Abstract
Introduction
Medullary thyroid carcinoma (MTC) is a neuroendocrine thyroid carcinoma with parafollicular C cell differentiation. It can occur in either sporadic or hereditary form. Surgery is still the only curative treatment. The efficacy of chemotherapy and radiotherapy is poor.
Methods
This was a retrospective study of 31 patients treated surgically for MTC in our oncology centre at Mansoura University between January 2008 and February 2019.
Results
The mean age at diagnosis was 39.9 years. The median pathological size was 4cm. Multifocal disease was found in 12 patients and extrathyroid extension in 3 cases. Twenty patients were pathologically node positive. The median number of positive lymph nodes was four. Seven cases were metastatic at diagnosis. Local recurrence occurred in six individuals while distant recurrence occurred only in one. The median time from surgery to local recurrence was 12 months. The estimated mean disease free survival was 56.5 months. Disease free survival was significantly related to age, metastasis and side of nodal spread.
Conclusions
In our study cohort, the disease occurred predominantly in women and younger patients. Age, distant metastasis and nodal spread were the most significant prognostic factors. This study has also demonstrated that prognosis is not only affected by nodal involvement but also by side of involvement. The role of hemithyroidectomy in node negative unifocal disease with a small tumour size warrants further investigation.
Keywords: Thyroid cancer, Medullary carcinoma, Neck dissection, Survival, Recurrence
Introduction
Medullary thyroid carcinoma (MTC) is the third most common subtype of thyroid carcinoma and it carries the second worst prognosis after anaplastic thyroid cancer. It is a neuroendocrine thyroid carcinoma with parafollicular C cell differentiation.1–4
Macroscopically, the tumour is well circumscribed and non-capsulated. It has a firm consistency and a chalky white or red colour. Microscopically, MTC shows nests, sheets or trabeculae of cells with round, polygonal or spindle morphology, separated by fibrovascular stroma with characteristic amyloid deposition. The cells have round or oval nuclei with coarse chromatin as well as finely granular cytoplasm. Immunohistochemical staining shows positivity for calcitonin in most cases. Chromogranin A, synaptophysin and calcitonin gene related peptide are mostly positive as well.2,5–9
Patients with MTC should undergo proper initial evaluation. Thorough history taking and physical examination must be performed. Details of symptoms of hyperparathyroidism, pheochromocytoma and carcinoid syndrome should be elicited, and a thorough family history should be obtained.
Neck ultrasonography is performed to evaluate the thyroid gland and cervical lymph nodes. MTC exhibits ultrasonographic features that are similar to other thyroid malignancies (eg calcifications, hypoechogenicity, spiculated margins and internal vascularity). However, in MTC cases, nodules tend to be larger and more cystic in nature with a more homogeneous solid component. The calcifications may be coarser than in papillary carcinomas. MTC appears cold on iodine imaging because it does not concentrate iodine. The role of fluorodeoxyglucose positron emission tomography is debatable. Neck and chest computed tomography (CT) as well as abdominal triphasic CT or magnetic resonance imaging should be performed in patients with nodal metastasis or elevated serum calcitonin (>400pg/ml). Biochemical evaluation involves serum calcitonin, carcinoembryonic antigen, calcium and urine or plasma metanephrine to exclude other components of MEN type 2 syndrome.1,4,9,10–14
Methods
This was a retrospective study. The institutional registry at the oncology centre at Mansoura University was searched for medullary thyroid cancer patients who underwent surgical intervention between January 2008 and February 2019. Patients unfit for operative treatment were excluded. The objectives of the study were assessment of disease epidemiology, pattern of recurrence and metastasis. The primary outcome measure was disease free survival while the secondary outcome measures were development of recurrence and associated metastasis.
The data were analysed using SPSS® version 22 (IBM, New York, US). Survival analysis was performed using Kaplan–Meier curves and significance was determined by employing the logrank test. A p-value of <0.05 was considered statistically significant.
Results
Thirty-one patients were operated on during the study period. Two-thirds of these (61.3%) were female. The mean age at diagnosis was 39.9 years. Three cases had a family history of MTC. All of the patients underwent total thyroidectomy and 25 underwent selective bilateral neck dissection (II, III, IV, Va, VI).
The median hospital stay was four days. The median pathological size was 4cm. Multifocal disease was found in 12 while extrathyroid extension was detected in 3 cases. The median number of lymph nodes retrieved was 24 and the median number of positive nodes was 4. Twenty patients were pathologically node positive (ipsilateral in 15 of these cases). Seven cases were metastatic at diagnosis. Adjuvant chemotherapy was used for seven patients while adjuvant radiotherapy was employed for nine. There was local recurrence in three cases and one individual developed distant recurrence. In addition, another three metastatic patients with complete local control encountered local failure. The median time from surgery to local recurrence was 12 months (Table 1).
Table 1.
Basic patient demographics and treatment data
| Parameter | Data |
|---|---|
| Sex | 12 male (38.7%), 19 female (61.3%) |
| Mean age at diagnosis | 39.9 years (SD: 12.96 years) |
| Family history of MTC | 3 (9.7%) |
| Neck dissection | 25 SND (80.6%), 1 MBND (3.2%), 5 no lymphadenectomy (16.1%) |
| Median length of hospital stay | 4 days (range: 1–13 days) |
| Median pathological size | 4cm (range: 1–7cm) |
| Tumour site | 13 right, 10 left, 7 both |
| Tumour focality | 17 unifocal, 14 multifocal |
| Extrathyroid extension | 3 (9.7%) |
| Median number of retrieved nodes | 24 (range: 5–56) |
| Node positive patients | 20 (64.5%) |
| Median number of positive nodes | 4 (range: 0–32) |
| Side of positive nodes | 15 ipsilateral, 5 contra/bilateral |
| Site of positive nodes | 7 lateral, 13 lateral and central |
| Metastasis | 7 (22.6%) |
| Stage | 1 stage I, 6 stage II, 1 stage III, 20 stage IV |
| Adjuvant chemotherapy | 7 (22.6%) |
| Adjuvant radiotherapy | 9 (29.0%) |
| Recurrence | 3 local, 1 distal |
| Progression | 6 local, 1 distal |
| Median time to recurrence | 12 months (range: 3–50 months) |
MBND = modified bilateral neck dissection; MTC = medullary thyroid carcinoma; SD = standard deviation; SND = selective neck dissection
There was no incidental discovery of contralateral tumour foci. All of the patients had normal thyroid function. The postoperative course was uneventful in 30 cases. Only one individual developed haematoma with internal jugular vein thrombosis following surgery. There was no documented in-hospital mortality.
Disease free survival
The estimated mean disease free survival was 56.5 months. Disease free survival was significantly related to age, metastasis and side of nodal spread (Table 2, Fig 1).
Table 2.
Factors affecting disease free survival (DFS)
| Parameter | Estimated mean DFS | p-value |
|---|---|---|
| Sex | Male: 32.4 months Female: 56.6 months |
0.96 |
| Age | ≤40 years: 81.4 months >40 years: 21.0 months |
0.001 |
| Tumour focality | Unifocal: 65.6 months Multifocal: 29 months |
0.34 |
| Node side | Ipsilateral: 67.9 months Contra/bilateral: 2.4 months |
0.001 |
| Node site | Lateral: 45.2 months Lateral and central: 48.0 months |
0.73 |
| Nodal spread | No: 30.7 months Yes: 49.9 months |
0.24 |
| Metastasis | No: 73.0 months Yes: 0 months |
0.00 |
Figure 1.
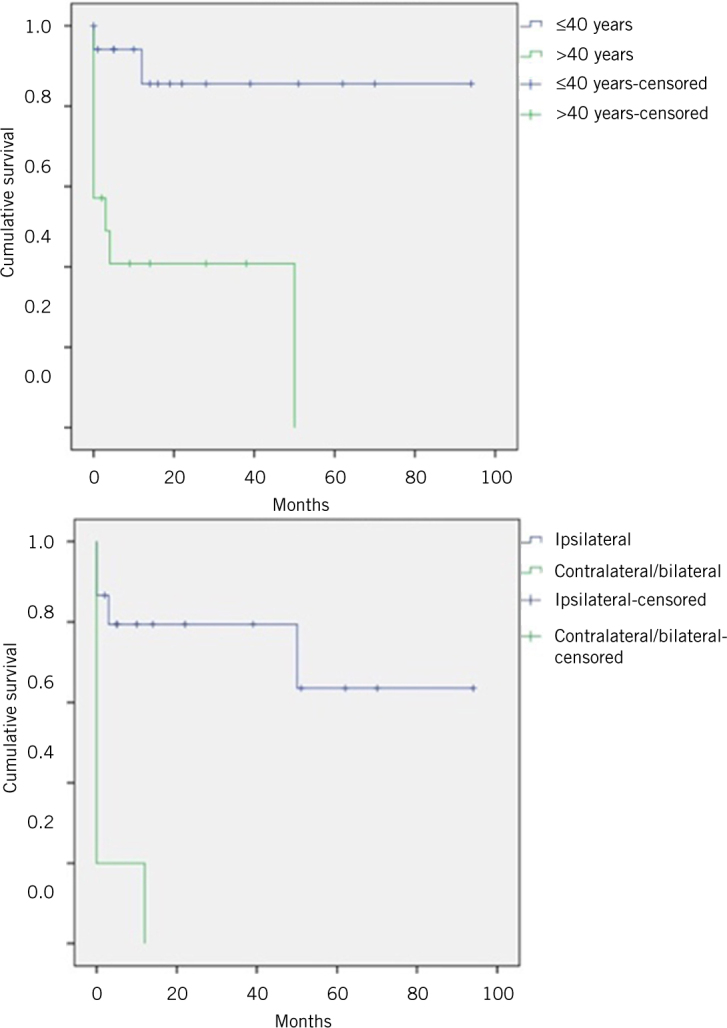
Kaplan–Meier curves showing disease free survival of medullary thyroid cancer patients by age group and distribution of node positive disease
Recurrence
True recurrence (defined by reappearance of medullary cancer either locally or at distant sites after clinical cure) was encountered in only four cases. However, none of the studied variables correlated significantly with recurrence.
Metastasis
Metastasis was found in seven patients; in six of these, it was present at the time of primary diagnosis and treatment. Factors that correlated significantly with metastasis included age >40 years, which carried a 12-fold increased risk, and presence of contralateral/bilateral nodal spread, with a risk that was 26 times higher. Those who developed metastasis were 35 times more likely than other patients to receive adjuvant chemotherapy (Table 3). Three individuals had single site metastasis (bony, hepatic and suprarenal metastasis) and four had metastasis at multiple sites (bone, lung, axillary node, suprarenal and liver).
Table 3.
Comparison between patients who developed distant metastasis and those who remained without remote disease
| Non-metastatic | Metastatic | Odds ratio | p-value | |
|---|---|---|---|---|
| Sex | 0.8 | 1.0 | ||
| Male | 9 | 3 | ||
| Female | 15 | 4 | ||
| Age | 12 | 0.028 | ||
| ≤40 years | 16 | 1 | ||
| >40 years | 8 | 6 | ||
| Tumour site | 3.7 | 0.30 | ||
| One lobe | 20 | 4 | ||
| Two lobes | 4 | 3 | ||
| Tumour focality | 1.1 | 1.0 | ||
| Unifocal | 13 | 4 | ||
| Multifocal | 9 | 3 | ||
| Extrathyroid extension | 2 | 1.0 | ||
| Absent | 8 | 2 | ||
| Present | 2 | 1 | ||
| Side of positive nodes | 26 | 0.014 | ||
| Ipsilateral | 13 | 2 | ||
| Contra/bilateral | 1 | 4 | ||
| Site of positive nodes | 1.1 | 1.0 | ||
| Lateral | 5 | 2 | ||
| Lateral and central | 9 | 4 | ||
| T stage | – | 0.18 | ||
| 1 | 3 | 3 | ||
| 2 | 8 | 2 | ||
| 3 | 9 | 1 | ||
| N stage | 4.2 | 0.37 | ||
| 0 | 10 | 1 | ||
| 1 | 14 | 6 | ||
| Median number of positive nodes | 3 | 12 | – | 0.14 |
| Median lymph node ratio | 0.15 | 0.58 | – | 0.17 |
| Adjuvant chemotherapy | 35 | 0.004 | ||
| No | 14 | 1 | ||
| Yes | 2 | 5 | ||
| Adjuvant radiotherapy | 0.9 | 1.0 | ||
| No | 9 | 3 | ||
| Yes | 7 | 2 |
Discussion
MTC is a unique form of neuroendocrine thyroid carcinoma, which arises from parafollicular C cells. C cells account for 1% of thyroid cells and are responsible for calcitonin production. MTC makes up 3–5% of thyroid carcinoma cases. It usually presents with a thyroid nodule, in the upper to middle third of the right or left lobes. Distant metastasis usually spreads to the lung, brain or liver and it has a profound negative impact on survival.
The disease can occur either in sporadic or hereditary form (20–30%)15 as a part of MEN type 2 syndrome as well as non-MEN familial MTC. Nearly all patients with hereditary MTC have RET proto-oncogene mutation, which is also observed in up to 50% of sporadic cases.2,16 Sporadic MTC with no RET mutation may show HRAS, KRAS or, rarely, NRAS mutations. MTC neither concentrates radioactive iodine, nor is it suppressed by levothyroxine treatment.1,2,9,14,15–20
Sporadic MTC generally affects patients aged 40–60 years.7,14 In our study, the mean age at diagnosis for the total cohort was 39.9 years. It tends to arise as a unilateral solitary tumour while hereditary MTC usually occurs in a bilateral multicentric pattern. Unifocal disease existed in just over half of our patients (n=17, 54.8%) and the disease was limited to one lobe (even if multifocal) in three-quarters (n=24, 77.4%).
Surgery is the main treatment for MTC and it can be cured if no residue is left behind. All patients with MTC should undergo total thyroidectomy and at least prophylactic central neck dissection, which offers a better cure rate than thyroidectomy alone, as well as better biochemical control. If the central nodes are involved and in cases with bulky tumours, the patient should also undergo prophylactic lateral neck dissection. Therapeutic lateral neck dissection is indicated if there is clinical or radiological lateral compartment involvement. Postoperative radiotherapy is indicated if there is residual gross neck disease. Vandetanib and cabozantinib are the preferred treatment for non-resectable local recurrence or metastatic disease. The efficacy of chemotherapy and radiotherapy is poor.1,5,9,12,16,18,21–25
Usually, 70% of patients with clinically detectable MTC have cervical lymph node involvement while the incidence of distant metastasis is nearly 10%.14 In our study, 64.5% of patients had positive nodes and 22.6% had metastasis. For cases in which ultrasonography showed disease confined to a single lobe, no pathological carcinoma foci were found in the other lobe. This suggests that hemithyroidectomy may be a feasible option in a certain group of patients.
Age, sex, local invasion, angioinvasion, nodal and distant metastasis, postoperative calcitonin and completeness of surgical resection are the most significant prognostic factors for MTC.7,14,17–19,26 In our study cohort, not only the presence of nodal deposits but also the side of involvement was of prognostic importance. It was evident that deposits in contralateral lymph nodes indicate a worse prognosis despite there being no difference in staging category using the eighth edition of the American Joint Committee on Cancer staging system.27 In our series, those aged >40 years had significantly lower disease free survival.
The incidence of skip lateral cervical nodal metastasis in the absence of central node involvement is 10%, being 25% for upper pole tumours.14 Another interesting finding among our patients was that there were no cases of central node metastasis in the absence of lateral neck disease.
It is acknowledged that this study has limitations. These include the small number of patients and the study cohort being confined to only surgical candidates. Furthermore, it was a retrospective study and there was no routine preoperative calcitonin measurement.
Conclusions
MTC is a rare but unique form of thyroid cancer, with a distinct origin and a specific treatment strategy. In our population, it occurred predominantly in women and younger patients (mean age: 39 years). Age, distant metastasis and nodal spread are the most significant prognostic factors for MTC. This study has also demonstrated that prognosis is not only affected by presence nodal involvement but also by side of involvement. The role of hemithyroidectomy as sufficient surgical treatment for node negative unifocal disease with a small tumour size warrants further investigation.
References
- 1.Konstantinidis A, Stang M, Roman SA, Sosa JA. Surgical management of medullary thyroid carcinoma. Updates Surg 2017; : 151–160. [DOI] [PubMed] [Google Scholar]
- 2.Matias-Guiu X, De Lellis R. Medullary thyroid carcinoma: a 25-year perspective. Endocr Pathol 2014; : 21–29. [DOI] [PubMed] [Google Scholar]
- 3.Simões-Pereira J, Bugalho MJ, Limbert E, Leite V. Retrospective analysis of 140 cases of medullary thyroid carcinoma followed-up in a single institution. Oncol Lett 2016; : 3870–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tyer NM, Yu R. Medullary Thyroid Carcinoma. : Braunstein GD. Thyroid Cancer. New York: Springer; 2012. 171–187. [Google Scholar]
- 5.Chakravarthy S, Jacob PM. Medullary Thyroid Carcinoma. : Parameswaran R, Agarwal A. Evidence-based Endocrine Surgery. Singapore: Springer; 2018. 141–150. [Google Scholar]
- 6.DeLellis RA. Medullary Thyroid Carcinoma. : Hunt JL. Molecular Pathology of Endocrine Diseases. New York: Springer; 2010. 103–121. [Google Scholar]
- 7.Hughes MS, Assadipour Y. Medullary Thyroid Carcinoma. : Pasieka JL, Lee JA. Surgical Endocrinopathies. Cham, Switzerland: Springer; 2015. 69–77. [Google Scholar]
- 8.Pitman MB, Oertel YC, Geisinger KR. Medullary Thyroid Carcinoma. : Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology. Cham, Switzerland: Springer; 2010. 117–128. [Google Scholar]
- 9.Raue F, Frank-Raue K. Epidemiology and Clinical Presentation of Medullary Thyroid Carcinoma. In: Raue F. Medullary Thyroid Carcinoma. Cham, Switzerland: Springer; 2015. 61–90. [DOI] [PubMed] [Google Scholar]
- 10.Ganeshan D, Paulson E, Duran C et al. Current update on medullary thyroid carcinoma. Am J Roentgenol 2013; : W867–W876. [DOI] [PubMed] [Google Scholar]
- 11.Heilo A, Sigstad E, Grøholt K. Medullary Thyroid Carcinoma. : Heilo A, Sigstad E, Grøholt K. Atlas of Thyroid Lesions. New York: Springer; 2011. 183–193. [Google Scholar]
- 12.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Thyroid Carcinoma. Version 2.2019. Fort Washington, PA: NCCN; 2019. [Google Scholar]
- 13.Valderrabano P, Klippenstein DL, Tourtelot JB et al. New American Thyroid Association sonographic patterns for thyroid nodules perform well in medullary thyroid carcinoma: institutional experience, systematic review, and meta-analysis. Thyroid 2016; : 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells SA, Asa SL, Dralle H et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; : 567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pacini F, Castagna MG, Cipri C, Schlumberger M. Medullary thyroid carcinoma. Clin Oncol 2010; : 475–485. [DOI] [PubMed] [Google Scholar]
- 16.Griebeler ML, Gharib H, Thompson GB. Medullary thyroid carcinoma. Endocr Pract 2013; : 703–711. [DOI] [PubMed] [Google Scholar]
- 17.Erovic BM, Kim D, Cassol C et al. Prognostic and predictive markers in medullary thyroid carcinoma. Endocr Pathol 2012; : 232–242. [DOI] [PubMed] [Google Scholar]
- 18.Moley JF. Medullary thyroid carcinoma: management of lymph node metastases. J Natl Compr Canc Netw 2010; : 549–556. [DOI] [PubMed] [Google Scholar]
- 19.Pazaitou-Panayiotou K, Chrisoulidou A, Mandanas S et al. Predictive factors that influence the course of medullary thyroid carcinoma. Int J Clin Oncol 2014; : 445–451. [DOI] [PubMed] [Google Scholar]
- 20.Waguespack SG, Rich TA, Perrier ND et al. Management of medullary thyroid carcinoma and MEN2 syndromes in childhood. Nat Rev Endocrinol 2011; : 596–607. [DOI] [PubMed] [Google Scholar]
- 21.Elisei R, Schlumberger MJ, Müller SP et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013; : 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim BH, Kim IJ. Recent updates on the management of medullary thyroid carcinoma. Endocrinol Metab 2016; : 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maia AL, Wajner SM, Vargas CV. Advances and controversies in the management of medullary thyroid carcinoma. Curr Opin Oncol 2017; : 25–32. [DOI] [PubMed] [Google Scholar]
- 24.Polistena A, Sanguinetti A, Lucchini R et al. Timing and extension of lymphadenectomy in medullary thyroid carcinoma: a case series from a single institution. Int J Surg 2017; : S70–S74. [DOI] [PubMed] [Google Scholar]
- 25.Prokopakis E, Doulaptsi M, Kaprana A et al. Treating medullary thyroid carcinoma in a tertiary center. Current trends and review of the literature. Hippokratia 2014; : 130–134. [PMC free article] [PubMed] [Google Scholar]
- 26.Valderrabano P, Simons S, Montilla-Soler J et al. Medullary Thyroid Carcinoma. In: Nasir A, Coppola D. Neuroendocrine Tumors: Review of Pathology, Molecular and Therapeutic Advances. New York: Springer; 2016. 117–140. [Google Scholar]
- 27.Amin MB, Edge S, Greene F et al. AJCC Cancer Staging Manual. 8th edn New York: Springer; 2017. [Google Scholar]


