Abstract
Rationale
Pavlovian conditioning with a discrete reward-predictive visual cue can elicit two classes of behaviors: “sign-tracking” (approach toward and contact with the cue) and “goal-tracking” (approach toward the site of reward delivery). Sign-tracking has been proposed to be linked to behavioral disorders involving compulsive reward-seeking, such as addiction. Prior exposure to psychostimulant drugs of abuse can facilitate reward-seeking behaviors through enhancements in incentive salience attribution. Thus, it was predicted that a sensitizing regimen of amphetamine exposure would increase sign-tracking behavior.
Objective
The purpose of these experiments was to determine how a regimen of exposure to amphetamine affects subsequent sign-tracking behavior.
Materials and methods
Male Long-Evans rats were given daily injections of d-amphetamine (2.0 mg/kg) or saline for 5 days, then given a 7-day drug-free period followed by testing in a Pavlovian conditioning task. In experiment 1, rats were presented with a visual cue (simultaneous illumination of a light and extension of a lever) located either to the left or right of a centrally located food trough. One cue (CS+) was always followed by food delivery, whereas the other (CS−) was not. In experiment 2, rats were tested in a nondiscriminative (CS+ only) version of the task.
Results
In both experiments, amphetamine-exposed rats showed less sign-tracking and more goal-tracking compared to saline controls.
Conclusions
Contrary to predictions, prior amphetamine exposure decreased sign-tracking and increased goal-tracking behavior. However, these results do support the hypothesis that psychostimulant exposure and incentive sensitization enhance behavior directed toward reward-proximal cues at the expense of reward-distal cues.
Keywords: Autoshaping, Sign-tracking, Goal-tracking, Conditioned approach, Pavlovian conditioning, Amphetamine, Sensitization, Incentive salience, Behavior, Associative learning, Conditioning, Learning and memory
Introduction
Stimuli associated with primary rewards (incentives), such as food, water, sex, and drugs of abuse, can acquire some of the same rewarding properties as the primary rewards themselves. This acquisition of incentive salience by reward-predictive cues is thought to underlie reward-related psychopathologies such as drug addiction, in that the acquired incentive properties of drug-predictive cues may compel drug-using behavior and precipitate relapse (Robinson and Berridge 1993). Reward-predictive cues’ acquisition of incentive salience can be demonstrated using a Pavlovian “sign-tracking” (often termed autoshaping) paradigm, in which presentation of a discrete visual cue (the conditioned stimulus or “sign”) followed by reward delivery leads to development of conditioned approach behavior toward the cue (Brown and Jenkins 1968). However, in addition to sign-tracking behavior directed toward discrete reward-predictive visual cues, conditioned behavior in the presence of such cues may also take the form of “goal-tracking,” that is, behaviordirected toward the reward itself [or the site of impending reward delivery (Boakes 1977; Silva et al. 1992)]. There is considerable individual variability in the distribution of sign- vs. goal-tracking behavior in response to reward-predictive cues, and there is current interest in understanding the relationship between this variability, other reward-related behaviors, and the underlying neurobiology (Cunningham and Patel 2007; Flagel et al. 2007a, b, 2008a, b; Tomie et al. 2000).
Chronic administration of drugs of abuse can induce a range of alterations in cognition and motivation (Briand et al. 2008; George et al. 2008; Mendez et al. 2008; Olausson et al. 2007; Schoenbaum et al. 2006; Simon et al. 2007). In particular, regimens of psychostimulant drugs (such as amphetamine and cocaine) can cause long-lasting enhancements in behavior directed by and toward rewards and reward-predictive cues (Harmer and Phillips 1998; Nordquist et al. 2007; Taylor and Jentsch 2001; Wyvell and Berridge 2001). This enhanced incentive salience attribution, or “incentive sensitization” may contribute to drug addiction by increasing behavior directed toward gaining access to drugs and drug cues (Robinson and Berridge 2003). However, it is not clear how incentive sensitization is related to the distribution of sign- vs. goal-tracking behavior.
On the one hand, it has been proposed that elevated sign-tracking may be related to vulnerability to drugs of abuse [as is conferred by manipulations such as prior psychostimulant exposure (Ferrario and Robinson 2007; Flagel et al. 2008a, b; Horger et al. 1992)]. In support of this idea, enhanced incentive salience attribution resulting from lesions of the subthalamic nucleus increases sign-tracking behavior, and greater sign-tracking has been associated with greater alcohol self-administration, as well as increases in both cocaine-induced psychomotor sensitization and alterations in dopaminergic markers implicated in addiction (Flagel et al. 2007b, 2008a, b; Tomie et al. 2008; Uslaner and Robinson 2006; Uslaner et al. 2005). Such data suggest that drug-induced incentive sensitization might result in increased sign-tracking behavior.
On the other hand, other data suggest that incentive sensitization resulting from drug administration could have the opposite effect—i.e., lead to an increase in behavior directed toward rewards (goals) themselves, with a concomitant decrease in sign-tracking behavior (Berridge 2007; Nocjar and Panksepp 2002; Nordquist et al. 2007). In particular, prior amphetamine exposure causes a shift in the activity of neurons in ventral pallidum from preferential encoding of reward-distal to reward-proximal cues (Tindell et al. 2005), suggesting that drug-induced incentive sensitization could shift the focus of behavior from sign-tracking (reward-distal) to goal-tracking (reward-proximal). The experiments described below were designed to determine how prior amphetamine exposure affects the distribution of sign- vs. goal-tracking behavior in a rat model. Experiment 1 used a discriminative (CS+/CS−) design, and experiment 2 used a simpler single CS design to verify the results from experiment 1.
Materials and methods
Subjects
Sixty-four male Long-Evans rats (Charles River Laboratories, Raleigh, NC, USA) weighing 275–300 g upon arrival were used for these experiments. Rats were individually housed and kept on a 12-h light/dark cycle (lights on at 0800 hours) with free access to food and water except as noted. All procedures were conducted in accordance with principles of laboratory animal care as laid out by the Texas A&M University Laboratory Animal Care and Use Committee and NIH guidelines.
Apparatus
Pavlovian conditioning task
Behavioral testing was conducted in six identical standard rat test chambers (Coulbourn Instruments, Allentown, PA, USA). Each test chamber was equipped with a recessed food pellet delivery trough fitted with a photobeam to detect head entries. The trough, into which 45 mg grain-based food pellets (PJAI, Test Diet: Richmond, IN, USA) were delivered, was located 2 cm above the floor in the center of the front wall. Retractable levers were located to the left and right of the food delivery trough, 11 cm above the floor, and a 1.12-W lamp was located 2.5 cm above each of these levers. Simultaneous lever extension and illumination of the corresponding lamp served as the CSs. Both levers were used in experiment 1, but only one (either the left or the right, counterbalanced across drug groups) was used in experiment 2. Each chamber was equipped with an overhead infrared activity monitor for measuring locomotion (Coulbourn Instruments) and was housed in a sound-attenuating cubicle. A 1.12-W lamp located on the inside back wall of the cubicle (31.5 cm above the floor of the cubicle) provided constant background illumination. The chambers were controlled and data were collected by a computer running Graphic State software (Coulbourn Instruments).
Locomotor assessment
Sensitization to the acute locomotor stimulant effects of amphetamine was tested in eight identical activity monitoring chambers (Versamax System, Accuscan Instruments, Columbus, OH, USA). Each chamber (40×40×30 cm) contained an array of photobeams raised 0.5 cm above the floor to detect movement in the horizontal plane. The activity chambers were connected to a computer running Versamap software (Accuscan Instruments) which recorded photobeam breaks.
Amphetamine exposure
Rats were randomly assigned to amphetamine or saline control groups. The amphetamine group received daily i.p. injections in the home cage of 2 mg/kg D-amphetamine sulfate (calculated as the weight of the salt) dissolved in 0.9% saline at a volume of 1 ml/kg for 5 consecutive days [D-amphetamine was obtained from the Drug Supply Program at the National Institute on Drug Abuse (experiment 1) and Sigma (experiment 2)]. This dose and injection regimen (as well as less intensive regimens) can produce lasting sensitization to the locomotor stimulant effects of amphetamine as well as lasting enhancements in incentive motivation for rewards and reward-predictive cues (Harmer and Phillips 1998; Vanderschuren et al. 1999). The saline group received an identical schedule of injections of 0.9% saline vehicle (1 ml/kg). Following injections, rats were left undisturbed for 3 days, after which they began food restriction to bring them to 85% of their free-feeding weight.
Behavior
Behavioral training began 7 days after the final injection. The procedure was modified from Di Ciano et al. (2001). On the day before the start of behavioral testing, each rat was given five of the 45 mg food pellets used in the sign-tracking task in its home cage to increase neophagia. Training began with a 64-min session of magazine training consisting of 38 deliveries of a single food pellet with an intertrial interval (ITI) of 100±40 s. The Pavlovian conditioning task began on the following day.
Experiment 1
Rats (n = 10/group) were trained for seven consecutive sessions in the discriminative task. Each 60-min session consisted of 50 trials (25 CS+, 25 CS−) with a 52±31-s ITI. On each trial, a 10-s visual CS (consisting of simultaneous extension of a retractable lever and illumination of the lamp located above the lever) was presented. On CS+ trials, the CS+ (either the left or right lever + lamp, counterbalanced across drug groups) was immediately followed by delivery of a single 45 mg food pellet into the food trough. On CS− trials, the CS− (the lever + lamp not used as the CS+) was not followed by food delivery. These CS− trials provided a control for pseudoconditioning (responding to the CS stimuli not due to pairing with the US). Lever presses and head entries into the food trough were recorded but had no programmed consequences.
Experiment 2
Rats (n = 17/group) were trained for seven consecutive sessions in the single-CS task. Training proceeded as in experiment 1, except that only a single CS (either the left or right lever + lamp combination, counterbalanced across drug groups) was used, and each session consisted of 25 CS trials with an ITI of 124±57 s.
Test for locomotor sensitization
Under red light and white noise, rats were placed in the chambers for a 15-min habituation period, after which they were briefly removed, injected with amphetamine (2 mg/kg), and returned to the chambers for an additional 30-min.
Data analysis
Statistical analyses were conducted in SPSS 12.0. The primary measures of interest were the percentages of time during CS presentations in which the rats engaged in lever-pressing (i.e., sign-tracking) and food trough entries (i.e., goal-tracking). In experiment 1, responding during the CS + relative to the CS− provided the primary measure of conditioned behavior. Additional measures included the percentage of trials on which at least one lever-press or food trough entry occurred, and the number of lever presses or food trough entries per trial (Boakes 1977; Di Ciano et al. 2001; Flagel et al. 2007b; Holland 1977; Simon and Setlow 2006). These data were analyzed using multifactor repeated measures analysis of variance (ANOVA). Comparisons of body weight prior to food restriction and magazine training performance were conducted using independent samples t tests. In all cases, p values less than 0.05 were considered significant. Outliers, defined as values outside the range of 1.5 x the interquartile range from the first or third quartile of the data distribution, were excluded prior to statistical analysis. Data from one rat in the saline group in experiment 1 were excluded for this reason.
In the test of locomotor sensitization in the activity chambers, horizontal activity (number of photobeam breaks) was collapsed into 5-min bins across the 15-min before and 30-min after amphetamine injections. Data in each post-amphetamine bin were normalized to baseline activity by calculating the percent change from the mean of the three baseline bins. These normalized values were analyzed using repeated measures ANOVA, with bin as the within-subjects variable and prior drug treatment as the between-subjects variable. In addition, correlations were conducted between this locomotor activity measure and measures of sign- and goal-tracking. Data from one outlier in the saline group were excluded prior to analysis.
Results
Experiment 1
There were no differences between saline control and amphetamine-exposed rats in either body weight prior to the start of food restriction (t(6)=.07, n.s.) or performance during magazine training (percentage of trials on which rats retrieved the food within 5 s of delivery; t(14)=.61, n.s.). Data from several rats on each measure were lost due to clerical errors.
Sign-tracking
The percentage of time spent lever-pressing (sign-tracking) during CS+ and CS− trials in each session was analyzed using a three-factor repeated measures ANOVA (session × CS type × drug condition), which revealed a main effect of session (F(6, 102)=3.79, p<.01), such that overall sign-tracking increased across the seven sessions, and a main effect of CS type (F(1, 17)=40.99, p<0.01), such that responding was greater on CS + than on CS− trials. There was also a significant interaction between the factors of session and CS type (F(6, 102)=10.11, p<.01), indicating that responding to the two CS types changed across sessions (Fig. 1a). Most importantly, there was a main effect of drug condition (F(1, 17)=4.73, p<.05), such that amphetamine-exposed rats displayed significantly less sign-tracking than saline controls, as well as a significant interaction between drug condition and CS type (F(1, 17)=4.87, p<.05), indicating that sign-tracking to the CS+ vs. CS− differed between groups.
Fig. 1.
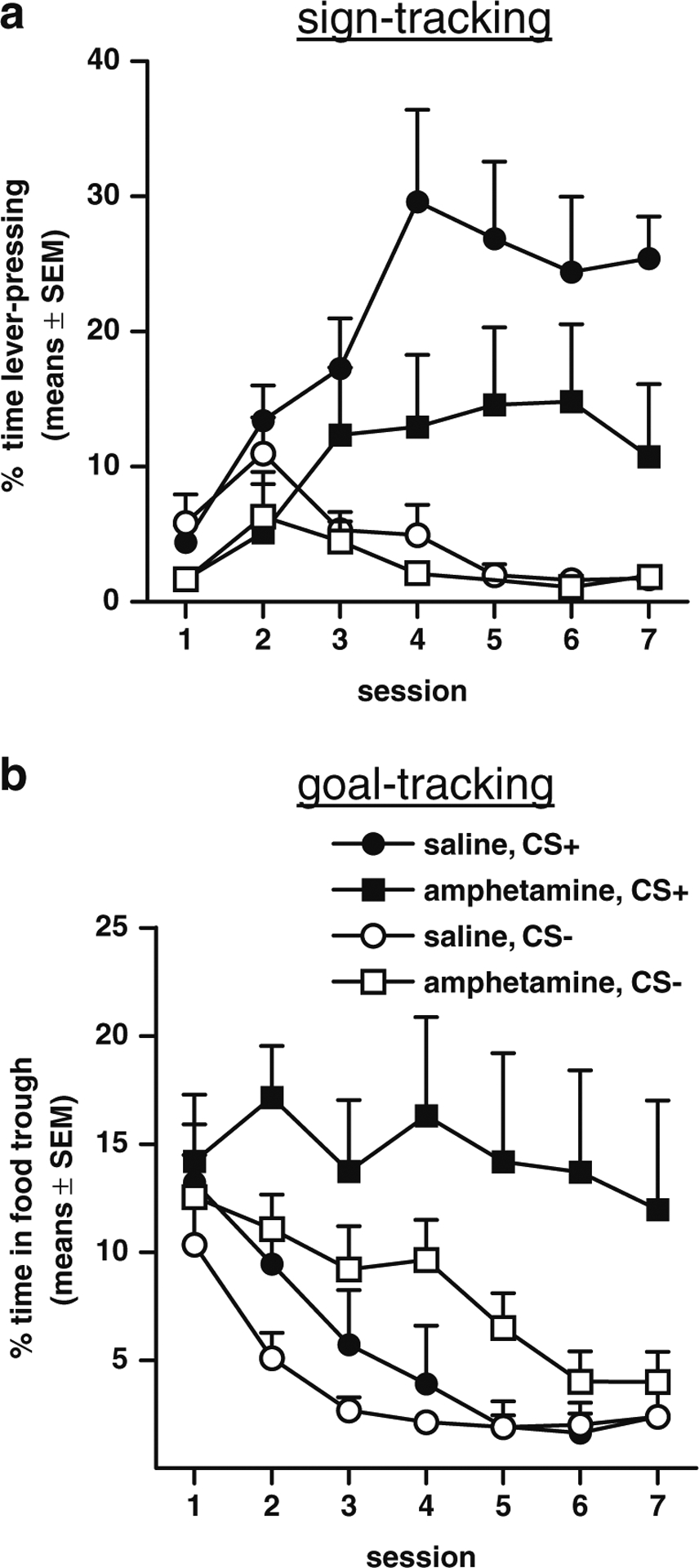
Sign-tracking and goal-tracking in amphetamine-exposed and saline control rats across the seven sessions of discriminative Pavlovian conditioning in experiment 1. a Sign-tracking (percentage of time spent lever-pressing) to the CS+ (closed symbols) and CS− (open symbols). b Goal-tracking (percentage of time in the food trough) to the CS+ (closed symbols) and CS− (open symbols)
To further assess discriminative sign-tracking in this experiment, we computed a discrimination ratio by dividing the percentage of time spent lever-pressing during the CS + by the sum of the percentages of time spent lever-pressing during the CS + and CS− (Fig. 2a). A two-factor ANOVA (session × drug condition) revealed results similar to those obtained in the analyses described above, with main effects of session (F(6, 102)=5.23, p<.01) and drug condition (F(1, 17)=15.59, p<0.01).
Fig. 2.
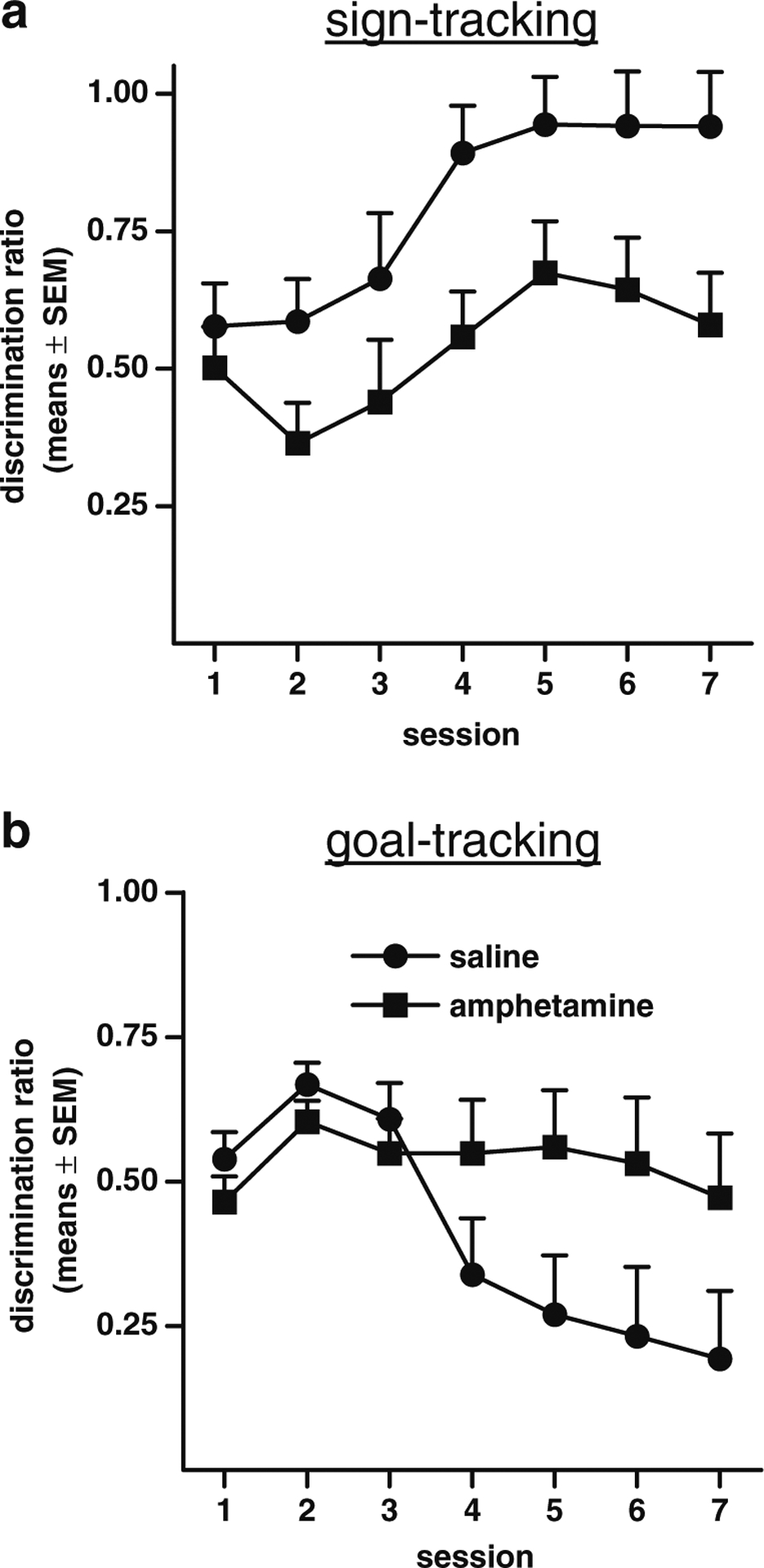
Sign-tracking and goal-tracking in amphetamine-exposed and saline control rats in Experiment 1 expressed as discrimination ratios (percentage of time engaged in behavior during the CS + DIVIDED BY percentage of time engaged in behavior during the CS+ PLUS percentage of time engaged in behavior during the CS−). a Discrimination ratios for the sign-tracking measure. b Discrimination ratios for the goal-tracking measure
In addition to the analysis of the percentage of time spent lever-pressing, sign-tracking was also assessed by computing the percentage of trials on which at least one lever-press occurred (Di Ciano et al. 2001) and the mean number of lever-presses per CS presentation (Flagel et al. 2007b). The results of these analyses were similar to those described above, and the most relevant are presented in Table 1.
Table 1.
Experiment 1
| Main effect of drug | Interaction | |
|---|---|---|
| Percent of trials with.. | ||
| Drug × CS type | ||
| Sign-tracking | F=8.23, p<0.05 | F=10.65, p<0.01 |
| Goal-tracking | F=5.68, p<0.05 | F=2.20, p=0.16 |
| Discrimination ratios | ||
| Drug × Session | ||
| Sign-tracking | F=11.63, p<0.01 | F=1.55, p=0.17 |
| Goal-tracking | F=2.96, p=0.l0 | F=l.25, p=0.29 |
| Number of behaviors/CS | ||
| Drug × CS type | ||
| Sign-tracking | F=2.63, p=0.l2 | F=1.61, p=0.22 |
| Goal-tracking | F=6.00, p<0.05 | F=2.45, p=0.14 |
| Discrimination ratios | ||
| Drug × Session | ||
| Sign-tracking | F=7.36, p<0.05 | F=1.20, p=0.31 |
| Goal-tracking | F=l.47, p=0.24 | F=3.49, p<0.01 |
Goal-tracking
The percentage of time rats spent in the food trough (goal-tracking) during CS+ and CS− trials in each session was analyzed using a three-factor repeated measures ANOVA (session × CS type × drug condition). This analysis revealed a main effect of session (F(6, 102)=7.19, p<0.01), such that overall goal-tracking decreased across the seven sessions, as well as a main effect of CS type (F(1, 17)=6.92, p<0.05), indicating that responding was greater on CS + than on CS− trials (Fig. 1b). There was also a main effect of drug condition (F(1, 17)=8.17, p<0.05), such that, across both CS types, amphetamine-exposed rats displayed more goal-tracking than saline controls. However, there were no significant interactions between drug condition and either of the other two factors.
To further assess discriminative goal-tracking, we computed a discrimination ratio by dividing the percentage of time spent in the food trough during the CS+ by the sum of the percentage of time spent in the food trough during the CS+ and CS− (Fig. 2b). A two-factor ANOVA (session × drug condition) revealed results similar to those obtained in the analyses described above, in that there was a main effect of session (F(6, 102)=4.69, p<0.01), and a significant interaction between session and drug condition (F(6, 102)=3.29, p<0.01) although the main effect of drug condition did not reach significance (F(1, 17)=2.39, p<0.14). It should be noted that the absence of an increase in the discrimination ratio in either group was likely due to the overall low level of goal-tracking behavior in this experiment, such that small numerical differences between responding to the CS+ vs. the CS− in some rats were magnified by the ratio measure. The results of additional analyses of goal-tracking behavior using measures of the percentage of trials with at least one food trough entry and the mean number of entries per CS presentation are presented in Table 1.
Comparison of sign-tracking vs. goal-tracking
To determine how amphetamine exposure affected the balance of sign-tracking vs. goal-tracking behavior, the relative percentage of time engaged in each behavior (the “lever-trough difference”) was calculated by subtracting the percentage of time spent in the food trough from the percentage of time spent lever-pressing during CS+ trials (Fig. 3) (Boakes 1977). A repeated measures ANOVA (drug condition × session) revealed a main effect of session, such that sign-tracking became more prevalent than goal-tracking across sessions (F(6, 102)=6.94, p<0.01), and a main effect of drug condition, such that amphetamine-exposed rats showed relatively less sign-tracking and more goal-tracking than saline controls (F(1, 17)=7.09, p<0.05).
Fig. 3.

“Lever-trough differences” (percentage of time during the CS+ spent lever-pressing MINUS percentage of time during the CS+ spent in the food trough) in amphetamine-exposed and saline control rats in experiment 1
Behavior during intertrial intervals
Repeated measures ANOVAs revealed no main effects or interactions involving drug condition on locomotor activity (as assessed with the overhead activity monitors) during either the pre-CS period (a 5-s block immediately prior to CS+ presentations) or the ITI (the intertrial interval excluding the pre-CS period, Fs<1.67, ps>0.14). However, repeated measures ANOVAs did reveal significant main effects of drug condition on the percentage of time in the food trough during both the pre-CS period (F(1, 17)=7.80, p<0.05) and the ITI (F(1, 17)=12.47, p<0.01). As such differences between drug conditions in responding at the food trough in the absence of the CSs could account for the differences in behavior observed in the presence of the CSs, a range of correlational analyses comparing food trough behavior during the pre-CS period and CS+ were conducted. However, there were no significant relationships between the percentage of time in the food trough during the pre-CS period and either the percentage of time in the food trough or the percentage of time lever pressing during the CS+ (rs<0.46, ps>0.17).
Experiment 2
As in Experiment 1, there were no differences between saline control and amphetamine-exposed rats in either body weight prior to the start of food restriction (t(32)=1.44, n.s.) or performance during magazine training [t(29)=1.39, n.s., data from three rats were lost].
Sign-tracking
In this experiment, only a single CS (lever + light combination) was used to determine whether the decreased sign-tracking and increased goal-tracking in amphetamine-exposed rats in experiment 1 was specific to the discriminative design. The percentage of time spent lever-pressing (sign-tracking) during the CS was analyzed using a two-factor repeated measures ANOVA (session × drug condition). This analysis revealed a main effect of session (F(6, 192)=5.46, p<0.01), such that overall sign-tracking increased across the seven sessions (Fig. 4a). Most importantly, as in experiment 1, there was a main effect of drug condition (F(1, 32)=14.04, p<0.01), such that amphetamine-exposed rats showed significantly less sign-tracking than saline controls. The results of additional analyses of sign-tracking behavior are presented in Table 2.
Fig. 4.

Sign tracking and goal-tracking in amphetamine-exposed and saline control rats across the seven sessions of Pavlovian conditioning in experiment 2. a Sign-tracking (percentage of time spent lever-pressing during the CS). b Goal-tracking (percentage of time in the food trough during the CS)
Table 2.
Experiment 2
| Main effect of drug | Interaction | |
|---|---|---|
| Percent of trials with.. | ||
| Drug × Session | ||
| Sign-tracking | F=5.56, p<0.05 | F=2.29, p<0.05 |
| Goal-tracking | F=3.58, p=0.07 | F=2.28, p<0.05 |
| Number of behaviors/CS | ||
| Drug × Session | ||
| Sign-tracking | F=6.28, p<0.05 | F=2.26, p<0.05 |
| Goal-tracking | F=l.65, p=0.21 | F=.l5, p=0.99 |
Goal-tracking
The percentage of time rats spent in the food trough during the CS presentation (goal-tracking) was analyzed using a two-factor repeated measures ANOVA (session × drug condition). This analysis revealed a main effect of session (F(6, 192)=3.74, p<0.01), such that overall goal-tracking increased across the seven sessions (Fig. 4b). Importantly, as in experiment 1, there was a main effect of drug condition (F(1, 32)=4.59, p<0.05), such that amphetamine-exposed rats showed significantly more goal-tracking than saline controls, as well as a significant interaction between drug condition and session (F(6, 192)=2.64, p<0.05), showing that goal-tracking across sessions differed between drug conditions. The results of additional analyses of goal-tracking behavior are presented in Table 2.
Comparison of sign-tracking vs. goal-tracking
A repeated measures ANOVA conducted on the “lever-trough difference” measure (see experiment 1) revealed a main effect of drug condition, such that amphetamine-exposed rats showed relatively less sign-tracking and more goal-tracking than saline controls (F(1, 32)=6.58, p<0.05), and a significant interaction between the factors of drug condition and session (F(6, 192)=3.27, p<0.01), such that amphetamine-exposed rats showed a greater shift than saline controls in the distribution of these behaviors across sessions (Fig. 5).
Fig. 5.
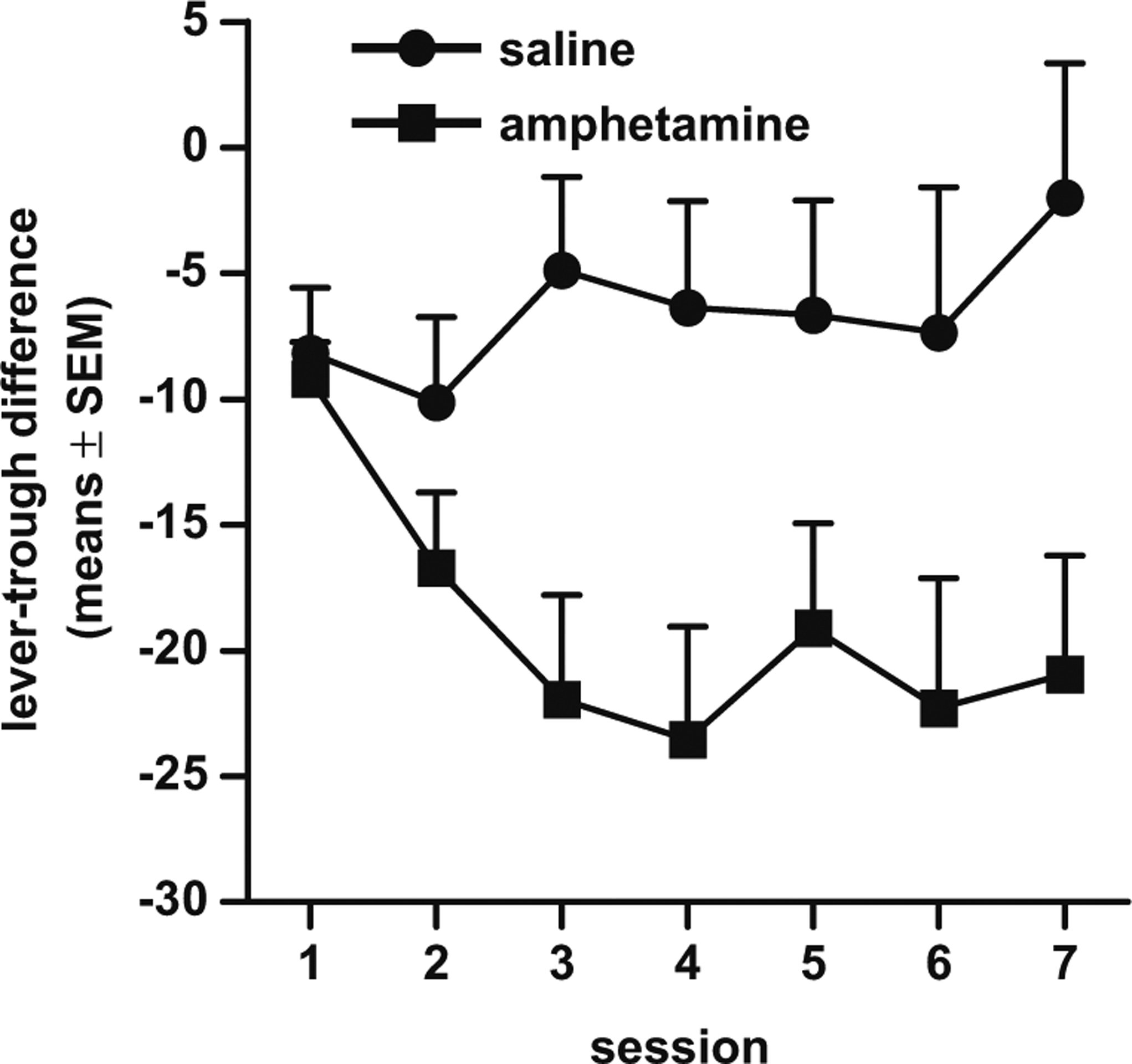
“Lever-trough differences” (percentage of time during the CS spent lever-pressing MINUS percentage of time during the CS spent in the food trough) in amphetamine-exposed and saline control rats in experiment 2
Behavior during intertrial intervals
Activity data from six rats were lost due to equipment failure. There were no main effects or interactions involving drug condition on locomotor activity during either the pre-CS period or the ITI (Fs<2.57, p>0.12), with the exception of a significant interaction between drug condition and session during the ITI, such that amphetamine-exposed rats showed less of an increase in locomotion across sessions than saline controls (F(6, 156)=2.21, p<0.05). However, repeated measures ANOVA did reveal significant main effects of drug condition during both the pre-CS period (F(1, 32)=6.19, p<0.05) and the ITI (F(1, 17)=5.62, p<0.05). In contrast to experiment 1 there were significant correlations between the percentage of time in the food trough during the pre-CS period and the percentage of time in the food trough during the CS (rs>0.59, ps<0.05), although not between the percentage of time in the food trough during the pre-CS period and the percentage of time lever pressing (sign-tracking) during the CS (rs<0.23, ps>0.17).
Test for locomotor sensitization
One cohort of rats in experiment 2 (n=9/group) was tested for the presence of sensitization to the locomotor stimulant effects of amphetamine. There were no activity differences between groups during a 15-min baseline period prior to amphetamine administration (Fs<1.74, ps>0.19). Following acute amphetamine, a repeated measures ANOVA (time bin × prior drug condition) revealed both a main effect of time bin, such that activity increased across time (F(5, 75)=18.37, p< 0.01), and a main effect of prior drug condition (F(1, 15)=4.75, p<0.05), such that rats that received prior amphetamine exposure displayed significantly greater activity compared to saline controls (Fig. 6). These data indicate that the amphetamine exposure regimen was sufficient to induce locomotor sensitization. However, there were no significant correlations between this sensitization measure and measures of sign- and goal-tracking, using either partial correlations to account for the effects of prior amphetamine exposure or bivariate correlations conducted separately on rats in each of the two drug conditions (rs<0.43, ps>0.12).
Fig. 6.
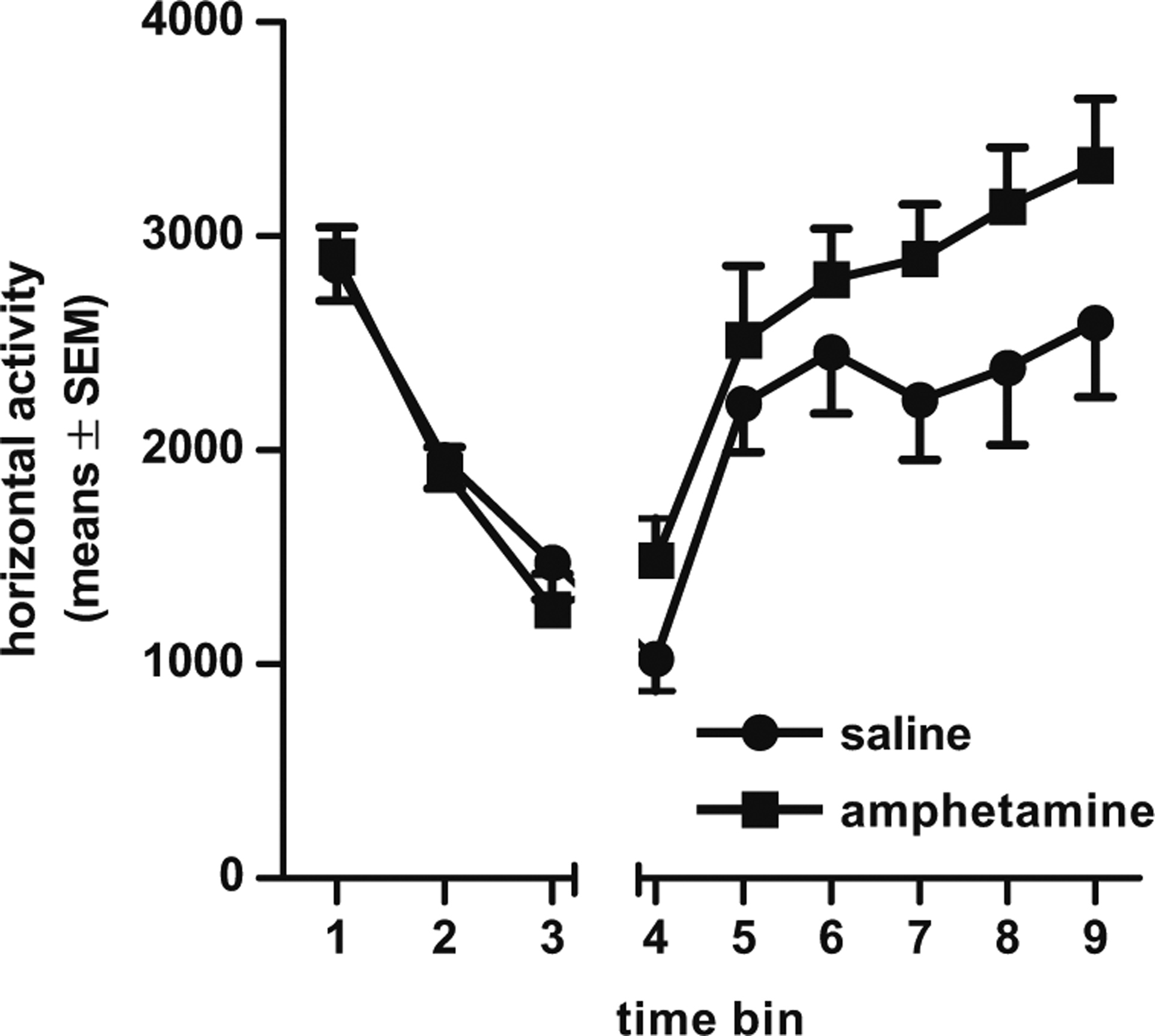
Locomotor activity in amphetamine-exposed and saline control rats in experiment 2 (in 5- min bins) during a 15-min pre-drug baseline period (bins 1–3) and 30 min (bins 4–9) after an amphetamine challenge injection (2 mg/kg, i.p.)
Discussion
Approach toward and contact with a cue predictive of reward (sign tracking) have been proposed to be an index of incentive salience attribution, or the degree to which a reward-predictive cue is attractive or “wanted” (Berridge 2001, 2007; Cardinal et al. 2002). Chronic exposure to psychostimulant drugs of abuse (such as amphetamine) can cause long-lasting enhancements in incentive salience attribution (Harmer and Phillips 1998; Nordquist et al. 2007; Wyvell and Berridge 2001), and lesions of the subthalamic nucleus, which appear to similarly enhance incentive salience, increase sign-tracking behavior (Uslaner et al. 2005, 2008). The present experiments, however, show that a regimen of amphetamine exposure which induced locomotor sensitization and which has been shown in other settings to enhance incentive salience attribution (Harmer and Phillips 1998; Mendez et al. 2007) caused a decrease in sign-tracking (contact with the CS), while at the same time causing an increase in goal-tracking (approach to the site of reward delivery during the CS).
Although perhaps surprising, the observation that amphetamine exposure reduced sign-tracking and increased goal-tracking behavior is consistent with several other lines of research. Regimens of amphetamine administration and drug-free periods similar to those used here have been shown to increase goal-tracking in Pavlovian conditioning tasks in which a combination visual/auditory CS is followed by water or sucrose reward (Harmer and Phillips 1998; Taylor and Jentsch 2001). Chronic amphetamine also alters reward-related neural activity in ventral pallidum in a Pavlovian conditioning task in which one CS (CS1) predicts a second CS (CS2) which predicts sucrose reward (Tindell et al. 2005). In this experiment, following a sensitizing regimen of chronic amphetamine exposure, ventral pallidal activity “shifted” from preferentially encoding CS1 (the reward-distal cue) to CS2 (the reward-proximal cue). In the Pavlovian conditioning task used in the present experiments, the CS itself (presentation of the lever + light combination) and the food trough could similarly be considered reward-distal and reward-proximal cues, respectively, as the CS is both more spatially and, necessarily, more temporally distant from the food reward than is the food trough. Thus, the decrease in sign-tracking and increase in goal-tracking behavior resulting from amphetamine exposure could be considered to be a similar “shift” in the distribution of behavioral focus from reward-distal to reward-proximal cues. The shift in ventral pallidal activity resulting from amphetamine exposure in the study by Tindell et al. (2005) is thought to reflect increased incentive salience attributed to the more reward-proximal cue. Similarly, the present results could stem from relatively greater incentive salience attributed to the reward-proximal food trough compared to the reward-distal lever + light CS (Flagel et al. 2008a, b). This interpretation would also account for the increase in food trough entries during the intertrial intervals, as an increase in incentive salience attributed to the food trough would be expected to influence behavior whenever that stimulus was available (i.e., during both CSs and intertrial intervals—see below for further discussion of this issue).
As mentioned above, previous work suggested that enhancements in incentive salience attributed to discrete reward-predictive visual cues could enhance sign-tracking behavior. Support for this view comes from experiments in which lesions of the subthalamic nucleus, which have been shown to enhance motivation for both food and drugs of abuse, enhance sign-tracking to visual cues paired with these rewards (Baunez et al. 2002; Uslaner et al. 2005, 2008). In the present experiments, however, amphetamine exposure, which has motivation-enhancing effects similar to those of subthalamic nucleus lesions (Ferrario and Robinson 2007; Mendez et al. 2007; Nordquist et al. 2007), had the opposite effect on sign-tracking. The reasons for this difference are not clear, although there are several possibilities. First, it is possible that subthalamic nucleus lesions and amphetamine exposure have effects aside from enhanced incentive salience attribution that alter the distribution of sign- and goal-tracking in opposite ways (e.g., through effects on prefrontal cortical function—see below). Second, it is possible that differences in the task apparatus used may account for the differences in the effects of these two manipulations. The retractable lever used as the CS by Uslaner et al. (2008) was 2.5 cm from the food trough, compared to 7 cm in the present experiments. The closer proximity of the lever CS to the food trough in the study by Uslaner et al. would render it more reward-proximal, and thus perhaps more likely to benefit from enhanced incentive salience attribution relative to the food trough itself. In addition, in an experiment in which Uslaner et al. (2008) showed increased sign-tracking to a lever CS paired with intravenous cocaine administration, the absence of a visual stimulus (equivalent to the food trough) that predicted reward delivery aside from the lever CS would have resulted in the bulk of incentive salience attributed to this sole predictive cue. Thus, the relative proximity and availability of reward-predictive visual cues may determine how changes in incentive salience attribution alter the frequency of sign-vs. goal-tracking conditioned behaviors (Holland 1977; Karpicke 1978; Wasserman and McCracken 1974).
Amphetamine administration can produce reductions in food intake and subsequent weight loss (Kornblith and Hoebel 1976; Leibowitz 1975; Wellman 1992), which could conceivably alter the distribution of sign-vs. goal-tracking. However, it is unlikely that such effects account for the observed results, as there were no differences in body weight between amphetamine-exposed and saline control rats. Acute amphetamine administration can also cause reductions in sign-tracking similar to those observed here (Messing et al. 1982), although it is not clear whether these effects are due to nonspecific disruptions in ongoing behavior. Importantly, the present results were not likely due to amphetamine-induced changes in general activity levels, as there were no differences in locomotion during either the Pavlovian conditioning task or under baseline conditions in the test for locomotor sensitization.
The finding in experiment 1 that goal-tracking decreased across sessions was unusual (Simon and Setlow 2006); however, similar results have been found in rats that displayed high levels of sign-tracking comparable to those in the present study (Flagel et al. 2007b, 2008a, b). It may be the case that high levels of sign-tracking (particularly in the presence of a discrete and accessible visual CS such as that used here and by Flagel and colleagues) preclude robust goal-tracking behavior due to response competition. In support of this idea, amphetamine-exposed rats in experiment 2 (which showed the lowest levels of sign-tracking across both experiments) did show an increase in goal-tracking across sessions. Importantly, the significant main effect of CS type on the goal-tracking measure in experiment 1 (Fig. 1b) indicates that this behavior was associative in nature.
Decreased sign-tracking and increased goal-tracking in amphetamine-exposed rats was evident as a shift in the relative frequencies of these two behaviors. This shift was revealed by significant differences between drug conditions in the relative proportions of the two behaviors [the “lever-trough difference” (Boakes 1977)]. Indeed, amphetamine exposure shifted the balance of behavior toward greater goal-tracking in both experiments 1 and 2, despite large differences between experiments in the levels of the two behaviors in saline controls. Of note, however, is the fact that in addition to spending more time than saline controls in the food trough during the CSs, amphetamine-exposed rats also spent more time in the food trough during intertrial intervals. This difference could be due to nonspecific effects of amphetamine exposure on food trough entries. However, several lines of evidence argue against this interpretation. First, the fact that amphetamine exposure did not affect locomotion during the intertrial intervals argues against a general behavioral activating effect of amphetamine. Second, there were no significant correlations between food trough entry during the pre-CS and CS periods in experiment 1 (although they were present in experiment 2), nor were there any significant correlations between food trough entry during the pre-CS period and sign-tracking in either experiment. These data suggest, at the very least, that the effects of amphetamine on sign-tracking were not due to general drug-induced changes in baseline food trough entry, and additionally, that drug-induced changes in baseline food trough entry do not necessarily account for changes in food trough entry (goal-tracking) during the CSs. Moreover, as suggested above, it may be the case that the greater baseline food trough behavior in amphetamine-exposed rats was due to greater incentive salience attributed to the food trough. In support of this argument, differences between drug groups in intertrial interval food trough behavior did not emerge until the third session of training (similar to the development of such differences in goal-tracking during the CS), suggesting that these differences were due to incentive motivational and/or learning factors, rather than to nonspecific drug effects.
The finding that amphetamine-exposed rats showed sensitization to the locomotor stimulant effects of acute amphetamine is consistent with the idea that the observed alterations in the pattern of conditioned behavior resulted from incentive sensitization, as locomotor and incentive sensitization are thought to be mediated by similar neural adaptations (Robinson and Berridge 2001) (although notably there were no correlations between locomotor sensitization and measures of sign- or goal-tracking). An alternative (or complementary) explanation arises from the observation that lesions of orbitofrontal cortex reduce sign-tracking behavior (Chudasama and Robbins 2003). As orbitofrontal cortex function can be compromised by chronic psychostimulant exposure (Schoenbaum et al. 2004; Schoenbaum and Setlow 2005; Schoenbaum and Shaham 2008), the effects of amphetamine in the current experiments may have been due in part to effects on this structure. Although this interpretation is consistent with the idea that drugs of abuse may simultaneously both enhance incentive salience attribution and impair prefrontal cortical-mediated behavioral control (Everitt et al. 2007; Jentsch and Taylor 1999), more work is needed to dissociate the relative contributions of these two mechanisms to the changes in sign- and goal-tracking resulting from amphetamine exposure.
Acknowledgements
We thank the Drug Supply Program at the National Institute on Drug Abuse for kindly providing D-amphetamine sulfate, and Dr. Jim Grau and three anonymous reviewers for comments on the manuscript. This work is supported by NIH DA018764 and the Office of the Vice President for Research at Texas A&M University (BS), NIH DA023331 (NWS), and NIH MH65728 (IAM). We have no conflicts of interest regarding the contents of this manuscript.
References
- Baunez C, Amalric M, Robbins TW (2002) Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. J Neurosci 22:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC (2001) Reinforcement, incentives and expectations In: DL Medin (ed) Psychology of Learning and Motivation. Academic Press, pp 223–278 [Google Scholar]
- Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 191:391–431 [DOI] [PubMed] [Google Scholar]
- Boakes RA (1977) Performance on learning to associate a stimulus with positive reinforcement In: Davis H, Hurwitz H (eds) Operant-Pavlovian Interactions. L. Erlbaum Associates, pp 67–97 [Google Scholar]
- Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE (2008) Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM (1968) Auto-shaping of the pigeon’s key-peck. J Exp Anal Behav 11:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26:321–352 [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW (2003) Dissociable contributions of the orbitofrontal and infralimbic cortex to Pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci 23: 8771–8780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Patel P (2007) Rapid induction of Pavlovian approach to an ethanol-paired visual cue in mice. Psychopharmacology (Berl) 192:31–41 [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ (2001) Differential involvement of NMDA, AMPA/Kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of Pavlovian approach behavior. J Neurosci 21: 9471–9477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Hutcheson DM, Ersche KD, Pelloux Y, Dalley JW, Robbins TW (2007) The orbital prefrontal cortex and drug addiction in laboratory animals and humans. Ann NY Acad Sci 1121:576–597 [DOI] [PubMed] [Google Scholar]
- Ferrario C, Robinson TE (2007) Amphetamine pretreatment accelerates the subsequent escalation of cocaine self-administration behavior. Eur Neuropsychopharmacol 17:352–357 [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clinton SM, Watson SJ, Robinson TE, Akil H (2007a) Rats selectively bred on the basis of a novelty-seeking trait exhibit individual differences in the propensity to approach signals vs. goals: implications for addiction. Soc Neurosci Abstr [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H (2007b) Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 191:599–607 [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE (2008a) Individual differences in the attribution of incentive salience to reward-related cues: implications for addiction. Neuropharmacology (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Akil H, Robinson TE (2008b) Individual differences in the attribution of incentive salience to a reward-related cue: influence on cocaine sensitization. Behav Brain Res 186:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Mandyam CD, Wee S, Koob GF (2008) Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 33:2474–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD (1998) Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol 9:299–308 [PubMed] [Google Scholar]
- Holland PC (1977) Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. J Exp Psychol: Animal Behav Process 3:77–104 [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S (1992) Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology (Berl) 107:271–276 [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 146:373–390 [DOI] [PubMed] [Google Scholar]
- Karpicke J (1978) Directed approach responses and positive conditioned suppression in the rat. Animal Learn Behav 6:216–224 [Google Scholar]
- Kornblith CL, Hoebel BG (1976) A dose-response study of anorectic drug effects on food intake, self-stimulation, and stimulationescape. Pharmacol Biochem Behav 5:215–218 [DOI] [PubMed] [Google Scholar]
- Leibowitz SF (1975) Catecholaminergic mechanisms of the lateral hypothalamus: their role in the mediation of amphetamine anorexia. Brain Res 98:529–545 [DOI] [PubMed] [Google Scholar]
- Mendez IA, Williams MT, Bhavsar A, Lu A, Bizon JL, Setlow B (2007) Amphetamine exposure and long-lasting sensitization of reward-directed behavior. Soc Neurosci Abstr [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez IA, Montgomery KS, LaSarge CL, Simon NW, Bizon JL, Setlow B (2008) Long-term effects of prior cocaine exposure on Morris water maze performance. Neurobiol Learn Mem 89:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing RB, Portoghese PS, Takenori AE, Sparber SB (1982) Antagonism of morphine-induced behavioral suppression by opiate receptor alkylators. Pharmacol Biochem Behav 16:621–626 [DOI] [PubMed] [Google Scholar]
- Nocjar C, Panksepp J (2002) Chronic intermittent amphetamine pretreatment enhances future appetitive behavior for drug- and natural-reward: interactions with environmental variables. Behav Brain Res 128:189–203 [DOI] [PubMed] [Google Scholar]
- Nordquist RE, Voorn P, de Mooij-van Malsen JG, Joosten RN, Pennartz CM, Vanderschuren LJ (2007) Augmented reinforcer value and accelerated habit formation after repeated amphetamine treatment. Eur Neuropsychopharmacol 17:532–540 [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Krueger DD, Tronson NC, Nairn AC, Taylor JR (2007) Orbitofrontal cortex and cognitive-motivational impairments in psychostimulant addiction: evidence from experiments in the non-human primate. Ann NY Acad Sci 1121:610–638 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of drug-addiction. Brain Res Brain Res Rev 18:247–291 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2001) Incentive-sensitization and addiction. Addiction 96:103–114 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (2003) Addiction. Annu Rev Psychol 54: 25–53 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B (2005) Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb Cortex 15:1162–1169 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Shaham Y (2008) The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol Psychiatry 63:256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Ramus SJ, Shaham Y, Saddoris MP, Setlow B (2004) Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci 19:1997–2002 [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA (2006) Orbitofrontal cortex, decision making, and drug-addiction. Trends Neurosci 29:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva FJ, Silva KM, Pear JJ (1992) Sign-versus goal-tracking: effects of conditioned-stimulus-to-unconditioned-stimulus distance. J Exp Anal Behav 57:17–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon NW, Setlow B (2006) Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: implications for drug addiction. Neurobiol Learn Mem 86:305–310 [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B (2007) Cocaine exposure causes long term increases in impulsive choice. Behav Neurosci 121:543–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD (2001) Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine, and 3,4-methylenedioxyamphetamine (“ecstasy”). Biol Psychiatry 50:137–143 [DOI] [PubMed] [Google Scholar]
- Tindell AJ, Berridge KC, Zhang J, Pecina S, Aldridge JW (2005) Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur J Neurosci 22: 2617–2634 [DOI] [PubMed] [Google Scholar]
- Tomie A, Aguado AS, Pohorecky LA, Benjamin D (2000) Individual differences in Pavlovian autoshaping of lever pressing in rats predict stress-induced corticosterone release and mesolimbic levels of monoamines. Pharmacol Biochem Behav 65:509–517 [DOI] [PubMed] [Google Scholar]
- Tomie A, Grimes KL, Pohorecky LA (2008) Behavioral characteristics and neurobiological substrates shared by Pavlovian sign-tracking and drug abuse. Brain Res Brain Res Rev 58:121–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Robinson TE (2006) Subthalamic nucleus lesions increase impulsive action and decrease impulsive choice - mediation by enhanced incentive motivation? Eur J Neurosci 24: 2345–2354 [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Yang P, Robinson TE (2005) Subthalamic nucleus lesions enhance the psychomotor-activating, incentive motivational, and neurobiological effects of cocaine. J Neurosci 25:8407–8415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Dell’Orco JM, Pevzner A, Robinson TE (2008) The influence of subthalamic nucleus lesions on sign-tracking to stimuli paired with food and drug rewards: facilitation of incentive salience attribution? Neuropsychopharmacology 33:2352–2361 [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Donne Schmidt E, De Vries TJ, Van Moorsel CAP, Tilders FJH, Schoffelmeer ANM (1999) A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neurosci 19:9579–9586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman EA, McCracken SB (1974) The disruption of autosphaped key pecking by food-tray illumination. J Expl Anal Behav 22:39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman PJ (1992) Overview of adrenergic anorectic agents. Am J Clin Nutr 55:193S–198S [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC (2001) Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J Neurosci 21:7831–7840 [DOI] [PMC free article] [PubMed] [Google Scholar]


