Abstract
Objective
The aim of this study was to identify the protective effects of Phellinus linteus mycelium (PLM) and its possible mechanisms in a model of monosodium iodoacetate- (MIA-) induced osteoarthritis (OA).
Methods
Intra-articular injection of MIA was injected to 50 μL with 80 mg/mL using a 0.3 mL insulin syringe into the right knee joint. Changes in hindpaw weight-bearing distribution between the right (osteoarthritic) and left (contralateral control) legs were used as an index of joint discomfort. PLM (50, 100, and 200 mg/kg body weight) was orally administered once daily for 14 days from day 7 after MIA treatment. And then, various factors associated with inflammatory response and cartilage degeneration in cartilage tissues detected by western blotting.
Results
PLM treatment showed a concentration-dependent elevation in change in hindpaw weight-bearing distribution (HWBD). PLM200 demonstrated the capacity to significantly increase HWBD, indicating that the change in weight-bearing distribution means the reduction of spontaneous pain. Our results indicate that PLM suppressed the inflammatory factors via NF-κB signaling pathway induced by p38 phosporlyation. Moreover, PLM200 exhibited a significant reduction of ROS produced by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. PLM100 and PLM200 inhibited the levels of matrix metalloproteinase (MMP)-1, one of proteinase that degrades extracellular matrix (ECM).
Conclusions
Taken together, our results indicated that PLM has a strong chondroprotective effect through the suppression both ROS production and inflammation.
1. Introduction
Osteoarthritis (OA) is nowadays one of the most prevalent chronic arthritis of human joint, and age and obesity are well known for its powerful risk factors [1, 2]. OA is characterized by cartilage degradation and inflammation and develops progressively over several years. Consequently, OA causes physical limitations, disability, mental stress, and socioeconomic burden [3]. Therefore, both bodily pain and physical functioning by OA are lower than that for cardiovascular conditions, chronic respiratiory diseases, and gastrointestinal conditions [4]. OA leads to much lower quality of life. Although OA was referred to as the noninflammatory arthritis historically, it is considered a low-grade inflammation disease [5, 6] triggered by factors like biomechanical stress associated with OA development [7]. Therefore, anti-inflammatory drugs like nonsteroidal anti-inflammatory drugs (NSAIDs) were given to patients with symptomatic OA to remove OA symptoms [8, 9]. However, these drugs led the gastrointestinal and cardiovascular side-effects [10]. Accordingly, new strategies about an effective and a safe herbal medicine for OA treatment is urgently needed.
Currently, many studies focused on describing the key factors responsible for the deepening of inflammatory processes [11]. An analysis of the ever-increasing reports showed the important role of the cytokine network in the pathogenesis of OA [12]. During the progression of OA, the degree of cytokine production can make changes to the duration and severity of OA [13]. Such inflammatory cytokines including tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 are important factors participating in the pathogenesis of OA [14] and intimately related to cartilage matrix degradation. Matrix metalloproteinases (MMPs), proteolytic enzymes, are a family of zinc-dependent endoproteases and possess multiple roles in degradation of various proteins and tissue remodeling in the extracellular matrix (ECM). Moreover, MMPs promote cell migration, proliferation, and differentiation and could play a role in cell apoptosis, tissue repair, and angiogenesis [15]. In addition, MMPs expression also increases during the inflammatory process. MMPs are secreted by inflammatory cells and promoted by inflammatory cytokines [16]. Whereas, it generally counteracts inhibitory actions of endogenous TIMPs [17, 18]. Hence, the inhibition of inflammatory cytokines and MMPs may be a valid approach for OA treatment.
Medicinal fungi could produce diverse bioactive metabolites including anticancer drugs, antibiotics, and immunosuppressants [19–21]. Many medically meaningful metabolites had been reported from various edible fungi. Phellinus linteus is a famous medicinal mushroom that is widely used in Korea, Japan, China, and other Asian countries [22]. The various components including polyphenols, pyrans, polysaccharides, and triterpenoids play a significant role in improving the health condition [23, 24]. Above all, Phellinus linteus mycelium (PLM) was used as medicines or healthy foods to treat several diseases such as infections, ulcer, cancer, and diabetes [25]. In the previous reports, PLM showed wide spectrum of bioactivities, such as antioxidant, anti-inflammatory, cytotoxic, antiviral, and antidiabetic effects [26–28]. Especially, β-glucan isolated from mushroom is a natural polysaccharide and possess various biological effects such as immune-modulating properties, antitumor, anti-infection, and lowering blood cholesterol [29, 30].
The hypothesis of the current study was PLM supplementation could have chondroprotective effect via the inhibition of cartilage matrix degradatin on the articular cartilage. So, the efficacy and underlying mechanism of PLM were examined using the MIA-induced OA model.
2. Materials and Methods
2.1. Materials
Monosodium iodoacetate (MIA), phenyl methyl sulfonyl fluoride (PMSF), dithiothreitol (DTT), and diethylenetriaminepentaacetic acid (DTPA) were purchased from Sigma Aldrich Co., Ltd (St. Louis, MO, USA). The protease inhibitor mixture solution and ethylene diamine tetraacetic acid (EDTA) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). ECL western blotting detection reagents and pure nitrocellulose membranes were supplied by GE Healthcare (Piscataway, NJ, USA). 0.3 mL insulin syringe was obtained from BD Medical-Diabetes Care (Holdrege, USA). Besides, all other chemicals and reagents were purchased from Sigma Aldrich Co., Ltd. (St Louis, MO, USA). Primary antibody rabbit polyclonal antibodies against p47phox, p22phox, catalase, glutathione peroxidase-1/2 (GPx-1/2), phospho-p38 (p-p38), goat polyclonal antibodies against tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), interleukin-1 beta (IL-1β), mouse monoclonal antibodies against nuclear factor-kappa B p65 (NF-kBp65), cyclooxygenase 2 (COX-2), inducible nitric oxide synthase (iNOS), matrix metalloproteinase-1 (MMP-1), tissue inhibitor matrix metalloproteinase 1 (TIMP-1), histone, β-actin, and secondary antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Moreover, rabbit polyclonal antireduced nicotinamide adenine dinucleotide phosphate oxidase 4 (NOX4) was obtained from LifeSpan BioSciences (Seattle, WA, USA). The BCA protein assay kit is supplied by Thermo Fisher Scientific (Waltham, MA, USA).
2.2. Preparation of PLM
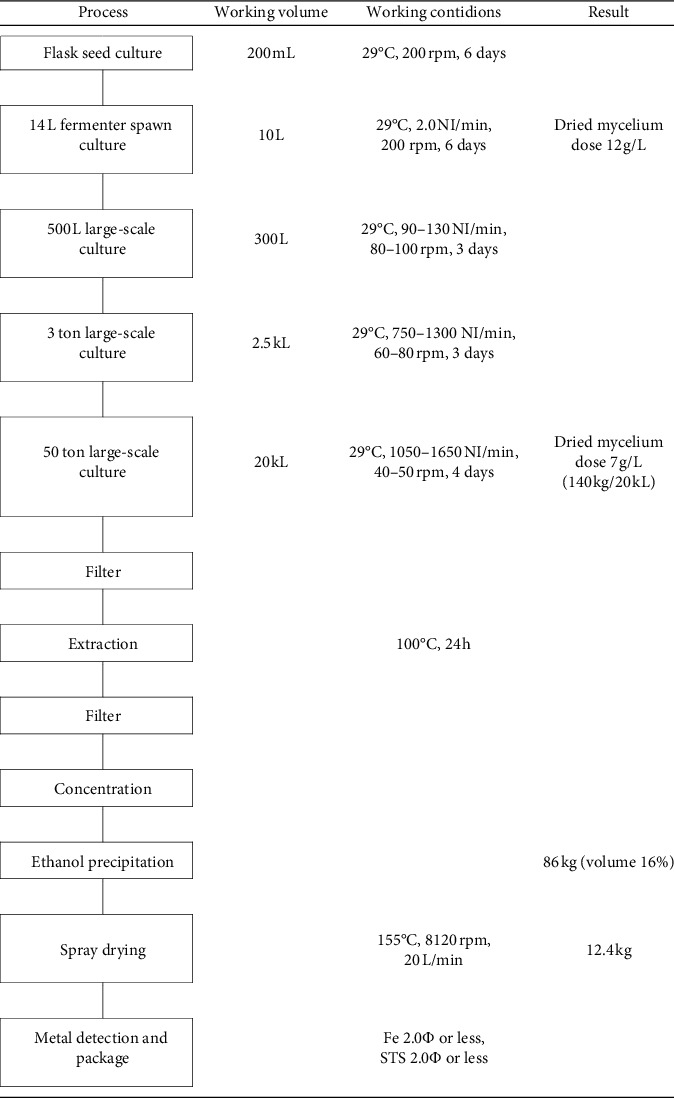
Phellinus linteus mycelium was obtained as a dried mycelium from Hankook Shinyak Corp. (Nonsan-si, Korea), and Green-lipped mussel powder was obtained from McFarlane Marketing (Aust) Pty Ltd. (Melbourne, Australia). Phellinus linteus mycelium was inoculated under optimal culture condition (at 30°C and pH 4-5) for 14 days on yeast malt extract glucose (YMG) agar. The extract condition was optimized for extraction temperature (100°C), extraction time (24 h), solvent amount (10 times purified water), and extraction frequency (1st). A 50 ton large-scale incubator was used to produce 7 g/L dry mycelium in 4 days after mass production of mushroom mycelium through the following manufacturing process (Table 1). The produced PLM analyzed the nutritional components in accordance with the method of food revolution at Korea Health Supplement Institute (Seongnam, Korea) [31]. The composition of PLM is shown in Table 2.
Table 1.
Powder manufacturing process of Phellinus linteus mycelium extract.
|
Table 2.
General nutrition compositions of Phellinus linteus mycelium.
| Calorie (kcal/100 g) | Carbohydrate (%) | Protein (%) | Fat (%) | Sodium (mg/100 g) | Sugar (mg/g) | Saturated fatty acid (g/100 g) | Transfat (g/100 g) | Cholesterol (mg/100 g) |
|---|---|---|---|---|---|---|---|---|
| 346.36 | 68.86 | 15.75 | 0.88 | 71.57 | 128.29 | 0.07 | 0 | 0 |
2.3. β-Glucan Measurement of PLM
β-Glucan content in PLM was analysed using the K-YBGL kit (Megazyme, Ireland) as the mushroom and yeast β-glucan assay procedure. Extraction, laboratory analysis, and calculation were performed in accordance with the manufacturer's instructions. β-glucan content was calculated as a percentage using the following equation:
| (1) |
Consequently, β-glucan content of PLM was 14.16 ± 6.27%.
2.4. Development of OA with MIA Injection and PLM Administration
Seven-week-old male Sprague Dawley rats weighing 200–250 g at the start of the experiment were purchased from DBL Co. (Eumseong, South Korea). The animals were housed three per cage in a room with controlled temperature conditions (23 ± 2°C), humidity (about 55 ± 5%), and lighting (12 h light/dark cycle) with free access to food and water. After adaptation (1 week), rats were randomly arranged in the descending order of weight and assigned into six groups of equal numbers (n = 7) without any statistical significance among the groups:
Group 1: Nor group included the normal rats
Group 2: Con group included the MIA control rats
Group 3: GLM200 group included the green-lipped mussel 200 mg/kg-administered and MIA rats
Group 4: PLM50 group included the PLM 50 mg/kg-administered and MIA rats
Group 5: PLM100 group included the PLM 100 mg/kg-administered and MIA rats
Group 6: PLM200 group included the PLM 200 mg/kg-administered and MIA rats
The normal and MIA control groups were administrated water using a stomach tube, while the other groups were orally administered green-lipped mussel 200 mg/kg or PLM 50, 100, and 200 mg/kg using a stomach tube for 2 weeks. OA was induced in SD rats; we employed the method of Wang with minor modifications [32]. After anesthetization with injection of Zoletil mixture (Vibrac, France) 0.75 mg/kg intraperitoneally, rats were injected with MIA 80 mg/mL in a 50 μL volume using a 0.3 mL insulin syringe (31G needle) inserted through the patellar ligament into the intra-articular space of the right knee; normal rats were injected with an equivalent volume of saline [33]. The rats in all groups were sacrificed after the end of experimental period. The rats were anesthetized using a mixture of Zoletil and xylazine and euthanized by isoflurane overdose. Right knee joint specimens were collected, and the muscles around the knee were joint quickly removed. The femoral cartilage of rat was removed through circular incision along the femoral edge of the articular capsule. In addition, the femur and tibial fibula were cut off along each bone surface. And then, the joint capsule was cut longitudinally and the synovial tissue was separated. The cartilage specimens were stored at −80°C until further study. The terminal blood samples were centrifuged at 3000 rpm for 20 min at 4°C and the serum was collected and stored at −80°C until analysis.
2.5. Measurements of Hindpaw Weight-Bearing Distribution
Hindpaw weight-bearing distribution (HWBD) was assessed using an incapacitance meter that measures the distribution of weight bearing across each hindlimb. The balance in the weight-bearing capability of the hindpaws was disrupted after OA induction. A significant shift in weight from the arthritic site to the contralateral limb, i.e., a weight bearing deficit, was considered to be an index of pain. Pain was measured via the weight bearing of the paw load using an incapacitance tester (Linton Instrumentation, Norfolk, UK) [34]. The hinpaw weight bearing distribution ratio (%) was calculated using the following equation:
| (2) |
2.6. Preparation of Cytosol and Nuclear Fractions
Protein extraction was performed according to the method of Komatsu with minor modifications [35]. The cytosol fraction was homogenized with ice-cold lysis buffer A (250 mL) containing 10 mM HEPES (pH 7.8), 10 mM KCl, 2 mM MgCl2, 1 mM DTT, 0.1 mM EDTA, 0.1 mM PMSF, and 1,250 μL protease inhibitor mixture solution. The homogenate was incubated at 4°C for 20 min. And then, 10% NP-40 was added and mixed well. After centrifugation (13,400 ×g for 2 min at 4°C) using Eppendorf 5415R (Hamburg, Germany), the supernatant liquid (cytosol fraction) was separated into a new e-tube. The left pellets were washed twice by buffer A, and the supernatant was discarded. Next, the pellets were suspended with lysis buffer C (20 mL) containing 50 mM HEPES (pH 7.8), 50 mM KCl, 300 mM NaCl, 1 mM DTT, 0.1 mM EDTA, 0.1 mM PMSF, 1% (v/v) glycerol, and 100 μL protease inhibitor mixture solution suspended and incubated at 4°C for 30 min. After centrifugation (13,400 ×g for 10 min at 4°C), the nuclear fraction was prepared to collect the supernatant. The protein concentration was determined using BCA protein assays by Thermo Fisher Scientific (Waltham, USA). Both cytosol and nuclear fractions were kept at −80°C before the analysis.
2.7. Immunoblotting Analysis
For the estimation of NF-κBp65 and histone (1 : 1000; Santa Cruz Biotechnology, Dallas, TX, USA), 10 μg of proteins from each nuclear fraction was electrophoresed through 8–10% sodium dodecylsulfate polyacrylamide gel (SDS-PAGE). Separated proteins were transferred to a nitrocellulose membrane, blocked with 5% (w/v) skim milk solution for 1 h, then incubated with primary antibodies to NF-κBp65 and histone, respectively, overnight at 4°C. After the blots were washed, they were incubated with anti-mouse IgG HRP-conjugated secondary antibody (1 : 3000; Santa Cruz Biotechnology) for 1.5 h at room temperature. In addition, 10–12 μg proteins of each cytosol fraction was eletrophoresed in 10–14% sodium dodecylsulfate polyacrylamide gel (SDS-PAGE) for immunodetection of p-p38/COX-2/iNOS/TNF-α/IL-6/IL-1β/catalase/GPx-1/2/MMP-1/TIMP-1/β-actin (1 : 1000; Santa Cruz, USA). Each antigen-antibody complex was visualized using ECL western blotting detection reagents and detected by chemiluminescence with Sensi-Q 2000 Chemidoc (Lugen Sci Co., Ltd., Gyeonggi-do, Korea). Band densities were measured using ATTO Densitograph software (ATTO Corporation, Tokyo, Japan) and quantified as the ratio to histone or β-actin. The protein levels of the groups are expressed relative to those of the normal rat (represented as 1). We followed the methods of Mi-rae Shin et al. 2017 [36].
2.8. Statistical Analysis
Data are expressed as mean ± SEM. Statistical comparisons were assessed by one-way ANOVA followed by the least-significant differences (LSD) test (SPSS 22.0 for Windows, SPSS Inc., NY, USA). Values of P < 0.05 were considered significant.
3. Results
3.1. Change in Hindpaw Weight-Bearing Distribution
The MIA model is the most often used, being commonly chosen to evaluate the efficacy of pharmacological agents for pain management. Pain assessment assay commonly used is the incapacitance test, which measures the weight distribution between both hind limbs [37]. The pain of knee joint leads to decrease of hindpaw weight-bearing distribution (HWBD), which has been proved in the previous study [34]. The HWBD on 0, 1, and 2 weeks is measured in Figure 1. On day 0, the HWBD of MIA-treated groups was lower compared with that of the normal group (P < 0.05). After 2 weeks, the decreased HWBD was significantly increased at GLM200 and PLM200 groups (P < 0.05). The pain of knee joint could alleviate though PLM supplementation.
Figure 1.
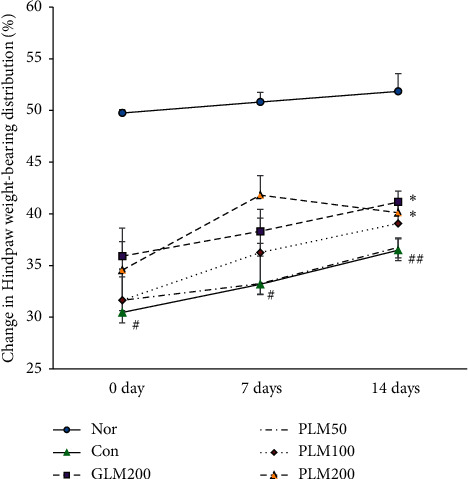
Change in Hindpaw weight-bearing distribution. Values are the mean ± SEM (n = 7). Nor: normal rats; Con: MIA-induced osteoarthritis rats; GLM200: MIA-induced osteoarthritis rats administrated with green-lipped mussel 200 mg/kg body weight; PLM50: MIA-induced osteoarthritis rats administrated with PLM 50 mg/kg body weight; PLM100: MIA-induced osteoarthritis rats administrated with PLM 100 mg/kg body weight; PLM200: MIA-induced osteoarthritis rats administrated with PLM 200 mg/kg body weight. Significance: #P < 0.05, ##P < 0.01 vs. normal rat values. ∗After 2 weeks, the decreased HWBD was significantly increased at GLM200 and PLM200 groups (P < 0.05).
3.2. NOX4, p47phox, and p22phox in Cartilage Tissues
The NADPH oxidase family including NOX4, p47phox, and p22phox is one of the potent cellular sources in the overproduction of reactive oxygen species (ROS) [38]. The expressions of NOX4, p47phox, and p22phox were examined, as shown in Figure 2. The treatment of MIA significantly led to upregulation of both NOX4 and p22phox in the cartilage tissue; however, PLM200 administration significantly suppressed the levels of all markers (NOX4; P < 0.05, p47phox; P < 0.05, and p22phox; P < 0.01).
Figure 2.
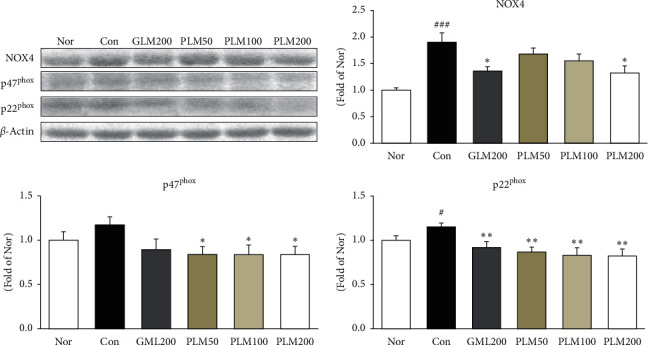
NADPH oxidases: NOX4, p47phox, and p22phox in cartilage tissues. Values are the mean ± SEM (n = 6). Nor: normal rats; Con; MIA-induced osteoarthritis rats; GLM200: MIA-induced osteoarthritis rats administrated with green-lipped mussel 200 mg/kg body weight; PLM50: MIA-induced osteoarthritis rats administrated with PLM 50 mg/kg body weight; PLM100: MIA-induced osteoarthritis rats administrated with PLM 100 mg/kg body weight; PLM200: MIA-induced osteoarthritis rats administrated with PLM 200 mg/kg body weight. Significance: #P< 0.05, ###P < 0.001 vs. normal rat values and ∗P < 0.05, ∗∗P < 0.01 vs. MIA control rat values.
3.3. p38-MAPK and NF-κBp65 in Cartilage Tissues
The previous study reported that p38-MAPK activity attenuates the transcriptional activity of the proinflammatory transcription factor, NF-κB [39, 40]. Activation of p38-MAPK affected the NF-κBp65 protein expression (Figure 3). The injection of MIA resulted in a significant increase of p38-MAPK compared with the normal group. In contrast, the treatment of all drugs significantly reduced the expression of p38-MAPK. Besides that, the NF-κBp65 expression in MIA control was elevated, whereas it was attenuated by GLM200, PLM100, and PLM200 administration (P < 0.05).
Figure 3.
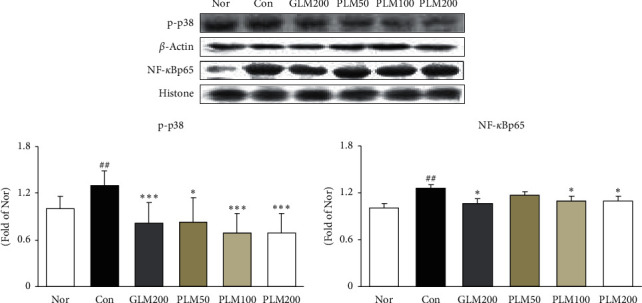
p-p38 and NF-κBp65 protein expressions in cartilage tissues. Values are the mean ± SEM (n = 6). Nor: normal rats; Con: MIA-induced osteoarthritis rats; GLM200: MIA-induced osteoarthritis rats administrated with green-lipped mussel 200 mg/kg body weight; PLM50: MIA-induced osteoarthritis rats administrated with PLM 50 mg/kg body weight; PLM100: MIA-induced osteoarthritis rats administrated with PLM 100 mg/kg body weight; PLM200: MIA-induced osteoarthritis rats administrated with PLM 200 mg/kg body weight. Significance: ##P < 0.01 vs. normal rat values and ∗P < 0.05, ∗∗∗P < 0.001 vs. MIA control rat values.
3.4. TNF-α, IL-6, IL-1β, COX-2, and iNOS in Cartilage Tissues
Activated NF-κB regulates the expression of inflammatory mediators and many cytokines [14]. Figure 4 displays the effect of PLM on the expression of inflammation-related mediators such as COX-2, iNOS, and cytokines including TNF-α, IL-6, and IL-1β. The MIA treatment significantly augmented these protein expressions. On the other hand, PLM200 treatment significantly decreased all factors. Especially, the suppression effects of PLM200 was near the levels of the normal group (iNOS, IL-6, and IL-1β; P < 0.05, COX-2; P < 0.01, TNF-α; P < 0.001).
Figure 4.
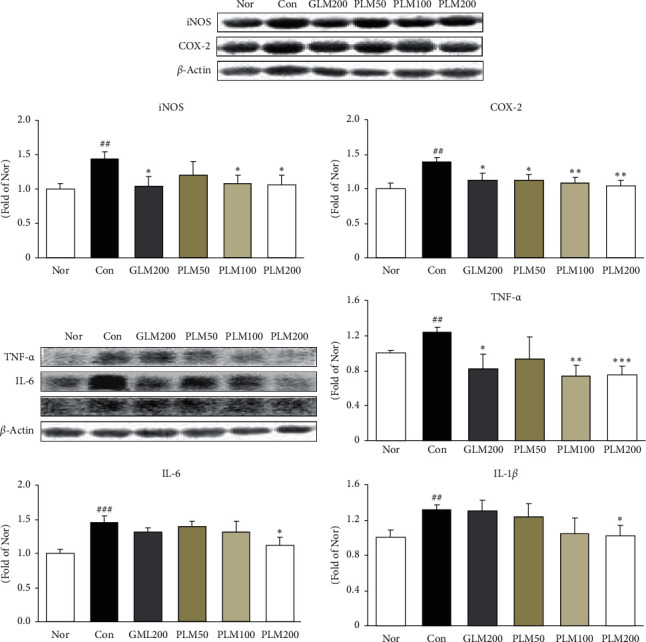
iNOS, and COX-2, TNF-α, IL-6, and IL-1β protein expressions in cartilage tissues. Values are the mean ± SEM (n = 6). Nor: normal rats; Con: MIA-induced osteoarthritis rats; GLM200: MIA-induced osteoarthritis rats administrated with green-lipped mussel 200 mg/kg body weight; PLM50: MIA-induced osteoarthritis rats administrated with PLM 50 mg/kg body weight; PLM100: MIA-induced osteoarthritis rats administrated with PLM 100 mg/kg body weight; PLM200: MIA-induced osteoarthritis rats administrated with PLM 200 mg/kg body weight. Significance: ##P < 0.01: ###P < 0.001 vs. normal rat values and ∗P < 0.05: ∗∗P < 0.01: ∗∗∗P < 0.001 vs. MIA control rat values.
3.5. MMP-1 and TIMP-1 in Cartilage Tissues
Cytokines upregulate metalloproteinase (MMPs) gene expression, and such prolytic enzymes degrade extracellular matrix, whereas TIMP-1 inhibits MMP-induced ECM degradation for the maintenance of homostasis [41]. Our results in this study revealed that PLM200 significantly suppressed the expression of MMP-1 (P < 0.01), otherwise elevated TIMP-1 without a significance (Figure 5).
Figure 5.
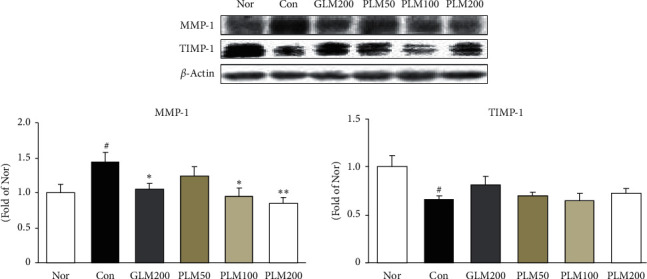
MMP-1 and TIMP-1 protein expressions in cartilage tissues. Values are the mean ± SEM (n = 6). Nor: normal rats; Con: MIA-induced osteoarthritis rats; GLM200: MIA-induced osteoarthritis rats administrated with green-lipped mussel 200 mg/kg body weight; PLM50: MIA-induced osteoarthritis rats administrated with PLM 50 mg/kg body weight; PLM100: MIA-induced osteoarthritis rats administrated with PLM 100 mg/kg body weight; PLM200: MIA-induced osteoarthritis rats administrated with PLM 200 mg/kg body weight. Significance: #P< 0.05 vs. normal rat values and ∗P < 0.05, ∗∗P < 0.01 vs. MIA control rat values.
3.6. Catalase and Gpx-1/2 in Cartilage Tissues
The activity of ROS is balanced by enzymatic antioxidants such as catalase and GPx-1/2 [42]. Antioxidant properties could reinforce the cellular antioxidant status. In our results, MIA control rats showed decreased expressions of catalase and GPx-1/2 compared with those of normal rats; however, PLM administration effectively upregulated these levels. Herein, antioxidant enzyme catalase significantly increased compared with MIA control rats by GLM200, PLM100, and PLM200 supplementation (P < 0.05) (Figure 6).
Figure 6.
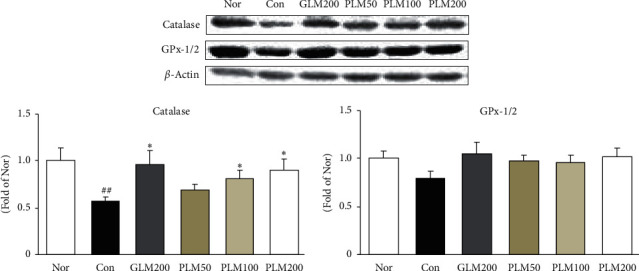
Catalase and GPx-1/2 protein expressions in cartilage tissues. Values are the mean ± SEM (n = 6). Nor: normal rats; Con: MIA-induced osteoarthritis rats; GLM200: MIA-induced osteoarthritis rats administrated with green-lipped mussel 200 mg/kg body weight; PLM50: MIA-induced osteoarthritis rats administrated with PLM 50 mg/kg body weight; PLM100: MIA-induced osteoarthritis rats administrated with PLM 100 mg/kg body weight; PLM200: MIA-induced osteoarthritis rats administrated with PLM 200 mg/kg body weight. Significance: ##P < 0.01 vs. normal rat values and ∗P < 0.05 vs. MIA control rat values.
4. Discussions
Osteoarthritis (OA) is a chronic and degenerative joint disease characterized by intra-articular inflammation and cartilage degradation [43]. Thus, inhibition of inflammation has been proposed as an effective strategy for improving the symptom or delaying the progression of OA. NSAIDs are the typical prescribed medications for treating disorders such as OA. But, the adverse effects about long-term uses of NSAIDs still existed [44]. Phytochemicals can modulate inflammatory response and treat OA [45]. Such nature-derived compounds have recently been paid attention to ideal drugs for OA due to their excellent anti-inflammatory activities and limited adverse effects [46, 47].
Phellinus linteus mycelium (PLM), a well-known medicinal mushroom, has been used in Asian countries for many centuries to prevent or treat diseases such as gastroenteric dysfunction, hemorrhage, diarrhea, rheumatoid arthritis, and cancers [48]. Our findings revealed that PLM inhibited inflammatory responses in MIA-induced osteoarthritis rats. To the best of our knowledge, this is regarded the first report to demonstrate the chondroprotective effect of PLM- on MIA-induced OA. Above all, hindpaw weight-bearing distribution (HWBD) is measured. HWBD, as a measure of OA progression, was measured as the difference between MIA-injected and contralateral hind limbs. The groups with GML200 and PLM administration displayed a higher HWBD compared with that of the MIA control group. Especially, PLM200 showed a significant increase. The pain of cartilage leads to decrease of HWBD, which has been proved by several studies [49, 50]. These results demonstrate a balance and relief of cartilage discomfort in the PLM-treated group.
As mentioned earlier, the degradation of cartilage results from the imbalance between catabolic activity and the mechanical stresses, and the latter is primarily controlled by ROS and MMPs. Thus, our results can be explained that the antioxidant defense of PLM decreased the cartilage damage by inhibition of the formation of ROS and MMPs. Disruption of the cartilage homeostasis leads to cartilage destruction. ROS-induced damage is significantly greater in OA cartilage. ROS in the OA process is focused in particular on NADPH oxidase, which is heme-containing transmembrane proteins and especially includes Nox4, p47phox, and p22phox. Ultimately, NADPH oxidase catalyses the transfer of the electron from NADPH and generates superoxide anion (O2−), giving rise to ROS [38, 51]. We measured protein expressions of NADPH oxidases associated with ROS production to investigate the role of PLM. Herein, excessive production of ROS damages nucleic acids, protein, lipids, and matrix components [52]. Mitochondria are essential for cellular bioenergetics and regarded as the major cellular site for ROS production [38]. Our present data indicate that treatment with PLM200 decreased NOX4, p47phox, and p22phox production in the cartilage. H2O2 is regarded as a representative form of ROS, and accumulation of H2O2 is linked closely to the progression of OA [32]. In the previous study, H2O2-induced oxidative stress microenvironment and catalase and GPx-1/2 neutralize H2O2 to form H2O [52]. After MIA injection, the antioxidant enzymes including catalase and GPx-1/2 were increased than those of the MIA control group. PLM treatment showed a significant upregulation of catalase, which alleviated the degradation of ECM and protected the cartilage from oxidative stress. As the result, this suggests that the antioxidant activity of PLM may contribute to reducing pain in the MIA rat model of OA.
In addition, we examined the expression of matrix metalloproteinase (MMP)-1 and TIMP-1. The extracellular matrix is composed of proteoglycans (aggrecans) that retain water and of collagen II (95%). MMPs are a class of proteolytic enzymes involved in the occurrence, development, and progression of OA that promote the degradation of cartilage matrix components such as collagen II, which is a crucial factor in balancing the synthesis and degradation of the ECM of articular cartilage [16, 53]. Among MMPs, MMP-1 is a soluble proteinase known as collagenase-1 and degrades the extracellular matrix of cartilage and plays a main role in the occurrence, development, and progression of OA [16]. Meanwhile, activated MMP-1 is mainly regulated by tissue inhibitor of metalloproteinase (TIMP)-1 as the chief MMP regulator. Moreover, TIMP-1 is produced by connective tissue and resists against collagenase, gelatinase, and stromelysin [54, 55]. In this study, MIA control expressed an increased MMP-1 expression, and it also suggested that MMP-1 is associated with destruction of cartilage. However, PLM supplementation significantly blocked the MMP-1 upregulation of the MIA control group. These results suggest that the anticatabolic effect of PLM may be due to the inhibition of MMP-1.
Mitogen-activated protein kinases (MAPKs), which are a family of serine/threonine kinases, integrate and process various extracellular signals or cytokines [56]. The p38 MAPK corresponds to an integral component of the proinflammatory signaling pathway about external stress signals and is associated with inflammation, cell differentiation, cell growth, and cell death [56, 57]. Several studies have shown that blocking the p38 activity attenuates the transcriptional activity of the proinflammatory transcription factor NF-κB without altering its DNA-binding activity. The NF-κB signaling pathway is involved in the regulation of inflammatory mediators and cytokines in the progression of OA [58]. NF-κB is a transcription factor localized to the cytoplasm and is rendered inactive by a constitutive interaction with the inhibitory protein IκB [38]. NF-κBp65 is separated from IκB and translocates to the nucleus to regulate the expression of inflammatory mediators and cytokines [59]. Many studies have demonstrated inflammatory cytokines such as IL-1β, TNF-α, and IL-6 play vital roles in OA pathogenesis [37, 51], which is intimately related to degradation of the extracellular matrix (ECM). Besides, COX is a key proinflammatory enzyme that converts arachidonic acid into prostaglandins. The excessive iNOS induces the overproduction of NO. NO generates ONOO− through reacting with O2−, and this can accelerate OA [60]. Inhibition of NF-κB activation is associated with the downregulation of COX-2 expression and synthesis. Moreover, higher levels of these cytokines in the initiation and progression of articular cartilage destruction have been shown to correlate with pain and physical function of patients with OA [61]. Our results suggest that suppression of NF-κB activation may underlie the mechanism by which PLM inhibits the inflammatory response in cartilage tissues. That is, the elevated expressions of COX-2 and iNOS were markedly attenuated by both PLM100 and PLM200 treatments, and three cytokines such as IL-1β, TNF-α, and IL-6 significantly decreased by PLM200.
5. Conclusions
Taken together, it suggests that PLM could inhibit excessive production of ROS and elevate anti-oxidant effect through catalase upregulation. Moreover, PLM could lead to NF-κB inactivation through inhibition of p38 MAPK and it will suppress sequentially both inflammation factors and components related to degradation of cartilage matrix (Figure 7). As the result, PLM treatment may be the potential therapeutic candidate in patients with osteoarthritis.
Figure 7.
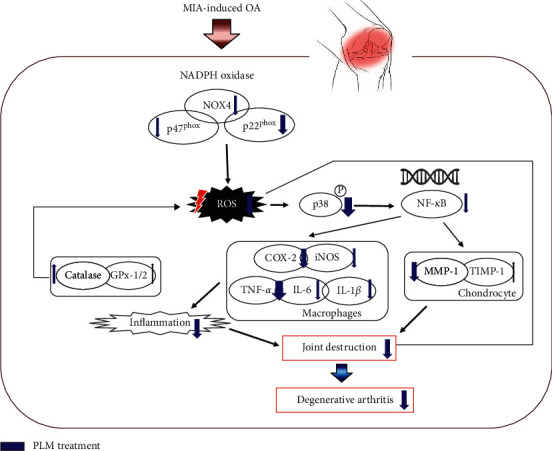
Possible mechanism of Phellinus linteus mycelium (PLM) in MIA-induced osteoarthritis rats.
Acknowledgments
This research was supported by the Ministry of Trade, Industry & Energy (MOTIE), Korea Institute for Advancement of Technology (KIAT) through the Encouragement Program for the Industries of Economic Cooperation Region (No. P0006194), and the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIT) (No .2018R1A5A2025272).
Data Availability
The data used to support the findings of this study are included within the article.
Ethical Approval
All animal procedures were approved by the Animal Research Ethics Committee of the Daegu Haany University (Permit Number: DHU2019-095).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
J.A. Lee and M.J. Kim performed the experiments and were responsible for data acquisition. B.W. Park and S.B. Seo made PLM through a manufacturing process of mass production. M.R. Shin performed data analysis and drafted the article. H.J. Park and S.S. Roh were responsible for the conception and design of the study. All authors have participated actively in carrying out and improving the study, and all have approved the submission of this article.
References
- 1.Pereira D., Ramos E., Branco J. Osteoarthritis. Acta Médica Portuguesa. 2015;28(1):99–106. doi: 10.20344/amp.5477. [DOI] [PubMed] [Google Scholar]
- 2.French H. P., Galvin R., Horgan N. F., Kenny R. A. Prevalence and burden of osteoarthritis amongst older people in Ireland: findings from the Irish longitudinal study on ageing (TILDA) The European Journal of Public Health. 2016;26(1):192–198. doi: 10.1093/eurpub/ckv109. [DOI] [PubMed] [Google Scholar]
- 3.Elders M. J. The increasing impact of arthritis on public health. The Journal of Rheumatology Supplement. 2000;60:6–8. [PubMed] [Google Scholar]
- 4.Reginster J. Y. The prevalence and burden of arthritis. Rheumatology. 2002;41(suppl_1):3–6. doi: 10.1093/rheumatology/41.s1.3. [DOI] [PubMed] [Google Scholar]
- 5.Griffin T. M., Scanzello C. R. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clinical and Experimental Rheumatology. 2019;37(Suppl 120, no. 5):57–63. [PMC free article] [PubMed] [Google Scholar]
- 6.Millerand M., Berenbaum F., Jacques C. Danger signals and inflammaging in osteoarthritis. Clinical and Experimental Rheumatology. 2019;37(Suppl 120, no. 5):48–56. [PubMed] [Google Scholar]
- 7.Chow Y. Y., Chin K. Y. The role of inflammation in the pathogenesis of osteoarthritis. Mediators of inflammation. 2020;2020:p. 16. doi: 10.1155/2020/8293921.8293921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bijlsma J. W., Berenbaum F., Lafeber F. P. Osteoarthritis: an update with relevance for clinical practice. The Lancet. 2011;377(9783):2115–2126. doi: 10.1016/s0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W., Moskowitz R. W., Nuki G., et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis and Cartilage. 2008;16(2):137–162. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 10.Lohmander L. S., McKeith D., Svensson O., et al. A randomised, placebo controlled, comparative trial of the gastrointestinal safety and efficacy of AZD3582 versus naproxen in osteoarthritis. Annals of the Rheumatic Diseases. 2005;64(3):449–456. doi: 10.1136/ard.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lange-Brokaar B. J. E., Ioan-Facsinay A., van Osch G. J. V. M., et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis and Cartilage. 2012;20(12):1484–1499. doi: 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 12.Vangsness C. T., Jr., Burke W. S., Narvy S. J., et al. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis—a pilot study. Bulletin of the NYU Hospital for Joint Diseases. 2011;69(2):122–127. [PubMed] [Google Scholar]
- 13.Wojdasiewicz P., Poniatowski Ł. A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators of Inflammation. 2014;2014:p. 19. doi: 10.1155/2014/561459.561459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes J. C., Martel-Pelletier J., Pelletier J. P. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39(1-2):237–246. [PubMed] [Google Scholar]
- 15.Zheng W., Zhang H., Jin Y., et al. Butein inhibits IL-1β-induced inflammatory response in human osteoarthritis chondrocytes and slows the progression of osteoarthritis in mice. International Immunopharmacology. 2017;42:1–10. doi: 10.1016/j.intimp.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Zeng G. Q., Chen A. B., Li W., Song J. H., Gao C. Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genetics and Molecular Research. 2015;14(4):14811–14822. doi: 10.4238/2015.november.18.46. [DOI] [PubMed] [Google Scholar]
- 17.Bode W., Fernandez-Catalan C., Grams F., et al. Insights into MMP-TIMP interactions. Annals of the New York Academy of Sciences. 1999;878:73–91. doi: 10.1111/j.1749-6632.1999.tb07675.x. [DOI] [PubMed] [Google Scholar]
- 18.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovascular Research. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Nicoletti R., Ciavatta M. L., Buommino E., Tufano M. A. Antitumor extrolites produced by Penicillium species. International journal of analytical, pharmaceutical and biomedical sciences. 2008;2:1–23. [Google Scholar]
- 20.Ng T. B., Wang H. X. Pharmacological actions of cordyceps, a prized folk medicine. The Journal of Pharmacy And Pharmacology. 2005;57(12):1509–1520. doi: 10.1211/jpp.57.12.0001. [DOI] [PubMed] [Google Scholar]
- 21.Han J. Y., Im J., Choi J. N., et al. Induction of IL-8 expression by Cordyceps militaris grown on germinated soybeans through lipid rafts formation and signaling pathways via ERK and JNK in A549 cells. Journal of Ethnopharmacology. 2010;127(1):55–61. doi: 10.1016/j.jep.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 22.Zhu T., Kim S. H., Chen C. Y. A medicinal mushroom: Phellinus linteus. Current Medicinal Chemistry. 2008;15(13):1330–1335. doi: 10.2174/092986708784534929. [DOI] [PubMed] [Google Scholar]
- 23.Chen W., Tan H., Liu Q., et al. A review: the bioactivities and pharmacological applications of Phellinus linteus. Molecules. 2019;24(10):p. 1888. doi: 10.3390/molecules24101888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojima K., Ohno T., Inoue M., Mizukami H., Nagatsu A. Phellifuropyranone a: a new furopyranone compound isolated from fruit bodies of wild Phellinus linteus. Chemical & Pharmaceutical Bulletin. 2018;56(2):173–175. doi: 10.1248/cpb.56.173. [DOI] [PubMed] [Google Scholar]
- 25.Huang S. C., Wang P. W., Kuo P. C., et al. Hepatoprotective principles and other chemical constituents from the mycelium of Phellinus linteus. Molecules. 2018;23(7):p. 1705. doi: 10.3390/molecules23071705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park H. J. Anti-allergic and anti-inflammatory activity of Phellinus linteus grown on Panax ginseng. Food Science and Biotechnology. 2017;26(2):467–472. doi: 10.1007/s10068-017-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura T., Matsugo S., Uzuka Y., Matsuo S., Kawagishi H. Fractionation and anti-tumor activity of the mycelia of liquid-cultured Phellinus linteus. Bioscience, Biotechnology, and Biochemistry. 2004;68(4):868–872. doi: 10.1271/bbb.68.868. [DOI] [PubMed] [Google Scholar]
- 28.Kim S.-H., Song Y.-S., Kim S.-K., Kim B.-C., Lim C.-J., Park E.-H. Anti-inflammatory and related pharmacological activities of the n-BuOH subfraction of mushroom Phellinus linteus. Journal of Ethnopharmacology. 2004;93(1):141–146. doi: 10.1016/j.jep.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 29.Lee D. H., Kim H. W. Innate immunity induced by fungal β-glucans via dectin-1 signaling pathway. International Journal of Medicinal Mushrooms. 2014;16(1):1–16. doi: 10.1615/intjmedmushr.v16.i1.10. [DOI] [PubMed] [Google Scholar]
- 30.Chanput W., Reitsma M., Kleinjans L., Mes J. J., Savelkoul H. F. J., Wichers H. J. β-Glucans are involved in immune-modulation of THP-1 macrophages. Molecular Nutrition & Food Research. 2012;56(5):822–833. doi: 10.1002/mnfr.201100715. [DOI] [PubMed] [Google Scholar]
- 31.Korea food & drug administration. Food Code. 2019. http://www.foodsafetykorea.go.kr/foodcode/01_02.jsp?idx=263.
- 32.Wang Z.-M., Chen Y.-C., Wang D.-P. Resveratrol, a natural antioxidant, protects monosodium iodoacetate-induced osteoarthritic pain in rats. Biomedicine & Pharmacotherapy. 2016;83:763–770. doi: 10.1016/j.biopha.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 33.Chin K. Y., Wong S. K., Japar Sidik F. Z., et al. The effects of annatto tocotrienol supplementation on cartilage and subchondral bone in an animal model of osteoarthritis induced by monosodium iodoacetate. International Journal of Environmental Research and Public Health. 2019;16(16):p. 2897. doi: 10.3390/ijerph16162897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y. M., Son E., Kim S. H., Kim O. S., Kim D. S. Anti-inflammatory and anti-osteoarthritis effect of Mollugo pentaphylla extract. Pharmaceutical Biology. 2019;57(1):74–81. doi: 10.1080/13880209.2018.1557700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Komatsu S. Extraction of nuclear protein. Methods in Molecular Biology. 2007;355:73–77. doi: 10.1385/1-59745-227-0:73. [DOI] [PubMed] [Google Scholar]
- 36.Shin M. R., Kim K. J., Kim S. H., et al. Comparative evaluation between sulfasalazine alone and in combination with herbal medicine on DSS-induced ulcerative colitis mice. BioMed Research International. 2017;2017:p. 10. doi: 10.1155/2017/6742652.6742652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Sousa valente J. The pharmacology of pain associated with the monoiodoacetate model of osteoarthritis. Frontiers in Pharmacology. 2019;10(974) doi: 10.3389/fphar.2019.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drevet S., Gavazzi G., Grange L., Dupuy C., Lardy B. Reactive oxygen species and NADPH oxidase 4 involvement in osteoarthritis. Experimental Gerontology. 2018;111:107–117. doi: 10.1016/j.exger.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Saha R. N., Jana M., Pahan K. MAPK p38 regulates transcriptional activity of NF-κB in primary human astrocytes via acetylation of p65. The Journal of Immunology. 2007;179(10):7101–7109. doi: 10.4049/jimmunol.179.10.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown K. K., Heitmeyer S. A., Hookfin E. B., et al. P38 MAP kinase inhibitors as potential therapeutics for the treatment of joint degeneration and pain associated with osteoarthritis. Journal of Inflammation. 2008;5(1):p. 22. doi: 10.1186/1476-9255-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Endrinaldi E., Darwin E., Zubir N., Revilla G. The effect of mesenchymal stem cell wharton’s jelly on matrix metalloproteinase-1 and interleukin-4 levels in osteoarthritis rat model. Open Access Macedonian Journal of Medical Sciences. 2019;7(4):529–535. doi: 10.3889/oamjms.2019.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosseinzadeh A., Bahrampour Juybari K., Fatemi M. J., et al. Protective effect of ginger (zingiber officinale roscoe) extract against oxidative stress and mitochondrial apoptosis induced by interleukin-1β in cultured chondrocytes. Cells Tissues Organs. 2017;204(5-6):241–250. doi: 10.1159/000479789. [DOI] [PubMed] [Google Scholar]
- 43.Vila S. Inflammation in osteoarthritis. Puerto Rico Health Sciences Journal. 2017;36(3):123–129. [PubMed] [Google Scholar]
- 44.Simon L. S. Nonsteroidal anti-inflammatory drugs and their risk: a story still in development. Arthritis research & therapy. 2013;15(Suppl 3):p. S1. doi: 10.1186/ar4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim K. S., Oh D. H., Choi H. M., et al. Pyrrolidine dithiocarbamate, a NF-kappaB inhibitor, upregulates MMP-1 and MMP-13 in IL-1beta-stimulated rheumatoid arthritis fibroblast-like synoviocytes. European Journal of Pharmacology. 2009;613(1–3):167–175. doi: 10.1016/j.ejphar.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 46.Luo Y., Shang P., LiLuteolin D. A flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Frontiers in Pharmacology. 2017;8:p. 692. doi: 10.3389/fphar.2017.00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fei J., Liang B., Jiang C., Ni H., Wang L. Luteolin inhibits IL-1β-induced infl in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomedicine & pharmacotherapy. 2019;109:1586–1592. doi: 10.1016/j.biopha.2018.09.161. [DOI] [PubMed] [Google Scholar]
- 48.Su H. H., Chu Y. C., Liao J. M., et al. Phellinus linteus mycelium alleviates myocardial ischemia-reperfusion injury through autophagic regulation. Frontiers in Pharmacology. 2017;8:p. 175. doi: 10.3389/fphar.2017.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuelert N., McDougall J. J. Grading of monosodium iodoacetate-induced osteoarthritis reveals a concentration-dependent sensitization of nociceptors in the knee joint of the rat. Neuroscience Letters. 2009;465(2):184–188. doi: 10.1016/j.neulet.2009.08.063. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y., Wang Y., Kong Y., et al. Carnosine prevents type 2 diabetes-induced osteoarthritis through the ROS/NF-κB pathway. Frontiers in Pharmacology. 2018;9:p. 598. doi: 10.3389/fphar.2018.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rousset F., Hazane-Puch F., Pinosa C., et al. IL-1beta mediates MMP secretion and IL-1beta neosynthesis via upregulation of p22phox and NOX4 activity in human articular chondrocytes. Osteoarthritis and Cartilage. 2015;23(11):1972–1980. doi: 10.1016/j.joca.2015.02.167. [DOI] [PubMed] [Google Scholar]
- 52.Chuang C. Y., Degendorfer G., Davies M. J. Oxidation and modification of extracellular matrix and its role in disease. Free Radical Research. 2014;48(9):970–989. doi: 10.3109/10715762.2014.920087. [DOI] [PubMed] [Google Scholar]
- 53.Kieseier B. C., Schneider C., Clements J. M., et al. Expression of specific matrix metalloproteinases in inflammatory myopathies. Brain. 2001;124(2):341–351. doi: 10.1093/brain/124.2.341. [DOI] [PubMed] [Google Scholar]
- 54.McDonnell S., Morgan M., Lynch C. Role of matrix metalloproteinases in normal and disease processes. Biochemical Society Transactions. 1999;27(4):734–740. doi: 10.1042/bst0270734. [DOI] [PubMed] [Google Scholar]
- 55.Gho W. G., Choi Y., Park K.-H., Huh J.-K. Expression of collagenases (matrix metalloproteinase-1, 8, 13) and tissue inhibitor of metalloproteinase-1 of retrodiscal tissue in temporomandibular joint disorder patients. Journal of the Korean Association of Oral and Maxillofacial Surgeons. 2018;44(3):120–127. doi: 10.5125/jkaoms.2018.44.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumar S., Boehm J., Lee J. C. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nature Reviews Drug Discovery. 2003;2(9):717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- 57.Kyriakis J. M., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological Reviews. 2001;81(2):807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- 58.de Andrés M. C., Takahashi A., Oreffo R. O. C. Demethylation of an NF-κB enhancer element orchestrates iNOS induction in osteoarthritis and is associated with altered chondrocyte cell cycle. Osteoarthritis and Cartilage. 2016;24(11):1951–1960. doi: 10.1016/j.joca.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Pan T., Chen R., Wu D., et al. Alpha-mangostin suppresses interleukin-1β-induced apoptosis in rat chondrocytes by inhibiting the NF-κB signaling pathway and delays the progression of osteoarthritis in a rat model. International Immunopharmacology. 2017;52:156–162. doi: 10.1016/j.intimp.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Ostojic M., Soljic V., Vukojevic K., Dapic T. Immunohistochemical characterization of early and advanced knee osteoarthritis by NF-κB and iNOS expression. Journal of Orthopaedic Research. 2017;35(9):1990–1997. doi: 10.1002/jor.23504. [DOI] [PubMed] [Google Scholar]
- 61.Marcu K. B., Otero M., Olivotto E., Maria Borzi R., Goldring M. B. NF-κB signaling: multiple angles to target OA. Current Drug Targets. 2010;11(5):599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


