Abstract
Objective
Tuberculosis (TB) incidence shows a seasonal trend. The purpose of this study was to explore seasonal trends in TB cases in Jiangsu Province.
Methods
TB case data were collected from the TB registration system from 2014 to 2018. The X12-ARIMA model was used to adjust the Jiangsu TB time series. Analysis of variance was used to compare TB seasonal amplitude (SA) between subgroups and identify factors responsible for seasonal variation.
Results
The TB incidence in Jiangsu showed a seasonal trend. Confirmed active TB peaked in March and reached a minimum in February. The amplitude of the peak-to-bottom difference was 38.15%. The SAs in individuals 7 to 17 years old (80.00%) and students (71.80%) were significantly different than those in other subgroups. Among bacterial culture positive individuals, the SAs among female patients, individuals aged 7 to 17 years and students were significantly different from those in the reference group. Among culture-negative patients, the SA among individuals aged 7 to 17 years was significantly different those in other subgroups.
Conclusions
The TB incidence in Jiangsu Province displayed a seasonal trend. Factors related to seasonal variation were age and occupation. Our results highlight the importance of controlling Mycobacterium tuberculosis transmission during winter.
Keywords: Tuberculosis, seasonality, distribution, X12-ARIMA, modeling, Jiansu Province
Introduction
According to Global Tuberculosis (TB) Reports, TB has become one of the top 10 causes of death.1 About 10 million people contract TB each year worldwide, and 1.2 million died from it in 2018. In 1996, Douglas et al.2 identified a seasonal trend in TB incidence. Seasonal trends in TB incidence have attracted wide attention as other respiratory diseases do not show the same seasonality. In the northern hemisphere,3–6 the peak months for TB incidence occur from March to June, and a trough occurs from November to February. Studies in the southern hemisphere showed that the TB incidence in Australia7 peaked in September and reached a trough in June. In South Africa,8 the peak of TB incidence occurred from October to December and the trough occurred from April to June.
More recently, studies on the seasonality of TB have been conducted in several areas of China including Wuhan,9 Chongqing,10 Yunnan11 and Xinjiang.12 These studies demonstrated that the peak of TB incidence in China occurred from March to September, and the trough occurred from October to December. Nonetheless, few studies have been conducted in the eastern part of China where the four seasons are distinct. For example, Jiangsu Province, located in the eastern part of China, currently reports around 30,000 new TB cases annually. Although a study13 conducted in China discussed the potential seasonality of TB in Jiangsu Province, the data from 2005 to 2012 could not accurately describe current incidence rates because of continuous improvement in TB detection methods in recent years. In addition, Liu et al.14 focused on the selection of mathematical models suitable to predict the incidence of TB in Jiangsu Province. However, their models made no detailed analysis of seasonality. Accordingly, we conducted this study to explore seasonal variation of TB incidence in eastern China, and to identify factors potentially influencing TB seasonal variation.
Materials and methods
Data collection
Data on notified TB cases from January 2014 to December 2018 were obtained from the TB Registration and Management System of Jiangsu Province. Basic information on TB cases was collected, including age, gender, occupation, and the date of diagnosis. All patients registered in the management system were enrolled in this study. There were no missing values for the variables included in the analysis.
Statistics
Commonly used time series analysis methods include spectral analysis, exponential smoothing methods and the autoregressive integrated moving average model (ARIMA)15 The ARIMA model is the most commonly used model for studies of TB seasonality.3,10,12 In this study, the X12-ARIMA model was used to seasonally adjust the sequence of monthly TB case notifications in Jiangsu Province from 2014 to 2018.
The X12-ARIMA model was used to decompose the original time series. X12-ARIMA encompasses two sub-procedures: X11 and RegARIMA. X11 is used to decompose the original time sequence and RegARIMA is used to build the ARIMA model. The premise for the ARIMA model is a steady sequence.16 Non-steady sequences are first smoothed according to differences, where the order of differences is given by the parameter “d”. The other two parameters of the ARIMA model are the autoregressive order “p” and the moving average “q”. These two parameters are estimated via the autocorrelation function and the partial autocorrelation function, respectively.17 The key process of the X11 model is to decompose the original sequence into trend-cyclic (Ct), seasonal (St) and irregular (It) components.18
Annual seasonal amplitude (SA) was used to represent variation in the number of TB cases in a year.5,12 The SA was calculated according to the following principle: the differences in the numbers of cases between the maximum monthly number and the minimum monthly number in a year were divided by the average numbers of cases in that year.5 The SA value represented the average level of seasonal variation over 5 years. The range was used to describe the variation in seasonal amplitude.
We divided the study population into subgroups according to age, gender, occupation, domiciliary registration status (transient or local) and the results of bacteriological tests. Manual laborers included builders and migrant workers; service staff included public attendants, nursery governesses and others with similar professions; and others included kindergarten children and patients with missing occupational information. Transient populations were defined as those whose domiciliary registration status indicated they were migrants in a given city, migrants in a given province, or migrants among provinces. A laboratory-confirmed case was defined as a patient with any positive result of a sputum smear, sputum culture, molecular diagnostic, or pathological test.
Analysis of variance and the Student–Newman–Keuls method were used to compare the SA in different subgroups. All analyses were performed using SAS 9.4 software (SAS Institute, Inc., Cary, NC, USA).
Ethics and consent statement
The study was approved by the Institutional Review Board of the Jiangsu Provincial Center for Disease Control and Prevention [JSCDCLWLL[2019]001]. Because this was a retrospective study, and detailed personal information was not included in the analysis, informed consent was exempted by the Institutional Review Board of Jiangsu Provincial Center for Disease Control and Prevention.
Results
We first evaluated seasonal trends in TB incidence in Jiangsu Province from 2014 to 2018. During this period, 150,151 TB cases were notified. In Table 1, the number of TB cases were calculated per month. The median number of cases per month was 2549 (inter-quartile range: 2180–2719). The month with the highest notified number of TB cases was March (14,515 cases notified, accounting for 9.67% of the total number of patients with TB). The month with the fewest notified TB cases was February (10,191 cases notified, accounting for 6.79% of total patients).
Table 1.
Monthly notified TB cases in Jiangsu Province from 2014 to 2018.
|
2014 |
2015 |
2016 |
2017 |
2018 |
total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | N | % | N | % | N | % | N | % | N | % | N | % |
| Jan | 2721 | 7.92% | 2555 | 7.93% | 2165 | 7.37% | 1967 | 7.08% | 2122 | 8.03% | 11,529 | 7.68% |
| Feb | 2220 | 6.46% | 2047 | 6.35% | 2140 | 7.29% | 2121 | 7.63% | 1663 | 6.29% | 10,191 | 6.79% |
| Mar | 3160 | 9.20% | 3329 | 10.33% | 2918 | 9.93% | 2536 | 9.13% | 2572 | 9.73% | 14,515 | 9.67% |
| Apr | 3129 | 9.11% | 2913 | 9.04% | 2762 | 9.40% | 2394 | 8.62% | 2537 | 9.60% | 13,735 | 9.15% |
| May | 3120 | 9.09% | 2806 | 8.71% | 2603 | 8.86% | 2593 | 9.33% | 2625 | 9.93% | 13,747 | 9.16% |
| Jun | 3219 | 9.37% | 2884 | 8.95% | 2626 | 8.94% | 2544 | 9.16% | 2392 | 9.05% | 13,665 | 9.10% |
| Jul | 2865 | 8.34% | 2690 | 8.35% | 2594 | 8.83% | 2404 | 8.65% | 2371 | 8.97% | 12,924 | 8.61% |
| Aug | 2644 | 7.70% | 2621 | 8.14% | 2581 | 8.79% | 2556 | 9.20% | 2360 | 8.93% | 12,762 | 8.50% |
| Sep | 3044 | 8.86% | 2958 | 9.18% | 2534 | 8.63% | 2482 | 8.93% | 2182 | 8.25% | 13,200 | 8.79% |
| Oct | 2718 | 7.91% | 2353 | 7.30% | 2076 | 7.07% | 1898 | 6.83% | 1931 | 7.30% | 10,976 | 7.31% |
| Nov | 2589 | 7.54% | 2416 | 7.50% | 2216 | 7.54% | 2174 | 7.82% | 1940 | 7.34% | 11,335 | 7.55% |
| Dec | 2912 | 8.48% | 2640 | 8.20% | 2157 | 7.34% | 2117 | 7.62% | 1745 | 6.60% | 11,572 | 7.71% |
| 34,341 | 100.00% | 32,212 | 100.00% | 29,372 | 100.00% | 27,786 | 100.00% | 26,440 | 100.00% | 150,151 | 100.00% | |
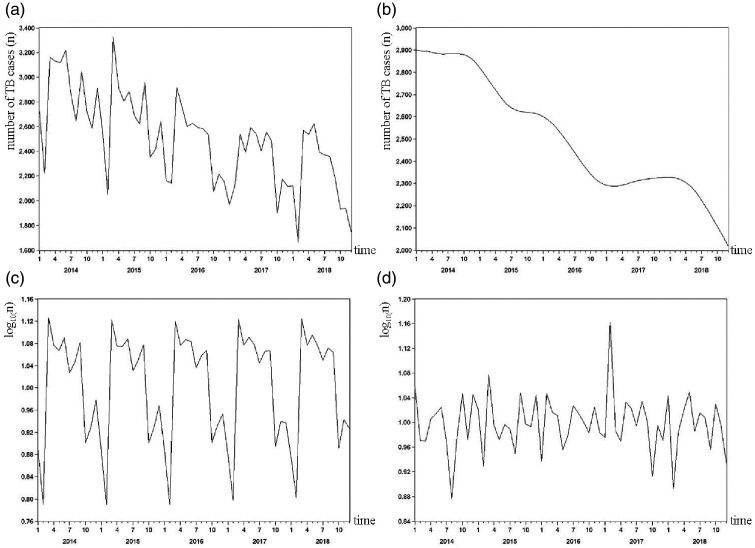
A time series diagram of TB incidence from 2014 to 2018 is shown in Figure 1a. The original sequence was decomposed into the trend (Figure 1b) as well as seasonal (Figure 1c) and irregular (Figure 1d) factors by the X12-ARIMA model. Figure 1b shows a steadily decreasing trend over the 5-year period after excluding the influences of seasonal and irregular factors. Figure 1c shows a significant seasonal trend in the number of notified TB cases. The annual SA remained stable from 2014 to 2018. The number of TB cases reached a peak in March and dropped to its minimum in February. Figure 1d shows the influences of irregular factors on the sequence of TB cases, reflecting errors that regular factors could not explain or variation produced by random factors.
Figure 1.
Original series (a), trend cycle (b), seasonal component (c), and irregular component (d) for notified TB cases from 2014 to 2018.
There were 108,682 men (72.4%) and 41,469 women (27.6%) among total TB cases. The male/female ratio was 2.62. The population was divided into five age groups: 0 to 6 years, 7 to 17 years, 18 to 40 years, 41 to 65 years and more than 65 years. Patients 41 to 65 years old were the most numerous, accounting for 38.9% of all cases, followed by those aged 18 to 40 years, accounting for 32.4% of the total. Most patients were peasants (96,813 cases, accounting for 64.5% of the total). The details of notified TB cases in Jiangsu Province from 2014 to 2018 are shown in Table 2.
Table 2.
Basic characteristics of TB cases in Jiangsu from 2014 to 2018.
| Group | 2014 | 2015 | 2016 | 2017 | 2018 | total |
|---|---|---|---|---|---|---|
| All TB cases | 34,341 | 32,212 | 29,372 | 27,786 | 26,440 | 150,151 |
| Sex | ||||||
| Male | 24,696 | 23,341 | 21,311 | 20,256 | 19,078 | 108,682 |
| Female | 9645 | 8871 | 8061 | 7530 | 7362 | 41,469 |
| Age (years) | ||||||
| 0–6 | 7 | 12 | 9 | 15 | 15 | 58 |
| 7–17 | 603 | 580 | 669 | 590 | 651 | 3093 |
| 18–40 | 10,998 | 10,244 | 9415 | 9103 | 8822 | 48,582 |
| 41–65 | 13,892 | 12,594 | 11,417 | 10,653 | 9923 | 58,479 |
| ≥66 | 8841 | 8782 | 7862 | 7425 | 7029 | 39,939 |
| Occupation | ||||||
| Students | 954 | 1034 | 1021 | 983 | 1226 | 5218 |
| Manual workers | 2519 | 2480 | 2250 | 2220 | 2262 | 11,731 |
| Peasants | 23,628 | 21,418 | 19,049 | 17,332 | 15,386 | 96,813 |
| Job-waiting | 3203 | 3546 | 3024 | 3320 | 3719 | 16,812 |
| Service staff | 1029 | 897 | 927 | 970 | 986 | 4809 |
| Retiree | 1859 | 2123 | 1887 | 1936 | 1787 | 9592 |
| Others | 1150 | 713 | 1214 | 1025 | 1074 | 5176 |
| Lab results | ||||||
| Positive | 10,144 | 9950 | 9550 | 9804 | 12,991 | 52,439 |
| Negative | 22,752 | 21,083 | 18,726 | 16,919 | 12,004 | 91,484 |
| Others | 1454 | 1179 | 1096 | 1063 | 1445 | 6228 |
| Residential status | ||||||
| Local | 27,615 | 23,421 | 21,602 | 20,187 | 18,736 | 111,561 |
| Transient | 6726 | 8791 | 7770 | 7599 | 7704 | 38,590 |
The SAs associated with patient subgroups are shown in Table 3. The average SA over the 5-year period was 38.15% (range: 30.02% to 47.76%). The SAs among 7- to 17-year-olds and students were significantly different from those in other groups. SAs among different age groups were significantly different (F = 9.771, P = 0.001). Seasonal variation in TB cases in those aged 7 to 17 years was significantly higher than in other age groups. The number of patients aged 0 to 6 years was too small to calculate the SA. The most common occupations of TB patients were students, manual laborers, peasants, job-waiting, service staff, retiree and other. SAs were significantly different in different occupational groups (F = 3.531, P = 0.010). The SA among students was significantly higher than among peasants and retirees. No associations were detected between SA and gender, transient populations and laboratory results.
Table 3.
Comparisons of seasonal variation in TB cases among different subgroups in Jiangsu Province.
| Group | Peak/Trough | Mean SA (range) | P value |
|---|---|---|---|
| All TB cases | Mar/Feb | 38.15% (30.02–47.76) | |
| Sex | |||
| Male | Mar/Feb | 39.73% (31.81–51.05) | F = 0.010 P = 0.925 |
| Female | Sep/Feb | 39.21% (30.28–54.77) | |
| Age (years) | |||
| 7–17 | Apr/Feb | 80.00% (63.68–118.39) | F = 9.771 P = 0.001 |
| 18–40 | Mar/Feb | 43.20% (29.53–62.98) | |
| 41–65 | Mar/Oct | 37.40% (30.23–44.02) | |
| ≥66 | Mar/Feb | 42.40% (35.42–56.17) | |
| Occupation | |||
| Students | Apr/Feb | 71.80% (50.05–92.84) | F = 3.531 P = 0.010 |
| Manual workers | Jul/Feb | 54.88% (41.62–81.17) | |
| Peasants | Mar/Oct | 44.18% (28.90–57.32) | |
| Job-waiting | Jul/Feb | 52.68% (38.89–63.89) | |
| Service staff | Sep/Feb | 62.76% (51.78–72.30) | |
| Retiree | Jul/Feb | 48.94% (43.39–52.29) | |
| Residential status | |||
| Local | Mar/Oct | 41.20% (35.84–45.60) | F = 1.950 P = 0.200 |
| Transient | Jul/Feb | 66.40% (33.00–133.63) | |
| Lab results | |||
| Positive | Jun/Feb | 38.80% (31.21–47.43) | F = 0.975 P = 0.352 |
| Negative | Mar/Feb | 46.20% (33.54–67.68) |
Note: The number of patients aged 0 to 6 years was too small to calculate SA.
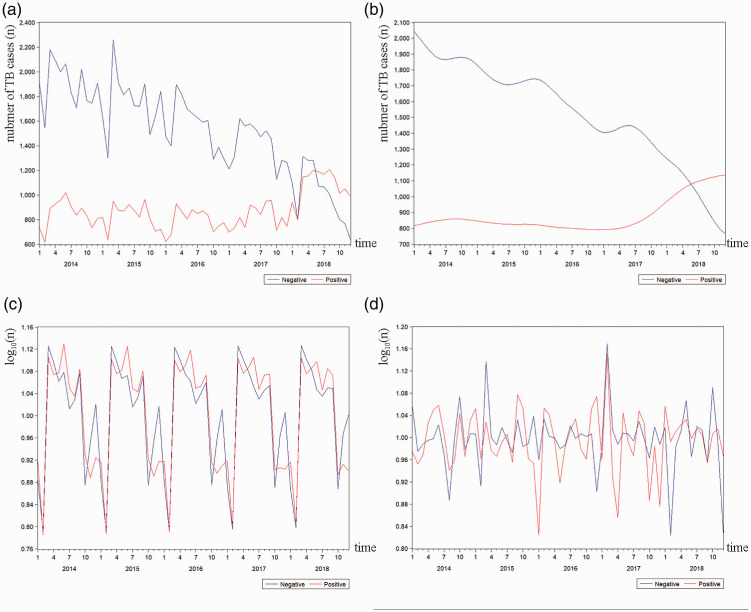
Patients with bacterial culture-confirmed TB are more likely to transmit Mycobacterium tuberculosis.19 Thus, we stratified the study population according to the status of laboratory tests. Figure 2 demonstrated the time series of the two groups of patients (culture positive and culture negative). The number of culture-negative patients showed a steady decline from 2014 to 2018 (Figure 2b) with a trend similar to that of the overall time series (Figure 1b). The number of culture-positive patients remained stable between 2014 and 2017, but 2018 saw a slight upward trend. As shown in Figure 2c, the number of culture-positive TB patients reached a peak in June, and that of culture-negative patients reached a peak in March. The month with the fewest numbers of culture-positive and culture-negative cases was February. Among culture-positive patients, the SAs in women (F = 13.505, P = 0.006), patients aged 7 to 17 years (F = 36.511, P < 0.001) and students (F = 6.430, P = 0.001) were significantly different from those in the reference group. Among culture-negative patients, the SA in patients aged 7 to 17 years (F = 5.792, P = 0.007) was significantly different from that in the reference group.
Figure 2.
Original series (a), trend cycle (b), seasonal component (c), and irregular component (d) for culture-positive and culture-negative TB cases from 2014 to 2018.
Discussion
The results of this study support the idea that TB incidence shows seasonal variation in Jiangsu Province. Notified TB cases reached their highest numbers in March and their lowest numbers in February. The peak month for TB incidence in Jiangsu Province was slightly earlier than other countries in the Northern Hemisphere, such as Iran3 and the United Kingdom.6 Our results are similar to those that have been reported in other areas of China such as Wuhan city,9 Chongqing city10 and Xinjiang autonomous region.12 In Jiangsu Province, the trough of TB incidence appeared later than in some countries such as the United States,5 Kuwait,20 Japan,21 and Portugal.22 Furthermore, the peak month in Jiangsu Province occurred in spring, whereas peaks occurred in spring and summer in other regions of China.11,23,24
A previous study conducted in Xinjiang autonomous region12 found that the SA of TB patients was 77.31%. In Wuhan, Hubei Province, located in the central region where seasonal variations in climate are more minor than in Xinjiang, the SA of TB patients was 43.40%.9 The climate of Jiangsu Province is similar to that of Wuhan, and the SA in Jiangsu Province was 38.15%. Difference between SAs in the central (Wuhan) and northwest (Xinjiang) regions of China may be related to the degree of seasonal variation in climate. A study13 indicated that the SA of TB was higher in temperate continental zones (Xinjiang), and lower in subtropical monsoon climate zones (Wuhan). This variation may result from the effects of temperature, humidity and pressure on the survival of M. tuberculosis in droplets.25 Mabaera et al.26 found that seasonal variation in notified TB cases was positively correlated with the degree of climate change by season. The greater the climate changes that occur over the year, the greater the fluctuation in the number of TB cases notified. Another study in Japan27 showed that the number of TB cases notified peaked after extreme weather. In addition to climate, a study showed that air quality has an impact on the seasonality of TB incidence. Siming et al. found that outdoor PM2.5 concentrations were related to the seasonality of TB. However, because we did not collect the relevant data, the impact of air quality on TB could not be evaluated in our study.
During the winter, indoor ventilation is reduced and close contact is increased, resulting in a significantly increased risk of M. tuberculosis transmission.28 In addition, the risk of progression from infection to active disease is higher during the first several months of infection. Therefore, we speculate that the peak incidence of TB in the spring was related to the peak transmission of M. tuberculosis in the winter. In our study, the SA of TB among students was 71.80%, significantly different from that of other occupational groups. This difference indicated that TB incidence among students had a more obvious seasonal trend. Additionally, a recent systematic review29 showed that the index case in about half TB outbreaks in schools occurred in winter, with an average outbreak duration of 4 months. Outbreaks occurred frequently in winter and spring because of long-term contacts in poorly ventilated rooms during cold weather. These results illustrate the importance of controlling M. tuberculosis transmission in winter.
In addition to the incubation period, the interval between the onset of symptoms and diagnosis of TB, called the total delay, depends on patients, doctors and the diagnostic technique. Of these three factors, the main cause of the total delay was patient behavior, resulting in long delays in diagnosis.30 At the individual level factors influencing total delay includes socioeconomic status, educational level, and cultural background.31 In our study, the peak of female cases appeared later than that of male cases, which may be explained by the significantly longer total delay of female cases than male cases in developing countries.32 The availability of health services also has an impact on diagnosis delay.33 It can be difficult for migrants to access health services, and thus the peak incidence of the transient population in this study appeared 4 months later than that of local residents. Social customs and habits also affect delays in diagnosis. For example, it is not auspicious in China to visit a hospital during the Spring Festival, and this festival can be regarded as one of the reasons for delays.13 Our results showed that the peak month for TB incidence was March and the trough month was February. This finding may be explained by the Lunar New Year causing patients to delay a visit to the doctor. When we replaced the original data with the averages of the maximum and minimum values, the peak incidence in Jiangsu Province occurred from April to September, and the trough occurred from October to January.
In 2018, molecular diagnostic technology was vigorously promoted in Jiangsu Province. This effort increased capacity for positive case finding. Our results showed that the numbers of positive patients climbed significantly in 2018 by up to 49.13% of the total number of patients. The peak periods for incidence among culture-positive patients occurred in spring and summer and were not as obvious as those of culture-negative patients. This finding was similar to that of a study in Wuhan and the reason was ascribed to the summer flu.23 Another study19 showed that culture-positive patients are more likely to transmit infection, which may lead to a longer duration of the peak incidence among positive patients. Moreover, Chen et al.34 suggested that culture-positive patients were more likely to have higher risks of diagnostic delays. However, the relationships among these factors the seasonal trends among positive patients in Jiangsu Province will require further research.
In addition to the above-mentioned factors (climatic effects, M. tuberculosis transmission in winter and delays in diagnosis), other factors responsible for the seasonality of TB may include latitude, sunshine and vitamin D. Many studies have reported the influence of latitude on TB seasonality, but conclusions have been contradictory. A nationwide study5 in the United States found that latitude had no effect on TB seasonality. However, studies in Australia7 and India35 indicated that TB had stronger seasonality in high latitude regions compared with low latitude regions. More detailed information is needed to analyze the relationship between latitude and TB seasonality. However, latitude may be a confounding factor, because of latitude influences sunshine, climate, and other natural factors.
There were several limitations of this study. First, detailed information on temperature and humidity were not available, which might have provided more specific information on the impact of seasonal changes on TB incidence. Second, individual factors, such as vitamin D level and sunshine exposure, might help to explain the impact of season on TB incidence at the individual level. Third, the number of children aged 0 to 6 years was too small to assess seasonal changes in the incidence of this group. Finally, the data used in this study was based on all cases entered in the online Tuberculosis Management Information System. In addition to pulmonary tuberculosis, the system also included a small number of extrapulmonary tuberculosis patients (4.03%). Seasonal patterns may differ between pulmonary and extrapulmonary TB. Further studies on the seasonal variation of extrapulmonary TB are warranted.
In summary, we found that the SA of TB incidence among students was significantly different from other groups. This finding emphasizes the importance of preventing M. tuberculosis transmission in winter. We also found that the Lunar New Year was an important factor that causes patients to postpone diagnosis, and may be responsible for the peak of TB notified cases in March. In addition, the peak incidence of TB incidence among women, transient populations, and manual laborers appeared later, which may be related to the availability of health services. This finding demonstrates the importance of ensuring fair access to health services.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Jiangsu Commission of Health [lgy2017083], the Youth Project of the Center for Disease Control and Prevention of Jiangsu Province [JKRC2016006] and The National Major Science & Technology Projects for Infectious Disease Control and Prevention [grant number 2018ZX10715002].
ORCID iD
Haitao Yang https://orcid.org/0000-0001-9831-1007
References
- 1. World Health Organization. Global tuberculosis report 2019, https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf? (2019, accessed 1 August 2020).
- 2.Douglas AS, Strachan DP, Maxwell JD. Seasonality of tuberculosis: the reverse of other respiratory diseases in the UK. Thorax 1996; 51: 944–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moosazadeh M, Khanjani N, Bahrampour A. Seasonality and temporal variations of tuberculosis in the north of Iran. Tanaffos 2013; 12: 35. [PMC free article] [PubMed] [Google Scholar]
- 4.Margalit I, Block C, Mor Z. Seasonality of tuberculosis in Israel, 2001-2011. Int J Tuberc Lung Dis 2016; 20: 1588–1593. [DOI] [PubMed] [Google Scholar]
- 5.Willis MD, Winston CA, Heilig CM, et al. Seasonality of tuberculosis in the United States, 1993-2008. Clin Infect Dis 2012; 54: 1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koh GCKW, Gemma H, Turner AM, et al. Tuberculosis incidence correlates with sunshine: an ecological 28-year time series study. PLoS One 2013; 8: e57752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maclachlan JH, Lavender CJ, Cowie BC. Effect of latitude on seasonality of tuberculosis, Australia, 2002-2011. Emerg Infect Dis 2012; 18: 1879–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ballif M, Zürcher K, Reid SE, et al. Seasonal variations in tuberculosis diagnosis among HIV-positive individuals in Southern Africa: Analysis of cohort studies at antiretroviral treatment programmes. BMJ Open 2018; 8: e017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X, Duan Q, Wang J, et al. Seasonal variation of newly notified pulmonary tuberculosis cases from 2004 to 2013 in Wuhan, China. PLoS One 2014; 9: e108369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liao Z, Zhang X, Zhang Y, et al. Seasonality and trend forecasting of tuberculosis incidence in Chongqing, China. Interdiscip Sci 2019; 11: 77–85. [DOI] [PubMed] [Google Scholar]
- 11.Huang L, Li XX, Abe EM, et al. Spatial-temporal analysis of pulmonary tuberculosis in the northeast of the Yunnan province, People’s Republic of China. Infect Dis Poverty 2017; 6; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wubuli A, Li Y, Xue F, et al. Seasonality of active tuberculosis notification from 2005 to 2014 in Xinjiang, China. PLoS One 2017; 12: e0180226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XX, Wang LX, Zhang H, et al. Seasonal variations in notification of active tuberculosis cases in China, 2005–2012. PLoS One 2013; 8: e68102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, Li Z, Ji Y, et al. Forecasting the seasonality and trend of pulmonary tuberculosis in Jiangsu Province of China using advanced statistical time-series analyses. Infect Drug Resist 2019; 12: 2311–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Box GEP, Jenkins GM, Reinsel GC, et al. (eds). Time series analysis: Forecasting and control. 5th Ed Hoboken: Wiley, 2016. [Google Scholar]
- 16.Wang YW, Shen ZZ, Jiang Y. Comparison of autoregressive integrated moving average model and generalised regression neural network model for prediction of haemorrhagic fever with renal syndrome in China: A time-series study. BMJ Open 2019; 9: e025773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anwar MY, Lewnard JA, Parikh S, et al. Time series analysis of malaria in Afghanistan: using ARIMA models to predict future trends in incidence. Malar J 2016; 15: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silhan PA. Income smoothing from a Census X-12 perspective. Adv Accounting 2014; 30: 106–115. [Google Scholar]
- 19.Nebenzahl-Guimaraes H, Verhagen L, Borgdorff M, et al. Transmission and progression to disease of Mycobacterium tuberculosis phylogenetic lineages in the Netherlands. J Clin Microbiol 2015; 53: 3264–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akhtar S, Mohammad HG. Seasonality in pulmonary tuberculosis among migrant workers entering Kuwait. BMC Infect Dis 2008; 8: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagayama N, Ohmori M. Seasonality in various forms of tuberculosis. Int J Tuberc Lung Dis 2006; 10: 1117–1122. [PubMed] [Google Scholar]
- 22.Bras AL, Gomes D, Filipe PA, et al. Trends, seasonality and forecasts of pulmonary tuberculosis in Portugal. Int J Tuberc Lung Dis 2014; 18: 1202–1210. [DOI] [PubMed] [Google Scholar]
- 23.Luo T, Sumi A, Zhou D, et al. Seasonality of reported tuberculosis cases from 2006 to 2010 in Wuhan, China. Epidemiol Infect 2014; 142: 2036–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge E, Zhang X, Wang X, et al. Spatial and temporal analysis of tuberculosis in Zhejiang Province, China, 2009-2012. Infect Dis Poverty 2016; 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tedijanto C, Hermans S, Cobelens F, et al. Drivers of seasonal variation in tuberculosis incidence: Insights from a systematic review and mathematical model. Epidemiology 2018; 29: 857–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mabaera B, Naranbat N, Katamba A, et al. Seasonal variation among tuberculosis suspects in four countries. Int Health 2009; 1: 53–60. [DOI] [PubMed] [Google Scholar]
- 27.Onozuka D, Hagihara A. The association of extreme temperatures and the incidence of tuberculosis in Japan. Int J Biometeorol 2015; 59: 1107–1114. [DOI] [PubMed] [Google Scholar]
- 28.Wingfield T, Schumacher SG, Sandhu G, et al. The seasonality of tuberculosis, sunlight, vitamin D, and household crowding. J Infect Dis 2014; 210: 774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bao H, Liu K, Wu Z, et al. Tuberculosis outbreaks among students in mainland China: A systematic review and meta-analysis. BMC Infect Dis 2019; 19: 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen S, Wei C, Shiyu Z, et al. Factors causing delay of access to tuberculosis diagnosis among new, active tuberculosis patients: a prospective cohort study. Asia Pac J Public Health 2014; 26: 33–41. [DOI] [PubMed] [Google Scholar]
- 31.Fuge TG, Bawore SG, Solomon DW, et al. Patient delay in seeking tuberculosis diagnosis and associated factors in Hadiya Zone, Southern Ethiopia. BMC Res Notes 2018; 11: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng HJ, Zheng YH, Zhang YY, et al. [ Study on factors causing the delay of access to tuberculosis diagnosis and its influencing factors in migrating tuberculosis patients in Putuo district, Shanghai]. Zhonghua Liu Xing Bing Xue Za Zhi 2006; 27: 311–315. [PubMed] [Google Scholar]
- 33.Bele S, Jiang W, Lu XH, et al. Population aging and migrant workers: Bottlenecks in tuberculosis control in rural China. PLoS One 2014; 9: e88290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Wang T, Liu L, et al. Trend in risk of delay in diagnosis of new pulmonary tuberculosis in Northwest China from 2008 to 2017. BMC Infect Dis 2019; 19: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narula P, Sihota P, Azad S, et al. Analyzing seasonality of tuberculosis across Indian states and union territories. J Epidemiol Glob Health 2015; 5: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]