Key Points
Question
Does telmisartan reduce the growth of small abdominal aortic aneurysms?
Findings
In this placebo-controlled randomized trial of 210 participants, a significant effect of telmisartan on abdominal aortic aneurysm growth rates was not shown. Telmisartan had no effect on requirement for abdominal aortic aneurysm repair or aneurysm rupture.
Meaning
Further adequately powered trials are needed to assess the efficacy of medical therapies to slow abdominal aortic aneurysm growth.
Abstract
Importance
Currently there is no drug therapy for abdominal aortic aneurysm (AAA).
Objective
To test the efficacy of the angiotensin receptor blocker telmisartan in slowing AAA growth in the Telmisartan in the Management of Abdominal Aortic Aneurysm (TEDY) trial.
Design, Setting, and Participants
A randomized, double-blind, placebo-controlled trial recruited participants between September 6, 2011, and October 5, 2016, to evaluate the efficacy of telmisartan treatment in patients with AAA. Participants with 35- to 49-mm AAAs recruited from Australia, the Netherlands, and the US were randomized 1:1 to receive telmisartan, 40 mg, or identical placebo. Analyses were conducted according to intention-to-treat principles. Final follow-up was conducted on October 11, 2018, and data analysis was performed between June and November 2019.
Intervention
Telmisartan, 40 mg, or identical placebo.
Main Outcomes and Measures
The primary outcome of the difference in AAA growth, assessed on core imaging laboratory-read ultrasonographic scanning, was tested with linear mixed-effects models. Other outcomes included effects on blood pressure, computed tomographic (CT)–measured AAA diameter and volume, time to AAA-related events (AAA repair or mortality due to AAA rupture), and health-related quality of life.
Results
Of 300 intended participants, 210 were enrolled and randomized to receive telmisartan (n = 107) or placebo (n = 103). Of patients included in the intention-to-treat analysis (telmisartan: n = 106, placebo: n = 101), 183 were men (88%); mean (SD) age was 73.5 (7.9) years. At 1 year, participants receiving telmisartan had mean lower systolic (8.9; 95% CI, 4.1-13.8 mm Hg; P < .001) and diastolic (7.0; 4.3-9.8 mm Hg; P < .001) blood pressure levels compared with participants receiving placebo. A total of 188 participants (91%) received at least 2 ultrasonographic scans and 133 participants (64%) had at least 2 CT scans. There was no significant difference in ultrasonographic-assessed AAA growth rates among those assigned telmisartan (1.68 mm/y) or placebo (1.78 mm/y): mean difference, −0.11 mm/y (95% CI, −0.60 to 0.38 mm/y; P = .66). Telmisartan had no significant effects on AAA growth assessed by CT-measured AAA diameter (mean difference, −0.01 mm/y; 95% CI, −0.02 to 0.01 mm/y; P = .23) or volume (mean difference, −0.02 cm3/y; 95% CI, −0.04 to 0.00 cm3/y; P = .11), AAA-related events (relative risk, 1.35; 95% CI, 0.54-3.35; P = .52), or health-related quality of life (mean difference in physical component score at 24 months, 0.4; 95% CI, 0.4-0.4; P = .80). Hypotensive symptoms (eg, syncope) were twice as common among participants receiving telmisartan compared with placebo (28 [26%] vs 13 [13%]; P = .02), but overall adverse event rates were otherwise similar for both groups.
Conclusions and Relevance
This underpowered study did not show a treatment effect for telmisartan on small AAA growth. Future trials will need to ensure adequate sample size and duration of follow-up.
Trial Registrations
anzctr.org.au Identifier: ACTRN12611000931976; ClinicalTrials.gov Identifier: NCT01683084
This randomized clinical trial examines the efficacy of telmisartan therapy slowing the growth of abdominal aortic aneurysm in patients with that condition.
Introduction
Abdominal aortic aneurysm (AAA) rupture is estimated to cause approximately 200 000 deaths worldwide annually.1 Surgery is currently the only treatment for AAA, but previous randomized clinical trials have reported that early elective open or endovascular repair does not reduce mortality of people with small asymptomatic AAAs.2 Current guidelines recommend that small asymptomatic AAAs (<55 mm in men and <50 mm in women) are simply monitored.3,4 Most small AAAs grow progressively during monitoring and subsequently undergo surgical repair.2 To our knowledge, there is currently no evidence to support any drug treatment for small AAAs.5,6
Angiotensin II has been implicated in AAA pathogenesis through studies in animal models.5 Angiotensin II appears to induce aortic aneurysm in mice by stimulating the angiotensin receptor-1 (ATR-1)7 and promoting aortic inflammation and matrix degradation.8 Blockade9,10,11 or deficiency of the ATR-112 limits AAA induced by high-fat feeding9 and elastase infusion10,11,12 in rodents. Despite promising findings in animal research, there have been limited randomized clinical trials testing the efficacy of medications inhibiting angiotensin II to slow AAA growth.6
One randomized clinical trial reported that the angiotensin converting enzyme (ACE) inhibitor perindopril did not slow AAA growth.13 To our knowledge, no previous randomized clinical trial has tested the efficacy of an angiotensin receptor blocker (ARB) to slow AAA growth. Experimental findings suggest that an ARB may be more effective than an ACE inhibitor in limiting AAA growth. Angiotensin receptor blockers, unlike ACE inhibitors, directly block the ATR-1 and thereby direct angiotensin II to stimulate the ATR-2, which has been reported to inhibit AAA pathogenesis in animal models.14 ACE inhibitors, but not ARBs, increase levels of kinins,15 which have been reported to promote AAA growth and rupture in animal models.16 Also, a number of non-ACE angiotensin-II–producing enzymes have been identified within human AAA samples,17,18 suggesting that ACE inhibitors might incompletely block the effects of angiotensin II.
Telmisartan is an ARB commonly used to control blood pressure. Five separate studies have reported that telmisartan limits pathologic aortic remodeling and AAA development within rodent models.9,10,11,12,19 In addition to blocking the ATR-1, telmisartan has been reported to stimulate the peroxisome proliferator activator receptor-γ,20 an effect that has been reported to limit AAA development within rodent models.21,22 It was hypothesized that telmisartan would limit AAA growth in people with small AAAs through its established efficacy in lowering blood pressure and its suggested ability to favorably affect pathologic aortic remodeling. The primary aim of the Telmisartan in the Management of Abdominal Aortic Aneurysm (TEDY) trial was to test the efficacy of telmisartan compared with placebo in slowing the growth of small AAAs over 2 years.
Methods
Design
TEDY was a parallel, randomized, multicenter, double-blind, placebo-controlled trial in which participants were randomized to receive identical capsules containing either telmisartan, 40 mg, or placebo once daily for 2 years in a 1:1 ratio. A detailed trial protocol has been previously published.23 The trial protocol is available in Supplement 1. The trial complied with the tenets of the Declaration of Helsinki24 and was approved by the relevant ethics and governance committees before initiation. All participants provided written informed consent; financial compensation was not provided. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
TEDY included men and women who had an AAA measuring 35 to 49 mm on computed tomographic (CT) or ultrasonographic imaging and no anticipated requirement for AAA repair within 1 year of recruitment. Participants were excluded if they were already prescribed an ARB or ACE inhibitor, had a known contraindication to telmisartan use (kidney impairment, defined as serum creatinine level >1.5-fold the upper limit of the reference range; a previously documented >70% stenosis of either renal artery; liver dysfunction, defined as any liver transaminase level >1.5-fold the upper limit of the reference range; or active gout), had undergone previous abdominal aortic surgery, had a mycotic AAA, or were currently enrolled in any other drug trial. Participants were recruited from vascular departments at sites in Australia, the Netherlands, and the US (the eAppendix in Supplement 2).
A total sample size of 300 participants was estimated as needed to test our hypothesis with a power of 80% (α = 0.05), allowing for a 20% dropout rate.23 This estimate was based on testing an AAA growth reduction of 30% and an annual increase in AAA diameter of 1.2 mm with an SD of 1.2. Owing to difficulty identifying eligible participants, recruitment was ended after a total of 210 participants had been randomized.
Patients were randomized by a blinded health professional through the secure trial website, coordinated by an independent trial center. Drug allocation was determined by a computer-generated random number sequence that was stratified by study site and initial aortic diameter (35-39, 40-44, and 45-49 mm). Random number sequences corresponded to serial numbers on concealed study packaging.
Identical-appearing capsules containing telmisartan, 40 mg (active drug), or inert cellulose powder (placebo) were identically packaged and dispensed to participants every 6 months during the trial. Other aspects of the participants’ treatment, such as control of blood pressure level, cholesterol level, and other care, was under the control of the treating vascular specialists according to current guidelines.3,4
Age; sex; history of diabetes, hypertension, and cardiovascular disease; smoking; medications; and waist-to-hip ratio were recorded at recruitment using previously published definitions.23 Estimated glomerular filtrate rate was calculated using serum creatinine level by the Chronic Kidney Disease Epidemiology Collaboration equation.
Outcomes
The primary study outcome was the between-treatment group difference in AAA growth over 2 years as estimated by the maximum anterior-posterior outer-to-outer orthogonal AAA diameter measured from centrally read ultrasonographic scans performed every 6 months. Ultrasonographic scans were performed by experienced sonographers using ultrasonographic machines present in vascular laboratories at each center. Each sonographer was formally trained and registered with specialized experience in vascular ultrasonography. All participants were scanned in the supine position. The length of the abdominal aorta was examined to identify the site of maximum AAA diameter. Static images in the transverse plane acquired in systole were obtained at the point of maximal diameter of the infrarenal abdominal aorta perpendicular to the central vessel line, stored in digital imaging and communications in medicine format, and transferred to the core imaging reading center. The quality of all images was confirmed on receipt. At the end of the trial, the maximum anterior-posterior outer-to-outer AAA diameter was measured from all images by one highly trained assessor (J.P.) blinded to group allocation. The intraobserver reproducibility of this assessor was examined by repeat measurement of 50 nontrial images 1 week apart and found to be high (reproducibility coefficient [95% limits of agreement] = 2.31 mm, ie, the SD of the mean difference between readings 1 and 2 multiplied by 1.96).
Computed tomographic angiography was performed at baseline, 1 year, and/or 2 years using scanners at each participating hospital, as previously described.25 The ethics boards’ approval for TEDY at the Netherlands and US sites allowed CT scans to be performed only at entry and at 2 years. Computed tomographic images were transferred to the core imaging reading center where they were imported to a workstation for analysis (Philips Intellispace Workstation, Advanced Vessel Analysis application [version 7]; Koninklijke Philips NV).25 Maximum orthogonal AAA diameter and infrarenal aortic volume were measured by a single highly trained assessor using methods that have previously been reported.25 Intraobserver reproducibility was assessed before initiating the readings on 10 images measured 1 week apart, and reproducibility coefficients were less than 3 mm. Blood pressure was measured at recruitment and every 6 months at the participating site using a digital monitor (Omron Intellisense, HEM-907) after participants had rested for 20 minutes supine.26 Recordings were measured 3 times and averaged. Serum C-reactive protein (CRP) level was measured at baseline, 3 months, and annually in a subset of participants, as previously described.27 Since peripheral artery disease is common in people with AAA, ankle brachial pressure index was measured at baseline and follow-up visits, as previously described.23 Health-related quality of life was assessed using the Short Form-36 questionnaire and assessed annually. Treatment adherence was assessed by pill counts performed at 3, 6, 12, 18, and 24 months.
Adverse Events
Adverse events and serious adverse events, defined according to Good Clinical Practice guidelines, were ascertained at interviews performed every 3 months. Serious adverse events were reviewed by the safety committee quarterly throughout the trial.
Statistical Analysis
All analyses were conducted according to intention-to-treat principles; that is, participants were analyzed according to their randomized treatment irrespective of adherence. The distribution of baseline characteristics and drug adherence were compared between groups using standard parametric methods as appropriate or with nonparametric tests (Wilcoxon rank sum test) for nonnormally distributed continuous variables. The Fisher exact test was used to assess for differences in the number of adverse events, allowing for low frequencies of uncommon adverse events. Assessment of the effects of telmisartan on AAA growth was performed using linear mixed models. Core laboratory imaging measurements were included until the time of AAA repair or death. The interaction of treatment allocation and time was modeled with a random coefficient and random slope of time to allow participants to differ in their rate of AAA growth. Mean AAA diameters and volumes were plotted within each group across the times included, and summary results are presented as mean differences and corresponding 95% CIs. Differences in blood pressure level, serum CRP level, and CT-determined AAA diameter and volume during the course of the trial were also assessed with linear mixed models. Any nonnormally distributed variables were transformed to a logarithmic scale and shown to be log-normally distributed before applying these models.
The effects of telmisartan on blood pressure were reported at 1 year (trial midpoint), consistent with reporting of previous blood pressuring–lowering trials, by post hoc analysis of the linear mixed models. The secondary outcome of time to AAA repair or death from AAA rupture was assessed with Cox proportional hazards methods and illustrated by a Kaplan-Meier graph. Short Form-36 summary scores were transformed to norm-based T scores with a mean (SD) score of 50 (10) in the general population, with differences assessed at the end of the trial. Analyses were performed using Stata, version 15.1 (StataCorp LLC), and graphics were produced with R, version 3.5 01 (The R Project for Statistical Computing). All statistical tests were 2-sided, and a P value <.05 was considered significant.
Results
Participants
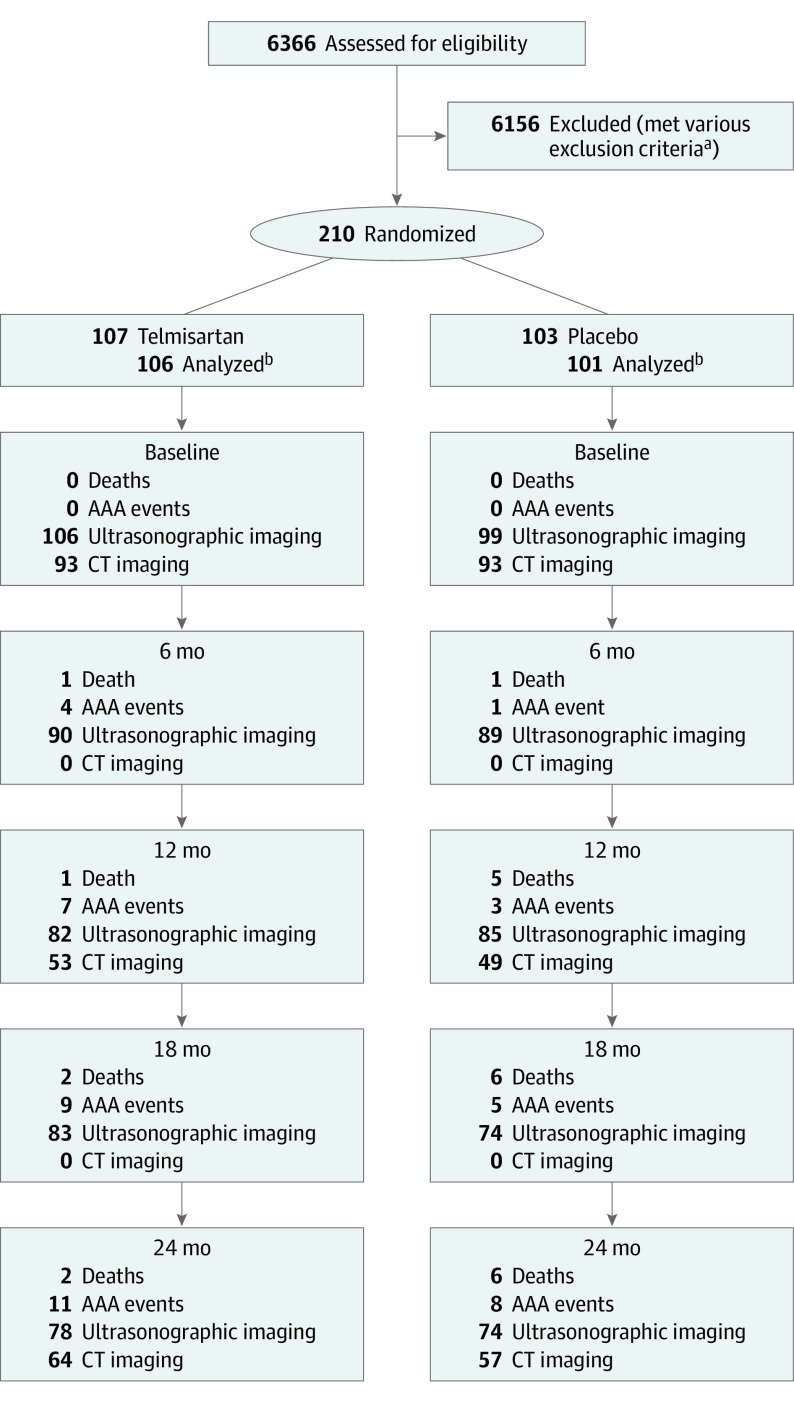
Between September 6, 2011, and October 5, 2016, 210 people with small AAAs, recruited from Australia (n = 118), the Netherlands (n = 70), and the US (n = 22), were randomized to receive telmisartan (n = 107) or placebo (n = 103). The trial was stopped before meeting the recruitment target of 300 participants owing to difficulties in identifying eligible people. Final follow-up was conducted on October 11, 2018, and data were analyzed from June to November 2019. Of the 6366 potential participants prescreened, 6156 individuals (96.7%) were excluded due to previous aortic surgery (n = 2412), inability to contact (n = 900), already prescribed an ACE inhibitor or ARB (n = 815), AAA diameter not within entry range (n = 803), declined involvement (n = 409), kidney (n = 102) or liver (n = 14) dysfunction, awaiting AAA repair (n = 39), active gout diagnosis (n = 11), documented kidney artery stenosis (n = 4), and miscellaneous other reasons (n = 647), such as miscoding (Figure 1). Of the participants randomized, 2 withdrew owing to being ineligible (infrarenal aortic diameter <30 mm) or no longer wishing to take part and requesting that all data be destroyed. A further participant was a duplicate entry. These 3 participants were excluded from the intention-to-treat analysis, which was limited to 207 people (telmisartan, n = 106; placebo, n = 101). A total of 183 of the participants were men (88%) and 24 were women (12%); mean (SD) age was 73.5 (7.9) years. The numbers of participants attending each follow-up ultrasonographic and CT scan are shown in Figure 1, with a total of 188 participants (91%) attending at least 2 ultrasonographic scans. One hundred thirty-three participants (64%) had at least 2 CT scans performed (baseline, n = 186; 1 year, n = 102; and 2 years, n = 121).
Figure 1. Flow of Included Participants.
Numbers of patients assessed for eligibility, randomized, follow-up events (cumulative), and participants having ultrasonographic and computed tomographic (CT) imaging of their abdominal aortic aneurysm (AAA). ACE indicates angiotensin converting enzyme; ARB, angiotensin receptor blocker.
aThe 6156 exclusions are enumerated in the Results section.
bThree patients withdrawn after randomization owing to ineligibility, complete withdrawal, and duplicate randomization.
The distribution of risk factors and medications was similar among participants randomized to receive telmisartan and placebo (Table). During follow-up, 89% or more of participants assessed took at least 80% of allocated capsules (eTable 1 in Supplement 2). Adherence to telmisartan or placebo capsules was similar over the 2 years of follow-up.
Table. Characteristics of Participants at Baseline.
| Characteristic | No. (%)a | P value | |
|---|---|---|---|
| Telmisartan (n = 106) | Placebo (n = 101) | ||
| AAA diameter, mmb | |||
| Mean (SD) | 43.3 (4.8) | 43.1 (5.2) | .71 |
| Level | |||
| <40 | 26 (25) | 28 (28) | .87 |
| 40 to <45 | 41 (39) | 38 (38) | |
| ≥45 | 39 (37) | 35 (35) | |
| Age, y | |||
| Mean (SD) | 73.2 (8.1) | 73.8 (7.8) | .61 |
| Group, y | |||
| <70 | 39 (37) | 35 (35) | .69 |
| 70 to <80 | 46 (43) | 41 (41) | |
| ≥80 | 21 (20) | 25 (25) | |
| Sex | |||
| Male | 92 (87) | 91 (90) | .46 |
| Female | 14 (13) | 10 (10) | |
| Region | |||
| Australia | 61 (58) | 55 (54) | .89 |
| The Netherlands | 35 (33) | 35 (35) | |
| US | 10 (9) | 11 (11) | |
| Prior disease | |||
| Cardiovascular diseasec | 59 (56) | 48 (48) | .24 |
| Diabetes | 11 (10) | 12 (12) | .73 |
| Smoking status | |||
| Current | 23 (22) | 27 (27) | .70 |
| Previous | 75 (71) | 67 (66) | |
| Never | 8 (8) | 7 (7) | |
| Systolic blood pressure, mm Hgd | |||
| Mean (SD) | 139 (17) | 136 (17) | .14 |
| Level | |||
| <130 | 33 (31) | 42 (42) | .10 |
| 130 to <140 | 22 (21) | 25 (25) | |
| ≥140 | 51 (48) | 34 (34) | |
| Diastolic blood pressure, mm Hg | |||
| Mean (SD) | 80 (10) | 78 (9) | .18 |
| Level | |||
| <80 | 55 (52) | 54 (53) | .45 |
| 80 to <90 | 32 (30) | 35 (35) | |
| ≥90 | 19 (18) | 12 (12) | |
| Waist-to-hip ratioe | |||
| Mean (SD) | 1.00 (0.07) | 1.00 (.07) | .55 |
| Level | |||
| ≤0.95 | 21 (20) | 17 (17) | .85 |
| >0.95 to <1.0 | 26 (25) | 25 (25) | |
| ≥1.0 | 59 (56) | 59 (58) | |
| Glomerular filtration rate, mL/min/1.73 m2 | |||
| Mean (SD) | 75 (17) | 77 (16) | .34 |
| Level | .63 | ||
| <60 | 15 (14) | 12 (12) | |
| ≥60 | 91 (86) | 89 (88) | |
| Medications | |||
| Antiplatelet agent | 65 (61) | 57 (56) | .48 |
| Statin | 69 (65) | 66 (65) | .97 |
| β-Blocker | 24 (23) | 21 (21) | .75 |
| Calcium channel blocker | 12 (11) | 14 (14) | .58 |
| Diuretic | 13 (12) | 9 (9) | .43 |
Abbreviation: AAA, abdominal aortic aneurysm.
Percentages may not total 100 because of rounding.
Maximum infrarenal aortic diameter as reported by participating sites (rather than core lab-measured AAA diameter).
Includes participants with a documented history of coronary heart disease, stroke, or peripheral artery disease.
P value calculated using nonparametric testing.
Not measured in 3 participants (1.4%).
Outcomes
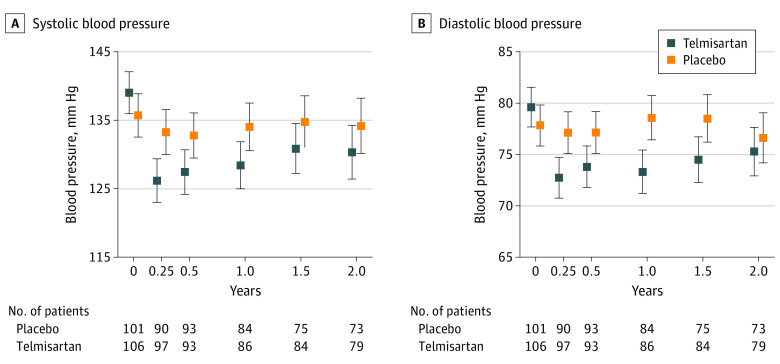
Participants randomized to receive telmisartan had lower blood pressure than those randomized to receive placebo (Figure 2). At 1 year, participants receiving telmisartan had mean lower systolic (8.9; 95% CI, 4.1-13.8 mm Hg; P < .001) and diastolic (7.0; 4.3-9.8 mm Hg; P < .001) blood pressure levels compared with participants receiving placebo. The CRP level was measured throughout the trial (baseline, n = 207; 3 months, n = 176; 1 year, n = 168; and 2 years, n = 151) and was not affected by allocation to telmisartan or placebo (mean difference in logCRP, −0.06/y; 95% CI, −0.20 to 0.07) (eFigure 1 in Supplement 2).
Figure 2. Effect of Telmisartan Compared With Placebo on Blood Pressure.
Blood pressure among patients randomized to receive telmisartan vs placebo for systolic (A) or diastolic (B) blood pressure (8.9; 95% CI, 4.1-13.8 mm Hg vs 7.0; 4.3-9.8 mm Hg). Summary mean differences reported at 1 year (trial midpoint). Error bars represent 95% CI.
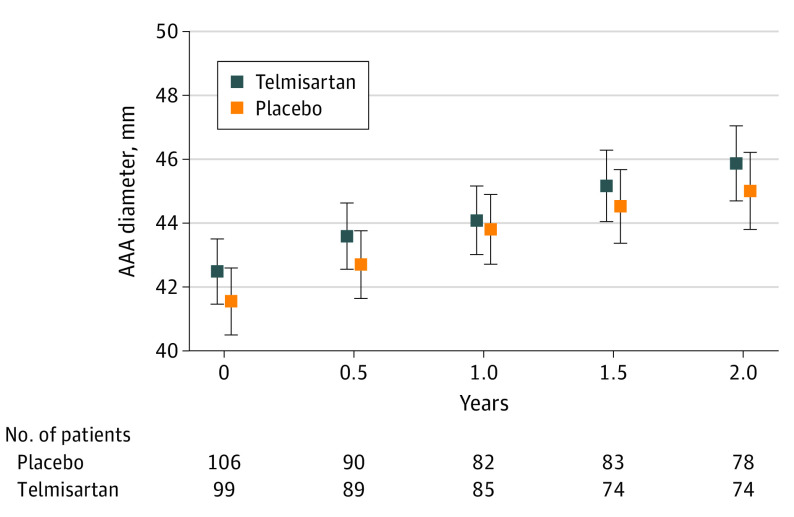
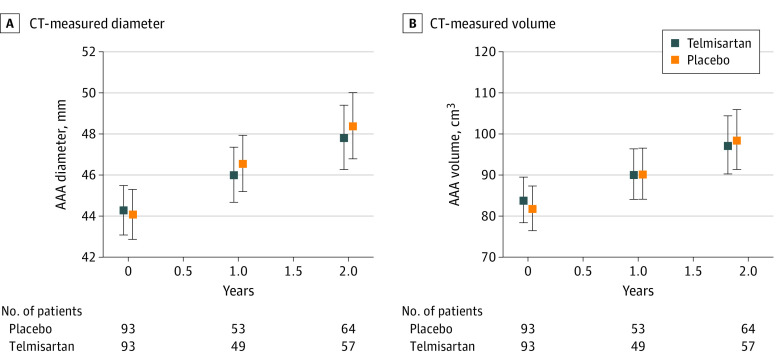
Estimated mean annual AAA growth rates were 1.68 mm/y (95% CI, 1.33-2.02 mm/y) in participants randomized to receive telmisartan and 1.78 mm/y (95% CI, 1.44-2.13 mm/y) in those receiving placebo (Figure 3). This finding represented a mean slower annual AAA growth, with a mean difference of −0.11 mm/y (−0.60 to 0.38 mm/y; P = .66) for participants randomized to receive telmisartan compared with placebo. There was no interaction between initial AAA diameter group and the effect of telmisartan on AAA growth (eFigure 2 in Supplement 2). Increases in CT-measured AAA diameter (mean difference, −0.01 mm/y; 95% CI, −0.02 to 0.01 mm/y; P = .23) and infrarenal aortic volume (mean difference, −0.02 cm3/y (95% CI, −0.04 to 0.00 cm3/y; P = .11) were similar in both groups (Figure 4).
Figure 3. Effect of Telmisartan on Small Abdominal Aortic Aneurysm (AAA) Growth Measured Using Ultrasonographic Imaging.
Core imaging laboratory-read AAA diameter measured as the maximum infrarenal outer-to-outer aortic diameter in the anteroposterior plane for telmisartan (1.68 mm/y; 95% CI, 1.33-2.02 mm/y) and placebo (1.78 mm/y; 95% CI, 1.44-2.13 mm/y). Mean difference, −0.11 (95% CI, −0.60 to 0.38 mm/y). Error bars represent 95% CI.
Figure 4. Effect of Telmisartan on Small Abdominal Aortic Aneurysm (AAA) Growth Measured Using Computed Tomographic (CT) Imaging.
AAA diameter and volume measured in a central core laboratory. Measurements recorded for 186 participants at baseline, 102 participants at 1 year, and 121 participants at 2 years. Error bars represent 95% CI.
During the course of the trial, 1 participant had an asymptomatic retroperitoneal AAA leak detected on routine ultrasonographic follow-up imaging and confirmed on a CT angiographic scan. The participant underwent emergency successful endovascular AAA repair. No other AAA ruptures were reported. A total of 18 other participants underwent AAA repair (open, n = 3; endovascular, n = 16). Allocation to telmisartan had no significant effect on AAA repair (relative risk, 1.35; 95% CI, 0.54-3.35; P = .52) (eFigure 3 in Supplement 2).
Allocation to telmisartan did not affect ankle brachial pressure index (eFigure 4 in Supplement 2). There was no significant effect of telmisartan on health-related quality of life as measured by the Short Form-36 questionnaire at the end of the trial (mean difference in physical component score at 24 months, 0.4 (95% CI, 0.4-0.4; P = .80) (eTable 2, eFigure 5, and eFigure 6 in Supplement 2).
Adverse Events
A total of 8 deaths and 60 hospitalizations occurred during the course of the study, with no significant differences between the telmisartan vs placebo groups (death: 2 [2%] vs 6 [6%], P = .16; hospitalization: 25 [25%] vs 33 [33%], P = .29) (eTable 3 in Supplement 2). Reports of syncope or hypotensive symptoms (eg, feeling faint) were twice as common among participants randomized to receive to telmisartan as those randomized to receive placebo (28 [26%] vs 13 [13%], P = .02) (eTable 3 in Supplement 2).
Discussion
To our knowledge, TEDY is the first randomized clinical trial to examine the effect of an ARB on AAA growth. Despite prescreening more than 6000 people, TEDY recruited only 70% of its planned enrollment (210 of 300 patients). Furthermore, a number of participants did not attend each AAA scan, with 91% having at least 2 ultrasonographic scans and only 64% at least 2 CT scans. TEDY was designed to test the efficacy of telmisartan to slow AAA growth by at least 30%.23 Given the failure to meet the recruitment target and the lack of 100% imaging follow-up, TEDY was underpowered to test this treatment effect. Recalculating the sample size estimate post hoc after considering the mean (SD) annual AAA growth within the placebo group (1.87 [1.87] mm) and the fact that 188 participants had at least 2 ultrasonographic scans, TEDY had approximately 80% power to test a 40% reduction in AAA growth. Thus, the findings of TEDY do not exclude a small treatment effect of telmisartan. Overall, TEDY highlights the challenges of completing randomized clinical trials testing medications for AAA. In TEDY, recruitment was particularly challenged because of the number of people already receiving ARBs or ACE inhibitors. To test a plausible treatment effect, larger trials with longer follow-up are needed.
It was hypothesized that telmisartan would slow AAA growth via favorably modifying multiple mechanisms implicated in AAA, including lowering blood pressure, reducing aortic peak wall stress, and limiting aortic inflammation.23,28 Since telmisartan did not significantly affect the primary end point, finding for the other outcomes in TEDY should be interpreted cautiously. With this caveat, telmisartan significantly reduced blood pressure, although this effect attenuated over time. Telmisartan had no effect on serum concentration of CRP. Very few participants underwent open AAA repair and therefore it was not possible to directly examine the effect of telmisartan on the aortic wall.
Strengths and Limitations
The findings of the TEDY trial should be interpreted after considering its strengths and weaknesses. As reported in a recent systematic review, TEDY is 1 of only 6 published randomized drug trials on AAA that have enrolled at least 200 participants and the first to test the effect of an ARB.6 Central reading of images by highly trained and reproducible assessors was performed to achieve high-quality outcome assessment. Complete blinding of investigators, participants, and assessors was maintained by identical appearance and packaging of drug and placebo. An important limitation of TEDY was that the planned sample size was not achieved. The trial did, however, include a sample size comparable with the largest AAA drug trials previously reported.6 In addition, the results of TEDY cannot be generalized to people not meeting the entry criteria for the trial. While telmisartan reduces blood pressure, participants in the placebo group maintained blood pressure that was well controlled as part of their standard care. Thus, the results of TEDY do not negate the need to control blood pressure to limit other cardiovascular events, such as myocardial infarction and stroke, in people with an AAA.
Conclusions
To our knowledge, the TEDY trial is the first randomized clinical trial to test the efficacy of an ARB in people with small AAAs. The findings of this underpowered trial showed no effect of telmisartan in slowing AAA growth.
Trial Protocol
eTable 1. Adherence to Allocated Medication
eTable 2. Effect of Telmisartan on Health-Related Quality of Life at the End of the Treatment Period
eTable 3. Effect of Telmisartan on Safety Outcomes
eFigure 1. Effect of Telmisartan on C-reactive Protein
eFigure 2. Lack of Interaction Between Initial AAA Diameter Grouping and Effect f Telmisartan on AAA Growth Measured With Ultrasound
eFigure 3. Effect of Telmisartan on Risk of AAA Repair or Death From AAA Rupture
eFigure 4. Effect of Telmisartan on Ankle Brachial Pressure Index
eFigure 5. Effect of Telmisartan on Short Form-36 Physical Component Score
eFigure 6. Effect of Telmisartan on Short Form-36 Mental Component Score
eAppendix. TEDY Team, Collaborating Sites, and Investigators
Data Sharing Statement
References
- 1.GBD 2013 Mortality and Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117-171. doi: 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filardo G, Powell JT, Martinez MA, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2015;2(2):CD001835. doi: 10.1002/14651858.CD001835.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaikof EL, Dalman RL, Eskandari MK, et al. . The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2-77.e2. doi: 10.1016/j.jvs.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 4.Wanhainen A, Verzini F, Van Herzeele I, et al. ; Esvs Guidelines Committee . European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8-93. doi: 10.1016/j.ejvs.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 5.Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. 2019;16(4):225-242. doi: 10.1038/s41569-018-0114-9 [DOI] [PubMed] [Google Scholar]
- 6.Golledge J, Moxon JV, Singh TP, Bown MJ, Mani K, Wanhainen A. Lack of an effective drug therapy for abdominal aortic aneurysm. J Intern Med. 2020;288(1):6-22. doi: 10.1111/joim.12958 [DOI] [PubMed] [Google Scholar]
- 7.Sakaue T, Suzuki J, Hamaguchi M, et al. . Perivascular adipose tissue angiotensin II type 1 receptor promotes vascular inflammation and aneurysm formation. Hypertension. 2017;70(4):780-789. doi: 10.1161/HYPERTENSIONAHA.117.09512 [DOI] [PubMed] [Google Scholar]
- 8.Cassis LA, Gupte M, Thayer S, et al. . ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol. 2009;296(5):H1660-H1665. doi: 10.1152/ajpheart.00028.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krueger F, Kappert K, Foryst-Ludwig A, et al. . AT1-receptor blockade attenuates outward aortic remodeling associated with diet-induced obesity in mice. Clin Sci (Lond). 2017;131(15):1989-2005. doi: 10.1042/CS20170131 [DOI] [PubMed] [Google Scholar]
- 10.Iida Y, Xu B, Schultz GM, et al. . Efficacy and mechanism of angiotensin II receptor blocker treatment in experimental abdominal aortic aneurysms. PLoS One. 2012;7(12):e49642. doi: 10.1371/journal.pone.0049642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaschina E, Schrader F, Sommerfeld M, et al. . Telmisartan prevents aneurysm progression in the rat by inhibiting proteolysis, apoptosis and inflammation. J Hypertens. 2008;26(12):2361-2373. doi: 10.1097/HJH.0b013e328313e547 [DOI] [PubMed] [Google Scholar]
- 12.Xuan H, Xu B, Wang W, et al. . Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms. J Vasc Surg. 2018;67(2):573-584.e2. doi: 10.1016/j.jvs.2016.12.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bicknell CD, Kiru G, Falaschetti E, Powell JT, Poulter NR; AARDVARK Collaborators . An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomized placebo-controlled trial (AARDVARK). Eur Heart J. 2016;37(42):3213-3221. doi: 10.1093/eurheartj/ehw257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lange C, Sommerfeld M, Namsolleck P, Kintscher U, Unger T, Kaschina E. AT2R (Angiotensin AT2 receptor) agonist, compound 21, prevents abdominal aortic aneurysm progression in the rat. Hypertension. 2018;72(3):e20-e29. doi: 10.1161/HYPERTENSIONAHA.118.11168 [DOI] [PubMed] [Google Scholar]
- 15.Cruden NL, Witherow FN, Webb DJ, Fox KA, Newby DE. Bradykinin contributes to the systemic hemodynamic effects of chronic angiotensin-converting enzyme inhibition in patients with heart failure. Arterioscler Thromb Vasc Biol. 2004;24(6):1043-1048. doi: 10.1161/01.ATV.0000129331.21092.1d [DOI] [PubMed] [Google Scholar]
- 16.Moran CS, Rush CM, Dougan T, et al. . Modulation of kinin β2 receptor signaling controls aortic dilatation and rupture in the angiotensin II–infused apolipoprotein E–deficient mouse. Arterioscler Thromb Vasc Biol. 2016;36(5):898-907. doi: 10.1161/ATVBAHA.115.306945 [DOI] [PubMed] [Google Scholar]
- 17.Nishimoto M, Takai S, Fukumoto H, et al. . Increased local angiotensin II formation in aneurysmal aorta. Life Sci. 2002;71(18):2195-2205. doi: 10.1016/S0024-3205(02)01998-7 [DOI] [PubMed] [Google Scholar]
- 18.Furubayashi K, Takai S, Jin D, et al. . The significance of chymase in the progression of abdominal aortic aneurysms in dogs. Hypertens Res. 2007;30(4):349-357. doi: 10.1291/hypres.30.349 [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto S, Kamide K, Banno F, et al. . Impact of RGS2 deficiency on the therapeutic effect of telmisartan in angiotensin II–induced aortic aneurysm. Hypertens Res. 2010;33(12):1244-1249. doi: 10.1038/hr.2010.184 [DOI] [PubMed] [Google Scholar]
- 20.Imayama I, Ichiki T, Inanaga K, et al. . Telmisartan downregulates angiotensin II type 1 receptor through activation of peroxisome proliferator–activated receptor gamma. Cardiovasc Res. 2006;72(1):184-190. doi: 10.1016/j.cardiores.2006.07.014 [DOI] [PubMed] [Google Scholar]
- 21.Jones A, Deb R, Torsney E, et al. . Rosiglitazone reduces the development and rupture of experimental aortic aneurysms. Circulation. 2009;119(24):3125-3132. doi: 10.1161/CIRCULATIONAHA.109.852467 [DOI] [PubMed] [Google Scholar]
- 22.Golledge J, Cullen B, Rush C, et al. . Peroxisome proliferator–activated receptor ligands reduce aortic dilatation in a mouse model of aortic aneurysm. Atherosclerosis. 2010;210(1):51-56. doi: 10.1016/j.atherosclerosis.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris DR, Cunningham MA, Ahimastos AA, et al. . Telmisartan in the management of abdominal aortic aneurysm (TEDY): the study protocol for a randomized controlled trial. Trials. 2015;16:274. doi: 10.1186/s13063-015-0793-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 25.Parr A, Jayaratne C, Buttner P, Golledge J. Comparison of volume and diameter measurement in assessing small abdominal aortic aneurysm expansion examined using computed tomographic angiography. Eur J Radiol. 2011;79(1):42-47. doi: 10.1016/j.ejrad.2009.12.018 [DOI] [PubMed] [Google Scholar]
- 26.Thomas Manapurathe D, Moxon JV, Krishna SM, et al. . Cohort study examining the association between blood pressure and cardiovascular events in patients with peripheral artery disease. J Am Heart Assoc. 2019;8(6):e010748. doi: 10.1161/JAHA.118.010748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moxon JV, Ng E, Lazzaroni SM, et al. . Circulating biomarkers are not associated with endoleaks after endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 2018;67(3):770-777. doi: 10.1016/j.jvs.2017.06.090 [DOI] [PubMed] [Google Scholar]
- 28.Rowbotham SE, Krishna SM, Moran CS, Golledge J. Fenofibrate and telmisartan in the management of abdominal aortic aneurysm. Curr Drug Targets. 2018;19(11):1241-1246. doi: 10.2174/1389450119666171227224655 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Adherence to Allocated Medication
eTable 2. Effect of Telmisartan on Health-Related Quality of Life at the End of the Treatment Period
eTable 3. Effect of Telmisartan on Safety Outcomes
eFigure 1. Effect of Telmisartan on C-reactive Protein
eFigure 2. Lack of Interaction Between Initial AAA Diameter Grouping and Effect f Telmisartan on AAA Growth Measured With Ultrasound
eFigure 3. Effect of Telmisartan on Risk of AAA Repair or Death From AAA Rupture
eFigure 4. Effect of Telmisartan on Ankle Brachial Pressure Index
eFigure 5. Effect of Telmisartan on Short Form-36 Physical Component Score
eFigure 6. Effect of Telmisartan on Short Form-36 Mental Component Score
eAppendix. TEDY Team, Collaborating Sites, and Investigators
Data Sharing Statement