. A similar trend was observed for industry-sponsored trials started per year, whereas the number of NIH- and US government-sponsored trials started per year decreased. Part of the decrease may have been associated with differential uptake of registration across sponsor types, which was faster for NIH- and US government-sponsored trials, within the first 5 years of its launch, accounting for most new registrations.
Differences were observed in clinical trial design characteristics over time, including different distributions across trial phases, intervention types, use of randomization or masking, and number of centers involved over time. There was a decrease in the percentages of phase 3 to 4 trials and drug trials being conducted over time compared with an increase in the percentages of nondrug trials and trials without an FDA-defined phase. The rate in difference between early-phase trials (phase 1-2) and phase 3 trials decreased by almost half by the end of 2019. This shift may be explained by the increased uptake in registration for these trials, expansion of the clinical trial definition, or increasing interest in other intervention types (eg, behavioral interventions, imaging, biologic, and devices) in recent years.15,16,17 The decreasing percentages of trials that involved drugs may also be associated with increasing costs and complexity of conducting phase 3 to 4 drug trials.
Overall, trial sample sizes decreased over time and took less time to complete. Median times to trial completion varied by sponsor type and phase. Industry completed trials at faster rates compared with the NIH and US government and other funders, possibly in association with more efficient trial startup processes and higher recruitment rates. Reasons for this trend may include changes in the types of outcomes being used (eg, surrogate outcomes and biomarkers as well as patient-reported outcomes), increasing trial-associated costs, and greater budget constraints. With an overall median sample size of 60 persons per trial, the ability to generate meaningful, reproducible differences with such a sample size remains questionable.10,18 Reports from almost 10 years ago had similar conclusions, without any evidence of change or improvement.10,12,19 The original planned sample sizes were not met and were often smaller compared with the actual sample size when the trial was completed.20 Reasons for not achieving the planned sample size, other than meeting the scientific goals of the trial, include recruitment and retention difficulties, business decisions, and unavailability or discontinuation of funding.21,22,23 At time of analysis, there were 21 455 trials started in 2019 and registered in ClinicalTrials.gov. If we assume registrations in ClinicalTrials.gov account for 70% of all trials registered and that the median cost (direct and indirect) per trial from start to finish is $1 000 000 at a minimum, the total cost for trials in 2019 would be approximately $31 billion. The median cost for comparative efficacy trials (phase 3-4) is closer to $19 million per trial, with larger trials ranging up to $53 million per trial.24,25 Thus, increased funding for larger randomized clinical trials may be warranted to inform clinical decision-making and answer important clinical and health policy questions.
Opportunities for Improvement
The findings suggest that registration and reporting systems could be further improved. There appears to be a need for ClinicalTrials.gov to modify its registration system to accommodate the broader range of trials being conducted and the collaborative arrangements involved. For instance, there is currently no explicit data element for funding source in ClinicalTrials.gov. Thus, the lead sponsor variable was used to estimate trends by the different agencies and organizations as classified in ClinicalTrials.gov. The term lead sponsor refers to the primary organization that oversees study implementation and is responsible for conducting data analysis and is further used to determine the primary funding source for the study.1 The impetus for ClincalTrials.gov was the FDA. That history is reflected in the emphasis on drug trials, US funding, and intermixing sponsor as funder and holder of Investigational New Drug applications. Because trials are largely collaborative and can involve several funders, there is potential for misclassification, underreporting, or overreporting of estimates of funding sources. Although methods have been described to estimate the probable funding source from ClinicalTrials.gov, this information cannot be easily exported or analyzed using the publicly available data set or derived without extensive data manipulation and assumptions.10 Thus, an explicit data element for the primary source or sources of funding with potential linkage to the study project number (eg, NIH Report Expenditures and Results Tool [RePORTER] for NIH-funded trials) and the total amount and duration of funding, if available, may improve the ability to compare trials across funding sources, reducing the risk of misclassification of trials in the other funder categories.
Additional updates that may be beneficial to the analysis of ClinicalTrials.gov registration data include further subdivisions for trial phases beyond the FDA-defined phases to reduce the number of trials lacking an FDA-defined phase, expansion of the trial typography as listed in ClinicalTrials.gov to better capture the different types of trial designs, discrete elements for the outcome types (eg, time to event, surrogate, composite, and patient reported) and for specific time points for the primary outcomes for analysis purposes, and reduction of free-text renders to prevent formatting issues when analyzing the database. This approach would allow for future comparisons of specific outcome types and adjust analyses by outcome duration. Improvements to the system for tracking publications related to the trial are needed, including a separate field for the publication associated with the primary outcome, to determine the fraction of trials registered that are published. Publication is the sine qua non of trials, but only a fraction of completed trials are published. Thus, continued efforts to enforce the timely and complete reporting of results are important to reduce the reporting biases associated with delayed publication or failure to publish.26,27,28,29,30
Limitations
This study has limitations. The analysis was limited to the available data as registered in ClinicalTrials.gov and thus may not provide a complete or accurate assessment of the clinical trials research enterprise. The ClinicalTrials.gov database structure and the individual study records have evolved since ClinicalTrials.gov was first launched in 2000, affected by the different registration and reporting requirements over time. Changes in required registration fields, data formats, and key definitions and terms (eg, clarification of applicable clinical trial and changes in phase categories) made it difficult to compare characteristics registered over time and resulted in missing data encountered for certain registration fields, such as enrollment, number of facilities, or overall status.12,32 ClinicalTrials.gov is only 1 of multiple registration sites where trials can be registered. The World Health Organization International Clinical Trials Registry Platform has 16 other places where trials can be registered with varying analytic capabilities. Although trials are required to meet ICMJE standards, it is difficult to obtain a single comprehensive evaluation of all registered trials. It also remains unclear how many duplicate registrations may exist, especially for non-US studies that may be registered in both ClinicalTrials.gov and a second or third registration registry.31 Thus, whether trials registered in ClinicalTrials.gov are representative of trials registered elsewhere may remain unknown until there is a system for merging registries into 1 or for establishing a universal and standardized data system for harvesting trial information from all registries through the World Health Organization International Clinical Trials Registry Platform.
Conclusions
Even with its limitations, ClinicalTrials.gov registration provides valuable insights into the massive clinical trials research enterprise. The findings suggest that the composition and design of trials changed over time and differed substantially by sponsor type. Increased funding toward larger randomized clinical trials may be warranted to inform clinical decision-making and guide future research.
eTable 1. Selected Terms and Definitions Used on ClinicalTrials.gov
eTable 2. Number of Trials Started by Year Between 2000 and 2019
eTable 3. Predictors of Sample Size for Completed Trials in Multivariable Regression by Phase
eTable 4. Anticipated and Actual Sample Sizes for Trials Started and Completed Between 2010 Through 2019 by Lead Sponsor and Phase
eFigure 1. Time to Study Completion by Lead Sponsor
eFigure 2. Median Time (Years) to Trial Completion by Lead Sponsor and Phase
eFigure 3. Anticipated vs Actual Sample Size of Trials Started and Completed Between 2010 Through 2019 by Lead Sponsor and Year
eAppendix. Example postgreSQL Code to Generate CT.gov Registration Dataset Used for Analysis
References
- 1.U.S. National Library of Medicine, National Institutes of Health ClinicalTrials.gov. Accessed January 1, 2020. https://clinicaltrials.gov/
- 2.McCray AT, Ide NC. Design and implementation of a national clinical trials registry. J Am Med Inform Assoc. 2000;7(3):313-323. doi: 10.1136/jamia.2000.0070313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laine C, Horton R, DeAngelis CD, et al. . Clinical trial registration. BMJ. 2007;334(7605):1177-1178. doi: 10.1136/bmj.39233.510810.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration Food and Drug Administration Modernization Act of 1997 (FDAMA): Pub L No. 105-115. Updated March 28, 2018. Accessed December 15, 2019. https://www.fda.gov/regulatory-information/selected-amendments-fdc-act/food-and-drug-administration-modernization-act-fdama-1997
- 5.Zarin DA, Tse T, Williams RJ, Rajakannan T. Update on trial registration 11 years after the ICMJE policy was established. N Engl J Med. 2017;376(4):383-391. doi: 10.1056/NEJMsr1601330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Angelis C, Drazen JM, Frizelle FA, et al. ; International Committee of Medical Journal Editors . Clinical trial registration: a statement from the International Committee of Medical Journal Editors. CMAJ. 2004;171(6):606-607. doi: 10.1503/cmaj.1041281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Food and Drug Administration US Food and Drug Administration Amendments Act (FDAAA) of 2007. 2007. Accessed January 10, 2020. https://www.fda.gov/regulatory-information/food-and-drug-administration-amendments-act-fdaaa-2007/fdaaa-certification-accompany-drug-biological-product-and-device-applications-or-submissions
- 8.Zarin DA, Tse T, Williams RJ, Carr S. Trial reporting in ClinicalTrials.gov—the final rule. N Engl J Med. 2016;375(20):1998-2004. doi: 10.1056/NEJMsr1611785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical Trials Transformation Initiative. Aggregate Analysis of ClinicalTrials.gov (AACT). 2020. Accessed January 1, 2020. https://aact.ctti-clinicaltrials.org/download
- 10.Califf RM, Zarin DA, Kramer JM, Sherman RE, Aberle LH, Tasneem A. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007-2010. JAMA. 2012;307(17):1838-1847. doi: 10.1001/jama.2012.3424 [DOI] [PubMed] [Google Scholar]
- 11.Ehrhardt S, Appel LJ, Meinert CL. Trends in National Institutes of Health funding for clinical trials registered in ClinicalTrials.gov. JAMA. 2015;314(23):2566-2567. doi: 10.1001/jama.2015.12206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gresham GK, Ehrhardt S, Meinert JL, Appel LJ, Meinert CL. Characteristics and trends of clinical trials funded by the National Institutes of Health between 2005 and 2015. Clin Trials. 2018;15(1):65-74. doi: 10.1177/1740774517727742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clinical Trials Transformation Initiative Connect to AACT. Accessed July 13, 2020. https://aact.ctti-clinicaltrials.org/connect
- 14.Clinical Trials Transformation Initiative AACT Data Dictionary. Accessed July 13, 2020. https://aact.ctti-clinicaltrials.org/data_dictionary
- 15.Mercieca-Bebber R, King MT, Calvert MJ, Stockler MR, Friedlander M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat Outcome Meas. 2018;9:353-367. doi: 10.2147/PROM.S156279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zagadailov E, Fine M, Shields A. Patient-reported outcomes are changing the landscape in oncology care: challenges and opportunities for payers. Am Health Drug Benefits. 2013;6(5):264-274. [PMC free article] [PubMed] [Google Scholar]
- 17.Bottomley A, Reijneveld JC, Koller M, Flechtner H, Tomaszewski KA, Greimel E; 5th EORTC Quality of Life in Cancer Clinical Trials Conference Faculty . Current state of quality of life and patient-reported outcomes research. Eur J Cancer. 2019;121:55-63. doi: 10.1016/j.ejca.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 18.Wittes J. Sample size calculations for randomized controlled trials. Epidemiol Rev. 2002;24(1):39-53. doi: 10.1093/epirev/24.1.39 [DOI] [PubMed] [Google Scholar]
- 19.Turner RM, Bird SM, Higgins JP. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One. 2013;8(3):e59202. doi: 10.1371/journal.pone.0059202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones CW, Safferman MR, Adams AC, Platts-Mills TF. Discrepancies between ClinicalTrials.gov recruitment status and actual trial status: a cross-sectional analysis. BMJ Open. 2017;7(10):e017719. doi: 10.1136/bmjopen-2017-017719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams RJ, Tse T, DiPiazza K, Zarin DA. Terminated trials in the ClinicalTrials.gov results database: evaluation of availability of primary outcome data and reasons for termination. PLoS One. 2015;10(5):e0127242-e0127242. doi: 10.1371/journal.pone.0127242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlisle B, Kimmelman J, Ramsay T, MacKinnon N. Unsuccessful trial accrual and human subjects protections: an empirical analysis of recently closed trials. Clin Trials. 2015;12(1):77-83. doi: 10.1177/1740774514558307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gul RB, Ali PA. Clinical trials: the challenge of recruitment and retention of participants. J Clin Nurs. 2010;19(1-2):227-233. doi: 10.1111/j.1365-2702.2009.03041.x [DOI] [PubMed] [Google Scholar]
- 24.Sertkaya A, Wong HH, Jessup A, Beleche T. Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials. 2016;13(2):117-126. doi: 10.1177/1740774515625964 [DOI] [PubMed] [Google Scholar]
- 25.Moore TJ, Zhang H, Anderson G, Alexander GC. Estimated costs of pivotal trials for novel therapeutic agents approved by the US Food and Drug Administration, 2015-2016. JAMA Intern Med. 2018;178(11):1451-1457. doi: 10.1001/jamainternmed.2018.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarin DA, Tse T, Sheehan J. The proposed rule for U.S. clinical trial registration and results submission. N Engl J Med. 2015;372(2):174-180. doi: 10.1056/NEJMsr1414226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeVito NJ, Bacon S, Goldacre B. Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study. Lancet. 2020;395(10221):361-369. doi: 10.1016/S0140-6736(19)33220-9 [DOI] [PubMed] [Google Scholar]
- 28.Ross JS, Mocanu M, Lampropulos JF, Tse T, Krumholz HM. Time to publication among completed clinical trials. JAMA Intern Med. 2013;173(9):825-828. doi: 10.1001/jamainternmed.2013.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross JS, Tse T, Zarin DA, Xu H, Zhou L, Krumholz HM. Publication of NIH funded trials registered in ClinicalTrials.gov: cross sectional analysis. BMJ. 2012;344:d7292. doi: 10.1136/bmj.d7292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simes RJ. Publication bias: the case for an international registry of clinical trials. J Clin Oncol. 1986;4(10):1529-1541. doi: 10.1200/JCO.1986.4.10.1529 [DOI] [PubMed] [Google Scholar]
- 31.van Valkenhoef G, Loane RF, Zarin DA. Previously unidentified duplicate registrations of clinical trials: an exploratory analysis of registry data worldwide. Syst Rev. 2016;5(1):116. doi: 10.1186/s13643-016-0283-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tse T, Fain KM, Zarin DA. How to avoid common problems when using ClinicalTrials.gov in research: 10 issues to consider. BMJ. 2018;361:k1452. doi: 10.1136/bmj.k1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Selected Terms and Definitions Used on ClinicalTrials.gov
eTable 2. Number of Trials Started by Year Between 2000 and 2019
eTable 3. Predictors of Sample Size for Completed Trials in Multivariable Regression by Phase
eTable 4. Anticipated and Actual Sample Sizes for Trials Started and Completed Between 2010 Through 2019 by Lead Sponsor and Phase
eFigure 1. Time to Study Completion by Lead Sponsor
eFigure 2. Median Time (Years) to Trial Completion by Lead Sponsor and Phase
eFigure 3. Anticipated vs Actual Sample Size of Trials Started and Completed Between 2010 Through 2019 by Lead Sponsor and Year
eAppendix. Example postgreSQL Code to Generate CT.gov Registration Dataset Used for Analysis