Abstract
Hamartomas commonly occur in respiratory and digestive organs, such as the lungs, pancreas, and liver; they rarely occur in the oral cavity, especially in the sublingual region. This report describes a 5-month-old boy who presented with a giant sublingual hamartoma and medial cleft tongue. He underwent corrective operations at 5 months, 11 months, and 31 months of age. Histopathological analysis revealed features suggestive of hamartoma. There have been no signs of recurrence. The boy exhibited normal speech development at 3 years of age; all other oral functions were unaffected at that time. This report includes a review of relevant literature. The findings in this report and previous literature suggest that a multidisciplinary approach, carefully planned staged surgery, and rehabilitation are needed to achieve favorable outcomes in patients with hamartoma in the oral cavity.
Keywords: Hamartoma, sublingual region, medial cleft tongue, pediatrics, oral floor, salivary gland, hyperplasia, ossification, cleft tongue, bifid tongue
Introduction
Hamartomas occur commonly in respiratory and digestive organs, such as the lungs, pancreas, and liver; they rarely occur in the oral cavity, especially in the sublingual region. Most hamartomas in the oral cavity occur in a localized manner or in combination with a systemic syndrome, but seldom involve malformation of adjacent organs.1,2 Moreover, medial cleft tongue is a rare type of tongue deformity that differs from bifid tongue in terms of the cause and extent of anomaly.3 To the best of our knowledge, few patients have been described with medial cleft tongue and intraoral hamartoma. Here, we describe an infant who presented with a giant sublingual tumor and medial cleft tongue. We discuss the patient’s clinical characteristics and the treatment procedures performed, along with a review of the relevant literature.
Case report
Patient history and initial examinations
A 5-month-old boy was referred to our department with a large mass in the oral floor and a split tongue. The mass slightly increased in size over time. The patient had been born at full-term by spontaneous vaginal delivery; at birth, he had exhibited a normal appearance, pulse, grimace, activity, and respiration (APGAR) score.
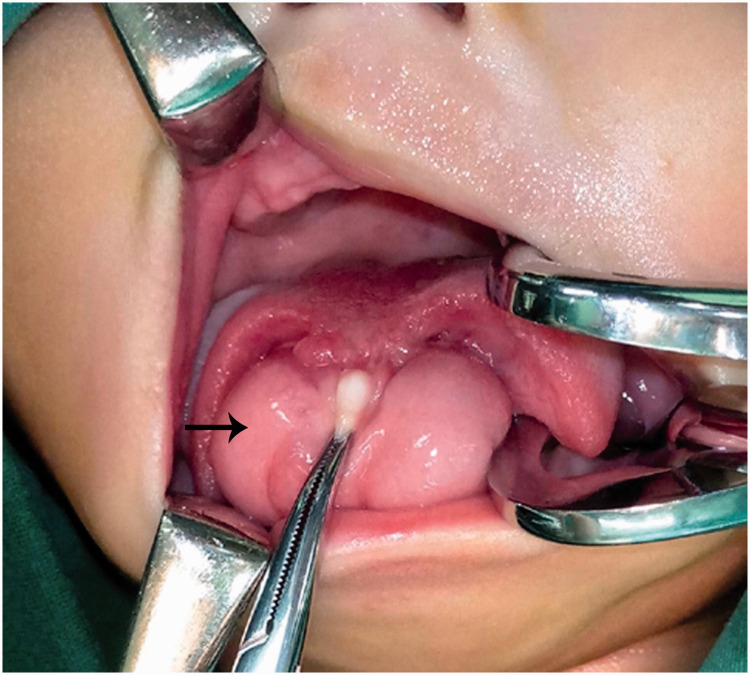
On clinical examination, the patient exhibited a giant, egg-shaped soft mass that occupied most of the space in the sublingual region. The surface of the mass was intact and smooth. A longitudinal depression resembling the shape of a lingual frenulum was present in the middle of the mass. At the midline, a long fissure extended from the foramen cecum to the tip of the tongue (Figure 1).
Figure 1.
Preoperative intraoral view: giant sublingual hamartoma (arrow) with medial cleft tongue.
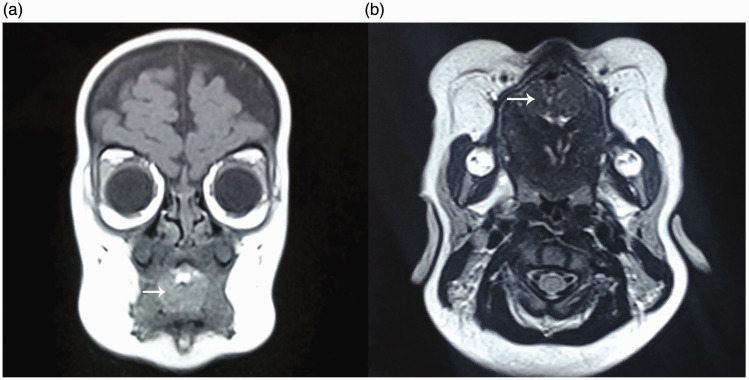
Coronal and transverse magnetic resonance images showed a space-occupying lesion in the anterior median of the tongue, with a homogeneous internal signal and clear boundary (Figure 2). A technetium-99m pertechnetate scan was performed to exclude the presence of a lingual thyroid. Echocardiography examination revealed patent foramen ovale, tricuspid regurgitation, and abnormal blood flow signal in the pulmonary artery, despite the absence of cardiac or circulatory symptoms.
Figure 2.
Magnetic resonance images: (a) coronal and (b) transverse sections show a space-occupying lesion (arrow in each panel) in the anterior median of the tongue, with homogeneous internal signal and clear boundary.
Treatment and follow-up
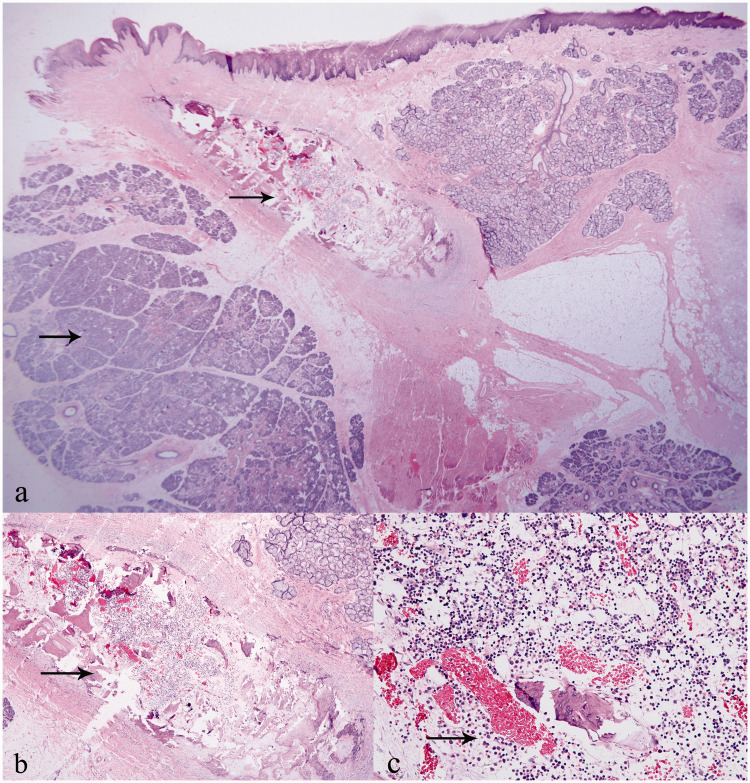
Three operations were performed on this baby. At 5 months of age, the sublingual mass was excised completely under general anesthesia with nasal endotracheal intubation. There was no obvious boundary between the sublingual mass and sublingual tissue. The sublingual glands were pushed to either side of the mass. The submandibular gland duct and lingual nerve were not exposed during surgery. After the operation, a traction wire was placed on the tip of the tongue to prevent asphyxia. The size of the mass was approximately 5.0 cm × 3.5 cm × 3.0 cm. Histopathological diagnosis comprised hamartoma; the microscopic features were significant hyperplasia of the salivary gland and localized fibrous tissue with ossification (Figure 3).
Figure 3.
Histopathological images of lingual hamartoma: (a) salivary gland hyperplasia and localized fibrous tissue with ossification (arrow) (HE, 12.5×); (b) ossification with bone marrow-like tissue formation (arrow) (HE, 40×); (c) bone marrow cells and dilated capillaries (arrow) (HE, 200×).
Abbreviation: HE, hematoxylin and eosin.
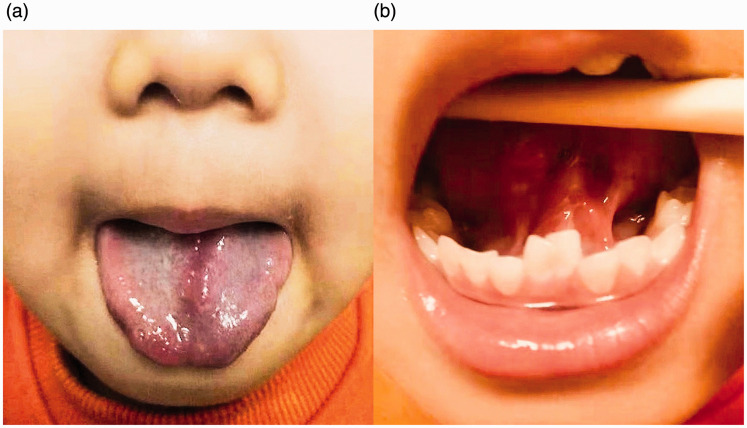
At 11 months of age, the patient underwent cleft tongue reconstruction. The edge of the tongue fissure was excised through and through in a zigzag manner, followed by primary closure of the mucosa and muscle layers. The patient recovered well and was discharged 3 days postoperatively. When the patient returned for follow-up 9 months later, the parents noted that the child's pronunciation was unclear. Clinical examination revealed that tongue adhesion to the oral floor had restricted tongue movement. At 31 months of age, an additional operation was performed to release the tongue from the oral floor and to reshape the tip of the tongue. The patient was then provided speech training for 1 year. At 1 year postoperatively, he exhibited normal speech and normal tongue motor function. There were no signs of tumor recurrence (Figure 4).
Figure 4.
Postoperative intraoral photographs at 3 months after the third operation: (a) good forward motion and satisfactory tongue appearance; (b) no sign of hamartoma recurrence after the third operation.
Discussion
Hamartomas are developmental tumor-like malformations that may occur in any organ, but most frequently occur in the gastrointestinal tract and respiratory system. The features of hamartomas were summarized by Patil et al.1 The patient described in the present report exhibited rare findings in terms of the location, size, and concomitant malformation. A review of the literature indicated that most hamartomas in the oral cavity are multifocal and relatively small, commonly comprising a manifestation of oral-facial-digital syndrome.2,4 The locations of hamartomas in the oral cavity have comprised the tongue and oral mucosa.5,6 To the best of our knowledge, no sublingual hamartomas with medial cleft tongue have been reported in the international literature, although partial duplication of the face was described in a patient with cleft palate, duplicated tongue, hamartoma of the oral floor, and lower cleft lip.7
Although medial cleft tongue differs from bifid tongue in terms of the extent of anomaly, the terms “cleft tongue” and “bifid tongue” are used interchangeably. There is only one report of a patient with medial cleft tongue, sublingual hamartoma, and cleft palate in the English language medical literature.3 Bifid tongue is an uncommon finding that can be syndromic or non-syndromic. It is usually encountered in combination with other orofacial findings and has been often reported as an associated finding in patients with oral-facial-digital syndrome.8,9 Takagi et al.5 suggested that non-syndromic congenital bifid tongue was caused by mechanical interference from the development of a congenital tumor during tongue development. Neoplastic growth could lead to hypoplasia and cleft formation. Furthermore, Takagi et al. reported that lingual hamartoma (64%) was the most common congenital tumor associated with bifid tongue. From 1985 to 2019, there were only 17 reports of patients with non-syndromic, congenital bifid tongue in the English language medical literature.5,10 Twelve of these 17 (69%) patients exhibited concomitant benign neoplasm. Eleven of the 12 patients had a cleft lip or palate; the other patient exhibited leiomyomatous hamartoma in the basal portion of the cleft, accompanied by tongue-tie. Only one of the 11 patients with cleft lip or palate exhibited a sublingual tumor. In contrast to previous studies, our patient had a large sublingual hamartoma, but exhibited no other oral deformity. Moreover, there was no obvious boundary between the sublingual hamartoma and sublingual tissue. The mass appeared to be two enlarged sublingual glands based on the presence of the lingual frenulum. Although the sublingual mass was very large, it did not affect palate formation or cause cleft palate. In previous reports, patients exhibited a small mass on the oral floor and short tongue fissure length; however, these patients exhibited a cleft palate.3,10,11 We presumed that, in our patient, the large mass on the oral floor had caused resistance to fusion from the foramen cecum during tongue development; however, this resistance was not transmitted to the palate and thus did not cause cleft palate. Alternatively, some unknown mechanisms may remain in the complex development of the tongue and palate. Our findings indicate the need for further analyses of the mechanisms underlying cleft tongue and palate.
Notably, histopathological analysis revealed salivary gland hyperplasia and localized fibrous tissue with ossification, which has not been mentioned in previous literature. Thus far, most hematomas in head and neck (including the oral cavity) are composed of neural elements (e.g., neurovascular hamartoma), lipomatous tissue, and salivary gland tissue (e.g., hamartomatous sialolipoma).12,13 As a specialized muscular organ, the tongue is responsible for taste, swallowing, and pronunciation. Early surgical intervention and long-term regular follow-ups may be useful for prevention of speech impairment and swallowing disorders later in life. In previous reports, the masses were excised and tongues/lips were repaired; however, those masses were smaller and were located on the body of the tongue, which differed from the mass characteristics in our patient.5 Notably, our patient’s tongue fissure length was shorter. Considering the patient’s young age, we planned a two-stage operation to reduce the risk of postoperative edema in the respiratory tract. The operation was successful; however, subsequent adhesion of the tongue to the oral floor resulted in restricted tongue movement in all directions. Therefore, a third operation was performed; this complication was not mentioned in previous literature, which suggests that oral floor adhesion may occur in patients with a long tongue fissure. Therefore, postoperative follow-up was important and speech training was necessary.
The appropriate timing for corrective surgery is unclear; however, early surgery can help infants with sublingual hamartoma and cleft tongue in terms of language and swallowing development during growth. To achieve favorable outcomes, a multidisciplinary approach, carefully planned staged surgery, and rehabilitation are needed.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics
The publication of this case report was approved by the Ethics Committee of Hebei General Hospital (approval no. 201929). It was registered in the institutional clinical trials database, in accordance with our hospital’s guidelines. Written informed consent was obtained from the patient's parents for publication of the patient’s medical information, as well as his clinical and histopathological images.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
References
- 1.Patil S, Rao R, Majumdar B. Hamartomas of the oral cavity. J Int Soc Prev Community Dent 2015; 5: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wushou A, Liu W, Bai XF, et al. Clinical analysis of 194 cases of head and neck hamartoma. Oral Surg Oral Med Oral Pathol Oral Radiol 2013; 115: 299–303. [DOI] [PubMed] [Google Scholar]
- 3.Jank S, Kelderer HW, Raubenheimer EJ, et al. Medial tongue cleft associated with intraoral hamartoma-case report and review of literature. Int J Oral Max Surg 2008; 37: 296–299. [DOI] [PubMed] [Google Scholar]
- 4.Kreiger PA, Ernst LM, Elden LM, et al. Hamartomatous tongue lesions in children. Am J Surg Pathol 2007; 31: 1186–1190. [DOI] [PubMed] [Google Scholar]
- 5.Takagi Y, Machida J, Sato H, et al. Congenital bifid tongue with lingual hamartoma: a case report and review of the literature. J Oral Maxillofac Surg Med Pathol 2016; 28: 133–137. [Google Scholar]
- 6.Elo JA, Sun HH, Laudenbach JM, et al. Multiple oral mucosal hamartomas in a 34-year-old female. Head Neck Pathol 2017; 11: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verdi GD, Hersh JH, Russell LJ. Partial duplication of the face: case report and review. Plast Reconstr Surg 1991; 87: 756–762. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqua A, Abubaker P, Saraswati FK, et al. Bifid tongue: differential diagnosis and a case report. J Oral Maxillofac Surg Med Pathol 2015; 27: 686–689. [Google Scholar]
- 9.Rai R, Rai AR, Rai R, et al. Prevalence of bifid tongue and ankyloglossia in south Indian population with an emphasis on its embryogenesis. Int J Morphol 2012; 30: 182–184. [Google Scholar]
- 10.Lee JY, Mohd Zainal H, Mat Zain MAB, et al. Bifid tongue and cleft palate with and without a Tessier 30 facial cleft: cases of rare congenital anomalies and a review of management and literature. Cleft Palate Craniofac J 2019; 56: 1243–1248. [DOI] [PubMed] [Google Scholar]
- 11.Bhattacharya V, Khanna S, Bashir SA, et al. Cleft palate associated with hamartomatous bifid tongue. Report of two cases. J Plast Reconstr Aesthet Surg 2009; 62: 1442–1445. [DOI] [PubMed] [Google Scholar]
- 12.Allon I, Allon DM, Hirshberg A, et al. Oral neurovascular hamartoma: a lesion searching for a name. J Oral Pathol Med 2012; 41: 348–353. [DOI] [PubMed] [Google Scholar]
- 13.Parente P, Longobardi G, Bigotti G. Hamartomatous sialolipoma of the submandibular gland: case report. Br J Oral Maxillofac Surg 2008; 46: 599–600. [DOI] [PubMed] [Google Scholar]