Abstract
Background:
Polysubstance use (PSU) is prevalent among individuals with substance use disorders, but the vast majority of preclinical substance use research has focused on individual substances in isolation. Cocaine has been prevalent in the repertoire of persons who use more than one illicit substance.
Methods:
We conducted a meta-analysis combining results from literature searches and secondary data analyses to estimate the prevalence of simultaneous and concurrent cocaine + alcohol and cocaine + cannabis use among cocaine users. We next summarized the small body of literature on behavioral, cognitive and neurobiological consequences of cocaine PSU across species, with a focus on alcohol and cannabis. Finally, we used systematic literature searches to assess the extent to which human and animal studies on the neurobiological consequences of cocaine include PSU subjects.
Results:
The estimated prevalence of simultaneous and concurrent alcohol use among human cocaine users was 74% and 77%, respectively. The estimated prevalence of simultaneous and concurrent cannabis use among cocaine users was 38% and 64%, respectively. Consumption of alcohol or cannabis with cocaine enhances subjective responses to cocaine, concomitant with changes in cocaine metabolism that increase blood cocaine levels, and, in the case of alcohol, produce the psychoactive metabolite cocaethylene. There is also consistent evidence for neurobiological effects of cocaine + alcohol combinations. However, animal PSU research with cocaine lags behind human research.
Conclusion:
Based on the prevalence and known consequences of PSU, consideration of PSU in both human and animal research is vital for understanding patterns of substance use.
Keywords: Cannabis, Alcohol, Cocaethylene, Neurocognitive, Neuroimage
1. Introduction
Polysubstance use (PSU; the ingestion of morethan one drug of abuse within a defined period) is the norm rather than the exception for individuals with substance use disorders. Such behavior no doubt reflects a variety of causes, including the desire for enhanced psychoactive effects, alleviation of negative side-effects of one substance with another, and substance accessibility. Most substance use research, however, (including the vast majority of preclinical research) has focused on individual substances in isolation or treated PSU as a “nuisance” variable. Limiting research to individual substances risks overlooking potentially critical interactions among substances, which may influence the patterns, consequences, and ultimately efficacy of treatment for substance use disorders.
In 2014 the National Survey on Drug Use and Health (NSDUH) reported that approximately 913,000 Americans met DSM-IV criteria for cocaine abuse or dependence in the past month. An analysis of data collected by the Tennessee Department of Mental Health and Developmental Disabilities showed that among 36,425 individuals who received treatment for substance use disorder between 1998 and 2004, 48.7% reported polysubstance use (Kedia et al., 2007). Of those reporting cocaine use, 77.8% (n = 21,970) were polysubstance users. The most common two-drug combinations (out of a possible 36 combinations) for the entire study population was cocaine + alcohol (12% of the population). The same study found that the third most common drug combination was cocaine + cannabis (3.4% of the population), while the second most common was alcohol + cannabis (7.4% of the population). Nicotine use was not recorded. Among participants who reported combining three drugs, the most common combination was cocaine + alcohol + cannabis (8.9% of the population).
The goal of this article is to highlight the importance of co-occurring substance use and to suggest that it should receive greater attention. We focus on cocaine use in combination with other substances (particularly alcohol and cannabis) because of the prevalence of these substance combinations, and availability of preclinical data. We begin with epidemiological evidence concerning the prevalence of cocaine PSU, followed by a discussion of data from basic research and clinical studies bearing on this topic.
2. Methods
2.1. Review and meta-analysis of studies with cocaine PSU prevalence
In order to estimate the prevalence of simultaneous and concurrent cocaine + alcohol and cocaine + cannabis use among cocaine users, we conducted a review combining results from both secondary data analyses and literature searches. For the purposes of these analyses, “concurrent use” refers to two or more substances used within a specified period (e.g., past 30 days), whereas “simultaneous use” refers to two or more substances used on the same occasion with overlapping consumption/effects.
Secondary analyses were conducted using five datasets: the 2014 NSDUH dataset, Women Teaching Women (WTW), Sisters Teaching Options for Prevention (STOP), Prescription Drug Misuse, Abuse, and Dependence (Rx), and HealthStreet. All but the NSDUH study were community-based in defined geographical locations. For the WTW (Ruger et al., 2010) and Rx (Nattala et al., 2011) studies, participants were recruited by community health workers through community outreach in the St. Louis, MO area during the study period from 2000 to 2006 and 2008 to 2010, respectively. The STOP study (Cottler et al., 2014) recruited participants from drug courts in St. Louis from 2005 to 2008. In our ongoing community engagement program HealthStreet, participants are recruited in north central Florida and asked about past 30-day substance use.
Literature searches were carried out in PubMed using the search term “cocaine AND (co-administration OR concomitant OR simultaneous OR concurrent) and with the restriction of studies conducted in humans and publication dates from January 1990 to June 2017. A total of 770 articles were identified through these search terms. The title and abstract of each article were screened using Abstrackr (Wallace et al., 2012); 636 articles were excluded due to irrelevancy to the topic. The remaining 134 articles went through further full-text review to determine their eligibility. Fifty-four articles were excluded after full-text screening because they did not include the drug combinations of interest (i.e., cocaine and alcohol, or cocaine and cannabis), 24 were excluded because they involved small clinical samples that could not estimate prevalence in a general population, 17 were excluded because the studies were not relevant to the prevalence of drug combinations, 18 were excluded either because the article was not retrievable or it was a case report/post-mortem/neurological study, 6 were excluded because they were review articles, 3 were excluded because the prevalence of specific drug combinations was not reported, and 1 was excluded because it was not published in English. Post-screening, 11 studies remained and first author, published year, country, sample description, PSU rate, and measurement of PSU information were extracted (see Table 1). The sample size of included studies ranged from 98 to 36,425. Two studies (Grant and Harford, 1990; Hedden et al., 2009; Hedden et al., 2010) used the National Survey on Drug Use and Health (formerly titled National Household Survey on Drug Abuse) but used different survey cohorts. Seven studies used community samples in different locations. Only four studies using clinically sampled data were included because they either had relatively large samples (n > 1000) (Kedia et al., 2007; Lindsay et al., 2009), included patients from multiple inpatient and outpatient clinics (Guindalini et al., 2006), or used an emergency department-based sampling scheme which allowed us to estimate the PSU prevalence in the general population (Vanek et al., 1996). Four studies reported only simultaneous use and 6 studies reported only concurrent use; one study reported both simultaneous and concurrent use.
Table 1.
Summary of the 16 epidemiological studies utilized in the meta-analysis.
| # | Study | Study period | Location | Sample | Polysubstance use rate | Measure |
|---|---|---|---|---|---|---|
| 1 | (Grant and Harford, 1990) | 1985 | US, national | Study: 1985 National Household Survey on Drug Abuse, n=8,083, national sample, female: 56%, non-white: % not reported; oversampled with Blacks and Hispanics mean age: not reported; age range: 12 and older |
Simultaneous use: Among general population: cocaine + alcohol: 2.8% (past month) and 6.1% (past year) Among cocaine users (n = 280): Alcohol: 82.7% Concurrent use: Among general population: Cocaine + alcohol: 2.4% (past month) and 4.7% (past year) Among cocaine users (n = 280): Alcohol: 96.5% |
Methods: self-administered paper answer
sheets) Concurrent: use both substances during the same time period (month or year) Simultaneous: use of cocaine with alcohol at the same time (or within a couple of hours) |
| 2 | (Olthuis et al., 2013) | Not reported | Canada, Halifax, Nova Scotia | Study: Not specified, n=226, community sample, female: % not reported, non-white: 11%, mean age: 27; age range 18–59 |
Simultaneous use: Among new powdered cocaine users (n = 124): Alcohol: 75.0% Cannabis: 58.1% Alcohol or cannabis or tobacco: 96.0% Among new crack cocaine users (n = 35): Alcohol: 42.9% Cannabis: 60.0% Alcohol, cannabis or tobacco: 82.9% |
Methods: Structured face-to-face
interview Simultaneous: co-administered any other licit or illicit substances the first time they used the cocaine |
| 3 | (Licht et al., 2012) | 2005–2008 | Denmark | Study: Danish MDMA and hallucinogen
study, n=98, community sample (hallucinogen and MDMA users), female = 13%, non-white: 5%, mean age = 26, age range: 1835 |
Simultaneous use: Among cocaine users (n = 91): Alcohol: 87% Cannabis:44% |
Methods: Structured interview using Customary
Drinking and Drug use Record (CDDR) Simultaneous use: ever combined specific substances or groups of substances in a single session of substance use |
| 4 | (Barrett et al., 2006) | 2003–2004 | Canada, Montreal, Quebec |
Study: not specified,
n=149, college student sample, female: 59%, non-white: 21%, mean age: 22, age range: not reported |
Simultaneous use: Among cocaine users (n = 64): Alcohol: 79.7% Cannabis: 40.0% Alcohol, cannabis or tobacco:95.3% |
Methods: Structured Interview Simultaneous: co-administered other substances during a session in which cocaine had been used |
| 5 | (Barrett et al., 2005) | 2002–2003 | Canada, Montreal, Quebec |
Study: not specified,
n=186, community sample (rave attendance) female: 50%, non-white: 12%, mean age: 24, age range: 1647 |
Simultaneous use: Among cocaine users (n = 17): Alcohol: 76.5% Cannabis:76.5% |
Methods: Structured Interview Simultaneous use: coadministration |
| 6 | (Hedden et al., 2009) | 2005 | US, national | Study: 2005 NSDUH n = 36,425, national sample, female: 54%, black: 12%; Hispanic: 15% mean age: not reported, age range: 18 and older |
Concurrent use: Among cocaine users (n = 394): Alcohol: 61.7% |
Methods: NSDUH survey Concurrent: past year concurrent use both substances |
| 7 | (Schwingel et al., 2014) | 2007–2009 | Brazil, Northeastern region of Brazil | Study: not specified, n= 145, recreational bodybuilders sample, female: 0%, non-white: % not reported, mean age: 27, age range: 18–42 |
Concurrent use: Among cocaine users (n = 33): Alcohol use: 75.8% |
Methods: structured face-to-face
interview Concurrent: not specified |
| 8 | (Booth et al., 2006) | 2002–2004 | US, eastern Arkansas, western Kentucky, and western Ohio |
Study: A prospective, 3-year “natural
history” study of rural stimulant users n= 710, multistate rural not-intreatment community sample, female: 39%, non-white: 32%, mean age: 33, age range: 18 and older |
Concurrent use: Among cocaine users (n = 613): Alcohol: 89.6% Cannabis: 85.3% |
Methods: structured interview Concurrent: use both substances in the past 6 months |
| 9 | (Lindsay et al., 2009) | Not reported | US, Houston, Texas | Study: behavioral and pharmacological
treatment study for cocaine dependence, n = 1183, clinical sample (treatment- seeking cocaine dependent individuals), female: 20%, black: 65%, mean age: 39, age range: 18–60 |
Concurrent use: Among cocaine dependent individuals (n = 1183): Cannabis: 46.4% |
Methods: Structured clinical
interview Concurrent: use both substances in the past 30 days |
| 10 | (Guindalini et al., 2006) | 1997–1998 | Brazil, Sao Paulo |
Study: not specified n= 699, clinical sample (six inpatients and one outpatient clinic), female: 4%, non-white: 29% mean age = 27, age range: 18 and older |
Concurrent use: Among cocaine users (n = 699): Heavy alcohol use: 36.6% Cannabis:93.7% |
Methods: Structured interview Concurrent: use
both substances in a lifetime Heavy alcohol use: > 50 units/ week |
| 11 | (Vanek et al., 1996) | 1991–1992 | US, Youngstown, Ohio | Study: not specified, n= 1287, emergency department sample, female: 35%, black:70%, mean age = 30, age range: not reported |
Concurrent use: Among cocaine users (n = 498): Alcohol: 34.9% |
Methods: medical records review Concurrent use: both substance tested positive in urine test |
| 12 | N/A | 2014 | US, national | Study: 2014 NSDUH, n= 55,271, National sample female: 52%, Non-white: 39%, Age: 12–17 :25% 18–25: 23% 25–34: 15% 35+: 37% age range: 12 and older |
Concurrent use: Among cocaine
users (n = 363): Alcohol: 95% Cannabis: 73% |
Methods: combined computer- assisted personal
Interviewing and audio computer-assisted
selfinterviewing Concurrent: use both substances in the past 30 days |
| 13 | Cottler LB. | 2000–2006 | US, St. Louis, Missouri | Study: Women Teaching Women (WTW), n= 501, Community sample, Female: 100%, Black: 81%, Mean age: 36, age range: 18 and older |
Simultaneous use: Among past 30-day cocaine users (n = 463): Alcohol: 23.5% Cannabis: 9.3% Concurrent use: Among past 30-day cocaine users (n = 463): Alcohol: 79.5% (past 30-day) Cannabis: 43.2% (past 30-day) Among lifetime cocaine users (n = 640) Alcohol: 90.8% (lifetime) Cannabis: 63.4% (lifetime) |
Methods: structured interview using Substance
Abuse Module (SAM) Simultaneous: In the past 30 days, used alcohol or cannabis to come down from a cocaine high Concurrent: use both substances in the past 30 days/ age range for using different substances overlapped (lifetime) |
| 14 | Cottler LB. | 2005–2008 | US, St. Louis, Missouri | Study: Sisters Teaching Options for Prevention
(STOP), n= 362, drug court sample, Female: 100%, Black: 69%, Mean age: 36, age range: 18–67 |
Simultaneous use: Among past 30-day cocaine users (n = 122): Alcohol: 36.1% Cannabis: 12.3% Concurrent use: Among past 30-day cocaine users (n = 122): Alcohol: 71.3% (past 30-day) Cannabis: 46.7% (past 30-day) Among: lifetime cocaine users (n = 292) Alcohol: 86.3% (lifetime) Cannabis: 70.9% |
Methods: structured interview using Substance
Abuse Module (SAM) Simultaneous: In the past 30 days, used alcohol or cannabis to come down from a cocaine high Concurrent: use both substances in the past 30 days/ age range for using different substances overlapped (lifetime) |
| 15 | Cottler LB. | 2008–2010 | US, St. Louis, Missouri |
Study: Prescription Drug Misuse, Abuse, and Dependence Study (Rx), n= 422, Community sample (prescription drug users) Female: 43%, Black: 50%, Mean age: 40, age range: 18–67 |
Simultaneous use: Among cocaine users who also simultaneous used prescription drug (n = 156): Alcohol: 82.7% Cannabis: 53.9% Concurrent use: Among past 30-day cocaine users (n = 72): Alcohol: 86.1% (past 30-day) Cannabis: 52.8% (past 30-day) Among lifetime cocaine users (n = 234) Alcohol: 98.3% (lifetime) Cannabis: 90.2% (lifetime) |
Methods: structured interview using Substance
Abuse Module (SAM) Simultaneous: In the last 12 months, used opioids/sedatives/ stimulants, cocaine and alcohol/ cannabis together Concurrent: use both substances in the past 30 days/ age range for using different substances overlapped (lifetime) |
| 16 | Cottler LB. | 2011–2017 | US, North Central Florida |
Study: HealthStreet, n= 9127, community sample Female: 59% Black: 60%, other minority:7%, Mean age: 44%, age range: 18–83 |
Concurrent use: Among general population (n = 9127): Cocaine + alcohol: 1.2% Cocaine + cannabis: 1.2% Cocaine + alcohol + cannabis: 0.8% Among past 30-day cocaine users (n = 174): Alcohol: 63.8% Cannabis: 61.5% Alcohol and cannabis: 43.7% |
Methods: Structured interview with Health Intake Form Concurrent: use both substances in the past 30 days |
In addition to the 11 studies from the literature search, we include 5 additional studies from secondary data analyses as mentioned above. In total, 16 studies with cocaine PSU prevalence were included. We summarized the geographical location and year of the study, total sample size of the study and number of cocaine users, demographics of the study sample (target sample population, gender composition, inclusion of racial/ethical minorities, mean age and age range), and measurement methods (concurrent or simultaneous, survey/interview/urine tests).
The meta-analysis was conducted in R, combining the results from 16 studies to estimate the confidence interval for the PSU rate in each study, the heterogeneity between studies and pooled estimate of cocaine PSU. Conservatively, if a study reported the prevalence of concurrent PSU using different time windows, then prevalence using the shorter time window was used in the analyses (e.g., if both past 30-day and lifetime prevalence were reported, past 30-day prevalence was used in the analyses). If the study reported PSU prevalence for both powder cocaine and crack cocaine use, the prevalence of powder cocaine use was used in the analyses. I2 statistics were calculated for each analysis and the value larger than 50% was considered substantial heterogeneity between included studies. Statistical significance of the heterogeneity was assessed by the P-value of the Chi-square test using a significance level of 0.1 (Higgins et al., 2003). In the case of significant heterogeneity, random effect models were used; otherwise, fixed effect models were used. A forest plot was used to illustrate the results of the meta-analysis. In addition, we reviewed findings regarding the temporal patterns of cocaine + alcohol and cocaine + alcohol PSU.
2.2. Review of clinical and preclinical literature on behavioral, cognitive and neurobiological consequences of cocaine PSU
Few studies in humans or animals have examined the behavioral, cognitive, and neurobiological consequences of PSU. For example, cocaine self-administration, in which an experimental animal makes an operant response to earn an intravenous (IV) infusion of cocaine, is the most common animal model of human cocaine use; however, of more than 3000 articles obtained using the PubMed search terms “cocaine AND self-administration”, fewer than 20 involve simultaneous or concurrent intake of a second substance. We limited our review of pre-clinical studies to those in which at least one substance was self-administered, and focused specifically on cocaine + alcohol and cocaine + cannabis PSU, as other substance combinations (particularly cocaine + heroin) have been the subject of previous reviews (Leri et al., 2003). We also excluded studies in which a period of intake of one substance preceded that of a second Substance (i.e., sequential self-administration as an investigation of the “gateway” hypothesis). In addition, we examined the relationship between cocaine PSU and the efficacy of cocaine use treatment.
2.3. Exploratory analysis of frequency of inclusion of PSU in human or animal cocaine studies
In order to investigate whether human and animal studies on the neurobiological consequences of cocaine use differ in the frequency with which PSU is identified and included, we conducted an exploratory analysis of recent literature on PubMed. For human studies, we used the search terms “fMRI AND cocaine” and selected the 40 most recent papers (as of January 1, 2018), in which the independent variable was cocaine use, and the dependent variable was a neuroimaging/ fMRI measure in human subjects. We recorded whether alcohol- or cannabis-dependent subjects or users were excluded and if not, how much of the substance was used and frequency of use. We then conducted a similar search for animal studies using the term “cocaine” and selected “Other animals” as subjects. We reviewed the results in reverse chronological order beginning with January 1, 2018, and selected only papers that utilized in vivo cocaine administration (in mammals) as an independent variable and a measure of neurobiology or behavior as a dependent variable (e.g., excluding toxicology studies).
3. Results
3.1. PSU prevalence and patterns of use
3.1.1. Review of studies including PSU prevalence
Among cocaine users, the prevalence of simultaneous alcohol use ranged from 24% to 98%; the prevalence of simultaneous cannabis use ranged from 12% to 76%; the prevalence of concurrent alcohol use ranged from 37% to 96%; the prevalence of concurrent cannabis use ranged from 43% to 94%. Heterogeneity was observed in study location, year, target population, and demographic composition (see Table 1). Ten studies were conducted in the US, three in Canada, two in Brazil and one in Denmark. The study period ranged from 1985 to 2017. Most studies used community samples recruited from metropolitan or urban areas; only one study targeted drug users in rural areas of the US (Booth et al., 2006). Three studies were NSDUH studies but with different study periods. Most studies included male and females and racial minorities. The average age ranged from 22 to 44. years. One study measured PSU through medical record review and urine tests (Vanek et al., 1996), while others used self-report from structured interviews. The target population varied between not-in-treatment drug users (Booth et al., 2006), treatment-seeking cocaine-dependent individuals (Lindsay et al., 2009), college students (Barrett et al., 2006), rave attendees (Barrett et al., 2005), recreational bodybuilders (Schwingel et al., 2014), and individuals who used specific drugs, such as hallucinogens (Licht et al., 2012), or prescription drugs (Rx study). All of these heterogeneities could contribute to the differences in the reported cocaine PSU rate.
In addition, the measures of simultaneous and concurrent PSU were not consistent between studies. For measures of simultaneous use, some studies asked participants to self-report use of both substances at the same time or within a couple of hours (Grant and Harford, 1990), some asked if both substances were co-administrated (Barrett et al., 2005); others asked if participants used alcohol/cannabis to come down from a cocaine high (WTW, STOP). The time window for concurrent PSU also differed. Most studies defined concurrent use as using both substances in the past 30 days, while other studies defined this as using both substances in the past 6 months (Booth et al., 2006), in the past year (Hedden et al., 2009), in a lifetime (Guindalini et al., 2006) or having both substances tested positive in a urine test (Vanek et al., 1996).
3.1.2. Meta-analysis results
The results of the meta-analysis of combining findings from literature searches and secondary data analyses are shown in Fig. 1. The I2 statistics in our analyses ranged from 97% to 99% which indicate substance heterogeneity between included studies. Chi-square test results indicated that the heterogeneity was statistically significant (p < 0.01) for all four groups of meta-analyses; therefore, a random effects model was used.
Fig. 1.
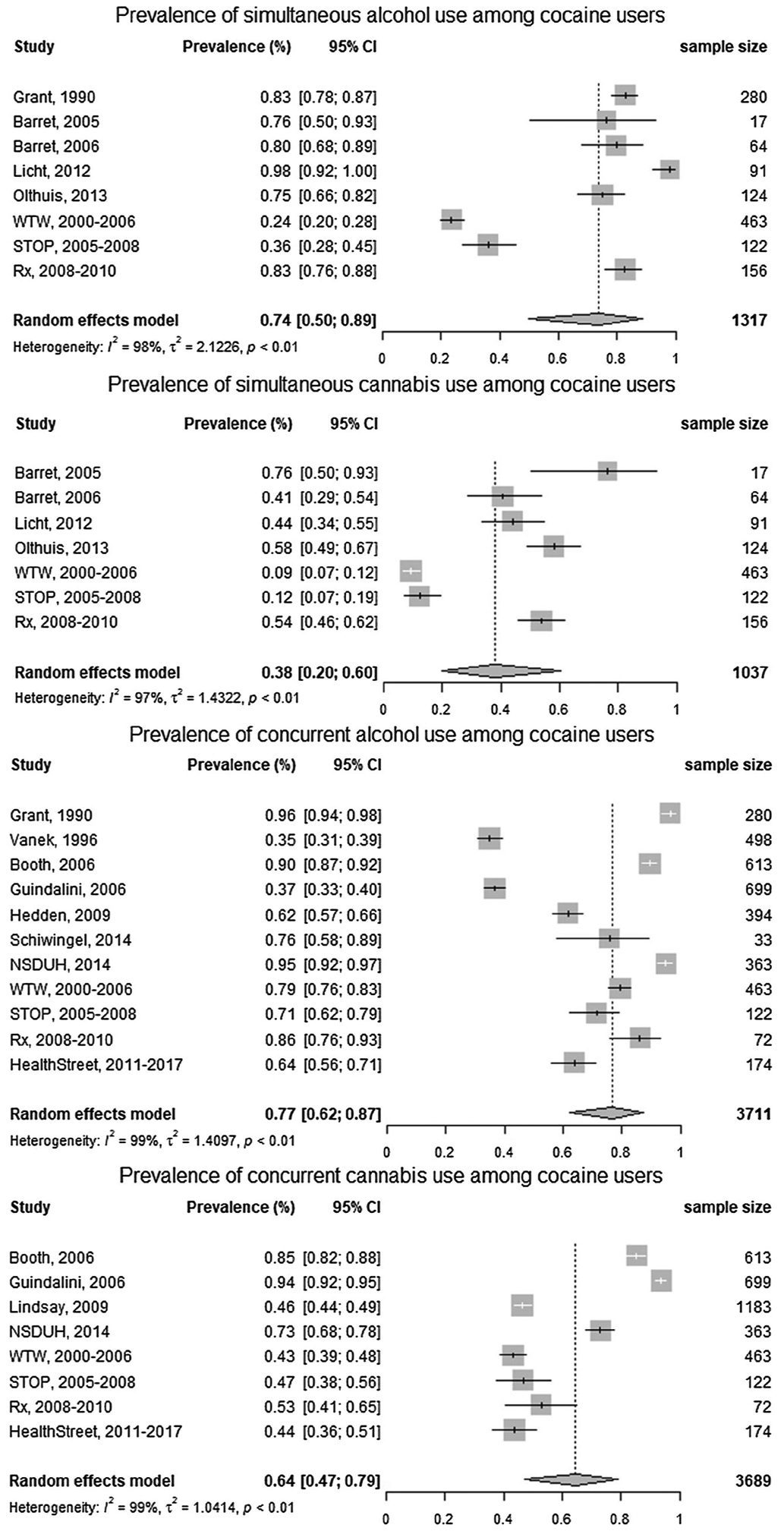
Forest plots show the prevalence of simultaneous and concurrent alcohol/cannabis use among cocaine users. Vertical lines in forest plots show the pooled estimates of prevalence from the random effect models. I2 values greater than 50% indicate substantial heterogeneity between studies.
The estimated prevalence of simultaneous alcohol use was 74% (95%CI: 50%, 89%) among cocaine users, and the prevalence of simultaneous cannabis use was 38% (95%CI: 20%, 60%). The estimated prevalence of concurrent alcohol use was 77% (95%CI: 62%, 87%) among cocaine users and the prevalence of concurrent cannabis use was 64% (95%CI: 47%, 79%). These findings were consistent with our hypothesis that alcohol and cannabis use are both prevalent among cocaine users and also indicate that the rate of concurrent/simultaneous alcohol use is higher than the rate of concurrent/simultaneous cannabis use.
3.1.3. Temporal patterns of substance use in cocaine PSU
Despite the availability of data on the prevalence of cocaine PSU, little is known regarding its temporal dynamics (e.g., whether cocaine is routinely consumed prior to another substance or vice versa). We identified only two studies that addressed this issue, both of which focused on cocaine and alcohol intake. In the first study, of 340 subjects who reported using cocaine and alcohol within 3 h of one another, subjects were asked to rate the likelihood of consuming alcohol prior to cocaine or vice versa using a 5-point scale (0 = never; 4 = practically all the time). Subjects reported a significantly higher prevalence of consuming alcohol prior to cocaine (mean frequency = 2.47) compared to cocaine prior to alcohol (mean frequency = 1.77) (MacDonald et al., 2015). The second study (Gossop et al., 2006) interviewed 102 subjects who reported having used both alcohol and cocaine in the previous month about the order of cocaine and alcohol use when the two substances were taken simultaneously. Participants were further characterized as exclusive crack cocaine users (n = 33) or powdered cocaine users (n = 69). For the participants who reported crack cocaine use, 21% reported consuming alcohol prior to crack cocaine, 36% reported consuming alcohol after crack cocaine, and 12% reported using both substances simultaneously. Nearly all the powdered cocaine users were likely to drink alcohol prior to, during and after cocaine use (number reporting alcohol prior to cocaine, 96%; alcohol after cocaine, 93%; simultaneously, 96%) (Gossop et al., 2006). In the same study, cocaine + alcohol users reported that when alcohol and cocaine are used simultaneously, the amount of each substance consumed during a typical substance use episode increases significantly compared to when alcohol or cocaine are used alone (Gossop et al., 2006). Thus, the limited evidence to date regarding the timing of simultaneous drug intake reveals the presence of a range of intake patterns. Further characterization of such patterns is particularly important in the case of simultaneous/concurrent cocaine and alcohol use, as alcohol can alter cocaine pharmacokinetics and metabolism (discussed in detail below).
3.2. Review of clinical and preclinical literature on behavioral, cognitive and neurobiological consequences of cocaine PSU
3.2.1. Rewarding and reinforcing effects of combined cocaine and alcohol administration
As described above, simultaneous or concurrent administration of alcohol with cocaine is the most prevalent 2-drug combination. McCance-Katz et al., (1998) compared subjective effects of cocaine, alcohol, and simultaneous cocaine + alcohol in patients meeting DSM-IV criteria for both cocaine and alcohol abuse in a double-blind randomized trial. Greater euphoria and increased perception of well-being were reported in the cocaine + alcohol condition relative to cocaine alone. In addition to the psychoactive effects of alcohol itself, two metabolic phenomena may underlie the enhanced subjective experience in the cocaine + alcohol condition. First, the combination of cocaine and alcohol increases plasma levels of cocaine, an effect also evident in rodents and caused by a reduction in the amount of cocaine metabolized to benzoylecgonine (Pan and Hedaya, 1999). Second, cocaethylene (CE), a psychoactive metabolite of cocaine formed only in the presence of alcohol, is detected in the plasma of subjects following cocaine + alcohol intake (McCance-Katz et al., 1998). Interestingly, the order in which cocaine and alcohol are administered appears to be critical for determining cocaine’s metabolite profile: cocaine administration before alcohol causes an increase in plasma cocaine levels, whereas cocaine administration after alcohol results in CE formation but no change in plasma cocaine levels (Perez-Reyes, 1994). These distinct consequences of the temporal sequence of drug intake support the need for more detailed data regarding the granular patterns of substance use in humans.
Unlike other cocaine metabolites, CE is a neuropharmacologically active stimulant and is self-administered by primates (Jatlow et al., 1991) and rodents (Raven et al., 2000). In rats, CE also produces conditioned place preference in a manner comparable to cocaine (Knackstedt et al., 2002). Moreover, self-reports indicate that IV and intranasal CE produce the same euphoric and stimulant effects as cocaine (Hart et al., 2000). These findings indicate that CE formation and its subsequent pharmacological effects may be reinforcing to those who co-administer alcohol and cocaine; however, the neurobiological effects of long-term CE administration have not been assessed. It is possible that CE produces neurochemical effects that differ from those produced by cocaine alone.
A small body of animal research supports the idea that the combination of cocaine and alcohol is more reinforcing/rewarding than either drug alone. Rats pre-treated with non-contingent IV cocaine immediately prior to access to a sweetened alcohol solution in the home cage consume greater amounts of alcohol relative to vehicle pre-treated rats (Knackstedt et al., 2006). Conversely, the effects of alcohol pre-treatment on IV cocaine self-administration have been investigated in rhesus monkeys with differing results. On average, alcohol pretreatment with doses in the range of 0.1–1.78 g/kg does not affect cocaine self-administration when alcohol is administered via a single non-contingent IV infusion 10 min prior to cocaine availability (Aspen and Winger, 1997). In two of the four monkeys in this study, however, responding for cocaine was increased after pretreatment with 1 g/kg of alcohol. In the same monkeys, self-administration of the dopamine reuptake inhibitor nomifensine was increased after alcohol pretreatment, while self-administration of the mu opioid receptor agonist alfentanil was not altered. In a similar study, noncontingent IV administration of 1 g/kg of alcohol administered immediately prior to a cocaine self-administration session reduced cocaine intake, whereas lower doses of alcohol had no effect (Czoty, 2015). In a different phase of the same study, monkeys were provided daily access to a sweetened alcohol solution beginning 4 h after the conclusion of operant cocaine self-administration. During this time, five of the six monkeys self-administered doses of cocaine that had not been reinforcing prior to alcohol experience. Upon discontinuing daily post-cocaine alcohol access, self-administration of the maintenance dose of cocaine was not affected; however, lower doses of cocaine, which had been reinforcing during the period of concurrent alcohol access, were no longer self-administered. Long-term self-administration of concurrent cocaine and alcohol may also have increased D3 dopamine receptor sensitivity (Czoty, 2015). Thus, simultaneous cocaine and alcohol administration increases the reinforcing qualities of dopamine agonists, potentially through enhancements in dopamine receptor sensitivity.
Both laboratory animals and humans experience post-cocaine anxiety, and humans report consuming alcohol to reduce the anxiety that remains after cocaine euphoria dissipates (Margolin et al., 1996). In rats, voluntary consumption of a sweetened alcohol solution after self-administration of a single cocaine infusion increases motivation to seek IV cocaine on subsequent days, while reducing anxiogenic behavior produced by cocaine in an operant runway self-administration task (Knackstedt and Ettenberg, 2005).
These findings, considered together, suggest that combined cocaine and alcohol use can enhance the respective reinforcing properties of each substance via both affective and pharmacokinetic interactions. Such interactions can increase intake, as well as the potential for adverse consequences.
3.2.2. Rewarding and reinforcing effects of combined cocaine and cannabis administration
Relative to cocaine + alcohol combinations, there is less known regarding the effects of cocaine + cannabis combinations. Pre-treatment with either of the major cannabinoid components of cannabis (cannabidiol or tetrahydrocannabinol; THC) increases blood and brain cocaine levels in mice (Reid and Bornheim, 2001). Similarly, individuals who smoke a THC cigarette prior to intranasal cocaine ingestion display increased plasma cocaine levels relative to those who do not ingest THC (Lukas et al., 1994). Participants in this study reported that the duration of the positive effects of cocaine was increased and the duration of the negative effects decreased. The latency to cocaine effects was also reduced. These data suggest that cocaine + cannabis users may combine these drugs to attain these subjective effects, although there is clearly a need for more research on this topic.
3.2.3. Neurocognitive correlates of cocaine PSU
A handful of studies in humans have focused explicitly on neural and cognitive integrity in PSU, and a more detailed review of such studies can be found in Meyerhoff (2017). The Meyerhoff group has conducted imaging and cognitive assessments in individuals dependent on cocaine only (cocaine use disorder; CUD), alcohol (alcohol use disorder; AUD), or AUD and other drugs (PSU) in a number of studies of the past two decades. In one study, subjects with AUD + CUD or CUD alone were assessed for neuronal viability via N-acetylaspartate (NAA) and white matter metabolite status via MRI and magnetic resonance spectroscopic (MRS) imaging (Meyerhoff et al., 1999). Subjects were recruited from the community (San Francisco, CA). Compared to non-dependent controls and CUD alone, CUD + AUD individuals displayed greater gliosis in the white matter of the frontal lobe that persisted through at least 4-months of abstinence. Other measures such as a reduction in NAA in the dorsolateral prefrontal cortex did not differ between CUD and CUD + AUD. A follow-up study from the same authors and study population assessed metabolite and grey and white matter alterations in abstinent individuals dependent on cocaine, alcohol, or both cocaine and alcohol. Relative to drug-naïve controls, both CUD and AUD individuals had decreased white matter in cortical regions, and CUD + AUD individuals had less prefrontal white matter relative to individuals with either CUD or AUD alone (O’Neill et al., 2001). This deficit in CUD + AUD was particularly pronounced in the anterior cingulate cortex (ACC). A more recent study by the same group (Pennington et al., 2015) compared subjects with AUD alone to those with PSU. PSU subjects had AUD and were also dependent on at least one psychostimulant (76% cocaine; 24% amphetamine). It should be noted that 10% of subjects were also dependent on opioids and 21% reported cannabis use. Participants were recruited from substance abuse treatment programs at the San Francisco VA Medical Center and Kaiser Permanente and were abstinent for approximately one month. PSU subjects were found to have thinner ACC than AUD subjects, in agreement with their previous study (O’Neill et al., 2001). Both AUD alone and PSU subjects displayed smaller left orbitofrontal cortex (OFC) volume and surface area compared to control subjects. More recently, the same group assessed 36 subjects with PSU and 69 with AUD alone at one month of abstinence from all substances except tobacco (Schmidt et al., 2017). Participants were recruited from substance abuse treatment programs at the San Francisco VA Medical Center and Kaiser Permanente; all 105 participants met DSM–IV–TR criteria for AUD. The 36 PSU subjects met DSM–IV–TR criteria for at least one other substance use disorder: 75% met criteria for cocaine; 33% for amphetamine; 19% for cannabis; 14% for opioids; 3% for anxiolytics; and 3% for hallucinogens. The demographics of the PSU group matched the epidemiological estimates of the prevalence of co-use described above, in that CUD and AUD were the most frequently co-morbid. Examining brain metabolite and amino acid transmitter levels in a similar patient population as the studies above, the same group has found that at 1 month of abstinence, PSU patients display lower choline, creatine, N-acetylaspartate and myo-inositol concentrations in the dorsolateral prefrontal cortex compared with AUD. Metabolite levels in the ACC and parieto-occipital cortex did not differ between groups (Abé et al., 2013a). This study found a trend for a greater decrease in GABA in the ACC of PSU relative to AUD. Another study (Ke et al., 2004) found decreased GABA concentrations in the left prefrontal cortex in all cocaine users, regardless of comorbid alcohol use, and a trend for greater decreases in the CUD + AUD subjects. Finally, (Abé et al., 2013b) found that PSU subjects had increased myo-inositol concentrations in temporal grey matter relative to both controls and AUD alone. Thus, relative to alcohol alone, concurrent dependence on cocaine and alcohol causes greater structural defects in white-matter, primarily in the frontal cortices, as well as in grey matter in the temporal lobe. Other deficits are similar for AUD and PSU relative to control subjects, including reduced OFC volume and decreased GABA levels.
Some studies from the Meyerhoff group also assessed cognitive function in AUD and PSU subjects. Pennington et al. (2015) administered a battery of cognitive tests to subjects in addition to the MRS scans detailed above. PSU subjects exhibited relationships between brain morphometry and processing speed, cognitive efficiency, working memory and inhibitory control that were not observed in controls or patients with AUD. Schmidt et al. (2017) administered a battery of neurocognitive assessments to all subjects at baseline and 17 PSU subjects were re-tested at four months of abstinence. At the baseline assessment, PSU subjects performed significantly worse than AUD on auditory–verbal memory and an inhibitory control measure of impulsivity but showed improvement on these measures at 4-months of abstinence. No group differences were observed in a number of other cognitive measures (e.g., general intelligence, working memory, fine motor skills). Thus, the presence of PSU with AUD is accompanied by worse deficits in some but not all cognitive domains.
3.2.4. Cocaine PSU and treatment efficacy
The data reviewed above suggest that the behavioral, cognitive, and neurobiological consequences of cocaine PSU can differ from those of individual substances. In light of this evidence, it is perhaps not surprising that the efficacy of pharmacological or behavioral therapies to attenuate cocaine use may depend on the concurrent use of other substances. For example, a placebo-controlled study of modafinil for the treatment of CUD found that modafinil decreased cocaine use only in patients without comorbid AUD (Anderson et al., 2009). In a sample of 94 patients with CUD, alcohol use was found to be a predictor of cocaine abstinence 12 months after completion of a treatment program for CUD (Carroll et al., 1993a). In a smaller sample of 27 patients, however, those with comorbid CUD + AUD showed similar reductions in substance use 24 weeks after outpatient relapse-prevention treatment compared to those with CUD alone (Schmitz et al., 1997). In a large cohort (n = 298) of treatment-seeking patients with and without co-morbid AUD, those with comorbidity displayed a greater number of DSM-III-R criteria for SUD and CUD itself, consistent with more severe dependence (Carroll et al., 1993b). A smaller study, however, (n = 74) found that CUD patients who also met DSM-III criteria for alcohol abuse (but not dependence) did not display a greater number of DSM-III cocaine dependence symptoms than those who did not abuse alcohol (Brady et al., 1995). Taken together, these studies indicate that there may be a relationship between alcohol use, as well as the degree of alcohol use, and CUD severity and treatment efficacy of interventions aiming to reduce cocaine use.
Cannabis use in CUD is associated with more frequent cocaine use (Budney et al., 1996; Lindsay et al., 2009), and some studies suggest effects on treatment outcomes. A study of 186 patients treated for CUD found no effect of cannabis use on treatment outcome (Budney et al., 1996). In a cohort of 250 patients discharged from inpatient SUD treatment, however, cannabis use reduced the time to first cocaine use and prevented stable remission (Aharonovich et al., 2005). Finally, concurrent cannabis use attenuated the efficacy of levodopa treatment to reduce cocaine use (Green et al., 2012). These studies suggest that, as is the case with alcohol, concurrent cannabis use may impact both cocaine use and treatment efficacy.
3.3. Exploratory analysis of the frequency of PSU in human and preclinical studies of neurobehavioral consequences of cocaine
From the search of recent human studies of neurobehavioral consequences of cocaine use, we found that no studies excluded users of alcohol or cannabis, although two excluded past week users of cannabis. It should be noted that for the majority of these studies, alcohol/ cannabis dependence did warrant exclusion for control subjects. Out of 40 neuroimaging studies of cocaine users, only 10 excluded alcohol-dependent and only 10 excluded cannabis-dependent subjects (Table 2), suggesting that a majority of studies on the effects of cocaine on fMRI measures include PSU subjects, likely influencing dependent measures. However, a literature search of animal studies reveals that only 2 of 40 studies used both alcohol and cocaine as independent variables in the same subjects, while the remainder did not include alcohol or cannabis co-administration. The first study involved alcohol delivered in utero as a model of fetal alcohol syndrome, and the second examined adolescent alcohol exposure followed by later cocaine administration (Ledesma et al., 2017; Macht et al., 2017). As both of these studies involved a developmental or gateway model, their results are not discussed here. Comparison of the frequency with which PSU subjects are considered between human and animal subjects indicates that cocaine PSU subjects are assessed significantly more often in human than animal studies (cocaine + alcohol, 75% and 5%; cocaine + cannabis, 72.5 and 0%; for both comparisons, 2-sided Fisher’s exact test, p < 0.0001). It should be noted that the point of this analysis is not necessarily that all animal studies should consider PSU, as there are valid reasons for excluding such subjects for some experimental questions. Rather, the point is to highlight that PSU has received very little consideration at the pre-clinical level, given its prevalence in both the general population and among human research subjects.
Table 2.
Summary of human studies investigating the neurobiological consequences of cocaine use and the inclusion of PSU subjects. The amount of alcohol and cannabis use reported is for the cocaine subjects. The exceptions are marked with * for studies where it could not be discerned whether the amount reported was for all subjects or only cocaine subjects. AUDIT = Alcohol Use Disorder Identification Test.
| # | Citation | Exclude Alcohol Dependent? | Exclude Alcohol Users? |
Amount of Alcohol Use | Exclude Cannabis Dependent? | Exclude Cannabis Users? |
Amount of Cannabis Use |
|---|---|---|---|---|---|---|---|
| 1 | (Ersche et al., 2017) | no | no | # with alc dependence: 3 of 44; mean AUDIT score: 4.0 (± 4.8) |
No | no | # with cannabis dependence: 16 of 44 |
| 2 | (Ray et al., 2017) | yes | no | 13/20 reported alcohol use; Mean of 1.9 days/month, 2.1 drinks/occasion | No | no | 10/20 reported infrequent cannabis use |
| 3 | (Garza-Villarreal et al., 2017) | no | no | # alcohol dependence (past 12 months): 13 of
36 # lifetime alc dependence: 17 of 36 # alc abuse (past 12 months): 17 of 36 # lifetime alc abuse: 22 of 36 |
No | no | 10/36 reported any use of cannabis -dependence not specified |
| 4 | (Worhunsky et al., 2017) | no | no | not reported | No | no | not reported |
| 5 | (Zhang et al., 2016) | yes | no | mean age = 40; mean of 17 years alcohol use |
Yes | no | mean of 10 years cannabis use |
| 6 | (Regier et al., 2017) | no | no | Group 1 No Abuse: mean age = 45 Avg years of use = 17.1 Group 2 Abuse: Mean age = 43 Avg years of use = 17.6 |
No | no | Group 1 No Abuse: 37% (10 subjects) used Group 2 Abuse: 45% (18 subjects) used |
| 7 | (Rosell-Negre et al., 2016) | no | no | not reported | No | no | not reported |
| 8 | (Rose et al., 2017) | no | no | not reported | no | no | not reported |
| 9 | (Hanlon et al., 2016) | yes | no | mean AUDIT score: 5.8 | yes | no | excluded past week users |
| 10 | (Ide et al., 2016) | yes | no | Mean age = 39.9; mean years alc. use = 16.1, 12.7 days drinking in prior month |
yes | no | mean years cannabis use=10.0 |
| 11 | (Kober et al., 2016) | no | no | # with current alc abuse/ dependence
diagnosis: 4/30 # with past alc abuse/ dependence diagnosis: 13 of 30 |
no | no | # with current cannabis abuse/ dependence
diagnosis: 3 of 30 # with past cannabis abuse/ dependence diagnosis: 7 of 30 |
| 12 | (Moeller et al., 2016b) | no | no | # with current alc use disorder: 1 of
22 # with past alc use disorder: 10 of 22 |
no | no | # with current cannabis use disorder:
1 of 22 # with past cannabis use disorder: 6 of 22 |
| 13 | (Kaag et al., 2016) | no | no | *19.9 ± 35 units/week | no | no | *Range 0–30 days/week |
| 14 | (Tobler et al., 2016) | no | no | mean g/week 259.6 ± 558.6; mean years of use: 14.4 ± 8.9 |
no | no | mean g/wk: 0.6 ± 1.5, mean years of use: 9.8 ± 9.6 |
| 15 | (Moeller et al., 2016a) | no | no | # subjects current/past/ none: 5/8/9 | no | no | # subjects current/past/ none: 1/5/16 |
| 16 | (Fotros et al., 2013) | no | no | # of users: 12 of 12 mean use per year (days):
77.1 ± 120.5 mean use in past 30 days: 7.5 ± 10.5 |
no | no | # of users: 12/12 mean use per year (days): 122.25 ± 153.8 mean use in past 30 days: 15.8 ± 12.8 |
| 17 | (Konova et al., 2016) | no (but none reported) | no | days use/ intoxication in past 30 days: 1
± 1; years use/intoxication: mean 7 ± 2 years for females and 7 ± 3 for males; 11 of 28 had past alcohol use disorder; 0 had current alc use disorder |
no | no | 11 of 28 had past cannabis use disorder;
1 of 28 had current cannabis use disorder |
| 18 | (Balodis et al., 2016) | no | no | past 28 day alc use (days): 6.45 ± 7.7 | no | no | past 28 day cannabis use (days): 1.21 ± 2.8 |
| 19 | Ramaekers et al., 2016) | yes | no | not reported; cutoff for inclusion was < 20 units/week | no | no | occasions used in past 3 months: 44.8 ± 26.8 |
| 20 | (Hanlon et al., 2015) | yes | no | mean AUDIT score: 7.14 ± 4.03 | yes | no | not reported (past week users excluded) |
| 21 | (Moreno-López et al., 2015) | no | no | median used: 20 units/month | yes | no | median used: 64 joints/month |
| 22 | (Crunelle et al., 2015) | no | no | 96.2% used; mean use/week: 19 ± 18 units | no | no | 46.2% used; mean use/ week: 4.2 ± 5.1 g |
| 23 | (Moeller et al., 2015) | no | no | current alcohol use disorder: 5/55
subjects Group 1 (PENK: C-Allele Carrier): 8/21 used; mean # intoxication days in past 30 days: 0.3 ± 0.7 Group 2 (PENK: T/T Genotype): 24/34 used; mean # intoxication days in past 30 days: 2.1 ± 6.0 |
no | no | current cannabis use disorder: 2/55
subjects Group 1 (PENK: C-Allele Carrier): 13/21 used; mean past 30 day use: 0.1 ± 0.3 days Group 2 (PENK: T/T Genotype): 23/34 used; mean past 30 day use: 0.1 ± 0.3 |
| 24 | (Ersche et al., 2015) | no | no | 29% dependent on alc | no | no | 20% dependent on cannabis; 68% reported occasional cannabis use |
| 25 | (Lai et al., 2015) | no | no | 81.3% used alc; mean # years using: 18 | no | no | not reported |
| 26 | (Liu et al., 2014) | no | no | current alcohol use disorder 6/13 subjects | no | no | current cannabis use disorder 1/13 subjects |
| 27 | (Liang et al., 2015) | yes | no | not reported | yes | no | not reported |
| 28 | (Hulka et al., 2016) | no | no | mean g/week: 262.00 ± 251.48 | yes | no | 33% of subjects tested positive for cannabis use; mean use:1.07 ± 1.92 g/week |
| 29 | (Worhunsky et al., 2014) | no | no | mean AUDIT score: 4.5 ± 6.7 | no | no | not reported |
| 30 | (Kaag et al., 2014) | no | no | mean units per week: 24 ± 21 | no | no | 28% used cannabis regularly |
| 31 | (Elton et al., 2015) | no | no | current/lifetime (total subjects =
38) Alc Abuse: 3/8 Alc dependence: 12/23 |
no | no | current/lifetime (total subjects = 38)
Cannabis abuse: 3/14 Cannabis dependence: 8/18 |
| 32 | (Tomasi et al., 2015) | yes | no | not reported | yes | no | not reported |
| 33 | (Moeller et al., 2014) | no | no | current alc use disorder: 0 of 21 past alc use disorder: 8 of 21 | no | no | past cannabis use disorder: 1 of 21 current cannabis use disorder: 9 of 21 |
| 34 | (Xu et al., 2014) | no | no | lifetime alc use disorder: 65%; avg days of alc use in 28 days prior to study: 7.1 ± 9.0 | no | no | not reported |
| 35 | (Prisciandaro et al., 2014) | no | no | # with alcohol dependence: 8 of 51 | no | no | # with cannbis dependence: 2 of 51 |
| 36 | (Zhang et al., 2014) | yes | no | years of alc use: 15 ± 8.9 | yes | no | years of cannabis use: 9 ± 3.8 |
| 37 | (McHugh et al., 2013) | no | no | Group 1 (non-relapsed at day 30): Avg # of standard drinks per week: 2.76 ± 2.28 Group 2 (relapsed at day 30): Avg # of standard drinks per week: 2.50 ± 2.30 |
no | no | Group 1 (non-relapsed at day 30): # of current users: 3 of 20 Group 2 (relapsed at day 30): # of current users: 3 of 24 |
| 38 | (Wisner et al., 2013) | yes | no | excluded men reporting > 14 drinks/week and women reporting > 10 drinks/week | yes | no | not reported |
| 39 | (Mitchell et al., 2013) | no | no | current alc use disorder: 1 of 15 (6.7%); past 28 day use: 3.40 ± 5.24 days |
no (but none sampled) | no | current cannabis use disorder: 0 of 15 (0%); past 28 day use:1.47 ± 3.9 days |
| 40 | (Kilts et al., 2014) | no | no | N = 42 # current/past alc dependence: 9/20 alc abuse: 7/10 |
no | no | N = 42 # current/ past Cannabis dependence: 2/13 Cannabis abuse: 7/17 |
4. Discussion
In this review, we report findings demonstrating that PSU is prevalent among cocaine users, with alcohol and cannabis used most frequently in combination with cocaine. Notably, despite the common understanding among researchers that many cocaine users engage in PSU, our review of the literature revealed few studies focused on cocaine PSU patterns. Indeed, many studies included in the meta-analysis did not directly report PSU rates among cocaine users; instead, rates had to be calculated from the data available in the articles (Booth et al., 2006; Hedden et al., 2009; Vanek et al., 1996). Even in those articles that reported cocaine PSU rates, only one paper (Grant and Harford, 1990) differentiated concurrent and simultaneous use. There was also a high degree of heterogeneity between included studies, possibly due to differences in sampling populations and measurements for PSU. Despite these caveats, even the lowest estimates of cocaine PSU represent a significant fraction of the cocaine-using population, arguing for additional research that, in particular, differentiates concurrent and simultaneous use.
The sparse literature that exists on this topic provides evidence that these drug combinations can alter subjective effects, intake patterns, and neurobiological outcomes relative to cocaine use alone. Of particular note, the combination of alcohol or cannabis with cocaine can alter cocaine’s metabolic profile, which could enhance its reinforcing effects. In addition, neuroimaging studies indicate the presence of qualitative and quantitative differences in the brains of individuals dependent on both cocaine and alcohol relative to either drug alone. Thus, the consequences of substance combinations cannot necessarily be predicted by those of individual substances. Finally, the comparison of human and animal research on the neurobiological consequences of cocaine use finds that cocaine + alcohol and cocaine + cannabis use is assessed at far lower rates in preclinical compared to human studies.
The data discussed in the present review highlight the need to better understand PSU at all levels of analysis, despite the challenges inherent in PSU research. Such challenges include matching subjects (both animal and human) for amounts consumed/duration of use for more than one drug. Preclinical research is hampered by the need to include numerous control groups that can be costly to employ. Furthermore, the synergistic effects of drug combinations on neurobiological changes can be difficult to dissect. A detailed discussion of the challenges inherent in studying neurobiological and behavioral consequences in PSU subjects can be found in (Meyerhoff, 2017).
We close with a call for future research in three areas. First, given shifting trends in substance use patterns (e.g., recent increases in opioid and cannabis use), there is an urgent need to better understand the prevalence and consequences of a broader range of substance combinations (e.g. (Fultz et al., 2017; Winkler et al., 2016). In addition, it is important to mention that the combination of nicotine with other substances, particularly alcohol, may occur with even greater frequency than cocaine + alcohol PSU (Romberger and Grant, 2004), but is not consistently recorded or considered in human studies that focus on illicit drug use (e.g. Kedia et al., 2007). Second, there is a need for more epidemiological data on temporal patterns of substance use combinations. The case of cocaine + alcohol combinations yielding different metabolites according to the order in which they are ingested (and the differential consequences of those metabolites) indicates potentially important differences in outcomes depending upon the temporal patterns with which these substances are ingested, but very few studies address this level of detail (e.g., data on daily, hourly, and even minute-to-minute patterns of intake). Third, there is a need to integrate these granular epidemiological data to establish animal models that better capture “real-world” human substance use. While the development of such models will require careful (and in many cases laborious) exploration of the effects of doses and timing of administration, ignoring PSU “complications” and focusing exclusively on individual substances impedes a full understanding of the neurobiology of substance use. The “back-translation” of human substance use patterns into accurate animal models will provide improved platforms for elucidating the neurobehavioral consequences of PSU, and, ultimately, development of therapies to decrease substance use and risk of relapse. Recent efforts such as the formation of the Collaborative Research on Addiction at NIH (CRAN) should be helpful in working toward these goals.
Role of funding source
Authors report National Institutes of Health funding (DA035167, TW009120, DA041106, T32DA035167, and R01DA027951 awarded to LBC; DA033436, DA037270 awarded to LAK; DA036534, DA039349, DA039701 awarded to BS). The HealthStreet program is supported by the Clinical and Translational Science Institute (CTSI) and the University of Florida (UF) College of Public Health & Health Professions and College of Medicine.
Footnotes
Conflict of interest
No conflict declared
References
- Abé C, Mon A, Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ, 2013a. Polysubstance and alcohol dependence: unique abnormalities of magnetic resonance-derived brain metabolite levels. Drug Alcohol Depend. 130, 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abé C, Mon A, Hoefer ME, Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ, 2013b. Metabolic abnormalities in lobar and subcortical brain regions of abstinent polysubstance users: magnetic resonance spectroscopic imaging. Alcohol Alcohol. 48, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Liu X, Samet S, Nunes E, Waxman R, Hasin D, 2005. Post discharge cannabis use and its relationship to cocaine, alcohol, and heroin use: a prospective study. Am. J. Psychiatry 162, 1507–1514. [DOI] [PubMed] [Google Scholar]
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, Ciraulo D, Dackis C, Roache JD, Salloum IM, Somoza E, Urschel HC, Elkashef AM, 2009. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 104, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspen JM, Winger G, 1997. Ethanol effects on self-administration of alfentanil, cocaine, and nomifensine in rhesus monkeys. Psychopharmacology (Berl.) 130, 222–227. [DOI] [PubMed] [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Carroll KM, Potenza MN, 2016. Neurofunctional reward processing changes in cocaine dependence during recovery. Neuropsychopharmacology 41, 2112–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP, Gross SR, Garand I, Pihl RO, 2005. Patterns of simultaneous polysubstance use in Canadian rave attendees. Subst. Use Misuse 40, 1525–1537. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Darredeau C, Pihl RO, 2006. Patterns of simultaneous polysubstance use in drug using university students. Hum. Psychopharmacol 21, 255–263. [DOI] [PubMed] [Google Scholar]
- Booth BM, Leukefeld C, Falck R, Wang J, Carlson R, 2006. Correlates of rural methamphetamine and cocaine users: results from a multistate community study. J. Stud. Alcohol 67, 493–501. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne S, Randall CL, Adinoff B, Malcolm R, 1995. Features of cocaine dependence with concurrent alcohol abuse. Drug Alcohol Depend. 39, 69–71. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Wong CJ, 1996. Marijuana use and treatment outcome in cocaine-dependent patients. Exp. Clin. Psychopharmacol 4, 396–403. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Power ME, Bryant K, Rounsaville BJ, 1993a. One-year follow-up status of treatment-seeking cocaine abusers. Psychopathology and dependence severity as predictors of outcome. J. Nerv. Ment. Dis 181, 71–79. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Bryant KJ, 1993b. Alcoholism in treatment-seeking cocaine abusers: clinical and prognostic significance. J. Stud. Alcohol 54, 199–208. [DOI] [PubMed] [Google Scholar]
- Cottler LB, O’Leary CC, Nickel KB, Reingle JM, Isom D, 2014. Breaking the blue wall of silence: risk factors for experiencing police sexual misconduct among female offenders. Am. J. Public Health 104, 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelle CL, Kaag AM, van den Munkhof HE, Reneman L, Homberg JR, Sabbe B, van den Brink W, van Wingen G, 2015. Dysfunctional amygdala activation and connectivity with the prefrontal cortex in current cocaine users. Hum. Brain Mapp 36, 4222–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, 2015. Effects of chronic binge-like ethanol consumption on cocaine self-administration in rhesus monkeys. Drug Alcohol Depend. 153, 278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Smitherman S, Young J, Kilts CD, 2015. Effects of childhood maltreatment on the neural correlates of stress- and drug cue-induced cocaine craving. Addict. Biol 20, 820–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Hagan CC, Smith DG, Jones PS, Calder AJ, Williams GB, 2015. In the face of threat: neural and endocrine correlates of impaired facial emotion recognition in cocaine dependence. Transl. Psychiatry 5, e570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Acosta-Cabronero J, Jones PS, Ziauddeen H, van Swelm RP, Laarakkers CM, Raha-Chowdhury R, Williams GB, 2017. Disrupted iron regulation in the brain and periphery in cocaine addiction. Transl. Psychiatry 7, e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotros A, Casey KF, Larcher K, Verhaeghe JA, Cox SM, Gravel P, Reader AJ, Dagher A, Benkelfat C, Leyton M, 2013. Cocaine cue-induced dopamine release in amygdala and hippocampus: a high-resolution PET [18F]fallypride study in cocaine dependent participants. Neuropsychopharmacology 38, 1780–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fultz EK, Martin DL, Hudson CN, Kippin TE, Szumlinski KK, 2017. Methamphetamine-alcohol interactions in murine models of sequential and simultaneous oral drug-taking. Drug Alcohol Depend. 177, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Villarreal EA, Chakravarty MM, Hansen B, Eskildsen SF, Devenyi GA, Castillo-Padilla D, Balducci T, Reyes-Zamorano E, Jespersen SN, Perez-Palacios P, Patel R, Gonzalez-Olvera JJ, 2017. The effect of crack cocaine addiction and age on the microstructure and morphology of the human striatum and thalamus using shape analysis and fast diffusion kurtosis imaging. Transl. Psychiatry 7, e1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossop M, Manning V, Ridge G, 2006. Concurrent use and order of use of cocaine and alcohol: behavioural differences between users of crack cocaine and cocaine powder. Addiction 101, 1292–1298. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC, 1990. Concurrent and simultaneous use of alcohol with cocaine: results of national survey. Drug Alcohol Depend. 25, 97–104. [DOI] [PubMed] [Google Scholar]
- Green C, Schmitz J, Lindsay J, Pedroza C, Lane S, Agnelli R, Kjome K, Moeller FG, 2012. The influence of baseline marijuana use on treatment of cocaine dependence: application of an informative-priors Bayesian approach. Front. Psychiatry 3, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindalini C, Vallada H, Breen G, Laranjeira R, 2006. Concurrent crack and powder cocaine users from Sao Paulo: do they represent a different group? BMC Public Health 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, DeVries W, Dowdle LT, West JA, Siekman B, Li X, George MS, 2015. A comprehensive study of sensorimotor cortex excitability in chronic cocaine users: integrating TMS and functional MRI data. Drug Alcohol Depend. 157, 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dowdle LT, Moss H, Canterberry M, George MS, 2016. Mobilization of medial and lateral frontal-striatal circuits in cocaine users and controls: an interleaved TMS/BOLD functional connectivity study. Neuropsychopharmacology 41, 3032–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Jatlow P, Sevarino KA, McCance-Katz EF, 2000. Comparison of intravenous cocaethylene and cocaine in humans. Psychopharmacology (Berl.) 149, 153–162. [DOI] [PubMed] [Google Scholar]
- Hedden SL, Malcolm RJ, Latimer WW, 2009. Differences between adult non-drug users versus alcohol, cocaine and concurrent alcohol and cocaine problem users. Addict. Behav 34, 323–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden SL, Martins SS, Malcolm RJ, Floyd L, Cavanaugh CE, Latimer WW, 2010. Patterns of illegal drug use among an adult alcohol dependent population: results from the National Survey on Drug Use and Health. Drug Alcohol Depend. 106, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG, 2003. Measuring inconsistency in meta-analyses. BMJ 327, 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulka LM, Scheidegger M, Vonmoos M, Preller KH, Baumgartner MR, Herdener M, Seifritz E, Henning A, Quednow BB, 2016. Glutamatergic and neurometabolic alterations in chronic cocaine users measured with (1) H-magnetic resonance spectroscopy. Addict. Biol 21, 205–217. [DOI] [PubMed] [Google Scholar]
- Ide JS, Hu S, Zhang S, Mujica-Parodi LR, Li CS, 2016. Power spectrum scale invariance as a neural marker of cocaine misuse and altered cognitive control. Neuroimage Clin. 11, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jatlow P, Elsworth JD, Bradberry CW, Winger G, Taylor JR, Russell R, Roth RH, 1991. Cocaethylene: a neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sci. 48, 1787–1794. [DOI] [PubMed] [Google Scholar]
- Kaag AM, Crunelle CL, van Wingen G, Homberg J, van den Brink W, Reneman L, 2014. Relationship between trait impulsivity and cortical volume, thickness and surface area in male cocaine users and non-drug using controls. Drug Alcohol Depend. 144, 210–217. [DOI] [PubMed] [Google Scholar]
- Kaag AM, Levar N, Woutersen K, Homberg J, van den Brink W, Reneman L, van Wingen G, 2016. Hyperresponsiveness of the neural fear network during fear conditioning and extinction learning in male cocaine users. Am. J. Psychiatry 173, 1033–1042. [DOI] [PubMed] [Google Scholar]
- Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, Awad LA, Rendall MJ, Gruber SA, Nason A, Mudrick MJ, Blank SR, Meyer AA, Knapp C, Ciraulo DA, Renshaw PF, 2004. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 130, 283–293. [DOI] [PubMed] [Google Scholar]
- Kedia S, Sell MA, Relyea G, 2007. Mono- versus polydrug abuse patterns among publicly funded clients. Subst. Abuse Treat. Prev. Policy 2, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Kennedy A, Elton AL, Tripathi SP, Young J, Cisler JM, James GA, 2014. Individual differences in attentional bias associated with cocaine dependence are related to varying engagement of neural processing networks. Neuropsychopharmacology 39, 1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Ettenberg A, 2005. Ethanol consumption reduces the adverse consequences of self-administered intravenous cocaine in rats. Psychopharmacology (Berl.) 178, 143–150. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Samimi MM, Ettenberg A, 2002. Evidence for opponent-process actions of intravenous cocaine and cocaethylene. Pharmacol. Biochem. Behav 72, 931–936. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Ben-Shahar O, Ettenberg A, 2006. Alcohol consumption is preferred to water in rats pretreated with intravenous cocaine. Pharmacol. Biochem. Behav 85, 281–286. [DOI] [PubMed] [Google Scholar]
- Kober H, Lacadie CM, Wexler BE, Malison RT, Sinha R, Potenza MN, 2016. Brain activity during cocaine craving and gambling urges: an fMRI study. Neuropsychopharmacology 41, 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Parvaz MA, Froböse MI, Alia-Klein N, Goldstein RZ, 2016. Converging effects of cocaine addiction and sex on neural responses to monetary rewards. Psychiatry Res. 248, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S, Gerstenblith G, Li J, Zhu H, Bluemke DA, Liu CY, Zimmerman SL, Chen S, Lai H, Treisman G, 2015. Chronic cocaine use and its association with myocardial steatosis evaluated by 1H magnetic resonance spectroscopy in African Americans. J. Addict. Med 9, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledesma JC, Aguilar MA, Giménez-Gómez P, Miñarro J, Rodríguez-Arias M, 2017. Adolescent but not adult ethanol binge drinking modulates cocaine withdrawal symptoms in mice. PLoS One 12, e0172956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J, 2003. Understanding polydrug use: review of heroin and cocaine co-use. Addiction 98, 7–22. [DOI] [PubMed] [Google Scholar]
- Liang X, He Y, Salmeron BJ, Gu H, Stein EA, Yang Y, 2015. Interactions between the salience and default-mode networks are disrupted in cocaine addiction. J. Neurosci 35, 8081–8090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht CL, Christoffersen M, Okholm M, Damgaard L, Fink-Jensen A, Knudsen GM, Erritzoe D, 2012. Simultaneous polysubstance use among Danish 3, 4-methylenedioxymethamphetamine and hallucinogen users: combination patterns and proposed biological bases. Hum. Psychopharmacol 27, 352–363. [DOI] [PubMed] [Google Scholar]
- Lindsay JA, Stotts AL, Green CE, Herin DV, Schmitz JM, 2009. Cocaine dependence and concurrent marijuana use: a comparison of clinical characteristics. Am. J. Drug Alcohol Abuse 35, 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Lu H, Filbey FM, Tamminga CA, Cao Y, Adinoff B, 2014. MRI assessment of cerebral oxygen metabolism in cocaine-addicted individuals: hypoactivity and dose dependence. NMR Biomed. 27, 726–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Kouri E, Fukuzako H, Mendelson JH, 1994. Marihuana smoking increases plasma cocaine levels and subjective reports of euphoria in male volunteers. Pharmacol. Biochem. Behav 48, 715–721. [DOI] [PubMed] [Google Scholar]
- MacDonald S, MacIntyre P, Joordens C, Stockwell T, Martin G, 2015. Factors related to simultaneous cocaine and alcohol use for clients in treatment. J. Alcohol. Drug Depend 2, 100–193. [Google Scholar]
- Macht VA, Kelly SJ, Gass JT, 2017. Sex-specific effects of developmental alcohol exposure on cocaine-induced place preference in adulthood. Behav. Brain Res 332, 259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin A, Avants SK, Kosten TR, 1996. Abstinence symptomatology associated with cessation of chronic cocaine abuse among methadone-maintained patients. Am. J. Drug Alcohol Abuse 22, 377–388. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Kosten TR, Jatlow P, 1998. Concurrent use of cocaine and alcohol is more potent and potentially more toxic than use of either alone–a multiple-dose study. Biol. Psychiatry 44, 250–259. [DOI] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA, 2013. Striatalinsula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am. J. Drug Alcohol Abuse 39, 424–432. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, 2017. Functionally relevant brain alterations in polysubstance users. Addictive Substances and Neurological Disease. Elsevier, pp. 217–237. [Google Scholar]
- Meyerhoff DJ, Bloomer C, Schuff N, Ezekiel F, Norman D, Clark W, Weiner MW, Fein G, 1999. Cortical metabolite alterations in abstinent cocaine and cocaine/alcohol-dependent subjects: proton magnetic resonance spectroscopic imaging. Addict. Biol 4, 405–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MR, Balodis IM, Devito EE, Lacadie CM, Yeston J, Scheinost D, Constable RT, Carroll KM, Potenza MN, 2013. A preliminary investigation of Stroop-related intrinsic connectivity in cocaine dependence: associations with treatment outcomes. Am. J. Drug Alcohol Abuse 39, 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Froböse MI, Konova AB, Misyrlis M, Parvaz MA, Goldstein RZ, Alia-Klein N, 2014. Common and distinct neural correlates of inhibitory dysregulation: stroop fMRI study of cocaine addiction and intermittent explosive disorder. J. Psychiatr. Res 58, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Beebe-Wang N, Schneider KE, Konova AB, Parvaz MA, Alia-Klein N, Goldstein RZ, 2015. Effects of an opioid (proenkephalin) polymorphism on neural response to errors in health and cocaine use disorder. Behav. Brain Res 293, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Fleming SM, Gan G, Zilverstand A, Malaker P, d’Oleire Uquillas F, Schneider KE, Preston-Campbell RN, Parvaz MA, Maloney T, Alia-Klein N, Goldstein RZ, 2016a. Metacognitive impairment in active cocaine use disorder is associated with individual differences in brain structure. Eur. Neuropsychopharmacol 26, 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Konova AB, Tomasi D, Parvaz MA, Goldstein RZ, 2016b. Abnormal response to methylphenidate across multiple fMRI procedures in cocaine use disorder: feasibility study. Psychopharmacology (Berl) 233, 2559–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-López L, Perales JC, van Son D, Albein-Urios N, Soriano-Mas C, Martinez-Gonzalez JM, Wiers RW, Verdejo-García A, 2015. Cocaine use severity and cerebellar gray matter are associated with reversal learning deficits in cocaine-dependent individuals. Addict. Biol 20, 546–556. [DOI] [PubMed] [Google Scholar]
- Nattala P, Leung KS, Abdallah AB, Cottler LB, 2011. Heavy use versus less heavy use of sedatives among non-medical sedative users: characteristics and correlates. Addict. Behav 36, 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Cardenas VA, Meyerhoff DJ, 2001. Separate and interactive effects of cocaine and alcohol dependence on brain structures and metabolites: quantitative MRI and proton MR spectroscopic imaging. Addict. Biol 6, 347–361. [DOI] [PubMed] [Google Scholar]
- Olthuis JV, Darredeau C, Barrett SP, 2013. Substance use initiation: the role of simultaneous polysubstance use. Drug Alcohol Rev. 32, 67–71. [DOI] [PubMed] [Google Scholar]
- Pan WJ, Hedaya MA, 1999. Cocaine and alcohol interactions in the rat: effect of cocaine and alcohol pretreatments on cocaine pharmacokinetics and pharmacodynamics. J. Pharm. Sci 88, 1266–1274. [DOI] [PubMed] [Google Scholar]
- Pennington DL, Durazzo TC, Schmidt TP, Abé C, Mon A, Meyerhoff DJ, 2015. Alcohol use disorder with and without stimulant use: brain morphometry and its associations with cigarette smoking, cognition, and inhibitory control. PLoS One 10, e0122505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes M, 1994. The order of drug administration: its effects on the interaction between cocaine and ethanol. Life Sci. 55, 541–550. [DOI] [PubMed] [Google Scholar]
- Prisciandaro JJ, Joseph JE, Myrick H, McRae-Clark AL, Henderson S, Pfeifer J, Brady KT, 2014. The relationship between years of cocaine use and brain activation to cocaine and response inhibition cues. Addiction 109, 2062–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers JG, van Wel JH, Spronk D, Franke B, Kenis G, Toennes SW, Kuypers KP, Theunissen EL, Stiers P, Verkes RJ, 2016. Cannabis and cocaine decrease cognitive impulse control and functional corticostriatal connectivity in drug users with low activity DBH genotypes. Brain Imaging Behav. 10, 1254–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven MA, Necessary BD, Danluck DA, Ettenberg A, 2000. Comparison of the reinforcing and anxiogenic effects of intravenous cocaine and cocaethylene. Exp. Clin. Psychopharmacol 8, 117–124. [DOI] [PubMed] [Google Scholar]
- Ray S, Biswal BB, Aya A, Gohel S, Srinagesh A, Hanson C, Hanson SJ, 2017. Modeling causal relationships among brain areas in the mesocorticolimbic system during resting-state in cocaine users utilizing a graph theoretic approach. J. Alcohol. Drug Depend 5, 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier PS, Monge ZA, Franklin TR, Wetherill RR, Teitelman A, Jagannathan K, Suh JJ, Wang Z, Young KA, Gawrysiak M, Langleben DD, Kampman KM, O’Brien CP, Childress AR, 2017. Emotional, physical and sexual abuse are associated with a heightened limbic response to cocaine cues. Addict. Biol 22, 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MJ, Bornheim LM, 2001. Cannabinoid-induced alterations in brain disposition of drugs of abuse. Biochem. Pharmacol 61, 1357–1367. [DOI] [PubMed] [Google Scholar]
- Romberger DJ, Grant K, 2004. Alcohol consumption and smoking status: the role of smoking cessation. Biomed. Pharmacother 58, 77–83. [DOI] [PubMed] [Google Scholar]
- Rose EJ, Salmeron BJ, Ross TJ, Waltz J, Schweitzer JB, Stein EA, 2017. Dissociable effects of cocaine dependence on reward processes: the role of acute cocaine and craving. Neuropsychopharmacology 42, 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell-Negre P, Bustamante JC, Fuentes-Claramonte P, Costumero V, Llopis-Llacer JJ, Barrós-Loscertales A, 2016. Reward contingencies improve goal-directed behavior by enhancing posterior brain attentional regions and increasing corticostriatal connectivity in cocaine addicts. PLoS One 11, e0167400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruger JP, Ben Abdallah A, Cottler L, 2010. Costs of HIV prevention among out-of-treatment drug-using women: results of a randomized controlled trial. Public Health Rep. 125, 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TP, Pennington DL, Cardoos SL, Durazzo TC, Meyerhoff DJ, 2017. Neurocognition and inhibitory control in polysubstance use disorders: Comparison with alcohol use disorders and changes with abstinence. J. Clin. Exp. Neuropsychol 39, 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Bordnick PS, Kearney ML, Fuller SM, Breckenridge JK, 1997. Treatment outcome of cocaine-alcohol dependent patients. Drug Alcohol Depend. 47, 55–61. [DOI] [PubMed] [Google Scholar]
- Schwingel PA, Zoppi CC, Cotrim HP, 2014. The influence of concomitant use of alcohol, tobacco, cocaine, and anabolic steroids on lipid profiles of Brazilian recreational bodybuilders. Subst. Use Misuse 49, 1115–1125. [DOI] [PubMed] [Google Scholar]
- Tobler PN, Preller KH, Campbell-Meiklejohn DK, Kirschner M, Kraehenmann R, Stämpfli P, Herdener M, Seifritz E, Quednow BB, 2016. Shared neural basis of social and non-social reward deficits in chronic cocaine users. Soc. Cogn. Affect. Neurosci 11, 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND, 2015. Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: association to striatal D2/D3 receptors. Hum. Brain Mapp 36, 120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanek VW, Dickey-White HI, Signs SA, Schechter MD, Buss T, Kulics AT, 1996. Concurrent use of cocaine and alcohol by patients treated in the emergency department. Ann. Emerg. Med 28, 508–514. [DOI] [PubMed] [Google Scholar]
- Wallace CB, Small K, Brodley EC, Lau J, Trikalinos AT, 2012. Deploying an interactive machine learning system in an evidence-based practice center: abstrackr. Proceedings of the ACM International Health Informatics Symposium (IHI). pp. 819–824. [Google Scholar]
- Winkler MC, Greager EM, Stafford J, Bachtell RK, 2016. Methamphetamine self-administration reduces alcohol consumption and preference in alcohol-preferring P rats. Addict. Biol 23, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KM, Patzelt EH, Lim KO, MacDonald AW, 2013. An intrinsic connectivity network approach to insula-derived dysfunctions among cocaine users. Am. J. Drug Alcohol Abuse 39, 403–413. [DOI] [PubMed] [Google Scholar]
- Worhunsky PD, Malison RT, Rogers RD, Potenza MN, 2014. Altered neural correlates of reward and loss processing during simulated slot-machine fMRI in pathological gambling and cocaine dependence. Drug Alcohol Depend. 145, 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worhunsky PD, Potenza MN, Rogers RD, 2017. Alterations in functional brain networks associated with loss-chasing in gambling disorder and cocaine-use disorder. Drug Alcohol Depend. 178, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kober H, Wang X, DeVito EE, Carroll KM, Potenza MN, 2014. Hippocampal volume mediates the relationship between measures of pre-treatment cocaine use and within-treatment cocaine abstinence. Drug Alcohol Depend. 143, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Bednarski SR, Erdman E, Li CS, 2014. Error-related functional connectivity of the thalamus in cocaine dependence. Neuroimage Clin. 4, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Sinha R, Potenza MN, Malison RT, Li CS, 2016. Cocaine dependence and thalamic functional connectivity: a multivariate pattern analysis. Neuroimage Clin. 12, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]


