Key Points
Question
Is myocardial injury, detected by troponin elevation, associated with mortality and higher cardiovascular and noncardiovascular complications in patients hospitalized with coronavirus disease 2019?
Findings
In this multicenter, cross-sectional study of 614 White Italian patients hospitalized with coronavirus disease 2019, elevated troponin values were associated with higher mortality and a greater risk of cardiovascular and noncardiovascular complications.
Meaning
In this study of patients with coronavirus disease 2019, elevated troponin levels on admission are associated with increased risk of in-hospital death and complications.
This cohort study of patients with coronavirus disease 2019 who were hospitalized in cardiology units in Italy assesses the association of troponin levels at admission with patient outcomes.
Abstract
Importance
Myocardial injury, detected by elevated plasma troponin levels, has been associated with mortality in patients hospitalized with coronavirus disease 2019 (COVID-19). However, the initial data were reported from single-center or 2-center studies in Chinese populations. Compared with these patients, European and US patients are older, with more comorbidities and higher mortality rates.
Objective
To evaluate the prevalence and prognostic value of myocardial injury, detected by elevated plasma troponin levels, in a large population of White Italian patients with COVID-19.
Design, Setting, and Participants
This is a multicenter, cross-sectional study enrolling consecutive patients with laboratory-confirmed COVID-19 who were hospitalized in 13 Italian cardiology units from March 1 to April 9, 2020. Patients admitted for acute coronary syndrome were excluded. Elevated troponin levels were defined as values greater than the 99th percentile of normal values.
Main Outcomes and Measures
Clinical characteristics and outcomes stratified as elevated or normal cardiac troponin levels at admission, defined as troponin T or troponin I at a level greater than the 99th percentile of normal values.
Results
A total of 614 patients with COVID-19 were included in this study (mean age [SD], 67 [13] years; 70.8% male), of whom 148 patients (24.1%) died during the hospitalization. Elevated troponin levels were found in 278 patients (45.3%). These patients were older (mean [SD] age, 64.0 [13.6] years vs 71.3 [12.0] years; P < .001) and had higher prevalence of hypertension (168 patients [50.5%] vs 182 patients [65.9%]; P < .001), heart failure (24 [7.2%]; 63 [22.8%]; P < .001), coronary artery disease (50 [15.0%] vs 87 [31.5%]; P < .001), and atrial fibrillation (33 [9.9%] vs 67 [24.3%]; P < .001). Elevated troponin levels were associated with an increased in-hospital mortality (37% vs 13%; HR, 1.71 [95% CI, 1.13-2.59]; P = .01 via multivariable Cox regression analysis), and this was independent from concomitant cardiac disease. Elevated troponin levels were also associated with a higher risk of in-hospital complications: heart failure (44 patients [19.2%] vs 7 patients [2.9%]; P < .001), sepsis (31 [11.7%] vs 21 [6.4%]; P = .03), acute kidney failure (41 [20.8%] vs 13 [6.2%]; P < .001), multiorgan failure (21 [10.9%] vs 6 [2.9%]; P = .003), pulmonary embolism (27 [9.9%] vs 17 [5.2%]; P = .04), delirium (13 [6.8%] vs 3 [1.5%]; P = .02), and major bleeding (16 [7.0%] vs 4 [1.6%]; P = .008).
Conclusions and Relevance
In this multicenter, cross-sectional study of Italian patients with COVID-19, elevated troponin was an independent variable associated with in-hospital mortality and a greater risk of cardiovascular and noncardiovascular complications during a hospitalization for COVID-19.
Introduction
Troponin levels are a well-established marker of myocardial injury.1 Early studies2,3,4,5 in patients with coronavirus disease 2019 (COVID-19) have demonstrated that elevated plasma troponin levels were common and associated with a more severe clinical course and higher mortality. However, these studies were mostly based on Chinese patients, were limited to 1 or 2 centers, and/or had a small sample size.2,3,4,5 Since COVID-19 reports from other countries are characterized by an older patient age, more comorbidities, and higher risk of death than those from China,6,7,8 the importance of elevated troponin levels in non-Chinese populations needs further investigation.
In Italy, the COVID-19 outbreak caused a major reorganization of the health care system in pandemic areas, with cardiology units admitting patients with COVID-19 almost exclusively, mostly those with associated cardiac disease.6,8,9 The old age of and the high burden of comorbidities in Italian patients account for their higher risk of death and complications.6,8 This population has therefore ideal characteristics to analyze the association of myocardial injury with the outcomes of patients with COVID-19. In this study, we investigated the prevalence and prognostic implications of myocardial injury, detected by elevated troponin levels, in consecutive patients with COVID-19 who were hospitalized in 13 cardiology units in Italy.
Methods
Study Population
This is a multicenter, cross-sectional study enrolling consecutive patients with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 infection who were referred to 13 Italian cardiology units from March 1 to April 9, 2020 (list of centers and investigators in the eAppendix in the Supplement). We included patients hospitalized with a laboratory-confirmed diagnosis of COVID-19 and high-sensitivity plasma troponin levels, either troponin I or troponin T, measured within 24 hours from the time of COVID-19 diagnosis. Patients hospitalized with a diagnosis of acute coronary syndrome were excluded.
Diagnoses of COVID-19 were made by real-time reverse transcriptase–polymerase chain reaction assays of nasal and pharyngeal swabs. Real-time reverse transcriptase–polymerase chain reaction assays of lower respiratory tract aspirates were also performed when indicated. Patients were followed up after the COVID-19 diagnosis, and all causes of in-hospital mortality or discharge were ascertained until April 23, 2020. This study complied with the Declaration of Helsinki and was approved by the ethical committee of Spedali Civili di Brescia, Brescia, Italy, and each recruiting center. This is a retrospective observational study. As such, a waiver for consent was granted by local ethics committees, provided the informed consent was collected at the follow-up visit for the patients who were still alive.
Patients’ data were extracted from the in-hospital medical records. Cardiac injury was defined by plasma levels of high-sensitivity troponin, either troponin T or troponin I, greater than the 99th percentile of normal values, as per manufacturer indications.
Statistical Analysis
Data are presented stratified by troponin level at admission. Comparisons between 2 independent groups were made, respectively, using t tests for normally distributed continuous variables, Wilcoxon tests for nonnormally distributed ones, and χ2 tests for proportions. For all variables with at least 1 expected count less than 5, Fisher exact tests instead of χ2 tests were used. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline checklist for data reporting. Accordingly, we showed the number of nonmissing values for each variable.
Cumulative incidence functions of death were computed, taking into account hospital discharge as a competing event. Comparison of cumulative incidence functions among subgroups was performed by means of the Gray test. Variables clinically relevant and significantly associated with the risk of death at the univariable analysis were tested in a multiple Cox regression model to identify independent risk factors using a complete-case approach (ie, observations of participants with missing data were omitted). The hazard ratios (HRs), 95% CIs, and P values from a Wald test are reported. A 2-tailed P value less than .05 was considered statistically significant. Statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc).
Results
In this analysis, 614 patients were included (mean age [SD], 67 [13] years; 435 male patients [70.8%]), of whom 148 patients (24.1%) died during a median hospital stay of 13 (interquartile range, 8-23) days. Elevated troponin levels were found in 278 of the 614 patients studied (45.3%). Compared with those with normal values, patients with increased troponin levels were older (mean [SD] age, 64.0 [13.6] years vs 71.3 [12.0] years; P < .001), had higher prevalence of cardiac comorbidities (hypertension: 168 patients [50.5%] vs 182 patients [65.9%]; P < .001, heart failure: 24 [7.2%] vs 63 [22.8%]; P < .001; coronary artery disease: 50 [15.0%] vs 87 [31.5%]; P < .001; atrial fibrillation: 33 [9.9%] vs 67 [24.3%]; P < .001), had lower left ventricular ejection fraction (median [interquartile range], 58.0% [53.8%-61.0%] vs 55.0% [40.0%-58.0%]; P < .001), and were more likely to be treated with angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) (110 patients [35.3%] vs 114 patients [44.0%]; P = .04), anticoagulants (29 patients [9.4%] vs 55 patients [21.7%]; P < .001), and statins (68 patients [21.7%] vs 101 patients [39.1%]; P < .001) (Table). Laboratory examinations (with values reported as median [interquartile ranges]) showed lower lymphocytes (973 [630-1400] cells/μL vs 880 [600-1140] cells/μL; P = .008 [to convert to cells × 109 per liter, multiply by 0.001]) and higher C-reactive protein (46 [13-118] mg/dL vs 70 [18-160] mg/dL; P = .007 [to convert to milligrams per liter, multiply by 10]), procalcitonin (0.13 [0.06-0.40] ng/mL; 0.30 [0.10-3.00] ng/mL; P < .001), D-dimer (789 [426-1699] μg/mL vs 1038 [496-3299] μg/mL; P = .02 [to convert to nanomoles per liter, multiply by 5.476]), creatinine (0.92 [0.75-1.11] mg/dL vs 1.12 [0.89-1.67] mg/dL; P < .001 [to convert to micromoles per liter, multiply by 88.4]), and N-terminal pro-brain natriuretic peptide (204 [85-554] pg/mL vs 882 [196-3170] pg/mL; P < .001) plasma levels in the patients with elevated troponin (eTable 1 in the Supplement). Factors associated with elevated troponin levels were estimated glomerular filtration rate (odds ratio [OR] per 10 mL/min, 0.82 [95% CI, 0.76-0.88]; P < .001), serum sodium (OR per 1 mEq/L, 1.06 [95% CI, 1.02-1.10]; P = .004 [to convert to millimoles per liter, multiply by 1.0]), C-reactive protein (OR per 1 mg/dL, 1.03 [95% CI, 1.01-1.05]; P = .002), a history of heart failure (OR, 2.01 [95% CI, 1.07-3.79]; P = .03), a history of coronary artery disease (OR, 2.04 [95% CI, 1.27-3.29]; P = .003), and prior anticoagulant therapy (OR, 2.01 [95% CI, 1.16-3.50]; P = .01) (eTable 2 in the Supplement).
Table. Demographic and Clinical Characteristics of the Study Population at Admission Stratified by Baseline Troponin Level (N = 614).
| Characteristic | Troponin | P value | |||
|---|---|---|---|---|---|
| Normal (n = 336) | Elevated (n = 278) | ||||
| No. Assessed | No. Affected (%) | No. Assessed | No. Affected (%) | ||
| Age, mean (SD), y | 336 | 64.0 (13.6) | 278 | 71.3 (12.0) | <.001 |
| Male | 336 | 234 (69.6) | 278 | 201 (72.3) | .53 |
| BMI ≥30 | 262 | 51 (19.5) | 213 | 46 (21.6) | .65 |
| Smoker (ever) | 292 | 78 (26.7) | 224 | 73 (32.6) | .18 |
| Hypertension | 333 | 168 (50.5) | 276 | 182 (65.9) | <.001 |
| Dyslipidemia | 333 | 71 (21.3) | 275 | 106 (38.5) | <.001 |
| Diabetes | 333 | 66 (19.8) | 276 | 82 (29.7) | .006 |
| Heart failure | 333 | 24 (7.2) | 276 | 63 (22.8) | <.001 |
| Atrial fibrillation | 333 | 33 (9.9) | 276 | 67 (24.3) | <.001 |
| Coronary artery disease | 333 | 50 (15.0) | 276 | 87 (31.5) | <.001 |
| Prior cardiac surgery or percutaneous valve treatment | 333 | 27 (8.1) | 276 | 39 (14.1) | .03 |
| Prior heart transplant/LVAD | 333 | 0 (0.0) | 276 | 4 (1.4) | .09 |
| COPD | 333 | 27 (8.1) | 276 | 31 (11.2) | .24 |
| Chronic kidney disease with eGFR <60 mL/min/m2 | 333 | 34 (10.2) | 276 | 76 (27.5) | <.001 |
| Prior ACEi or ARB therapy | 312 | 110 (35.3) | 259 | 114 (44.0) | .04 |
| Prior anticoagulant therapy | 309 | 29 (9.4) | 254 | 55 (21.7) | <.001 |
| Prior statin therapy | 313 | 68 (21.7) | 258 | 101 (39.1) | <.001 |
| Temperature, mean (SD), °C | 332 | 37.3 (1.0) | 269 | 37.2 (1.0) | .40 |
| Temperature ≥37.5 °C | 332 | 151 (45.5) | 269 | 111 (41.3) | .34 |
| Respiratory rate ≥22 breaths/min | 277 | 141 (50.9) | 176 | 107 (60.8) | .049 |
| Blood pressure, mean (SD), mm Hg | |||||
| Systolic | 330 | 129 (20) | 271 | 129 (24) | .97 |
| Diastolic | 330 | 75 (12) | 271 | 73 (15) | .06 |
| Heart rate, mean (SD), beats/min | 328 | 86 (16) | 272 | 87 (20) | .60 |
| Oxygen saturation, ambient air, median (IQR), % | 329 | 93 (88-96) | 271 | 92 (87-96) | .03 |
| Pao2/FiO2, median (IQR), mm Hg/% | 298 | 246 (127-319) | 238 | 233 (120-310) | .29 |
| Pao2/FiO2 <300 mm Hg/% | 298 | 207 (69.5) | 238 | 170 (71.4) | .69 |
| SOFA score | |||||
| Median (IQR) | 194 | 2 (1-3) | 188 | 3 (2-4) | <.001 |
| ≥3 | 194 | 70 (36.1) | 188 | 103 (54.8) | <.001 |
| ≥6 | 194 | 9 (4.6) | 188 | 24 (12.8) | .008 |
| COVID-19 score peak, median (IQR) | 64 | 4.5 (1.0-10.3) | 104 | 10.0 (3.8-14.0) | <.001 |
| LV ejection fraction, median (IQR), % | 104 | 58.0 (53.8-61.0) | 131 | 55.0 (40.0-58.0) | <.001 |
Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; FiO2, fraction of inspired oxygen; IQR, interquartile range; LV, left ventricular; LVAD, left ventricular assist device; Pao2, oxygen partial pressure at arterial gas analysis; SOFA, sequential organ failure assessment.
Elevated Troponin Levels at the Time of Admission and In-Hospital Outcomes
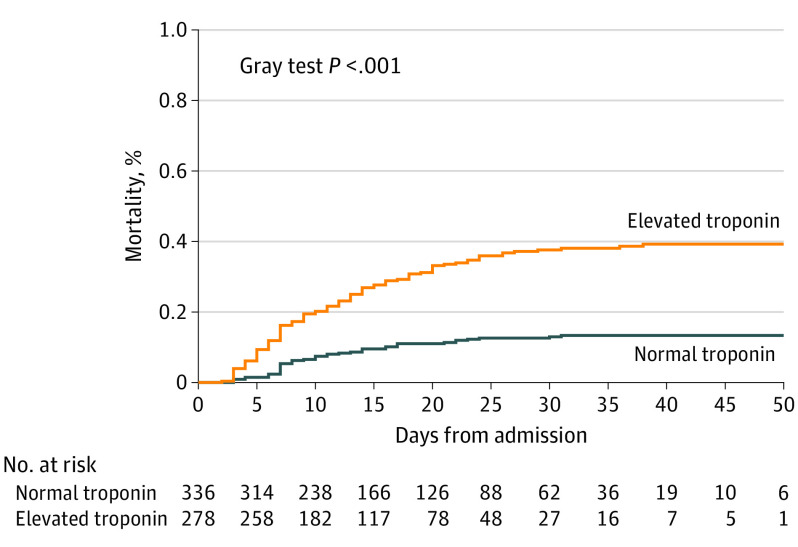
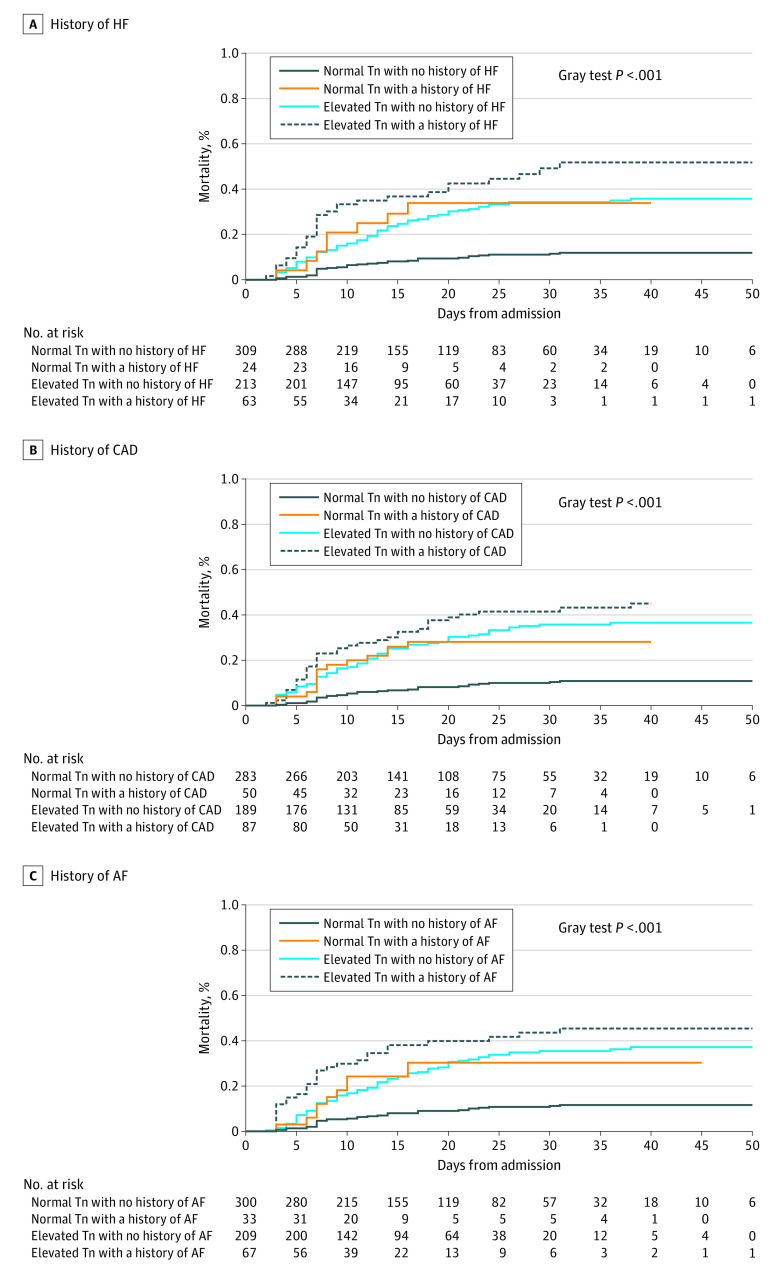
Elevated serum troponin was associated with increased in-hospital death (104 events [37.4%] vs 44 events [13.1%]; P < .001; Figure 1; eTable 3 in the Supplement). The mortality rate was lowest in the patients with no history of cardiac disease and low troponin levels, intermediate in those with either preexisting cardiac disease (hazard ratios [HRs]: heart failure [HF], 3.49 [95% CI, 1.62-7.53]; coronary artery disease [CAD], 2.96 [95% CI, 1.57-5.58]; atrial fibrillation [AF], 3.59 [95% CI, 1.77-7.29]) or elevated troponin levels (HRs: HF, 3.27 [95% CI, 2.19-4.88]; CAD, 3.60 [95% CI, 2.34-5.55]; AF, 3.49 [95% CI, 2.32-5.24]), and highest in those with both elevated troponin levels and one of the major cardiac comorbidities (HRs: HF, 5.28 [95% CI, 3.25-8.58], CAD, 5.22 [95% CI, 3.22-8.47]; AF, 5.09 [95% CI, 3.09-8.36]) (Figure 2). The association of elevated troponin levels and mortality remained significant after adjustment for comorbidities (adjusted HR, 1.71 [95% CI, 1.13-2.59]; P = .01) (eTable 4 in the Supplement). Exploratory analyses were performed when the percentage of complete-case observations was less than 80% (eTables 5, 6, and 7 in the Supplement). The HRs associated with elevated level of troponin remained similar to the HRs estimated in the main analysis when adjusted for lymphocytes and respiratory rate (n = 334, adjusted HR, 1.65 [95% CI, 1.03-2.62]; P = .04) and prior ACEi or ARB therapy and prior statin therapy (n = 473; adjusted HR, 1.61 [95% CI, 1.05-2.47]; P = .03) and similar but nonsignificant when adjusted for lymphocytes, respiratory rate, prior ACEi or ARB therapy, and prior statin therapy (n = 299; adjusted HR, 1.50 [95% CI, 0.92-2.45]; P = .11).
Figure 1. Cumulative Incidence of Death During Hospitalization Stratified by Baseline Troponin Level (N = 614).
Figure 2. Cumulative Incidence of Death During Hospitalization Stratified by Baseline Troponin Level and Histories of Heart Failure (HF) (A), Coronary Artery Disease (CAD) (B), Atrial Fibrillation (AF) (C) (n = 609).
Tn indicates troponin.
Elevated troponin levels were also associated with a complicated clinical course. Significant differences between patients with increased vs those with normal troponin levels were found for sepsis (31 patients [11.7%] vs 21 patients [6.4%]; P = .03), acute kidney failure (41 [20.8%] vs 13 [6.2%]; P < .001), multiorgan failure (21 [10.9%] vs 6 [2.9%]; P = .003), pulmonary embolism (27 [9.9%] vs 17 [5.2%]; P = .04), delirium (13 [6.8%] vs 3 [1.5%]; P = .02), and major bleeding (16 [7.0%] vs 4 [1.6%]; P = .008), as well as heart failure (44 [19.2%] vs 7 [2.9%]; P < .001) and non–ST-elevation myocardial infarction (16 [7.0%] vs 1 [0.4%]; P < .001) (eTable 3 in the Supplement).
Discussion
Our results show that myocardial injury, detected by high troponin levels, is present in a high proportion of patients hospitalized for COVID-19 and independently associated with mortality and cardiovascular and noncardiovascular complications. Elevated troponin levels were found in 45.3% of the patients and were associated with a 71% increase in the risk of in-hospital death and with a more than 2-fold increase in major complications, including sepsis, acute kidney failure, multiorgan failure, pulmonary embolism, and major bleeding. The incidence of heart failure and non–ST-elevation acute myocardial infarction were more than 6-fold in the patients with increased troponin levels compared with others.
To our knowledge, this is the first report regarding the clinical significance of elevated troponin levels in a large, multicenter cohort of consecutive White Italian patients who were admitted for COVID-19. Our results confirm and expand those observed in smaller, mostly single-center studies from Chinese patients,2,3,4,5 as well as the more diverse, multiethnic population of New York, New York.10 Compared with these studies, the prevalence of elevated troponin levels in this population was higher. This can be explained by the older mean age and high prevalence of cardiac comorbidities of this patient group. Accordingly, kidney function, history of heart failure and coronary artery disease, and oral anticoagulation, a variable associated with a history of atrial fibrillation, were independently associated with elevated troponin levels by multivariable logistic regression analysis. The older age and higher prevalence of comorbidities, compared with the Chinese series, were consistent with the characteristics of patients with COVID-19 from Europe and the United States.7,8,9
The mechanisms underlying cardiac involvement in COVID-19 are multiple and not entirely clear.11,12,13,14 They may include nonspecific mechanisms attributable to systemic infection, respiratory failure, and hypoxemia, as well as myocardial injury caused by the systemic inflammatory response.11,12,13,14 It has been hypothesized that one of the pivotal consequences of COVID-19 is an abnormal immune host response.12 Consistently, patients with myocardial injury have a more marked inflammatory response with higher C-reactive protein, D-dimer, fibrinogen, and procalcitonin levels in both this study and previous studies.2,4 It may therefore be hypothesized that increased troponin levels are the expression of the involvement of different organs and tissues, such as can be expected in a general hyperinflammatory disorder.12,14
Our study provides new data that may be of clinical utility in managing patients with COVID-19. Measurement of troponin levels at the time of hospital admission for COVID-19 might be included in the diagnostic workup to identify patients at increased risk of worse outcome and those who may require more intensive treatment. Whether the detection of myocardial injury is associated with abnormalities in cardiac structure and function has not been described. Only a small percentage of these patients underwent echocardiography, and their follow-up was too short to be able to determine whether troponin elevation had an association with cardiac function and postdischarge events, including heart failure.
Limitations
The main limitation of our study is the analytic accuracy of the prognosticative value of troponin, given the different assays used at each hospital. Because of the different assays used, we could only categorize these patients as those with normal or elevated plasma troponin levels, without the possibility of assessing the association between different troponin values and outcomes. Given the logistical limitations during this emerging outbreak, other laboratory data, such as natriuretic peptides, were not collected in all patients. Similarly, echocardiographic data were not collected routinely in most of the patients.
Conclusions
In conclusion, elevated plasma troponin levels were found in up to 45% of patients admitted for COVID-19 in Italian cardiology units. Elevated troponin levels on admission were associated with higher rates of in-hospital complications and in-hospital mortality, and these associations were independent from concomitant cardiac disease and other baseline variables. Diagnostic workup including markers of myocardial injury may be helpful to stratify patients with COVID-19 at hospital admission, so that patients in need of more intensive care may be identified.
eAppendix. List of Centers and Investigators.
eTable 1. Laboratory findings of the study population at admission stratified by baseline troponin level (N=614).
eTable 2. Multivariable logistic regression model for elevated level of troponin (N=533).
eTable 3. In-hospital management and outcomes of the study population stratified by baseline troponin level (N=614).
eTable 4. Univariable and multivariable Cox regression model for death.
eTable 5. Univariable and multivariable Cox regression model for death, including lymphocytes and respiratory rate.
eTable 6. Univariable and multivariable Cox regression model for death, including lymphocytes, respiratory rate, prior ACEi/ARB therapy and prior statin therapy.
eTable 7. Univariable and multivariable Cox regression model, including prior ACEi/ARB therapy and prior statin therapy.
References
- 1.McCarthy CP, Raber I, Chapman AR, et al. . Myocardial injury in the era of high-sensitivity cardiac troponin assays: a practical approach for clinicians. JAMA Cardiol. 2019;4(10):1034-1042. doi: 10.1001/jamacardio.2019.2724 [DOI] [PubMed] [Google Scholar]
- 2.Shi S, Qin M, Shen B, et al. . Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei JF, Huang FY, Xiong TY, et al. . Acute myocardial injury is common in patients with covid-19 and impairs their prognosis. Heart. 2020;heartjnl-2020-317007. doi: 10.1136/heartjnl-2020-317007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo T, Fan Y, Chen M, et al. . Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020. doi: 10.1001/jama.2020.4683 [DOI] [PubMed] [Google Scholar]
- 7.Richardson S, Hirsch JS, Narasimhan M, et al. ; and the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020. doi: 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inciardi RM, Adamo M, Lupi L, et al. . Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41(19):1821-1829. doi: 10.1093/eurheartj/ehaa388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020. doi: 10.1001/jama.2020.4031 [DOI] [PubMed] [Google Scholar]
- 10.Lala A, Johnson KW, Januzzi JL, et al. ; Mount Sinai Covid Informatics Center . Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;S0735-1097(20)35552-2. doi: 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.1286 [DOI] [PubMed] [Google Scholar]
- 12.Liu PP, Blet A, Smyth D, Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142(1):68-78. doi: 10.1161/CIRCULATIONAHA.120.047549 [DOI] [PubMed] [Google Scholar]
- 13.Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020. doi: 10.1001/jamacardio.2020.1105 [DOI] [PubMed] [Google Scholar]
- 14.Tomasoni D, Italia L, Adamo M, et al. . COVID-19 and heart failure: from infection to inflammation and angiotensin II stimulation, searching for evidence from a new disease. Eur J Heart Fail. 2020. doi: 10.1002/ejhf.1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. List of Centers and Investigators.
eTable 1. Laboratory findings of the study population at admission stratified by baseline troponin level (N=614).
eTable 2. Multivariable logistic regression model for elevated level of troponin (N=533).
eTable 3. In-hospital management and outcomes of the study population stratified by baseline troponin level (N=614).
eTable 4. Univariable and multivariable Cox regression model for death.
eTable 5. Univariable and multivariable Cox regression model for death, including lymphocytes and respiratory rate.
eTable 6. Univariable and multivariable Cox regression model for death, including lymphocytes, respiratory rate, prior ACEi/ARB therapy and prior statin therapy.
eTable 7. Univariable and multivariable Cox regression model, including prior ACEi/ARB therapy and prior statin therapy.