Key Points
Question
Is there an association of culprit lesion location with outcomes of culprit-lesion-only percutaneous coronary intervention (PCI) vs immediate multivessel PCI in patients with multivessel disease, myocardial infarction, and cardiogenic shock?
Findings
In this post hoc analysis of a randomized clinical trial, culprit-lesion-only vs immediate multivessel PCI was associated with lower 1-year death if the culprit lesion was located in the left main or proximal left anterior descending artery but not if located in other coronary segments.
Meaning
Patients with multivessel disease, myocardial infarction, and cardiogenic shock may especially benefit from culprit-lesion-only PCI if the culprit lesion is located in the left main or proximal left anterior descending artery.
Abstract
Importance
Myocardial infarction with a culprit lesion located in the left main or proximal left anterior descending artery compared with other coronary segments is associated with more myocardium at risk and worse clinical outcomes.
Objective
To evaluate the association of culprit lesion location with outcomes of culprit-lesion-only percutaneous coronary intervention with optional staged revascularization vs immediate multivessel percutaneous coronary intervention in patients with multivessel disease, myocardial infarction, and cardiogenic shock.
Design, Setting, and Participants
Post hoc analysis of the Culprit Lesion Only Coronary Intervention vs Multivessel Coronary Intervention in Cardiogenic Shock (CULPRIT-SHOCK), an investigator-initiated randomized, open-label clinical trial. Patients with multivessel disease, acute myocardial infarction, and cardiogenic shock were enrolled at 83 European centers from April 2013 through April 2017.
Interventions
Patients were randomized to culprit-lesion-only percutaneous coronary intervention with optional staged revascularization or immediate multivessel percutaneous coronary intervention (1:1). For this analysis, patients were stratified by culprit lesion location in the left main or proximal left anterior descending artery group and other-culprit-lesion location group.
Main Outcomes and Measures
End points included a composite of death or kidney replacement therapy at 30 days and death at 1 year.
Results
The median age of the study population was 70 (interquartile range, 60-78 years) and 524 of the study participants were men (76.4%). Of the 685 patients, 33.4% constituted the left main or proximal left anterior descending artery group and 66.6% the other-culprit-lesion location group. The left main or proximal left anterior descending artery group had worse outcomes compared with the other-culprit-lesion location group (56.8% vs 47.5%; P = .02 for the composite end point at 30 days and 59.8% vs 50.1%; P = .02 for death at 1 year). In both groups, culprit-lesion-only vs immediate multivessel percutaneous coronary intervention was associated with a reduced risk of the composite end point at 30 days (49.1% vs 64.3% and 44.1% vs 50.9%; P for interaction = .27). At 1 year, culprit-lesion-only vs immediate multivessel percutaneous coronary intervention was associated with a significantly reduced risk of death in the left main or proximal left anterior descending artery but not the other-culprit-lesion location group (50.0% vs 69.6%; P = .003 and 49.8% vs 50.4%; P = .89; P for interaction = 0.02).
Conclusions and Relevance
In patients with multivessel disease with myocardial infarction and cardiogenic shock, a culprit lesion located in the left main or proximal left anterior descending artery vs other coronary segments was associated with worse outcomes. These patients may especially benefit from culprit-lesion-only percutaneous coronary intervention with optional staged revascularization, although further investigation is needed to confirm this finding.
Trial Registration
ClinicalTrials.gov Identifier: NCT01927549.
This post hoc analysis of a randomized clinical trial evaluates the association of culprit lesion location with outcomes of culprit-lesion-only percutaneous coronary intervention with optional staged revascularization vs immediate multivessel percutaneous coronary intervention in patients with multivessel disease, myocardial infarction, and cardiogenic shock.
Introduction
Myocardial infarction (MI) complicated by cardiogenic shock (CS) is a life-threatening condition associated with high risk of early mortality.1,2 A culprit lesion (CL) located in the left main (LM) or proximal left anterior descending artery (pLAD), compared with other coronary segments, has been shown to be associated with higher risk for cardiac arrest, cardiogenic shock, and poor short-term and long-term survival.3,4 However, limited data exist on the association of the CL location with outcomes of patients presenting with multivessel coronary artery disease (MVD) and MI complicated by cardiogenic shock.5,6 In addition, it is uncertain whether there is an association of the CL location with outcomes of different revascularization strategies in these patients. The Culprit Lesion Only PCI vs Multivessel PCI in Cardiogenic Shock (CULPRIT-SHOCK) trial has found a significant benefit of culprit-lesion-only percutaneous coronary intervention with optional staged revascularization (CL-PCI) over immediate multivessel PCI (MV-PCI) with regards to risk of death or kidney replacement therapy at 30 days, which was consistent at 1-year follow-up.7,8
In this post hoc analysis of the CULPRIT-SHOCK trial, we aimed to determine short-term and long-term outcomes associated with LM/pLAD CL location vs other CL location and to evaluate the association of CL location with short-term and long-term clinical outcomes of the different revascularization strategies.
Methods
Study Population
The trial design and main results have been published elsewhere.7,8 In brief, CULPRIT-SHOCK was an investigator-initiated, multicenter, randomized, open-label clinical trial comparing CL-PCI with MV-PCI in patients with acute MI complicated by CS. Cardiogenic shock was defined as systemic blood pressure less than 90 mm Hg for at least 30 minutes or dependence on inotropes to maintain a systemic blood pressure of 90 mm Hg or greater, signs of pulmonary congestion, and signs of impaired organ perfusion with at least one of the following: altered mental status, cold, clammy skin, urine output less than 30 mL/h, or arterial lactate levels greater than 18.02 mg/dL (to convert to millimoles per liter, multiply by 0.111). Main trial inclusion criteria were planned early revascularization by means of PCI, MVD (defined as at least 2 major vessels [≥2 mm diameter] with >70% stenosis of the diameter), and an identifiable CL. The study was approved by the ethical committee in Leipzig, Germany, and by regional and national ethics review boards of all participating sites. Written informed consent was obtained with the use of a prespecified process that varied slightly according to country.8 Patients were randomized in a 1:1 fashion to undergo either CL-PCI or MV-PCI. The CL was treated first in all enrolled patients. Implantation of drug-eluting stents was recommended but not mandated. For patients randomized to CL-PCI, staged PCI was recommended on the basis of the patient’s clinical status and the presence of residual ischemia on objective testing.8 In patients from the MV-PCI group, all additional lesions (major coronary arteries with >70% stenosis of the diameter), including chronic total occlusions, were recommended to be treated with PCI immediately after treatment of the CL.
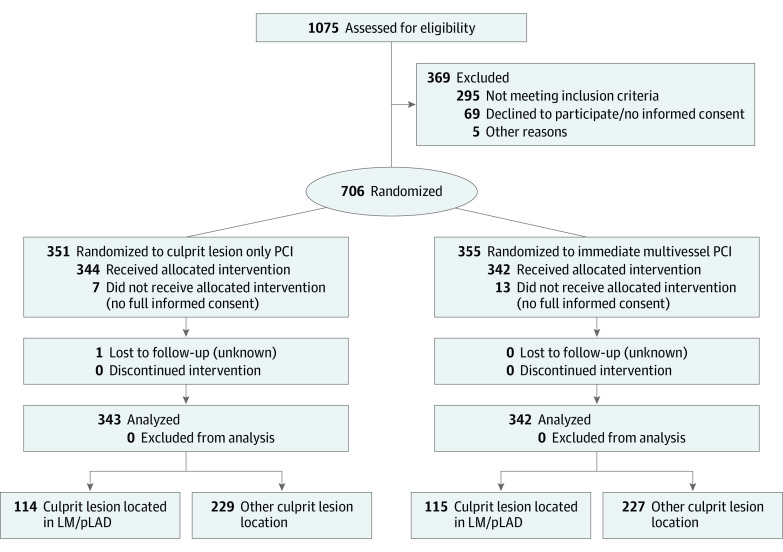
In this analysis, the study population was divided according to CL location in the LM/pLAD group and the other CL location (OCLL) group. With respect to the former, the CL was located either in the LM (ostial, shaft, or distal) or in the pLAD (pLAD was defined as the portion of LAD from the origin from LM up to the first septal perforator or first diagonal artery, whichever comes first). Regarding the latter, the CL was located in any other coronary segment. In each of these groups, the randomly assigned revascularization strategies (CL-PCI vs MV-PCI) were compared (Figure 1).
Figure 1. Study Flowchart.
PCI indicates percutaneous coronary intervention; LM, left main coronary artery; pLAD, proximal left anterior descending artery.
Clinical End Points
The primary end point of this analysis was a composite of death or kidney replacement therapy at 30 days. In addition, death, renal replacement, MI, rehospitalization for heart failure, staged or urgent repeated revascularization, stroke, and bleeding at 30 days and 1 year were evaluated. Furthermore, at 1 year, all-cause death and the composite end points of death or MI; death or rehospitalization for heart failure; and death, MI, or rehospitalization for heart failure were analyzed.
Statistical Analysis
All analyses were performed in the intention-to-treat population. Categorical variables are presented as percentages and were compared with the χ2 test. Continuous variables are presented as median with interquartile range (IQR) and compared with the Mann-Whitney-Wilcoxon test. Outcomes are presented as percentages and were compared by χ2 tests. Kaplan-Meier curves were used to show event rates over time according to group assignment (LM/pLAD vs OCLL and CL-PCI vs MV-PCI, respectively). Odds ratios (ORs) with 95% confidence intervals were generated using logistic regression analysis. Covariates to adjusted differences between CL location groups included history of hypertension, history of previous coronary artery bypass grafting, and TIMI III flow post PCI. Formal interaction testing was performed to evaluate possible interaction between revascularization strategy and CL location. All analyses were conducted with the use of SAS software, version 9.4 (SAS Institute). All tests were 2-sided, and P less than .05 was considered significant.
Results
Between April 2013 and April 2017, 1075 patients were screened for enrollment in the CULPRIT-SHOCK trial and 706 were randomized to either CL-PCI or MV-PCI. Informed consent and 1-year follow-up were available in 685 of these patients and constituted the study population. One patient was lost to follow-up within 30 days. Left main/pLAD CL was documented in 229 patients (33.4%), and OCLL was documented in 456 patients (66.6%) (Figure 1). Patients from the OCLL group had higher prevalence of hypertension and prior coronary artery bypass grafting as well as lower baseline left ventricular ejection fraction compared with the LM/pLAD group (eTable 1 in the Supplement). With regards to procedural characteristics, significantly lower TIMI flow before and after intervention was documented in the LM/pLAD vs the OCLL group. Mechanical circulatory support was significantly more often used in patients with LM/pLAD CL location compared with OCLL (eTable 1 in the Supplement). Baseline clinical and procedural characteristics of patients by randomized treatment allocation within the LM/pLAD group and OCLL group, respectively, are presented in Table 1.
Table 1. Baseline Clinical and Procedural Characteristics of Patients With Left Main or Proximal Left Anterior Descending Artery Culprit Lesion and Other Culprit Lesion Location According to Randomized Treatment Allocation.
| Characteristic | LM/pLAD (n = 229) | Other culprit lesion location (n = 456) | ||||
|---|---|---|---|---|---|---|
| No./total No. (%) | P value | No./total No. (%) | P value | |||
| Culprit-lesion-only PCI (n = 114) | Immediate MV-PCI (n = 115) | Culprit-lesion-only PCI (n = 229) | Immediate MV-PCI (n = 227) | |||
| Age, median (IQR), y | 68 (58-76) | 70 (60-79) | .15 | 71 (61-78) | 70 (60-77) | .17 |
| Male sex | 94/114 (82.5) | 87/115 (75.7) | .21 | 163/229 (71.2) | 180/227 (79.3) | .045 |
| Current smoking | 33/113 (29.2) | 28/110 (25.5) | .53 | 52/221 (23.5) | 61/215 (28.4) | .25 |
| Hypertension | 55/113 (48.7) | 66/111 (59.5) | .11 | 145/225 (64.4) | 140/224 (62.5) | .67 |
| Hypercholesterolemia | 37/112 (33) | 34/111 (30.6) | .70 | 75/225 (33.3) | 82/222 (36.9) | .42 |
| Diabetes mellitus | 30/112 (26.8) | 45/112 (40.2) | .03 | 72/224 (32.1) | 71/223 (31.8) | .95 |
| Family history of coronary artery disease | 14/111 (12.6) | 10/108 (9.3) | .43 | 25/217 (11.5) | 31/213 (14.6) | .35 |
| Previous myocardial infarction | 16/112 (14.3) | 20/112 (17.9) | .47 | 44/226 (19.5) | 33/223 (14.8) | .19 |
| Previous PCI | 19/112 (17) | 16/112 (14.3) | .58 | 45/226 (19.9) | 47/223 (21.1) | .76 |
| Previous CABG | 4/112 (3.6) | 1/112 (0.9) | .17 | 16/228 (7) | 12/225 (5.3) | .46 |
| Previous congestive heart failure | 9/112 (8) | 14/112 (12.5) | .27 | 22/228 (9.6) | 14/224 (6.3) | .18 |
| Previous stroke | 8/112 (7.1) | 12/113 (10.6) | .36 | 20/228 (8.8) | 8/223 (3.6) | .02 |
| Resuscitation before randomization | 62/114 (54.4) | 60/115 (52.2) | .74 | 115/227 (50.7) | 129/227 (56.8) | .19 |
| ST-segment elevation myocardial infarction | 69/111 (62.2) | 64/111 (57.7) | .49 | 136/223 (61) | 145/219 (66.2) | .25 |
| Mean arterial pressure, median (IQR), mm Hg | 75 (63-93) | 79 (63-93) | .59 | 76 (63-91) | 74 (63-95) | .88 |
| Creatinine clearance, median (IQR), mL/min | 64 (45-102) | 61 (39-89) | .19 | 64 (42-90) | 69 (46-94) | .33 |
| Number of affected vessels | ||||||
| 1 | 1/114 (0.9) | 0/115 | .71 | 2/229 (0.9) | 2/227 (0.9) | .72 |
| 2 | 40/114 (35.1) | 39/115 (33.9) | 82/229 (35.8) | 85/227 (37.4) | ||
| 3 | 73/114 (64) | 76/115 (66.1) | 145/229 (63.3) | 140/227 (61.7) | ||
| ≥1 chronic total occlusion | 31/114 (27.2) | 27/115 (23.5) | .52 | 48/229 (21.0) | 51/227 (22.5) | .70 |
| Left ventricular ejection fraction, median (IQR), % | 30 (20-40) | 30 (25-40) | .47 | 35 (28-45) | 30 (20-45) | .37 |
| Arterial access | ||||||
| Femoral | 89/114 (78.1) | 94/115 (81.7) | .49 | 198/229 (86.5) | 183/227 (80.6) | .09 |
| Radial | 26/114 (22.8) | 21/115 (18.3) | .39 | 35/229 (15.3) | 45/227 (19.8) | .20 |
| Brachial | 1/114 (0.9) | 1/115 (0.9) | >.99 | 1/229 (0.4) | 0/227 | .32 |
| TIMI flow III | ||||||
| Before culprit lesion PCI | 20/113 (17.7) | 25/114 (21.9) | .96 | 37/226 (16.4) | 39/223 (17.5) | .40 |
| After culprit lesion PCI | 91/114 (79.8) | 94/114 (82.5) | .71 | 198/228 (86.8) | 199/224 (88.8) | .52 |
| Immediate PCI of additional lesions | 16/114 (14) | 104/115 (90.4) | <.001 | 27/229 (11.8) | 26/227 (90.7) | <.001 |
| Successful complete revascularization | 10/16 (62.5) | 75/103 (72.8) | <.001 | 16/27 (59.3) | 154/204 (75.5) | <.001 |
| Total contrast volume, median (IQR), mL | 200 (147-250) | 250 (200-350) | <.001 | 190 (139-250) | 260 (200-350) | <.001 |
| Total duration of fluoroscopy, median (IQR), min | 12 (8-20) | 19 (12-31) | <.001 | 13 (7-20) | 18 (12-28) | <.001 |
| Mechanical circulatory support | 43/114 (37.7) | 36/115 (31.3) | .31 | 56/229 (24.5) | 59/227 (26) | .71 |
| Mechanical ventilation | 94/114 (82.5) | 96/113 (85) | .61 | 178/229 (77.7) | 186/226 (82.3) | .22 |
| Subsequent medication in those who survived until hospital discharge | ||||||
| Statin | 56/62 (90.3) | 40/42 (95.2) | .36 | 121/133 (91.0) | 110/122 (90.2) | .82 |
| β-Blocker | 57/62 (91.9) | 37/42 (88.1) | .51 | 119/133 (89.5) | 108/122 (88.5) | .81 |
| ACE/ARB | 54/62 (87.1) | 34/42 (81.0) | .39 | 111/133 (83.5) | 93/122 (76.2) | .15 |
| Aspirin | 57/62 (91.9) | 39/42 (92.9) | .67 | 121/133 (91.0) | 116/122 (95.1) | .20 |
| Clopidogrel | 24/62 (38.7) | 18/42 (42.9) | .67 | 53/133 (39.8) | 47/122 (38.5) | .83 |
| Prasugrel | 16/62 (25.8) | 12/42 (28.6) | .76 | 41/133 (30.8) | 38/122 (31.1) | .96 |
| Ticagrelor | 22/62 (35.5) | 11/42 (26.2) | .32 | 46/133 (34.6) | 43/122 (35.2) | .91 |
Abbreviations: ACE/ARB, angiotensin converting enzyme/angiotensin receptor blocker; CABG, coronary artery bypass grafting; IQR, interquartile range; LM, left main coronary artery; MV-PCI, multivessel percutaneous coronary artery intervention; PCI, percutaneous coronary intervention; pLAD, proximal left anterior descending artery.
Outcomes of Patients With LM/pLAD CL Location Compared With OCLL
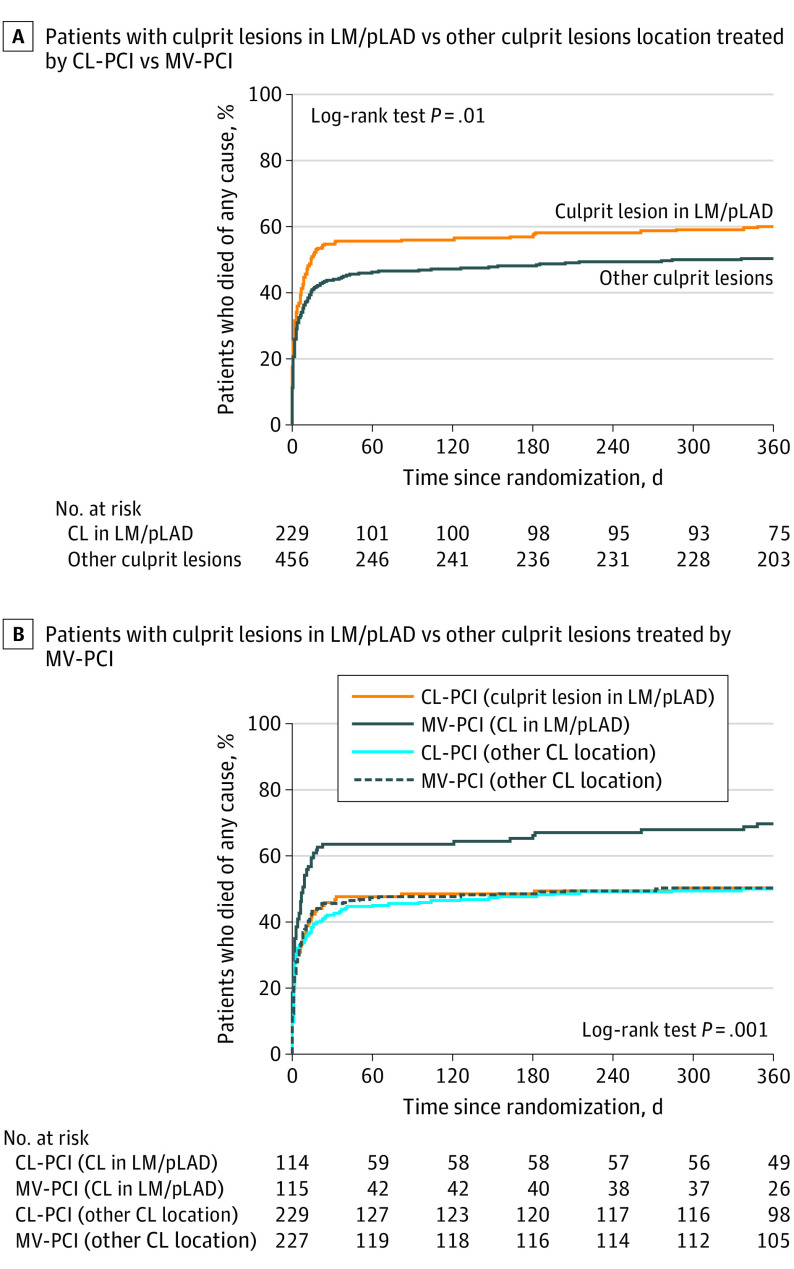
At 30 days, the composite of death or kidney replacement therapy occurred significantly more often in patients with LM/pLAD CL location vs OCLL (56.8% [n = 130 of 229] vs 47.5% [n = 216 of 455]; P = .02). Similarly, the individual end points of death (54.6% [n = 125 of 229] vs 43.7% [n = 199 of 455]; 0.007) and MI (2.6% [n = 6 of 229] vs 0.2% [n = 1 of 455]; P = .003) were increased in the LM/pLAD vs the OCLL group. There were no differences between groups with regards to the individual end points of kidney replacement therapy, stroke, and bleeding at 30 days (Table 2). At 1 year, LM/pLAD CL location vs OCLL was associated with higher risk of death (59.8% [n = 137 of 229] vs 50.1% [n = 228 of 455]; P = .02) and MI (3.5% [n = 8 of 229] vs 1.1% [n = 5 of 455]; P = .03) (Table 2, and Figure 2A).
Table 2. Clinical Outcomes at 30 Days and 1 Year After Randomization in Patients With Left Main or Proximal Left Anterior Descending Artery Culprit Lesion vs Other Culprit Lesion Location.
| Outcome | LM/pLAD, No./total No. (%) (n = 229) | Other culprit lesion location, No./total No. (%) (n = 456) | OR (95% CI) | P value |
|---|---|---|---|---|
| 30 d | ||||
| Primary end point: death from any cause or kidney replacement therapy | 130/229 (56.8) | 216/455 (47.5) | 1.45 (1.06-2.00) | .02 |
| Death | 125/229 (54.6) | 199/455 (43.7) | 1.55 (1.12-2.13) | .007 |
| Kidney replacement therapy | 33/229 (14.4) | 63/455 (13.8) | 1.05 (0.66-1.65) | .84 |
| Myocardial infarction | 6/229 (2.6) | 1/455 (0.2) | 12.22 (1.46-102.1) | .003 |
| Rehospitalization for heart failure | 2/229 (0.9) | 0/455 | NA | .046 |
| Stroke | 8/229 (3.5) | 15/455 (3.3) | 1.06 (0.44-2.54) | .89 |
| Staged or urgent repeated revascularization | 26/229 (11.4) | 60/455 (13.2) | 0.84 (0.52-1.38) | .49 |
| BARC bleeding 2-5 | 51/229 (22.3) | 97/455 (21.3) | 1.06 (0.72-1.55) | .78 |
| 1 y | ||||
| Death | 137/229 (59.8) | 228/455 (50.1) | 1.48 (1.07-2.05) | .02 |
| Kidney replacement therapy | 33/229 (14.4) | 63/455 (13.8) | 1.05 (0.66-1.65) | .84 |
| Myocardial infarction | 8/229 (3.5) | 5/455 (1.1) | 3.26 (1.05-10.1) | .03 |
| Rehospitalization for heart failure | 6/229 (2.6) | 16/455 (3.5) | 0.74 (0.28-1.91) | .53 |
| Stroke | 9/229 (3.9) | 20/455 (4.4) | 0.89 (0.40-1.99) | .78 |
| Staged or urgent repeated revascularization | 42/229 (18.3) | 101/455 (22.2) | 0.79 (0.53-1.18) | .24 |
| Any bleeding | 54/229 (23.6) | 107/455 (23.5) | 1.00 (0.69-1.46) | .99 |
| Death or myocardial infarction | 140/229 (61.4) | 233/455 (51.2) | 1.50 (1.09-2.07) | .01 |
| Death or rehospitalization for heart failure | 142/229 (62.0) | 242/456 (53.2) | 1.44 (1.04-1.99) | .03 |
| Death, myocardial infarction, or rehospitalization for heart failure | 145/229 (63.3) | 247/455 (54.3) | 1.45 (1.05-2.01) | .02 |
Abbreviations: BARC, bleeding academic research consortium; LM, left main coronary artery; NA, not applicable; OR, odds ratio; pLAD, proximal left anterior descending artery.
Figure 2. Kaplan-Meier Curves Comparing 1-Year Mortality.
A, Kaplan-Meier curve comparing 1-year mortality in left main (LM) or proximal left anterior descending artery (pLAD) culprit lesion (CL) location vs other CL location. B, Kaplan-Meier curve comparing 1-year mortality in patients with left main or pLAD CL location vs other CL location treated by culprit-lesion-only percutaneous coronary artery intervention (CL-PCI) with optional staged revascularization vs immediate multivessel percutaneous coronary artery intervention (MV-PCI).
There were no differences between groups with regards to kidney replacement therapy, rehospitalization for heart failure, stroke, and bleeding (Table 2). The composite end points of death or MI (61.4% [n = 56 of 114] vs 51.2% [n = 74 of 115]; P = .01); death or rehospitalization for heart failure (62.0% [n = 140 of 229] vs 53.2% [n = 233 of 455]; P = .03); and death, MI, or rehospitalization for heart failure (63.3% [n = 145 of 229] vs 54.3% [n = 247 of 456]; P = .02) were significantly higher in the LM/pLAD group compared with the OCLL group at 1 year (Table 2).
After adjustment for differences in clinical and procedural characteristics between groups, the risk for death or kidney replacement therapy associated with LM/pLAD CL location vs OCLL was attenuated (OR, 1.37; 95% CI, 0.98-1.90; P = .07) but remained significant for death at 30 days and 1 year (OR, 1.45; 95% CI, 1.04-2.02 and OR, 1.46; 95% CI, 1.05-2.04, respectively) (eTable 2 in the Supplement).
Outcomes of Patients With LM/pLAD CL Location and OCLL by Revascularization Strategy
At 30 days CL-PCI vs MV-PCI was associated with a reduced risk of the composite of death and kidney replacement therapy in the LM/pLAD group (49.1% [n = 56 of 114] vs 64.3% [n = 74 of 115]; P = .02) and in the OCLL group (44.1% [n = 101 of 229] vs 50.9% [n = 115 of 226]; P = .16), with no significant interaction between CL location and revascularization strategy (P for interaction = .27). With respect to individual end points, CL-PCI vs MV-PCI was associated with a reduced risk of death only in the LM/pLAD group (45.6% [n = 52 of 114] vs 63.5% [n = 73 of 115]; P = .007 in the LM/pLAD group and 41.9% [n = 96 of 229] vs 45.6% [n = 115 of 226]; P = .43 in the OCLL group; P for interaction = .07) (Table 3). Staged or urgent repeated revascularization was increased in the CL-PCI vs the MV-PCI group regardless of CL location (Table 3).
Table 3. Clinical Outcomes at 30 Days and 1 Year After Randomization in Patients With Left Main or pLAD Culprit Lesion and Other Culprit Lesion Location According to Randomized Treatment Allocation.
| Outcome | LM/pLAD | Other culprit lesion location | P value for interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No./total No. (%) | OR (95% CI) | P value | No./total No. (%) | OR (95% CI) | P value | ||||
| Culprit lesion-only PCI (n = 114) | Immediate MV-PCI (n = 115) | Culprit lesion-only PCI (n = 229) | Immediate MV-PCI (n = 227) | ||||||
| 30 d | |||||||||
| Primary end point: death from any cause or kidney replacement therapy | 56/114 (49.1) | 74/115 (64.3) | 0.53 (0.32-0.91) | .02 | 101/229 (44.1) | 115/226 (50.9) | 0.76 (0.53-1.10) | .15 | .27 |
| Death | 52/114 (45.6) | 73/115 (63.5) | 0.48 (0.28-0.82) | .007 | 96/229 (41.9) | 103/226 (45.6) | 0.86 (0.59-1.25) | .43 | .07 |
| Kidney replacement therapy | 14/114 (12.3) | 19/115 (16.5) | 0.71 (0.34-1.49) | .36 | 26/229 (11.4) | 37/226 (16.4) | 0.65 (0.38-1.12) | .12 | .87 |
| Myocardial infarction | 4/114 (3.5) | 2/115 (1.7) | 2.1 (0.37-11.45) | .40 | 0/229 | 1/226 (0.4) | NA | .31 | .21 |
| Rehospitalization for heart failure | 1/114 (0.9) | 1/115 (0.9) | 1.0 (0.06-16.33) | 1.0 | 0/229 | 0/226 | NA | NA | NA |
| Stroke | 6/114 (5.3) | 2/115 (1.7) | 3.14 (0.62-15.89) | .15 | 7/229 (3.1) | 8/226 (3.5) | 0.86 (0.31-2.41) | .77 | .18 |
| Staged or urgent repeat revascularization | 24/114 (21.1) | 2/115 (1.7) | 15.1 (3.47-65.45) | <.001 | 50/229 (21.8) | 10/226 (4.4) | 6.03 (2.97-12.24) | <.001 | .26 |
| Any bleeding | 24/114 (21.1) | 27/115 (23.5) | 0.87 (0.47-1.62) | .66 | 43/229 (18.8) | 54/226 (23.9) | 0.74 (0.47-1.16) | .18 | .67 |
| 1 y | |||||||||
| Death | 57/114 (50) | 80/115 (69.6) | 0.44 (0.25-0.75) | .003 | 114/229 (49.8) | 114/226 (50.4) | 0.98 (0.68-1.42) | .89 | .02 |
| Kidney replacement therapy | 14/114 (12.3) | 19/115 (16.5) | 0.71 (0.34-1.49) | .36 | 26/229 (11.4) | 37/226 (16.4) | 0.65 (37/226) | .12 | .87 |
| Myocardial infarction | 5/114 (4.4) | 3/115 (2.6) | 1.71 (0.40-7.34) | .46 | 1/229 (0.4) | 4/226 (1.8) | 0.24 (0.03-2.19) | .17 | .13 |
| Rehospitalization for heart failure | 4/114 (3.5) | 2/115 (1.4) | 2.05 (0.37-11.45) | .40 | 14/229 (6.1) | 2/226 (0.9) | 7.29 (1.64-32.47) | .002 | .26 |
| Stroke | 6/114 (5.3) | 3/113 (2.6) | 2.07 (.51-8.50) | .30 | 9/229 (3.9) | 11/226 (4.9) | 0.80 (0.32-1.97) | .63 | .26 |
| Staged or urgent repeated revascularization | 34/114 (29.8) | 8/115 (7) | 5.68 (2.50-12.94) | <.001 | 77/229 (33.6) | 24/226 (10.6) | 4.26 (2.58-7.06) | <.001 | .56 |
| BARC 2-5 | 26/114 (22.8) | 28/115 (24.3) | 0.92 (0.50-1.69) | .78 | 49/229 (21.4) | 58/226 (25.7) | 0.79 (0.51-1.22) | .28 | .69 |
| Death or myocardial infarction | 59/114 (51.8) | 81/115 (70.4) | 0.45 (0.26-0.78) | .004 | 115/229 (50.2) | 118/226 (52.2) | 0.92 (0.64-1.33) | .67 | .03 |
| Death or rehospitalization for heart failure | 60/114 (52.6) | 82/115 (71.3) | 0.45 (0.26-0.77) | .004 | 126/229 (55.0) | 116/226 (51.3) | 1.16 (0.80-1.68) | .43 | .004 |
| Death, myocardial infarction, or rehospitalization for heart failure | 62/114 (54.4) | 83/115 (72.2) | 0.45 (0.27-0.80) | .005 | 127/229 (55.5) | 120/226 (53.1) | 1.10 (0.76-1.59) | .61 | .01 |
Abbreviations: BARC, Bleeding Academic Research Consortium; LM, left main coronary artery; NA, not applicable; OR, odds ratio; pLAD, proximal left anterior descending artery.
At 1 year, CL-PCI vs MV-PCI resulted in a significantly lower risk of death in patients with LM/pLAD but not in patients with OCLL (50% [n = 57 of 114] vs 69.6% [n = 80 of 115]; P = .003 and 49.8% [n = 114 of 229] vs 50.4% [n = 114 of 226]; P = .89) with significant interaction between CL location and revascularization strategy (P for interaction = .02) (Table 3, Figure 2B). Similarly, the composite end points of death or MI, death or rehospitalization for heart failure and death, MI, or rehospitalization for heart failure were significantly lower in patients from the LM/pLAD group when treated with CL-PCI vs MV-PCI (Table 3). In both LM/pLAD and OCLL groups, staged or urgent repeated revascularization was performed more often in patients assigned to CL-PCI than in MV-PCI (28.9% vs 7%; P < .001 and 33.6% vs 10.6%; P < .001, respectively). Patients assigned to CL-PCI who underwent repeated revascularization had staged (vs urgent) revascularization in 61.7% (n = 21 of 34) and 54.5% (n = 42 of 77) in the LM-pLAD CL location group and the OCLL group, respectively. No significant differences were found in stroke and bleeding between CL-PCI and MV-PCI regardless of revascularization strategy (Table 3).
Discussion
The major findings of this analysis in patients presenting with MVD and acute MI complicated by CS are the following: (1) a CL located in the LM or pLAD vs other coronary segments is associated with worse short- and long-term outcomes, and (2) a strategy of CL-PCI with optional staged revascularization of non-CL as compared with immediate MV-PCI resulted in a reduced risk of death or kidney replacement therapy at 30 days regardless of CL location and in a 1-year mortality benefit in patients with LM/pLAD CL location. In consensus with these findings, previous studies documented worse outcomes associated with LM/pLAD CL location compared with OCLL.9,10,11,12,13 In hemodynamically stable patients, CL location in the LM or pLAD has been shown to be associated with higher risk of target lesion revascularization and MI.3 Moreover, in a real-world registry, infarctions in the LAD territory were associated with higher risk of death and heart failure compared with CL located in the left circumflex and right coronary artery.4 Data from the American College of Cardiology–National Cardiovascular Data Registry showed that in the setting of CS, CL location in the pLAD was an independent predictor of in-hospital mortality.5 These findings are not surprising because the LM and pLAD supply at least 50% of the left ventricular myocardium,12,14 and a CL located in these compared with other coronary segments results in more myocardium at risk.15 Nevertheless, a substudy of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial16 could not confirm an association of CL location and 1-year mortality, which was discussed to be attributed to advancements in interventional treatment of LM disease over the last decade. Furthermore, this analysis showed that a CL location in distal compared with proximal and mid coronary segments was associated with higher risk of mortality at 1 year.16 Authors hypothesized that the salvage of myocardium by PCI in patients with proximal and mid lesions was greater compared with patients with distal lesions. Because measurement of myocardial salvage by established methods, such as cardiac magnetic resonance imaging, had not been performed, these findings could not be fully explained. However, most importantly, more than 20% of the IABP-SHOCK II trial population had only single-vessel disease, which makes comparisons with our study results difficult.
Acute MI complicated by CS is associated with high morbidity and mortality.9,17 Early adequate treatment in an intensive cardiac care setting is crucial and should include invasive hemodynamic monitoring, supportive pharmacotherapy, and evaluation for the need of additional therapies including hypothermia and mechanical circulatory support.18 In addition, early revascularization has been shown to improve outcomes in these patients.17,19 With regards to patients presenting with MVD, the CULPRIT-SHOCK trial provided unique information and found lower risk of death or kidney replacement therapy for CL-PCI vs MV-PCI at 30 days and a persistent benefit at 1-year follow-up.7,8 According to the results of this analysis, patients with LM/pLAD CL location may especially benefit from a strategy focusing only on the CL with optional staged revascularization. In these patients, we found very high mortality at 1 year but a relative risk reduction of 47% (50.0% vs 69.6%) with CL-PCI vs MVI-PCI. As mentioned previously, the LM and pLAD supply a major part of the left ventricular myocardium. Revascularization of concomitant non-CL in other coronary segments during the primary PCI may not add more benefit but rather increase the risk of complications such as stent thrombosis, dissections, perforations, or slow flow.20 In addition, immediate MV-PCI increases the amount of contrast media use and thereby the risk of kidney failure.20,21 Furthermore, a prolonged coronary intervention may prolong the time out of the intensive care unit with suboptimal hemodynamic and respiratory management. Besides our data, only very limited information is available on this specific patient population. An analysis of the Korea Acute Myocardial Infarction Registry (KAMIR) registry22 on patients with MI and CS provided data on CL-PCI vs MV-PCI and analyzed the subgroup of patients with LM or LAD CL vs left circumflex or right coronary artery CL. In contrast to our results, the analysis of the KAMIR registry found favorable outcomes in patients treated with MV-PCI vs CL-PCI regardless of the CL location. These conflicting results may be explained by several differences of the patient populations and mainly by the study designs. The KAMIR study was an observational registry, and it cannot be excluded that selection bias has affected the results despite multivariate adjustment and sensitivity analyses. However, most importantly, the KAMIR registry included patients with staged revascularization in the MV-PCI group. In CULPRIT-SHOCK, patients with staged PCI were included in the CL-PCI group. In fact, staged PCI was encouraged in patients randomized to CL-PCI and represented 85.1% of patients who underwent repeated revascularization. Beneficial effects of complete revascularization performed as staged PCI were found by the COMPLETE trial23 and could also be an attractive strategy in patients presenting with CS, although special considerations apply. In patients with CS, longer periods until stabilization and recovery may have an effect on the timing of a staged approach.24
Interestingly, we found higher rates of rehospitalization for heart failure associated with CL-PCI vs MV-PCI within the OCLL group but not the LM/pLAD group; however, with no significant interaction between CL location and revascularization strategy. The underlying reasons for this finding are uncertain. It could be argued that these results might be attributed to the play of chance or a matter of competing risk. Patients from the CL-PCI survive more often and therefore have a higher likelihood to develop heart failure compared with those who underwent MV-PCI.7
Limitations
There are several limitations of this study that should be taken into account when interpreting the study results. First, this is a post hoc analysis of a randomized clinical trial. The analyses are possibly underpowered and results should be interpreted as hypothesis generating rather than conclusive. Second, there are various definitions of the pLAD, which makes comparisons between studies difficult. This analysis used the SYNTAX score definition of pLAD. Third, the CULPRIT-SHOCK trial enrolled patients with both STEMI and NSTEMI with CS. Results may differ between patients with STEMI and NSTEMI; however, analyses of these subgroups would have resulted in very small groups with no clinically meaningful results. Fourth, because there were no planned follow-up echocardiograms, we cannot rule out an effect of LVEF improvement on the lower risk of rehospitalization for heart failure in the OCLL group treated by MV-PCI as compared with CL-PCI.
Conclusions
In patients with multivessel disease presenting with acute MI and CS, a CL located in the LM and/or pLAD vs other coronary segments was associated with worse outcome. A strategy of CL-PCI with optional staged revascularization of non-CL compared with immediate MV-PCI resulted in a reduced risk of death or kidney replacement therapy at 30 days regardless of CL location. Patients with a CL located in the LM and/or pLAD may especially benefit from a CL-PCI strategy with regards to 1-year mortality, although further investigation is needed to confirm this finding.
eTable 1. Baseline clinical and procedural characteristics in patients with left main or proximal left anterior descending artery culprit lesion versus other culprit lesion location
eTable 2. Multivariate adjusted analysis for risk of 30-day and 1-year endpoints in patients with left main or proximal left anterior descending artery culprit lesion versus other culprit lesion location
References
- 1.Aissaoui N, Puymirat E, Tabone X, et al. Improved outcome of cardiogenic shock at the acute stage of myocardial infarction: a report from the USIK 1995, USIC 2000, and FAST-MI French nationwide registries. Eur Heart J. 2012;33(20):2535-2543. doi: 10.1093/eurheartj/ehs264 [DOI] [PubMed] [Google Scholar]
- 2.Backhaus T, Fach A, Schmucker J, et al. Management and predictors of outcome in unselected patients with cardiogenic shock complicating acute ST-segment elevation myocardial infarction: results from the Bremen STEMI Registry. Clin Res Cardiol. 2018;107(5):371-379. doi: 10.1007/s00392-017-1192-0 [DOI] [PubMed] [Google Scholar]
- 3.Roguin A, Camenzind E, Kerner A, et al. Long-term outcomes of stenting the proximal left anterior descending artery in the PROTECT trial. JACC Cardiovasc Interv. 2017;10(6):548-556. doi: 10.1016/j.jcin.2016.12.028 [DOI] [PubMed] [Google Scholar]
- 4.Entezarjou A, Mohammad MA, Andell P, Koul S. Culprit vessel: impact on short-term and long-term prognosis in patients with ST-elevation myocardial infarction. Open Heart. 2018;5(2):e000852. doi: 10.1136/openhrt-2018-000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein LW, Shaw RE, Krone RJ, et al. ; American College of Cardiology National Cardiovascular Data Registry . Mortality after emergent percutaneous coronary intervention in cardiogenic shock secondary to acute myocardial infarction and usefulness of a mortality prediction model. Am J Cardiol. 2005;96(1):35-41. doi: 10.1016/j.amjcard.2005.02.040 [DOI] [PubMed] [Google Scholar]
- 6.Zeymer U, Vogt A, Zahn R, et al. ; Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK) . Predictors of in-hospital mortality in 1333 patients with acute myocardial infarction complicated by cardiogenic shock treated with primary percutaneous coronary intervention (PCI); results of the primary PCI registry of the Arbeitsgemeinschaft Leitende Kardiologische Krankenhausärzte (ALKK). Eur Heart J. 2004;25(4):322-328. doi: 10.1016/j.ehj.2003.12.008 [DOI] [PubMed] [Google Scholar]
- 7.Thiele H, Akin I, Sandri M, et al. ; CULPRIT-SHOCK Investigators . One-year outcomes after PCI strategies in cardiogenic shock. N Engl J Med. 2018;379(18):1699-1710. doi: 10.1056/NEJMoa1808788 [DOI] [PubMed] [Google Scholar]
- 8.Thiele H, Akin I, Sandri M, et al. ; CULPRIT-SHOCK Investigators . PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017;377(25):2419-2432. doi: 10.1056/NEJMoa1710261 [DOI] [PubMed] [Google Scholar]
- 9.Kunadian V, Qiu W, Ludman P, et al. ; National Institute for Cardiovascular Outcomes Research . Outcomes in patients with cardiogenic shock following percutaneous coronary intervention in the contemporary era: an analysis from the BCIS database (British Cardiovascular Intervention Society). JACC Cardiovasc Interv. 2014;7(12):1374-1385. doi: 10.1016/j.jcin.2014.06.017 [DOI] [PubMed] [Google Scholar]
- 10.Puricel S, Adorjan P, Oberhänsli M, et al. Clinical outcomes after PCI for acute coronary syndrome in unprotected left main coronary artery disease: insights from the Swiss Acute Left Main Coronary Vessel Percutaneous Management (SALVage) study. EuroIntervention. 2011;7(6):697-704. doi: 10.4244/EIJV7I6A112 [DOI] [PubMed] [Google Scholar]
- 11.Gharacholou SM, Ijioma NN, Lennon RJ, et al. Characteristics and long term outcomes of patients with acute coronary syndromes due to culprit left main coronary artery disease treated with percutaneous coronary intervention. Am Heart J. 2018;199:156-162. doi: 10.1016/j.ahj.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 12.Harjai KJ, Mehta RH, Stone GW, et al. ; Primary Angioplasty In Myocardial Infarction (PAMI) Investigators . Does proximal location of culprit lesion confer worse prognosis in patients undergoing primary percutaneous coronary intervention for ST elevation myocardial infarction? J Interv Cardiol. 2006;19(4):285-294. doi: 10.1111/j.1540-8183.2006.00146.x [DOI] [PubMed] [Google Scholar]
- 13.Patel N, De Maria GL, Kassimis G, et al. Outcomes after emergency percutaneous coronary intervention in patients with unprotected left main stem occlusion: the BCIS national audit of percutaneous coronary intervention 6-year experience. JACC Cardiovasc Interv. 2014;7(9):969-980. doi: 10.1016/j.jcin.2014.04.011 [DOI] [PubMed] [Google Scholar]
- 14.Karha J, Murphy SA, Kirtane AJ, et al. ; TIMI Study Group . Evaluation of the association of proximal coronary culprit artery lesion location with clinical outcomes in acute myocardial infarction. Am J Cardiol. 2003;92(8):913-918. doi: 10.1016/S0002-9149(03)00969-X [DOI] [PubMed] [Google Scholar]
- 15.Velders MA, van Boven N, Boden H, et al. Association between angiographic culprit lesion and out-of-hospital cardiac arrest in ST-elevation myocardial infarction patients. Resuscitation. 2013;84(11):1530-1535. doi: 10.1016/j.resuscitation.2013.07.016 [DOI] [PubMed] [Google Scholar]
- 16.Fuernau G, Fengler K, Desch S, et al. Culprit lesion location and outcome in patients with cardiogenic shock complicating myocardial infarction: a substudy of the IABP-SHOCK II-trial. Clin Res Cardiol. 2016;105(12):1030-1041. doi: 10.1007/s00392-016-1017-6 [DOI] [PubMed] [Google Scholar]
- 17.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock, SHOCK investigators: should we emergently revascularize occluded coronaries for cardiogenic shock. N Engl J Med. 1999;341(9):625-634. doi: 10.1056/NEJM199908263410901 [DOI] [PubMed] [Google Scholar]
- 18.Thiele H, Ohman EM, de Waha-Thiele S, Zeymer U, Desch S. Management of cardiogenic shock complicating myocardial infarction: an update 2019. Eur Heart J. 2019;40(32):2671-2683. doi: 10.1093/eurheartj/ehz363 [DOI] [PubMed] [Google Scholar]
- 19.Acharya D. Predictors of outcomes in myocardial infarction and cardiogenic shock. Cardiol Rev. 2018;26(5):255-266. doi: 10.1097/CRD.0000000000000190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel B, Mehta SR, Mehran R. Reperfusion strategies in acute myocardial infarction and multivessel disease. Nat Rev Cardiol. 2017;14(11):665-678. doi: 10.1038/nrcardio.2017.88 [DOI] [PubMed] [Google Scholar]
- 21.Marenzi G, Assanelli E, Campodonico J, et al. Contrast volume during primary percutaneous coronary intervention and subsequent contrast-induced nephropathy and mortality. Ann Intern Med. 2009;150(3):170-177. doi: 10.7326/0003-4819-150-3-200902030-00006 [DOI] [PubMed] [Google Scholar]
- 22.Lee JM, Rhee TM, Hahn JY, et al. ; KAMIR Investigators . Multivessel percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction with cardiogenic shock. J Am Coll Cardiol. 2018;71(8):844-856. doi: 10.1016/j.jacc.2017.12.028 [DOI] [PubMed] [Google Scholar]
- 23.Mehta SR, Wood DA, Storey RF, et al. ; COMPLETE Trial Steering Committee and Investigators . Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381(15):1411-1421. doi: 10.1056/NEJMoa1907775 [DOI] [PubMed] [Google Scholar]
- 24.Thiele H, Desch S. CULPRIT-SHOCK (culprit lesion only PCI versus multivessel percutaneous coronary intervention in cardiogenic shock): implications on guideline recommendations. Circulation. 2018;137(13):1314-1316. doi: 10.1161/CIRCULATIONAHA.117.032907 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Baseline clinical and procedural characteristics in patients with left main or proximal left anterior descending artery culprit lesion versus other culprit lesion location
eTable 2. Multivariate adjusted analysis for risk of 30-day and 1-year endpoints in patients with left main or proximal left anterior descending artery culprit lesion versus other culprit lesion location