Abstract
Pulmonary sarcomatoid carcinoma (PSC) is a unique, highly invasive pulmonary malignancy with a poor prognosis, representing 0.1–0.4% of all malignant lung tumors. Because of its highly aggressive character and propensity for frequent metastasis, PSC shows low response rates to traditional treatments such as chemotherapy, radiotherapy, and neoadjuvant therapy. In recent years, considerable progress has been made in gene sequencing, targeted therapies, and immunotherapies. One of the most promising treatment approaches is the selection of mono-targeted or multi-targeted drugs according to tumor gene-mutation sites, such as epidermal growth factor receptor or vascular endothelial growth factor receptor 2 (EGFR/VEGFR2), anaplastic lymphoma kinase (ALK), and others. Another approach is the activation of therapeutic anti-tumor immunity via pathways including programmed cell-death protein-1/programmed cell-death ligand-1 (PD-1/PD-L1), which has been used in individual cases. In this review, we will introduce the clinicopathologic features, molecular typing, and traditional treatments. We will also review the biological characteristics and the latest therapies for PSC. These novel therapies show promise in the management of PSC, and the outcomes of investigative trials will hopefully reveal a variety of treatment options for patients with PSC.
Keywords: biological characteristics, clinicopathological characteristics, molecular typing, pulmonary sarcomatoid carcinoma (PSC), therapies
Introduction
Pulmonary sarcomatoid carcinoma (PSC) is a unique, highly invasive, biphasic type of pulmonary cancer with a poor prognosis, it represents 0.1–0.4% of all malignant tumors of the lung.1–3 PSC may represent an epithelial neoplasm derived from a single clonal tissue; other tumors of this type include cancerous interstitial malignancy, malignant transformation of epithelial and mesenchymal cells, hamartoma, tissues with carcinosarcomatoid changes, and sarcoma carcinogenesis.4 The morphological changes of the primary tumor foci are reminiscent of the phenomenon of epithelial–mesenchymal transition (EMT), and it is worth noting that biphasic PSCs are potentially derived from EMT, particularly tumors composed of spindle and giant cells,5–7 which are associated with highly aggressive and conventional treatment-refractory behavior.1 Arshi et al. reported a case of sarcomatoid transformation in a moderately differentiated squamous-cell carcinoma,8 which verified the EMT process. To further explore this process, related genes have been identified, including the Twist and ZEB genes, and the membrane protein kinase DDR2 gene.9 On the basis of the 2015 World Health Organization classification, PSC can be divided into five subgroups: pleomorphic carcinoma (PC), spindle-cell carcinoma (SCC), giant-cell carcinoma (GCC), carcinosarcoma, and pulmonary blastoma.10 In contrast to other types of lung cancers, recent retrospective studies demonstrated that PSC shows frequent genetic mutations.11 Patients are almost always in the middle or advanced stages when they are diagnosed; these patients often do not have the opportunity to undergo surgical treatment.12 Furthermore, PSC is highly resistant to first-line chemotherapy drugs.13,14 These features account for its poor overall survival (OS) of PSC,11 which is 20% lower than that of other types of non-small cell lung cancer (NSCLC), whose OS is 45%.15 There is a lack of randomized controlled trials and long-term follow-up data due to the rarity of PSC.16 The treatment of this rare cancer faces huge challenges, and it is difficult to match the current standard research methods. Sufficient knowledge about this tumor type is not available. Therefore, it is necessary to explore promising treatments, so that patients with this rare malignancy can benefit from the current scientific research advances in the treatment of common tumors and data from retrospective studies.6 This review summarizes the current research on the clinicopathological characteristics, genetic mutations, and comprehensive therapies for PSC.
Clinicopathological characteristics
Clinical features
Patients with PSC commonly present with symptoms of dyspnea, cough, hemoptysis, bloody sputum, chest pain, and weight loss, often present after a median duration of approximately 1 month for clinical evaluation and diagnosis.10,17 The median age of patients with PSC is 68 years and the median tumor size is 5 cm.18 Patients are more commonly male (32/53) and smokers (51/53)19 with a history of moderate-to-heavy tobacco consumption, which may promote the development of carcinoma, although smoking status has not been shown to affect OS (p > 0.05).7,20,21 These demographic features are the same for tumors of simple or mixed cell types.22 Clinically, patients are typically diagnosed at an advanced stage, with a mean age of onset ranging from 59 to 61 years.23 Li et al. reported 38 patients with PSC, the median survival was 21 months, while 1-year, 3-year, and 5-year survival rates were 68.4%, 31.6%, and 18.4%, respectively.24 Other studies reported similar overall 5-year survival rates, that is, approximately 11–25%,9,19,25 the median reported OS of 148 patients was 22.5 months (range from 0.9 months to 102.4 months).14 Some studies demonstrated a fairly low rate of 5-year survival in PSC (pathological stage [pStage] I: 16.3%, n = 49) compared with other NSCLCs (pStage I adenocarcinoma 81.8%, and pStage I squamous-cell carcinoma 70.2%).3
In patients with advanced disease, OS has no correlation with race, age, sex, smoking, tumor size, N stage, pleural invasion, or lymphatic metastasis.12,18,20 Ung et al. showed that adjuvant chemotherapy did not have a significant effect on prognosis.16 Other factors, such as the neutrophil-to-lymphocyte ratio (NLR) can also be used in patient evaluations.22 Histologic type, time from symptom onset to diagnosis, tumor invasiveness, tumor growth, distant metastases, P stage, extent of surgical resection, vascular/lymphatic invasion, and gene mutations such as programmed cell death ligand-1 (PD-L1), TLG-P, and KRAS mutations are independent prognostic factors in PSC.7,10,12,20,24,26–28 Patients with pStage >II showed a lower survival probability compared with the patients with lower-stage disease.3 Patients with pN0 disease had a high rate of vascular invasion (57.1%), indicating that distant metastases may often occur in the early stage; this phenomenon might explain why N stage does not affect prognosis.12,23,28,29 The presenting complications of PSC are similar to those of other lung cancers. In addition, tumor metastasis often includes various involved organs, such as the lung, adrenal glands, pleura, brain, bone, or liver, but may also include unusual anatomic sites, such as subdiaphragmatic nodes, kidney, peritoneum, pancreas, skin, and right ventricle.4,30 Approximately 62% of patients had more than two metastatic locations.17,31 When there are rare distant metastases, the survival rate is extremely low (survival time: 3–5 months for skin metastases), and only palliative treatments or the experimental and targeted drugs can be used for patients.32
Imaging characteristics
Computed tomography (CT) diagnosis of PSC by Qin et al. is summarized as follows:33
(1) Peripheral tumors are more common than central ones, and lesions are always confined to the upper lobe.
(2) Tumors are usually huge, with well-defined boundaries, and are round or quasi-circular. However, lobulated tumor lesions may occur due to an unbalanced tumor growth rate; burr sign is rare.
(3) Plain CT shows dense soft tissue in the tumor, which may be accompanied by large areas of patchy necrosis. Due to uneven tumor necrosis, irregular thick-walled cavities or multiple small, wall-less cavities within the areas of necrosis can also occur.
(4) In enhanced CT, most masses show edge-ring-shape enhancement or uneven patchy enhancement.
(5) Peritoneal masses with local or distant metastases are common, and subpleural lesions tend to invade the chest wall or pleura.
(6) Intratumoral calcification is rare. Therefore, CT is useful only for preliminary identification of tumors, and histopathology and immunohistochemistry are the gold standards for diagnosis.
Positron emission tomography CT (PET-CT) examination can be performed if the patient has multiple metastases or the indication for enhanced CT is unclear. There is no statistically significant difference between the central and peripheral maximal standard unit value (SUVmax) in patients with PSC. There is no significant difference in SUVmax of I, II, and III in tumor–node–metastasis staging either. In addition, there is no great correlation between SUVmax and sex/age/smoking history/serum tumor markers/tumor size in PSC.25
Some studies indicate that SUVmax of PET-CT is strongly correlated with the level of PD-L1 expression and KRAS mutation status in PSC, indicating that SUVmax can be used to evaluate PD-L1 expression and KRAS mutation status.25 PD-L1 expression is detected in 21 patients, with a positive rate of 87.5%, and the SUVmax in PET-CT of 17 samples with PD-L1 expression levels is equal to or greater than 50%, which is higher than that of tumors with expression levels less than 50%.25 Six patients with KRAS gene mutation showed a higher SUVmax than that of patients without mutation.25
Pathological classification
The biological behavior and clinicopathological characteristics of PSC are not well documented.27 The diagnosis of PSC is only used for surgically removed specimens.34 According to early studies, PSC with mesenchymal differentiation shows more aggressive clinical features of early local invasion and distant metastasis compared with other NSCLCs, despite being diagnosed at early stages.1,20 Most of the genes involved in EMT,6,7 such as vimentin, are overexpressed in PSCs. However, immumohistochemical staining of tumors should also include epithelial markers [epithelial membrane antigen (EMA), cytokeratin], TTF-1, P40, and negative markers (e.g. primary antibodies for mesenchymal cells, melanocytes, and sarcomatous cells).35,36 Other representative markers for sarcomatous elements to assist in making a differential diagnosis, include desmin and sarcomeric actin/smooth muscle.15,36 Based on the Ackerman Surgical Pathology, chapter: Lung and pleural37 and Clinicopathological diagnosis and differential diagnosis of tracheal, pulmonary, pleural and mediastinal diseases, chapter: Trachea and lung tumors,34 the characteristics of each sarcomatoid carcinoma types are described below.
Pleomorphic carcinoma
Pleomorphic carcinoma is the most common type of PSC, accounting for more than 50%.35 It accounts for 2–3% in all lung cancer specimens removed surgically.34 Two types of pleomorphic carcinoma exist, including a giant- and spindle-cell type,36 which are named similarly to those of thyroid tumors.37 Pleomorphic carcinoma consists of epithelial carcinoma and sarcomatoid elements. Epithelial carcinoma is composed of traditional NSCLCs (squamous-cell carcinoma, adenocarcinoma, adenosquamous-cell carcinoma, and large-cell undifferentiated carcinoma).35,38 Sarcoma-like cells usually account for at least 10% of the tumor tissue and consist of spindled or giant cells, which can present as one or a mixture of the two cell types.35,38 Pleomorphic carcinomas can also be composed entirely of spindled and giant tumor cells, without an epithelial component, and contain similar cell types to those of malignant fibrohistiocytomas.35,38 The microscopic characteristics of SCC are described in detail below. Immunohistochemistry will make the expression of a certain component in PC more significant. If the component of non-polymorphic cancer cells is obvious, the diagnosis can be made even if spindle cells or giant cells do not express CK.34 The polymorphic components express Vimentin and actin cross-linking protein (fascin) in addition to CK, and also express related markers (Napsin A, TTF-1, P63 and desmoglycin) in varying degrees.34
Spindle-cell carcinoma
SCC is mainly composed of malignant spindle cells;37 however, epithelial features can still be identified through morphologic, electron microscopic, and immunohistochemical methods.38 Spindle-cell elements consist of fusiform malignant cells that are positive for epithelial antibody markers. For example, EMA, cytokeratin 7 (CK7), cytokeratin (CK), carcinoembryonic antigen (CEA), and other antibodies are used.2,15 The spindle cell consists of spindle cells, whose cytoplasm is eosinophilic. Under the microscope, it can be seen that the cells are mainly arranged in sheets, bundles, and spindles. The larger the epithelial-like cells are, the larger the nucleoli are, and the chromatin is often blistered or rough. Spindle-shaped tumor cells can be mixed with mild-to-moderate lymphocytes as the main inflammatory cells.39 Malignant spindle cells are usually composed of spindle molecules and eosinophilic cytoplasm and are arranged in bundles and sheets on histopathology. However, morphologic variation can also be seen: long, thin cells with a fibroblastic appearance can be seen admixed with epithelioid components, and the cells have large nuclei with prominent nucleoli.37 Vascular infiltration is common in SCC, and vascular walls on histopathologic tissue sections can be stained by pan-cytokeratin, which is the key to making a diagnosis.35
Giant-cell carcinoma
Similarly, GCCs are composed entirely of giant cells.38 GCC is a morphologic rather than a pathologic phenotype, can occur in any lung lobe, and has a poor prognosis. The prevalence rates are the upper lobe (52.7%) is greater than lower lobe (21.7%), which is greater than middle lobe (4.9%), which is greater than main bronchus (4.3%), and which is greater than overlapping lesions (1.1%).40 Cells are usually composed of highly pleomorphic giant tumor cells arranged in a solid sarcoma-like pattern. Most tumors are located in the periphery, and the lesions are usually widespread at diagnosis. Some cases are associated with focal adenoid differentiation and mucous production, indicating that some tumors are dedifferentiated adenocarcinoma.37 Giant cells are usually abundant, with eosinophilic cytoplasm, large and irregular nuclei, lobed nuclei, or multiple nuclei,35,39 and rough or vesicular chromatin. Neutrophil infiltration, necrosis, bleeding, and vascular infiltration are common.34 It is important that these tumors should be distinguished from benign giant-cell tumors of bone.41
Carcinosarcoma
Carcinosarcoma is mainly gray–white with hemorrhage and necrotic masses.34 Carcinosarcoma consists of traditional NSCLC and heterologous sarcoma-like types. Traditional types are usually composed of squamous-cell carcinoma, followed by adenocarcinoma; the others are adenosquamous carcinoma and large-cell carcinoma.34 Sarcomas containing squamous-cell carcinoma components are often of a central type, growing in the bronchi, and carcinosarcoma containing adenocarcinoma components is often peripheral.34,37 These tumors can also have both giant osteoclast-like cells and a sarcomatous component with separations that can be clear or indistinct, similar to what is observed on histopathology of angiosarcomas, liposarcoma, osteosarcomas, chondrosarcomas, and rhabdomyosarcomas,38 the first two kinds of sarcoma are relatively rare.34,37 Cases identified by morphologic, immune-histochemical, and molecular biologic techniques have shown that these are different manifestations of the same biologic phenomenon; tumor cells can lose some or all epithelial markers and acquire mesenchymal tissue markers;37 thus, it is further confirmed that the tumor originated from EMT. Immunohistochemical staining showed that TTF-1, Napsin A, and CK labeled adenocarcinoma components. P63, P40, and CK5/6 labeled squamous-cell carcinoma components. S-100, desmin and myogenin mark chondrosarcoma and rhabdomyosarcoma components.34 For carcinosarcoma with high-grade fetal adenocarcinoma, β-catenin is expressed on the cell membrane.34
Pulmonary blastoma
In a study of biphasic sarcomatoid carcinomas of the lung, Manzotti et al. found that PSC could originate from EMT.6 Pulmonary blastomas are defined as biphasic tumors containing fetal adenocarcinomatous tissues (poorly differentiated or highly differentiated) with primitive mesenchymal stroma, and sometimes they also contain differentiated sarcomatous tissues.37 The epithelial component is characterized by an irregularly branched glandular structure lined with pseudostratified columnar cells, clear cytoplasm, and minimal nuclear atypia.37 Microscopically, pulmonary blastomas are characterized by well-differentiated fetal adenocarcinoma and abundant mesenchymal components that are typically composed of undifferentiated small, oval, or spindle cells.34 This is similar to what is seen in pseudoglandular fetal lungs. In up to one third of cases, cellular atypia consistent with fetal or conventional adenocarcinoma can be found, although the appearance is generally bland.35,38 The overall appearance is similar to that of 10~16-week-old fetal lung tissue and is similar to Wilms’ tumors. Subnuclear and supranuclear cytoplasmic vacuoles can be seen in many cells. Solidly cellular masses (mulberries) are commonly rich in cells with eosinophilic cytoplasm, accounting for about 50%.34,37 Scattered neuroendocrine cells occur in 67% of patients.34 However, the nuclei of these mulberries are often bright with a frosted hyaline appearance caused by the accumulation of biotin, and the epithelial cells are rich in glycogen. The stromal components can show skeletal muscle, cartilage, and bone differentiation. Gastrointestinal and yolk sac differentiation can be seen in individual cases, and occasionally malignant melanomatous tissues can be found.37 Immunohistochemical staining showed that CK, CK7, CEA, EMA and TTF-1 were diffusely positively expressed, and β-catenin was expressed on the nucleus. chromatin A, syn, vimentin and peptide hormones were focal positive. Mesenchymal embryonic cells express vimentin and specific actin, desmin, myogenin and S-100 are expressed in heterologous rhabdomyosarcoma and chondrosarcoma, respectively.34 Compared with females, carcinosarcomas are more common in males with a history of smoking.33,42 From 1995 to 2011, only one review reported 42 cases of biphasic pulmonary blastoma (CBPC), showing that it is extremely rare among lung carcinomas.43
Table 1 summarizes the above characteristics of this tumor type, as what has been previously reported by Yi and Zhang,44 Baldovini et al.,35 Rossi et al.,36 Rosai37 and Wang et al.34 In summary, the heterogeneity of PSC tissues determines the complexity of differential diagnoses when dealing with these tumors. There are also many genetic changes associated with the various tissue types.
Table 1.
Sarcoma components, non-pulmonary sarcoma components, genetic characteristics, and immunohistochemistry of PSC.
| classification | Components | Immunohistochemistry | |
|---|---|---|---|
| Pleomorphic carcinoma (PC) |
NSCLCs:
adenocarcinoma, squamous-cell carcinoma, adenosquamous-cell carcinoma, and large-cell undifferentiated carcinoma |
Sarcoma:
sarcoma-like components usually make up at least 10% of the tumor tissue and are composed of spindle or giant cells, either of the two, or a mixture of the two |
Typical representative:
CKs (CK5/6, CK, CK7, CK14,
CK18, CK19), EMA, TTF-1
and Naspin (in case of adenocarcinoma
differentiation), P40 and
high-molecular-weight CKs (in case of
squamous-cell carcinoma) Others: composition expression of: (1) squamous-cell carcinoma and adenocarcinoma: PCK, SPA (+) (2) sarcoma cells: vimentin (+) constantly, CK(+) focally, S-100, desmin, α-SMA, CD117 and PDGF-α(–) |
| Spindle-cell carcinoma | Malignant spindle cells are usually composed of spindle molecules and eosinophilic cytoplasm, arranging in bundles sheets |
Typical representative:
CKs (CK, CK5/6, CK7, CK14,
CK18, CK19, CK20), EMA,
TTF-1 and napsin (in case
of adenocarcinoma differentiation), P40
and high-molecular-weight CKs (in case
of squamous-cell carcinoma) Others: PCK, vimentin, P53(+) strongly, P63(+), Ki-67:70~90% Desmin, S-100, α-SMA, CD34 and CD117(–) |
|
| Giant-cell carcinoma | The cancer cells are composed of large, highly pleomorphic tumor cells arranged in a solid, sarcoma-like pattern; dense neutrophil infiltrations can be seen inside and between tumor cells, and can be associated with neutrophilia; most tumors are peripheral, and in some cases, focal adenoid differentiation and mucinous production are present |
Typical representative:
CKs (CK7, CK20),
EMA, TTF-1 (+/–) and
napsin (in case of adenocarcinoma
differentiation), P40 and
high-molecular-weight CKs (in case of
squamous-cell carcinoma) Others: PCK, keratin, vimentin, CD45 and NSE(+), GFAP(–) |
|
| Carcinosarcoma |
NSCLCs:
adenocarcinoma or squamous-cell carcinoma |
Sarcoma:
specific fibrosarcoma or malignant fibrohistiocytoma, or similar to chondrosarcoma, osteosarcoma, rhabdomyosarcoma, or angiosarcoma |
Typical representative:
CKs (CK5/6, CK7),
EMA, TTF-1 and
napsin (in case of adenocarcinoma
differentiation), P40 and
high-molecular-weight CKs (in case of
squamous-cell carcinoma), Markers of sarcomatous
differentiation: rhabdomyosarcoma myogenic marker(+);
osteosarcoma S-100(+), high-grade fetal
adenocarcinoma sarcoma: β-catenin is
expressed on the cell
membrane Others: PCK, P63, CAM5.2(+) |
| Pulmonary blastoma | Pulmonary blastoma is a bipolar tumor consisting of primitive epithelium similar to that of well-differentiated fetal adenocarcinoma and primitive mesenchymal matrix containing high nucleo-plasma ratio blastocytoid cells |
Typical representative:
CKs, CK7, TTF-1, and
napsin expression in the
adenocarcinoma; vimentin in
blastematous stroma (myogenic markers in case of
rhabdomyosarcoma component) Others: SP-A; mulberry-like structure: GATA-6, Syn, NSE, CgA, CD10 and biotin(+); uncertainties: vimentin, desim, SMA, MSA, myoglobin, S-100(+) possibly; germinoma: AFP, PLAP(+), β-catenin is expressed on the nucleus |
|
NSCLC, non-small-cell lung cancer; PSC, pulmonary sarcomatoid carcinoma.
Genetic characteristics
Although gene sequencing has been reported in detail for an ever increasing number of common lung cancers, the molecular typing and biological characteristics of lung sarcomatoid carcinoma, and the presence or absence of targeted gene mutations remain unclear.15,45 Studies have shown that chromosomes of sarcomatoid carcinomas (1q, 3q, 7, 8q, and 19), chromosomes of pleomorphic carcinomas and SCC (5p, 9q, 11q, 12p, 13q and 17q), and in giant-cell carcinomas, both chromosomes (13p and 15p) have changed.34 SCC and pleomorphic carcinoma have TP53, KRAS and EGFR mutations, MET and FGFR2 amplification, EML4-ALK gene rearrangement,34 etc. The most common change of gene in carcinosarcoma is TP53 mutation, while KRAS and EGFR mutations are relatively rare.34 Both low-grade fetal lung adenocarcinoma and lung blastoma are associated with missense mutations in exon 3 of the CTNNB1 gene, causing large expression of β-catenin protein in the cytoplasm to enter the nucleus to activate Wnt signaling pathway activity.46
Changzhou First People’s Hospital (Jiangsu province, China) enrolled 32 PSC patients retrospectively and conducted a cohort study. Among these patients, EGFR, KRAS, and MET mutation rates were 8%, 22%, and 16%, respectively, and the tumor suppressor genes and the top two mutations were TP53 (69%) and RB1 (25%). About 40.6% (13/32) of Chinese patients with PSC were shown to have high tumor mutational burdens (TMBs).47 Similarly, another study showed that the gene mutations rates of TP53, KRAS, and MET were 70%, 30–40%, and 13–20%, respectively.48 In the research by Lococo et al., 49 PSC cases were studied using next-generation sequencing (NGS) analysis of 26 potentially relevant genes in surgically resected tumor samples. A total of 39 PSC cases (80%) showed at least one mutation (with the highest mutation rates in TP53, KRAS, PTEN, KDR, ERBB2 and PIK3CA), and 69% of variants were somatic mutations.3 Transversions accounted for 60%, followed by transitions (33%), the least common gene mutation was indels, representing 7%.3 Frameshift variant accounted for 5.3%.3 In another experiment, 57 somatic mutation events were detected in the PSCs with a total of 1167 mutations. In these 1167 mutations, missense variants, splice variants and gain variants were accounted for 73.7%, 14% and 7%, respectively. TP53, KRAS and PTEN were the top three mutated genes with mutation rates of 80%, 35% and 35%, respectively.6 In a cohort of PSC cases, 69% carried at least one mutation, representing a higher rate of mutation compared with other NSCLCs. Two or more mutations have been reported to co-exist (co-mutated tumors) in retrospective studies. In addition, a large diversity of alterations has been reported, based on NGS, including mutations in TP53, KRAS, PIK3CA, EGFR, NOTCH1, CDKN2A, CDKN2B, STK11, PTEN, NF1, and BRAF. There are several novel mutated genes, including LAMB4, CDH4, RASA1, CDK4, LMTK2, CDH7, BRCA1, BRCA2, SCAF1 and SMARCA4), ALK translocations, and MET alterations, have also been described,3,11,13,15,21,49–52 along with EGFR, TP53, PI3KCA, and other mutations. Patients with PSC showing a gene mutation had a lower rate of survival than those without mutations;3 multi-gene mutations were associated with a higher mortality rate than single-gene mutations. Based on Fallet et al.’s research, KRAS mutations repel EGFR mutations, while EGFR and NOTCH1 mutations tended to correlate.21 Compared with biphasic PSCs, pure tumors more commonly showed mutations of APC and KRAS. TP53 showed the opposite trend, with a higher rate of mutation in biphasic tumors. PTEN and STK11 mutations occurred specifically in bipolar PSC.3 There were no EGFR, TP53, or KRAS mutations in non-tumor areas.21 Vieira et al. reported that blood vessel invasion had no correlation with PIK3CA, MET, or KRAS mutations.23 In Eastern Asian countries, some studies have demonstrated that EGFR, KRAS, and EML4-ALK fusion genes play vital roles in the nosogenesis of lung cancers.20 Therefore, we will describe several important gene mutations in detail.
TP53 and P53R2
TP53 mutations are much more frequent in pure PSC than in mixed type tumors,15 TP53 mutations were found in approximately three quarters of patients.19 P53R2 plays a dual role in the regulation of carcinoma, including tumor suppression, such as promoting cell apoptosis and inhibiting cell proliferation; these pathways rely on the P21 signal pathway. A high rate of P53R2 protein was associated with poor OS;53 we can conclude that the presence of P53R2 is an important factor associated with poor prognosis in patients with PSC,53 and P53R2 expression is closely associated with the occurrence, development, and progression of PSC.
KRAS
Surprisingly, the rate of KRAS mutation is higher in pure PSC, occurring at a rate of 56% among 49 cases of PSC.3 Codon 12/13 mutation in KRAS was the second most common mutation in the series by Mehrad et al. and it accounts for 43.4% of the entire cohort and representing a higher proportion of PSCs with adenocarcinoma components (approximately 46.6%);19 this indicates that PSCs are genetically more closely related to adenocarcinoma. In PSC cases, KRAS mutations seem to correlate with more aggressive tumor behavior,25 and were identified only in smokers, KRAS transversion mutations account for 79%.3 Chen et al. stated that KRAS mutations would reduce the efficacy of chemotherapy drugs and oral targeted drugs in NSCLC.54 However, whether the KRAS mutation is related to poor prognosis and drug resistance in PSC is still unclear. In the future, more studies on KRAS mutation should be conducted to better define its role in PSC.1
MET
In NSCLC, MET exon-14 skipping accounts for approximately 1–3% of cases, PSC shows a higher incidence (ranging from 9.5% to 22%).54 Activating MET signaling pathway participates in many oncogenic processes, including invasion, metastasis, and EMT in PSC, especially when the PSCs lack complete ALK and MET co-amplification.1,55 MET exon-14 skipping might be a driver of oncogenic mutation and contribute to aggressive clinical behavior and drug-sensitive mutations. Therefore, targeted therapy such as crizotinib,6,19,49 one type of MET inhibitor,13,16 may represent a positive development for the treatment of PSC with a MET mutation. Small interfering ribonucleic-acid silencing of MET or MET inhibition, like crizotinib, reduced tumor proliferation and viability. Furthermore, the drug can also reduce the downstream activation of AKT and mitogen-activated protein kinase in H596 cells and Hs746T.50 Though we predicted that it may be effectively targeted through specific tyrosine kinase inhibitors (TKIs), MET amplification remains an ‘Achilles heel’ in PSC.56
EGFR
VEGF expression in early precancerous lung lesions could facilitate tumor development.21 Previous cases have shown that EGFR mutations could be detected in 8.8% of patients with lung sarcomatoid carcinoma.51 Adenocarcinomas with EGFR mutations are less likely to have high-grade sarcomatoid transformation than those with KRAS or ALK mutations, possibly because adenocarcinomas with EGFR mutations typically have low-level properties, lepidic growth areas, and a more indolent course than EGFR(–) tumors.45 EGFR mutation plays an important role in the poor prognosis of NSCLC compared with classical NSCLC, which may explain the poorer outcome of PSC.57 However, the incidence of EGFR mutation in patients with PSC in China is low. Moreover, due to the lack of clinical and cytological studies to explore the significance of the mutation and the value of the application of this targeted drug further, the efficacy of related drugs is uncertain.
PD-1/PD-L1
Retrospective studies reported a high incidence of PD-L1 expression in patients with PSC.7 Velchet et al. reported that 69.2% (9/13) of patients were positive for PD-L1.58 Tobacco using, KRAS mutations, vascular invasion, CD163 positive cell level, and TTF-1 positivity were independent factors correlating with PD-L1 expression in lung cancer.13,59 A total of 54 patients (36.5%) were positive for PD-L1 and 36 of 148 patients showed CD47 and PD-L1 expression.14 Multivariate analysis showed that CD47 and PD-L1 co-expression is an independent negative prognostic factor for the assessment of OS.14,60 There is a significant correlation between PD-L1 expression and CD47 and PD-L1 co-expression, the density of CD8(+) T lymphocytes (p = 0.004, 0.012) and CD68(+) macrophages (p = 0.026, 0.034) is higher.14 Notably, in the sarcoma component, overexpression of PD-L1 is correlated with the presence of tumor-infiltrating macrophages and lymphocytes.59 In Vieira’s report, 25 out of 75 (27%) patients showed PD-L1 expression in both sarcomatoid and epithelial areas. The rate of expression in sarcomatous areas only is 13% (n = 10/75), the rate for simple epithelial areas is similar.59 PD-L1 overexpression provides a biological basis for immunotherapy in patients with PSC. However, due to the lack of relevant case reports, we could not identify whether it is a potential therapeutic option for this type of disease. Further in vitro and in vivo research should be conducted to demonstrate whether immunotherapy is efficient for treating PSC, with the aim of providing patients with alternative treatments.
Polygenic mutation or fusion
Previous case reports have reported the following combinations: EGFR together with TP53 mutations; EML4-ALK fusion with a TP53 mutation; a positive EGFR mutation combined with PD-L1 overexpression; KRAS mutations in conjunction with TP53 mutations; JAK3 variant with a mutation in TP53; PIK3CA mutation plus KRAS mutation; and PHF20-NTRK1 fusion.7,45,52 There also have case report of KRAS/TP53/STK11 co-mutations.61 It’s worth noting that the combination of KRAS and either TP53 or KRAS mutations independently correlated with OS in multivariable models. Both the degree of mutation in KRAS and the presence of TP53 or KRAS mutations are associated with recurrence, especially in the context of local metastasis at relapse.3 Some studies have suggested that substantial tumor angiogenesis may lead to mutations in multiple genes, such as NF1, TP53, and others.11 Mutations in the above genes lead to overexpression of proto-oncogenes and reduced expression of tumor-suppressor genes, which are involved in the occurrence and development of tumors. For patients with multiple gene mutations or gene fusion, it is necessary to explore the correlation between these genes so that we can combine these findings and apply them to the development of targeted drugs with multiple genes.
In this review, we described the characteristics of gene detection for PSCs and confirmed the high TMB of these tumors. For the genetic changes mentioned above, the therapeutic schedules, survival analyses, and prognoses of various targeted drug therapies are described in detail below.
Treatments
Because PSC is resistant to traditional chemotherapy and shows a low response to radiotherapy, complete tumor resection is a key for patients with PSC, especially those with localized tumors.4 Concerning comprehensive chemotherapy with radio- and neoadjuvant therapy, further exploration and support from evidence-based medicine and clinical evidence are needed.3 A low rate of mutations among Asians suggests that the clinical benefit from anti-EGFR drugs in PSC could be limited. It seems that the inhibition of ALK gene expression is an invalid strategy as well.50 However, biological targeted therapy may have broader potential application than traditional therapy.24 KRAS gene mutation may be sensitive to CDK 4/6 inhibitor (Abemaciclib) or MEK inhibitors (trametinib).62 TP53 mutations may be associated with the sensitivity of VEGF/VEGFR inhibitors. Immune-checkpoint inhibitors (ICIs) can also benefit patients with this disease.47 Antiangiogenic therapy is another critical therapy for PSC patients.
Traditional treatments and neoadjuvant therapies
PSC is an unconventional lung carcinoma that is currently managed with standard treatments used for conventional NSCLC (surgery, radiation therapy and chemotherapy),3 summarized as follows.
Patients with early-stage tumors should consider surgery first;54 tumor size is the most important prognostic factor.63 In one study, the median OS of the entire cohort (n = 37) was 5.69 years and the median disease-free survival (DFS) was 3.15 years; the rates for 5-year OS and DFS for the entire cohort were 50.3% and 45.2%, respectively.22 Early detection of tumors, combined with surgery and adjuvant treatment, may extend the survival of patients with lung cancer.4 Complete tumor excision in the early stages with proper follow-up care and perioperative chemotherapy are common therapies bringing satisfactory results.12,64 Compared with those treated with surgery, patients treated without surgery show an obviously worse prognosis.64 A clinical study by Kaira et al. showed that none of the patients enrolled in the study received adjuvant therapy; nine underwent surgery, and six of them did not relapse, as of the data analysis, two patients developed postoperative disease progression or recurrence and one patient died of non-cancer-related disease (pneumonia) after surgery.64 In the study by Maneenil et al., 45 patients underwent complete surgical resection and there was an exact recurrence; of the 45 patients, 33 developed disease recurrence. The median DFS of these patients was approximately 8.19 months, whereas the 1-year, 2-year, and 5-year DSF was 32%, 27%, and 21%, respectively.7 When analyzing radiation therapy variables (n = 2060), results showed that the median and 5-year OS of patients who had received radiation therapy versus patients who had not received radiation therapy (20.6%) were 5 months (8%) versus 6 months (20.6%; p < 0.001).18 Among patients who did not undergo surgery, 1396 (48.8%) patients received radiation therapy, and their median 5-year survival rate was 4 months (3.4%), patients who did not receive radiation therapy had a 2-month shorter survival time (4.8%).18 Liang et al. predicted the survival times of PSC patients by using the nomogram model, proving that radiotherapy could achieve a slightly better prognosis for patients with stage I–III disease who were not suitable candidates for surgery and did have not obvious disease (4%).65 Stereotactic whole body radiotherapy for primary tumors can only provide general control of local primary lesions but has no obvious advantages for distant metastases and prognoses.66 However, there are no clear data on the efficacy of radiotherapy for the treatment of PSC, the amount of clinical benefit of radiotherapy that can bring to patients is not known either.
Generally speaking, even in early stages, PSC tends to have a worse prognosis, and patients undergoing any currently known treatment options, such as platinum-based chemotherapy and radiotherapy, seem to have a poor prognosis.45,62 Previous studies showed that patients with early-stage disease (peripherally located tumors, without distant metastasis, with lower T stage) had significantly better OS.7,12 Unfortunately, due to the highly aggressive peculiarity and propensity for metastasis, patients are often in advanced disease stages when diagnosed with PSC, and surgery is not a viable treatment option. Palliative anti-tumor therapy is often the best choice to prolong OS.12 Drug resistance can occur with platinum plus pemetrexed chemotherapy in a short time, resulting in tumor recurrence.52 The efficacy of combined adriamycin and ifosfamide chemotherapy to treat patients with metastatic PSC has been limited.63 For PSC patients treated with chemotherapy, the median progression-free survival (PFS) and OS were 2 months and 4–6 months, respectively.16,17 Palliative chemotherapy is considered for patients in poor physical condition; however, this therapy has not shown satisfactory results. Moreover, unexpected benefits can occur in patients with advanced disease, who use traditional Chinese medicine during palliative treatment, such as a Ma-xing-shi-gan or Tianma Gouteng Decoctions. In one case report, some symptoms were relieved, and a better quality of life was obtained;67 however, the true effectiveness is questionable since there is only one case reported in the literature to date.
For patients considering surgery, preoperative adjuvant therapy is often required due to tumor size or other factors; the median OS and 5-year OS of patients receiving neoadjuvant or both neoadjuvant and adjuvant radiotherapy was 11 months (28.6%, n = 43) and 9 months (15.1%, n = 43), respectively.63 For patients with stage IIb–IIIa disease, DFS was significantly improved in patients undergoing perioperative chemotherapy.31 In Maneenil et al.’s study, the authors showed that 24 patients with PSC receiving neoadjuvant and/or adjuvant chemotherapy had the longest median survival time of 18.6 months. They stated that surgery and neoadjuvant or adjuvant chemotherapy/radiotherapy were interrelated factors influencing patient prognosis and survival.7 Thus, neoadjuvant or adjuvant chemotherapy may be beneficial for patients with PSC who can undergo surgical resection.43 A total of 77 patients were studied in Vieira’s research: 20 of them received neoadjuvant chemotherapy. Among them, 11 patients showed partial response (PR), 8 patients were stable without progression, and 1 patient deteriorated, but this proportion may be overestimated due to the small number of cases and selection bias.23 According to Martinez et al.’s research, one patient underwent neoadjuvant chemotherapy and one patient underwent radiotherapy and chemotherapy: results showed that the goal of lowering tumor stage was not achieved.28 In Hou et al.’s investigation, for 45 of 114 patients receiving neoadjuvant or adjuvant chemotherapy, the survival rate was significantly improved compared with patients who did not receive neoadjuvant chemotherapy.12 Due to the small number of patients and individual differences, these data do not fully prove that neoadjuvant chemotherapy is ineffective.
To our knowledge, first-line chemotherapy most commonly includes pemetrexed, paclitaxel, docetaxel, gemcitabine, and vinorelbine. Age, sex, histological subtypes, stage, and surgical therapies have not been shown to observably affect the efficacy of chemotherapy.17 Retrospective studies have demonstrated that PSC is often refractory to chemotherapy regimens because of the poor sensibility to these types of tumors.7,28,68 However, compared with monotherapy without platinum-based drugs, the use of platinum-based combination chemotherapy can significantly improve OS, univariate analysis showed that median survival with platinum-based combination therapy was 5.6 months, and that with monotherapy was only 0.4 months.16,17 Patients treated with platinum based or platinum-free chemotherapy had no significant statistical differences regarding the rate of advanced disease.16,17 Since there are no large sample statistics, only case reports can be described. Xiong et al.69 reported that all of 32 patients with PSC received chemotherapy with gemcitabine combined with cisplatin (GP) or paclitaxel combined with cisplatin (TP). They had a median OS of 14 months and a PFS of 5 months. The remission rate was 21.9%. Initial stage IV diagnosis and a tumor diameter larger than 6 cm were independent factors associated with poor prognosis. In addition, patients with platinum-based combination therapy had significantly better quality of life than patients treated with monotherapy.17 Kong et al.70 reported three men with PSC: they were diagnosed with stage III–IV and treated with carboplatin, albumin-bound paclitaxel, as well as apatinib, together. Two of them achieved a PR, and the other man experienced disease progression in the 7th month. Li et al. reported a male patient with a histopathologic diagnosis of giant-cell carcinoma of the lung. He was treated with combined chemotherapy (gemcitabine and cisplatin) every 3 weeks for four cycles, starting 2 months after surgery, and was still alive and free from disease progression at 7 years’ post-surgery.62 Tamura et al. also reported that patients’ disease was well controlled and had no progression after receiving cisplatin plus gemcitabine.68 In other reports, the chemotherapy regimen of platinum combined with gemcitabine or platinum plus paclitaxel has been found superior to other combinations. No factors associated with good prognosis were found in multivariate analysis.17 In Lin et al.’s study, 18 patients with metastatic/recurrent disease received palliative chemotherapy. One of them was diagnosed with PSC, fibrosarcoma, and adenocarcinoma: the chemotherapy regimen for this patient was docetaxel plus cisplatin. Another patient was diagnosed with SCC: the chemotherapy regimen for this patient was cisplatin plus paclitaxel and doxorubicin. Both patients had a PR. The remaining patients received doxorubicin, ifosfamide, MAID and CYVADTIC, they were also treated with gemcitabine as salvage treatment weekly, but no objective effects were observed.20 A study showed that one patient was diagnosed with early metastatic recurrence after 2 years of initial surgery. She received three rounds of treatments with the combination therapy of cisplatin (or carboplatin) and etoposide. Though she experienced alternating periods of disease control and recurrence, she remains alive 7 years after her initial surgery.43 Therefore, cisplatin combined with gemcitabine chemotherapy can be an effective choice for the treatment of pulmonary pleomorphic carcinoma.68 Maneenil et al. reported that docetaxel was initiated as subsequent treatment for a patient with PSC, and disease progression was demonstrated after four cycles.7 Comparatively, the use of platinum-based doublet chemotherapy has a significant curative effect in patients with PSC.
Targeted therapy
The inherent histological heterogeneity and low frequency of these tumors may be responsible for the limited data on driving oncogenes. However, discovery of a high proportion of mutant alleles in the same tumor leads to an expectation of good efficacy with the usage of targeted therapy.21 In an earlier survey, the authors noted that one in five patients had hemoptysis at the time of diagnosis of malignancy. Recently, TKI has been recommended for the treatment of patients with EGFR mutations in NSCLC. For patients with EGFR mutations who were initially treated with EGFR TKI, the mean OS was longer than that with platinum-based combination chemotherapy.10 Based on the highly aggressive peculiarity of these tumors, antiangiogenic therapy is also a good choice. New ICIs have also been recommended as first-line treatments in select patients.
Tyrosine kinase inhibitors
EGFR TKIs
Patients with PSC generally respond poorly to chemotherapy, and targeted drug therapy efficacies appear to be largely unknown, as there are only a few case reports that indicate a PR in patients receiving gefitinib. For patients with EGFR-mutated tumors, the US Food and Drug Administration (FDA) has approved gefitinib, afatinib, and osimertinib for clinical therapies.48 The benefits of successfully treating patients with mutations by using first-generation TKI drugs, including gefitinib, erlotinib, icotinib, are limited. The second- and third-generation TKIs, such as afatinib and osimertinib, respectively,45,48,64,71 provide better benefits to patients with rare mutations compared with first-generation TKIs. Research by He et al. showed that afatinib combined with crizotinib could provide partial remission in patients with PSC.72 However, EGFR mutation rates in PSC patients are different among countries, for example, based on race and ethnicity; the rates of EGFR mutation can differ from patient to patient.73 TKIs can be used in patients with EGFR-mutated tumors; however, the therapeutic effects are modest and controversial.15 Researchers, such as Hsieh et al., have used TKIs to treat patients with EGFR mutations (with or without other gene mutations) and found the MET copy numbers and gene expression were increased. There might also have been MET amplification before treatment, so perhaps, for these patients, the effect of monotherapy is limited, which could be the reason for the resistance of drugs mentioned above.74
In Lin et al.’s research, three patients received gefitinib (one type of EGFR TKI) as a palliative rescue treatment, and their condition was stable for 2 months, 3 months, and 5 months.20 From what Li et al. reported, one patient was positive for EGFR mutation, and gefitinib could be used. After treatment, the patient’s clinical symptoms disappeared and the tumor stabilized for 4 months.24 Tamura et al. reported that one patient with PSC underwent surgery, and postoperative genetic testing demonstrated the absence of EGFR exon 19. The patient was treated with gefitinib after recurrence, the complete remission period was approximately 35 months.68 Zou et al. also reported that following treatment with 150 mg erlotinib once daily, disease progression with distant metastasis occurred after 6 months.75 In Ikushima et al.’s report, an elderly woman was diagnosed with SCC (stage IVb, T4N2M1) with an EGFR exon 19 deletion, and gefitinib was administered; however, the patients responded poorly to this EGFR TKI and progressed rapidly after a short-term response.51 In a retrospective study, it was found that most patients with PSC were unlikely to benefit from anti-EGFR therapy due to low EGFR mutation rates and high KRAS mutation rates.57 The efficiency of EGFR TKIs varies from person to person, but the overall effectiveness in patients with PSC is not as good as those with adenocarcinoma.3,48 Thus, EGFR receptor antagonists are not necessarily effective. It is speculated that the occurrence and development of SCC are not completely dependent on the EGFR signaling pathway, and other mechanisms could play a more important role in the development of SCC.51,57
Therefore, accurate gene detection and research concerning mechanism of EGFR in PSC are necessary, and multi-gene-targeted therapy may be able to solve the deficiencies associated with single-gene targeted therapy.
ALK TKIs
For the rearrangement of rare genes, such as those of the ALK1 gene, patients can take a combination of crizotinib and ceritinib.48 There are few case reports of patients with ALK rearrangements with differing results, but most patients had invalid or partial responses;48 however, there have been individual reports of patients with PRs.76 New ALK–TKI drugs, such as ceritinib, are more effective in patients with crizotinib insensitivity or resistance. Another potent and selective ALK inhibitor, aletinib, has a significant effect in patients with ALK-positive advanced lung cancer that is insensitive or resistant to crizotinib treatment. A new generation of ALK tyrosine kinase inhibitors is currently under clinical research and development.45 The FDA has approved the new ALK inhibitor, brigatinib, which is effective in 55% of patients with lung cancer who have mutations that are drug resistant to crizotinib, with a control rate of 86%. The second part of the clinical trial showed good prospects for brigatinib. However, the application of brigatinib in PSC has not yet been reported. In Liu’s research, based on the presence of MET amplification, crizotinib brought about rapid dramatic clinical improvement.50 Lin et al. showed a patient with ALK-rearranged advanced PSC treated with crizotinib who achieved PR for 7 months.2 Valter et al. presented a patient with ALK-positive carcinosarcoma who showed a good response to crizotinib, but there are little data provided in this report.77 It has been reported that when patients with PSC have mutation in KRAS-ex2, as well as fusion in EML4-ALK, chemotherapy and crizotinib are not effective.54 Crizotinib extends patient survival when tumors show isolated ALK gene fusions.78 ALK-TKI may have more benefits compared with chemotherapy, and applications in PSC warrant further exploration.
MET TKIs
MET gene mutations include amplification, point-mutation activation, and MET exon-14 skipping. The currently targeted drugs for MET mutations are crizotinib, capmatinib, tepotinib, and savolitinib (also known as AZD6094, HMPL-504, and volitinib, respectively). Han et al. reported that an older male patient with a MET exon-14-skipping mutation had a mutant allele frequency of about 73.9%. The recommended therapy was to administer 600 mg of savolitinib orally once per day, and the patient obtained a PFS period of more than 8 months.54 As the patient’s condition progressed, the research team found EGFR, fibroblast growth factor receptor 1 (FGFR1), and KRAS gene amplifications in tumor biopsies, from which it was speculated that targeted drug resistance could have been caused by the above genetic changes.54
Antiangiogenic therapy
Multivariate analysis indicated that vascular invasion reduced the survival time of patients with PSC.23 A high frequency of vascular invasion suggests that we need to be cautious when using bevacizumab in these patients. Li et al. reported one case of PSC treated with apatinib (VEGFR2 TKI). Due to the Eastern Cooperative Oncology Group (ECOG) score standard of 3, the patient was precluded from chemotherapy and was prescribed apatinib (250 mg) orally once per day. After 5 days of treatment, the symptoms of hemoptysis disappeared, and the patient’s chest tightness was alleviated. At 10 days later, the patient was able to breathe normally. Tumor regression was observed up to 14 months after targeted therapy. Surprisingly, there were no evident complications associated with apatinib.79 Li et al. reported a case in which the patient was administered apatinib (250 mg) orally once per day. Sustained tumor regression was observed after treatment, and no severe complications were reported to be associated with apatinib therapy.11 Apatinib may be another fruitful option for patients with conventional chemotherapy-resistant PSC. Antiangiogenic therapy is also an alternative for patients with advanced PSC.
Immune-checkpoint inhibitors
Programmed cell-death protein-1 (PD-1)/PD-L1 inhibition significantly enhances CD4+ and CD8+ T-lymphocyte cytokine secretion, cell proliferation, cytotoxicity, antitumor activity, and improves the clinical prognoses of patients with a variety of tumor types. It has been used as an adjuvant treatment in patients with advanced lung cancer.80 In patients with NSCLC, immunotherapy targeting the PD-1/PD-L1 pathway correlated with total mutations, KRAS mutations, and the EMT process.26,33 Patients are often prescribed this therapy after failing in a chemotherapy/radiotherapy regimen. Sarcomatoid differentiation with high expression of PD-1/PD-L1 appears to be widespread; therefore, patients with PSC can benefit greatly from ICI therapy. Patients with lung PSC had significantly higher PD-L1 expression (9/13) than NSCLC patients. Thus, PD-1/PD-L1 immunotherapy might be one of the most effective ways to treat this disease.81 Some researchers believed that PSC patients had significant differential expression of PD-L1 compared with that of NSCLC patients. When PD-L1 is negative, immunotherapy can still be effective due to the interaction between the immune system and the tumor.82 Since PD-L1 and PD-L2 are highly expressed in patients with stage III PSC, pembrolizumab could be used as a therapeutic option for patients with drug resistance after radiotherapy and chemotherapy.83 When PD-1 is highly expressed (more than 50%), nivolumab or pembrolizumab provide an obvious treatment effect in the short term, and patients can obtain a higher quality of life after pain relief. The patients still recovered well after a year of follow up.66,76,81 However, another study showed one patient with stage IV disease, who had received first-line and second-line chemotherapy, gained no benefit after surgery. After treatment with nivolumab, this patient’s symptoms improved, the brain damage resolved, and reactions occurred at other metastatic sites.84 Rajdev et al. reported that a patient with PSC, who lacked PD-L1 expression, was treated with nivolumab, and although the patient did not quit smoking, a continued decrease in tumor size was observed.4 Anti-PD1 can effectively control brain-tumor metastases.84 In a KEYNOTE-024 clinical trial, patients with PD-L1 tumor expression and a tumor proportion score (TPS) of >50% had higher survival rates after receiving pembrolizumab therapy compared with after receiving platinum-based dual chemotherapy.85 In another case report, half the tumor cells expressed PD-L1. The patient was treated with pembrolizumab at the time of relapse and PR was documented after six cycles of therapy.7 From what Sukrithan et al. reported in papers before, several cases showed that pembrolizumab can have a positive effect (PR to complete response) in PSC with high PD-L1 TPS.49,86 Studies have also shown that when the expression level of tumor PD-L1 is increased to more than 50%, the anti-tumor effect of the drug clone 22C3 (Merck Sharp & Dohme Ltd, UK) used in the phase I study of pembrolizumab is significant. Tumor lymphocyte infiltration is associated with positive PD-L1 expression and is a pre-existing immune response marker that has been used as an important predictor of the efficacy of these drugs.59 Domblides et al. reported about 37 patients, who had been treated with immunotherapy inhibitors (nivolumab) as the main drug. The results showed that regardless of PD-L1 status, the overall response rate (ORR) was 15/37 (40.5%) and the disease control rate was 24/37 (64.8%). The median OS was 12.7 months (range: 0.3–45.7), and the median expression of PD-L1 was 70% (0–100). For PD-L1 positive patients, the ORR was 58.8%, and for PD-L1 negative patients, the ORR was 0% (p = 0.44). The median TMB was 18 mutations/megabase (MMb), accounting for 87.5% of cases with high TMB.61 However, some reports also documented disease progression with targeted therapy, although the expression lever of PD-L1>50% and the use of pembrolizumab can stabilize disease.76 Yang et al. believe that PD-L1 expression does not affect clinicopathologic characteristics (univariate analysis), nor does it affect prognoses. Some clinical patients do not show an effective anti-PD-1/PD-L1 monotherapeutic response. Although the drugs could have significant positive effects, there are several adverse reactions, including autoimmune hepatitis, diarrhea, interstitial pneumonia, and thyroiditis, of which some could even be fatal.61 Recently, new targets have been discovered, such as integrin-associated protein (IAP, also known as CD47), and its expression was related to clinical stages.14 In addition, stage IV patients had higher CD47 expression (seven out of eight, 87.5%) compared with patients at lower stages of the disease. Therefore, PD-L1/CD47 co-expression was shown to be useful as a novel predictive marker, combined with dual-targeted immunotherapy.14 There is also evidence that when a MET gene-skipping mutation on exon 14 is present, there can exist a PR of pembrolizumab therapy.49
Based on the data above, ICI therapy is a potentially effective targeted therapy. Its therapeutic effect is far better than that of traditional chemoradiotherapy when patients have indications for the drugs. However, no specific reports are available regarding the treatment cycle, and determining whether a treatment dose should be adjusted, when a patient can stop the drugs after complete remission, and whether drug resistance exists or not. Due to the lack of large clinical data analyses, the practical application of such drugs can only be documented in case reports, with great heterogeneity. The OS rate and the benefit to patients need to be demonstrated further in clinical trials.
Others
As the most effective inhibitor of DDR2, saracatinib has been used in lung cancer. It is a broad-spectrum TIK. Dasatinib inhibits BCR-ABL kinases and SRC family kinases, as well as many other selective oncogenic kinases, including c-kit, ephrin (EPH) receptor kinases, and PDGF receptors. Studies have shown that in alveolar epithelial cells, this drug can inhibit TGFβ-mediated EMT, thus, it can be used for the prevention of pulmonary fibrosis recurrence, reversing EMT-mediated resistance to EGFR inhibitors in lung cancer, and inhibition of s-like cell proliferation in PSC.6 For rare multi-loci genetic changes, Chen et al. believe that when KRAS mutations and EML4-ALK rearrangements co-exist, TKIs, and first-line chemotherapy can be very beneficial in treating patients with PSC.54 Karim et al. reported an EML4-ALK translocation in 2/25 patients; 1 patient received crizotinib and obtained ideal OS.87 Genetic testing has suggested that a new PHF20-NTRK1 fusion mutation could be a driver of sarcomatous proliferation in patients with postoperative recurrences.52 Entrectinib/larotrectinib can be used to treat patients with this NTRK fusion mutation in solid tumors.52 Cai et al. reported that patients with TPM3-ROS1 fusion mutations could benefit for long periods after crizotinib therapy. Targeted drugs for ROS gene therapy include ceritinib, brigatinib, lorlatinib, cabozantinib, and entrectinib.88 For the patients with TP53/KRAS/STK11 co-mutations, the focus on the targeted therapy against KRAS and STK11 is used. STK11 is mutated in ‘cold tumors,’ which can be manifested as cerebral ischemic resistance and reduced CD8+ T cell infiltrations. For patients with low PD-L1 expression (less than 50%), ICI as a first-line therapy combined with other treatments needs to be further verified.61 At the 2019 American Society of Clinical Oncology annual meeting, AMG 510 was described as a new small-molecule inhibitor that targets KRAS G12C mutations (13% of KRAS G12C mutations have been observed in cases of advanced NSCLC). Abstracts published at the European Society of Medical Oncology Immuno-Oncology Conference in Neva, Italy, on 12 December 2019, described first-line AMG 510 treatments that improved OS, PFS, and ORRs in patients with metastatic non-squamous NSCLC. Recently, the FDA granted priority review of a new drug, selpercatinib (loxo-292), an oral RET kinase inhibitor for the treatment of advanced RET fusion-positive NCSLC. No clinical trials have been reported on these targeted drugs in the treatment of PSCs with the above, rare, genetic mutations, and therefore, the therapeutic effect is unclear. Additionally, whether drug combinations could improve responses is not known.14 Similarly, whether there is a difference in the responses of PSC patients administered either PD-1 or PD-L1 inhibitors, is unclear.
Conclusion
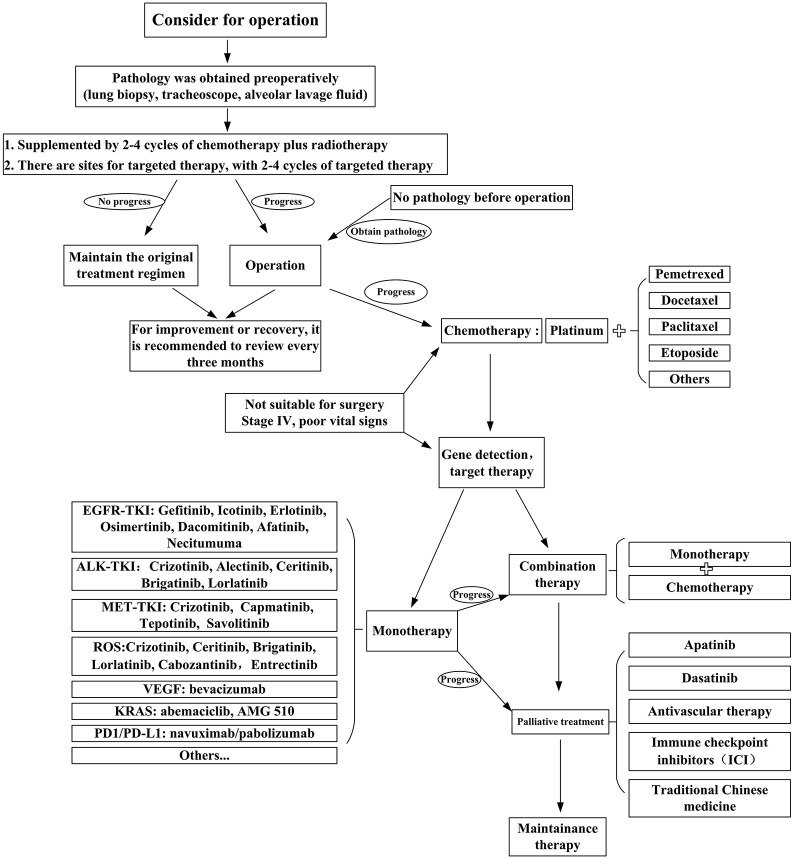
The poor prognosis of PSC is primarily due to its biological characteristics and high resistance to classical first-line chemotherapy. Based on a better understanding of the carcinogenic mechanisms of this rare tumor, the development of innovative therapeutic strategies is needed.17 Surgery is the key to OS. For patients with PSC, eligibility for surgical treatment, neoadjuvant or adjuvant chemotherapy based on the patient’s ECOG score assessment can also be applied,31 despite the fact that these tumors have high resistance to conventional chemotherapy. However, platinum-based dual-drug chemotherapy can provide PR for patients. Based on characteristics such as early-stage metastasis and chemotherapy resistance, it is also important to detect molecular alterations that may improve drug efficacy and prolong patient survival.89 Targeted therapy and immunotherapy are currently new research fields in PSC. Further in vivo and in vitro experiments are needed, as well as clinical trials. Patients with PSC should be treated with appropriately targeted drugs and immunosuppressive agents on the basis of gene detection and on the premise of meeting indications.7 Although the therapeutic options for these patients are limited, some benefits from crizotinib therapy compared with conventional chemotherapy have been observed. In the near future, second-generation ALK inhibitors, such as serratinib, alectinib, and brigatinib, and third-generation ALK inhibitors, such as laratinib, may prolong the survival time of patients with PSC with ALK rearrangement compared with the first-generation ALK inhibitors, such as crizotinib.2 For PSC patients, the early application of antiangiogenic therapy and ICIs can maximize patient treatment responses, significantly improving the quality of life of these patients, expanding the treatment regimens for PSC and other rare diseases, and provide a lifeline for desperate patients. Antiangiogenic targeted drugs like apatinib and monoclonal antibodies such as bevacizumab can be also used for crizotinib-resistant patients. PD-L1/PD1 overexpression and a high degree of vascular invasiveness provide rationales for the use of ICIs.16 MET can be targeted in PSC, which can guide the therapy of patients with a MET mutation. Furthermore, targeted therapies, such as TP53 mutation reversal drugs, CDK 4/6 inhibitor, and MEK inhibitor may become potent treatment options in the future.62 Figure 1 shows the flow chart of treatment for patients with PSC, which is summarized based on various articles and case analyses. Researchers in this field need to make joint efforts to improve the diagnosis and treatment of PSC in order to identify therapies that can bring maximal benefit to patients.
Figure 1.
Flow chart for the treatment of PSC.
The primary treatment of PSC is surgical resection. Patients can be treated preoperatively with radiotherapy or targeted therapy. Adjuvant chemotherapy and/or targeted therapy are recommended after surgery. Patients need to be reviewed every two to four cycles postoperatively. If the disease progresses, it is necessary to change the treatment plan. For patients with poor physical condition, palliative treatment should be provided to prolong the life of the patient as much as possible. Early use of antivascular therapy combined with immunotherapy maximizes patient treatment responses.
PD-1, programmed cell-death protein 1; PD-L1, programmed cell-death ligand 1; PSC, pulmonary sarcomatoid carcinoma; TKI, tyrosine kinase inhibitor.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclose receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by grants from the National Natural Science Foundation of China (81773207), the Natural Science Foundation of Tianjin (17YFZCSY00840, 18PTZWHZ00240, 19YFZCSY00040), and the Special Support Program for High Tech Leader and Team of Tianjin (TJTZJH-GCCCXCYTD-2-6). Funding sources had no role in study design, data collection, and analysis, in the decision to publish, or in the preparation of the manuscript.
Contributor Information
Xin Li, Department of Lung Cancer Surgery, Tianjin Medical University General Hospital, Tianjin, China.
Di Wu, Department of Lung Cancer Surgery, Tianjin Medical University General Hospital, Tianjin, China.
Hongyu Liu, Tianjin Key Laboratory of Lung Cancer Metastasis and Tumor Microenvironment, Tianjin Medical University General Hospital, Tianjin, China.
Jun Chen, Department of Lung Cancer Surgery, Tianjin Key Laboratory of Lung Cancer Metastasis and Tumor Microenvironment, Tianjin Lung Cancer Institute, Tianjin Medical University General Hospital, Anshan Road no.154, Heping District, Tianjin 300052, China.
References
- 1. Shum E, Stuart M, Borczuk A, et al. Recent advances in the management of pulmonary sarcomatoid carcinoma. Expert Rev Respir Med 2016; 10: 407–416. [DOI] [PubMed] [Google Scholar]
- 2. Lin L, Huang F, Chen F, et al. Anaplastic lymphoma kinase (ALK)-rearranged pulmonary pleomorphic carcinoma successfully treated with crizotinib. J Int Med Res 2018; 46: 3491–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lococo F, Gandolfi G, Rossi G, et al. Deep sequencing analysis reveals that KRAS mutation is a marker of poor prognosis in patients with pulmonary sarcomatoid carcinoma. J Thorac Oncol 2016; 11: 1282–1292. [DOI] [PubMed] [Google Scholar]
- 4. Rajdev K, Siddiqui AH, Ibrahim U, et al. Sarcomatoid carcinoma of the lung presenting as localized bronchiectasis: a case report and review of literature. Respir Med Case Rep 2018; 24: 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yoshida Y, Sibusa T, Ishii Y, et al. Granulocyte colony-stimulating factor- and interleukin-6-producing large-cell carcinoma of the lung with sarcomatoid changes suggestive of epithelial-mesenchymal transition: an autopsy case report. Intern Med 2019; 58: 3305–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manzotti G, Torricelli F, Benedetta D, et al. An epithelial-to-mesenchymal transcriptional switch triggers evolution of pulmonary sarcomatoid carcinoma (PSC) and identifies dasatinib as new therapeutic option. Clin Cancer Res 2019; 25: 2348–2360. [DOI] [PubMed] [Google Scholar]
- 7. Maneenil K, Xue Z, Liu M, et al. Sarcomatoid carcinoma of the lung: the Mayo clinic experience in 127 patients. Clin Lung Cancer 2018; 19: e323–e333. [DOI] [PubMed] [Google Scholar]
- 8. Arshi J, Sauer M, Yin F. Rapid sarcomatoid transformation of lung squamous cell carcinoma after neoadjuvant therapy: a case report. Anticancer Res 2020; 40: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 9. Yu Y, Zhang Q, Zhang J, et al. Prevalence of MET exon 14 skipping mutation in pulmonary sarcomatoid carcinoma patients without common targetable mutations: a single-institute study. J Cancer Res Ther 2019; 15: 909–913. [DOI] [PubMed] [Google Scholar]
- 10. Sim JK, Chung SM, Choi JH, et al. Clinical and molecular characteristics of pulmonary sarcomatoid carcinoma. Korean J Intern Med 2018; 33: 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen J, He Q, Liu J, et al. CD8+ tumor-infiltrating lymphocytes as a novel prognostic biomarker in lung sarcomatoid carcinoma, a rare subtype of lung cancer. Cancer Manag Res 2018; 10: 3505–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou J, Xing L, Yuan Y. A clinical analysis of 114 cases of sarcomatoid carcinoma of the lung. Clin Exp Med. Epub ahead of print 9 July 2018. DOI: 10.1007/s10238-018-0517-2. [DOI] [PubMed] [Google Scholar]
- 13. Pecuchet N, Vieira T, Rabbe N, et al. Molecular classification of pulmonary sarcomatoid carcinomas suggests new therapeutic opportunities. Ann Oncol 2017; 28: 1597–1604. [DOI] [PubMed] [Google Scholar]
- 14. Yang Z, Xu J, Li R, et al. PD-L1 and CD47 co-expression in pulmonary sarcomatoid carcinoma: a predictor of poor prognosis and potential targets of future combined immunotherapy. J Cancer Res Clin Oncol 2019; 145: 3055–3065. [DOI] [PubMed] [Google Scholar]
- 15. Li X, Wang D, Zhao Q, et al. Clinical significance and next-generation sequencing of Chinese pulmonary sarcomatoid carcinoma. Sci Rep 2017; 7: 3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ung M, Rouquette I, Filleron T, et al. Characteristics and clinical outcomes of sarcomatoid carcinoma of the lung. Clin Lung Cancer 2016; 17: 391–397. [DOI] [PubMed] [Google Scholar]
- 17. Vieira T, Girard N, Ung M, et al. Efficacy of first-line chemotherapy in patients with advanced lung sarcomatoid carcinoma. J Thorac Oncol 2013; 8: 1574–1577. [DOI] [PubMed] [Google Scholar]
- 18. Rahouma M, Kamel M, Narula N, et al. Pulmonary sarcomatoid carcinoma: an analysis of a rare cancer from the surveillance, epidemiology, and end results database. Eur J Cardiothorac Surg 2018; 53: 828–834. [DOI] [PubMed] [Google Scholar]
- 19. Mehrad M, Roy S, LaFramboise WA, et al. KRAS mutation is predictive of outcome in patients with pulmonary sarcomatoid carcinoma. Histopathology 2018; 73: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lin Y, Yang H, Cai Q, et al. Characteristics and prognostic analysis of 69 patients with pulmonary sarcomatoid carcinoma. Am J Clin Oncol 2016; 39: 215–222. [DOI] [PubMed] [Google Scholar]
- 21. Fallet V, Saffroy R, Girard N, et al. High-throughput somatic mutation profiling in pulmonary sarcomatoid carcinomas using the LungCarta panel: exploring therapeutic targets. Ann Oncol 2015; 26: 1748–1753. [DOI] [PubMed] [Google Scholar]
- 22. Seong YW, Han SJ, Jung W, et al. Perioperative change in neutrophil-to-lymphocyte ratio (NLR) is a prognostic factor in patients with completely resected primary pulmonary sarcomatoid carcinoma. Thorac Dis 2019; 11: 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vieira T, Antoine M, Ruppert AM, et al. Blood vessel invasion is a major feature and a factor of poor prognosis in sarcomatoid carcinoma of the lung. Lung Cancer 2014; 85: 276–281. [DOI] [PubMed] [Google Scholar]
- 24. Li Y, Zhang L, Jiang J, et al. Clinical characteristics and prognostic analysis of 38 patients with pulmonary sarcomatoid carcinoma. Zhongguo Fei Ai Za Zhi 2015; 18: 537–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu X, Huang Y, Li Y, et al. 18F-FDG PET/CT imaging in pulmonary sarcomatoid carcinoma and correlation with clinical and genetic findings. Ann Nucl Med 2019; 33: 647–656. [DOI] [PubMed] [Google Scholar]
- 26. Lococo F, Torricelli F, Rossi G, et al. Inter-relationship between PD-L1 expression and clinic-pathological features and driver gene mutations in pulmonary sarcomatoid carcinomas. Lung Cancer 2017; 113: 93–101. [DOI] [PubMed] [Google Scholar]
- 27. Rapicetta C, Lococo F, Stefani A, et al. Primary sarcomatoid carcinoma of the lung: radiometabolic (18F-FDG PET/CT) findings and correlation with clinico-pathological and survival results. Lung 2016; 194: 653–657. [DOI] [PubMed] [Google Scholar]
- 28. Avila Martínez RJ, Marrón Fernández C, Hermoso Alarza F, et al. Primary pulmonary sarcomatoid carcinomas. Arch Bronconeumol 2013; 49: 405–407. [DOI] [PubMed] [Google Scholar]
- 29. Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg 2007; 84: 973–980. [DOI] [PubMed] [Google Scholar]
- 30. Cote CL, Castonguay M, Baskett R. Resection of pulmonary sarcomatoid carcinoma metastasized to the right ventricle. J Card Surg 2020; 35: 1108–1109. [DOI] [PubMed] [Google Scholar]
- 31. Chaft JE, Sima CS, Ginsberg MS, et al. Clinical outcomes with perioperative chemotherapy in sarcomatoid carcinomas of the lung. J Thorac Oncol 2012; 7: 1400–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tambe A, Ramadas P, Williams M, et al. Pulmonary sarcomatoid carcinoma presenting as subcutaneous nodules. Proc (Bayl Univ Med Cent) 2020; 33: 67–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Qin Z, Huang B, Yu G, et al. Gingival metastasis of a mediastinal pulmonary sarcomatoid carcinoma: a case report. Cardiothorac Surg 2019; 14: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang EH, Zhang J, Da YP. et al. Clinicopathological diagnosis and differential diagnosis-trachea, lung, pleura and mediastinal diseases, chapter: trachea and lung tumors. Clinicopathological diagnosis and differential diagnosis, 2018, pp. 123–129. [Google Scholar]
- 35. Baldovini C, Rossi G, Ciarrocchi A. Approaches to tumor classification in pulmonary sarcomatoid carcinoma. Lung Cancer (Auckl) 2019; 10: 131–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rossi G, Cavazza A, Sturm N, et al. Pulmonary carcinomas with pleomorphic, sarcomatoid, or sarcomatous elements: a clinicopathologic and immunohistochemical study of 75 cases. Am J Surg Pathol 2003; 27: 311–324. [DOI] [PubMed] [Google Scholar]
- 37. Goldblum JR, Lamps LW, McKenney JK. et al. Rosai and Ackerman’s surgical pathology, 11th edition JAMA 2017; 1: 372-443. [Google Scholar]
- 38. Kitazawa R, Kitazawa S, Nishimura Y, et al. Lung carcinosarcoma with liposarcoma element: autopsy case. Pathol Int 2006; 56: 449–452. [DOI] [PubMed] [Google Scholar]
- 39. Fishback NF, Travis WD, Moran CA, et al. Pleomorphic (spindle/giant cell) carcinoma of the lung. A clinicopathologic correlation of 78 cases. Cancer 1994; 73: 2936–2945. [DOI] [PubMed] [Google Scholar]
- 40. Wang M, Liu Y, Qian X, et al. Giant cell carcinoma of the lung presenting as an isolated cyst containing air: a case report. Medicine (Baltimore) 2019; 98: e15689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lindholm KE, Kalhor N, Moran CA. Osteoclast-like giant cell-rich carcinomas of the lung: a clinicopathological, immunohistochemical, and molecular study of 3 cases. Hum Pathol 2019; 85: 168–173. [DOI] [PubMed] [Google Scholar]
- 42. Toyokawa G, Takenoyama M, Taguchi K, et al. The first case of lung carcinosarcoma harboring in-frame deletions at exon19 in the EGFR gene. Lung Cancer 2013; 81: 491–494. [DOI] [PubMed] [Google Scholar]
- 43. Le Caer H, Teissier E, Barriere JR, et al. Classic biphasic pulmonary blastoma: a case report and review of the literature. Crit Rev Oncol Hematol 2018; 125: 48–50. [DOI] [PubMed] [Google Scholar]
- 44. Yi M, Zhang Z. Pathology and diagnosis of sarcomatoid carcinoma of lung. Clin Exp Pathol 2014: 30: 56–60. [Google Scholar]
- 45. Terra SB, Jang JS, Bi L, et al. Molecular characterization of pulmonary sarcomatoid carcinoma: analysis of 33 cases. Mod Pathol 2016; 29: 824–831. [DOI] [PubMed] [Google Scholar]
- 46. Macher Goeppinger S, Penzel R, Roth W, et al. Expression and mutation analysis of EGFR, c-KIT, and β-catenin in pulmonary blastoma. J Clin Pathol 2011; 64: 349–353. [DOI] [PubMed] [Google Scholar]
- 47. Liang X, Li Q, Xu B, et al. Mutation landscape and tumor mutation burden analysis of Chinese patients with pulmonary sarcomatoid carcinomas. Int J Clin Oncol 2019; 24: 1061–1068. [DOI] [PubMed] [Google Scholar]
- 48. Sahay A, Kumar R, Janu A, et al. ALK1 gene rearranged pulmonary sarcomatoid carcinoma masquerading as tuberculosis in a young male. Turk Patoloji Derg 2020; 1: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sukrithan V, Sandler J, Gucalp R, et al. Immune checkpoint blockade is associated with durable responses in pulmonary sarcomatoid carcinoma. Clin Lung Cancer 2019; 20: e242–e246. [DOI] [PubMed] [Google Scholar]
- 50. Liu X, Jia Y, Stoopler MB, et al. Next-generation sequencing of pulmonary sarcomatoid carcinoma reveals high frequency of actionable MET gene mutations. Clin Oncol 2016; 34: 794–802. [DOI] [PubMed] [Google Scholar]
- 51. Ikushima H, Sakatani T, Masuda Y, et al. Lung spindle cell carcinoma harbouring a constitutively active epidermal growth factor receptor mutation. Respirol Case Rep 2019; 7: e00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ge J, Yao B, Huang J, et al. Molecular genetic characterization reveals linear tumor evolution in a pulmonary sarcomatoid carcinomas patient with a novel PHF20-NTRK1 fusion: a case report. BMC Cancer 2019; 19: 592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chen J, Xiao Y, Cai X, et al. Overexpression of p53R2 is associated with poor prognosis in lung sarcomatoid carcinoma. BMC Cancer 2017; 17: 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Han S, Fang J, Lu S, et al. Response and acquired resistance to savolitinib in a patient with pulmonary sarcomatoid carcinoma harboring MET exon 14 skipping mutation: a case report. Onco Targets Ther 2019; 12: 7323–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen CH, Chen WM, Tung SY, et al. Gastrointestinal metastasis from primary sarcomatoid carcinoma of the lung: a case report and review of the literature. World J Surg Oncol 2015; 13: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pelosi G, Gasparini P, Conte D, et al. Synergistic activation upon MET and ALK coamplification sustains targeted therapy in sarcomatoid carcinoma, a deadly subtype of lung cancer. J Thorac Oncol 2016; 11: 718–728. [DOI] [PubMed] [Google Scholar]
- 57. Italiano A, Cortot AB, Ilie M, et al. EGFR and KRAS status of primary sarcomatoid carcinomas of the lung: implications for anti-EGFR treatment of a rare lung malignancy. Int J Cancer 2009; 125: 2479–2482. [DOI] [PubMed] [Google Scholar]
- 58. Velcheti V, Rimm DL, Schalper KA. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1). J Thorac Oncol 2013; 8: 803–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vieira T, Antoine M, Hamard C, et al. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer 2016; 98: 51–58. [DOI] [PubMed] [Google Scholar]
- 60. Mu CY, Huang JA, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011; 28: 682–688. [DOI] [PubMed] [Google Scholar]
- 61. Domblides C, Leroy K, Monnet I, et al. Efficacy of immune checkpoint inhibitors in lung sarcomatoid carcinoma. J Thorac Oncol 2020; 15: 860–866. [DOI] [PubMed] [Google Scholar]
- 62. Li X, Zhang Z, Liu J, et al. Molecular features of giant-cell carcinoma of the lung: a case report and literature review. Onco Targets Ther 2018; 11: 751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Smadhi H, Boudaya MS, Abdannadher M, et al. Pulmonary sarcomatoid carcinoma: a surgical diagnosis and prognostic factors. La Tunisie medicale 2019; 97: 128–132. [PubMed] [Google Scholar]
- 64. Kaira K, Horie Y, Ayabe E, et al. Pulmonary pleomorphic carcinoma: a clinicopathological study including EGFR mutation analysis. J Thorac Oncol 2010; 5: 460–465. [DOI] [PubMed] [Google Scholar]
- 65. Liang X, Cheng Y, Yuan Z, et al. Clinical, pathological and treatment factors associated with the survival of patients with pulmonary sarcomatoid carcinoma. Oncol Lett 2020; 19: 4031–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Awobajo MD, Vaporciyan AA, Lu C, et al. Stereotactic body radiation therapy (SBRT) in the management of pulmonary spindle cell carcinoma. BMJ Case Rep 2020; 13:e234779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li W, Chen M, Zhao Y. Long-term survival in a patient with pulmonary spindle cell carcinoma treated with traditional Chinese medicine. BMJ Case Rep 2018; 2018: bcr2018225989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tamura Y, Fujiwara Y, Yamamoto N, et al. Retrospective analysis of the efficacy of chemotherapy and molecular targeted therapy for advanced pulmonary pleomorphic carcinoma. BMC Res Notes 2015; 8: 800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiong WJ, Zhang XX, Huang MJ, et al. Outcomes of treatment of 32 cases of advanced or relapsed post-surgery pulmonary sarcomatoid carcinoma. Sichuan Da Xue Xue Bao Yi Xue Ban 2014; 45: 320–323. [PubMed] [Google Scholar]
- 70. Kong FW, Wang WM, Liu L, et al. First-line albumin-bound paclitaxel/carboplatin plus apatinib in advanced pulmonary sarcomatoid carcinoma: a case series and review of the literature. Medicine 2020; 99: e20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Forest F, Yvorel V, Karpathiou G, et al. Histomolecular profiling of pleomorphic, spindle cell, and giant cell carcinoma of the lung for targeted therapies. Hum Pathol 2016; 49: 99–106. [DOI] [PubMed] [Google Scholar]
- 72. He Q, Shi X, Zhu H, et al. A case treated with Crizotinib after secondary MET amplification of a double rare L747S and G719S EGFR mutation pulmonary sarcomatoid carcinoma. Ann Oncol 2020; 31: 544–546. [DOI] [PubMed] [Google Scholar]
- 73. Xia L, Shao YW, Xia Y. Nkx2-4 Mutation confers resistance to EGFR-tyrosine kinase inhibitors in EGFR-mutant lung sarcomatoid carcinoma. J Thorac Oncol 2019; 14: e125–e126. [DOI] [PubMed] [Google Scholar]
- 74. Hsieh MS, Lin MW, Lee YH. Lung adenocarcinoma with sarcomatoid transformation after tyrosine kinase inhibitor treatment and chemotherapy. Lung Cancer 2019; 137: 76–84. [DOI] [PubMed] [Google Scholar]
- 75. Zou F, Xie G, Ma JA, et al. Epidermal growth factor receptor mutation heterogeneity analysis of pulmonary sarcomatoid carcinoma successfully treated with erlotinib: a case report. Oncol Lett 2015; 9: 2239–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. D’Antonio F, De Sanctis R, Bolengo I, et al. Pulmonary sarcomatoid carcinoma presenting both ALK rearrangement and PD-L1 high positivity: a case report on the therapeutic regimen. Medicine (Baltimore) 2019; 98: e16754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Valter A, Roosipuu R, Tamm H, et al. An anaplastic lymphoma kinase (ALK) fusion oncogene positive metastatic sarcomatoid carcinoma of the lung with good response to crizotinib. AME Case Rep 2018; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Niu HT, Dong P, Wang JN, et al. Expression of anaplastic lymphoma kinase fusion gene in patients with lung sarcomatoid carcinoma and treatment analysis. Zhonghua yi xue za zhi 2018; 98: 688–691. [DOI] [PubMed] [Google Scholar]
- 79. Li X, He Y, Zhu J, et al. Apatinib-based targeted therapy against pulmonary sarcomatoid carcinoma: a case report and literature review. Oncotarget 2018; 9: 33734–33738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tunger A, Sommer U, Wehner R, et al. The evolving landscape of biomarkers for anti-PD-1 or anti-PD-L1 therapy. J Clin Med 2019; 8: 1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Roesel C, Kambartel K, Kopeika U, et al. Lazarus-type tumour response to therapy with nivolumab for sarcomatoid carcinomas of the lung. Curr Oncol 2019; 26: e270–e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Babacan NA, Pina IB, Signorelli D, et al. Relationship between programmed death receptor-ligand 1 expression and response to checkpoint inhibitor immunotherapy in pulmonary sarcomatoid carcinoma: a pooled analysis. Clin Lung Cancer. Epub ahead of print 9 March 2020. DOI: 10.1016/j.cllc.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 83. Nishino K, Kunimasa K, Kimura M, et al. Favorable response to pembrolizumab after durvalumab failure in a stage III sarcomatoid carcinoma of the lung: a case report. BMC Pharmacol Toxicol 2020; 21: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Salati M, Baldessari C, Calabrese F, et al. Nivolumab-induced impressive response of refractory pulmonary sarcomatoid carcinoma with brain metastasis. Case Rep Oncol 2018; 11: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 86. Ota T, Niho S, Kirita K, et al. Impressive response to nivolumab of non-small-cell lung cancer containing sarcomatoid components. Respirol Case Rep 2019; 7: e00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Karim NA, Schuster J, Eldessouki I, et al. Pulmonary sarcomatoid carcinoma: University of Cincinnati experience. Oncotarget 2018; 9: 4102–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cai CL, Zhang MQ, Guo J, et al. Response to crizotinib in a patient with metastatic lung spindle cell carcinoma harboring TPM3-ROS1 fusion. Chin Med J (Engl) 2019; 132: 3003–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Weissferdt A, Moran CA. Primary giant cell carcinomas of the lung: a clinicopathological and immunohistochemical analysis of seven cases. Histopathology 2016; 68: 680–685. [DOI] [PubMed] [Google Scholar]