Abstract
Background:
Although alopecia areata is a common disorder, it has no US Food and Drug Administration–approved treatment and evidence-based therapeutic data are lacking.
Objective:
To develop guidelines for the diagnosis, evaluation, assessment, response criteria, and end points for alopecia areata.
Methods:
Literature review and expert opinion of a group of dermatologists specializing in hair disorders.
Results:
Standardized methods of assessing and tracking hair loss and growth, including new scoring techniques, response criteria, and end points in alopecia areata are presented.
Limitations:
The additional time to perform the assessments is the primary limitation to use of the methodology in clinical practice.
Conclusion:
Use of these measures will facilitate collection of standardized outcome data on therapeutic agents used in alopecia areata both in clinical practice and in clinical trials. (J Am Acad Dermatol 2018;79:470–8.)
Keywords: ALODEX score, alopecia areata, assessment measures, outcome measures, response criteria, SALT score
I. BACKGROUND
Alopecia areata (AA) is a common condition, affecting 1.7% to 2.1% of members of the general US population at some point during their lives.1,2 Multiple treatments are available, but none is currently approved by the US Food and Drug Administration for this indication and there is no consensus agreement on their use in clinical practice. The lack of standardized assessment methods and the necessity of taking into account the effect of extent, pattern, and duration of hair loss on regrowth have been barriers to clinical trials in AA. However, with the advent of electronic medical records and large collaborative databases, we now have an opportunity to collect data on large numbers of patients with AA seen in clinical settings. In addition, there is renewed interest in Food and Drug Administration approval of new medications for AA.
A necessary component of collecting comparative data on therapeutic outcomes from both clinical databases and clinical trials of AA is the creation and acceptance of standardized diagnostic criteria, assessment measures, response criteria, and end points. The following are recommendations by a group of dermatologists with expertise in hair disorders who first convened at Duke University on July 10, 2015, to address this issue.
II. DIAGNOSIS OF AA
A. CHARACTERISTIC FEATURES
Follicular ostia that are intact and visible by either direct visualization or by dermoscopic evaluation.
Complete loss of all terminal hair in at least 1 area of hair loss.
Increased hair shedding. Documentation by a gentle pull on a group of hairs at the periphery of patches of hair loss repeated in several areas of the scalp. The proximal ends of pulled hairs should be examined microscopically; telogen hairsalone, acombination of telogen and dystrophic anagen hairs, or broken hairs are typical findings.3–5 Finding multiple hairs on at least 5 pulls of the scalp hair is indicative of active progressive hair loss.
Lack of perifollicular erythema or scale, pustules, or other signs of active follicular inflammation.
B. SUPPORTIVE FEATURES
Exclamation point hairs. Aldersmith3 provided an excellent description of these hairs in 1897: “A few small broken hairs are generally to be seen at the edges of the patches; …club-shaped stumps about an eighth of an inch long…like a note of admiration (!) without the dot. These stumps are very easily extracted entire on traction instead of breaking off.” On microscopic examination, the distal ends of these exclamation point hairs show a trichorhexis nodosa–like change. Exclamation point hairs may also be seen in anagen effluvium6 and are another sign of active progressive hair loss.
Body hair loss in patches or loss of eyelashes or eyebrows.
Fine pitting of nails, trachyonychia (denoting the entire nail plate is rough or thin with or without longitudinal ridging, often likened to “sandpapered” nails), or 20-nail dystrophy.
Dermatoscopic findings of yellow and/or black dots.7
III. RECOMMENDATIONS FOR INITIAL (AND FOLLOW-UP) EVALUATION
(See Supplemental Material 1 and 2; available at http://www.jaad.org)
History. We have narrowed the data from the initial Alopecia Areata Investigational Guidelines8 to those factors that may affect either choice or response to treatment (Table I).9–23
- Physical examination
- Severity of hair loss. The classification of severity of AA, first published by Olsen in 199224 and 1997,25 formalized by the National Alopecia Areata Foundation Guidelines Committee in 1999,8 and revised in 2004,26 categorizes the scalp terminal hair (S) loss as follows: none (S0), 1% to 24% (S1), 25% to 49% (S2), 50% to 74% (S3), 75% to 99% (S4) (including 75% to 95% [S4a] and 96% to 99% [S4b]), and 100% (S5) (Table II). Severity also includes the extent of body hair (B) loss and is classified as none (B0), some (B1), or total (B2). Alopecia totalis can thus be S5B0 or S5B1, but alopecia universalis can only be S5B2.
- Pattern of hair loss. There are 4 main patterns of scalp hair loss in AA: patchy, diffuse (decrease in density diffusely over scalp), ophiasis (decrease in bandlike presentation in parietal and occipital areas), and totalis. The patchy subtype may remit quickly, be persistent with waxing and waning over time, or progress to total hair loss. The ophiasis pattern of hair loss and alopecia totalis)/universalis have a lower spontaneous remission rate and lower rate of response to therapy.9,10,12–17 The diffuse subtype is rare.
- Activity of hair loss. There are 2 quick methods of determining the overall activity of hair loss in AA.
- The presence of “exclamation point hairs” at the periphery of bald areas. These should be differentiated from new regrowing hairs, which have a tapered distal tip.
- Positive AA “hair pull.” Unlike the hair pull done in cases of telogen effluvium, where the number of hairs removed on a given hair pull may have implications for diagnosis and response to treatment, a hair pull in AA is primarily qualitative.
Scalp biopsy. Biopsy may not always show the classic perifollicular or peribulbar lymphocytic infiltrate but may instead show a very high percentage of miniaturized hairs and catagen/telogen hairs.27 A biopsy may be necessary to confirm a diagnosis of diffuse AA.
Table I.
Potential negative prognostic factors in alopecia areata AA, Alopecia areata.
|
Table II.
Scalp, Body, and Nail (SBN) classification of alopecia areata B, body hair; N, nail; S, scalp hair.
| Scalp hair loss (terminal hair only) |
|
| Body hair loss |
|
| Nail involvement |
|
Dense is defined as at least 2 pits per nail.
IV. ASSESSMENT OF AA
- One or more of the following methods should be chosen for use at the start of any given therapy and during follow-up visits. Table III details how each technique might best be utilized.
- Global assessment for those patients treated with systemic agents or whole scalp skin-directed therapy.
- Percentage scalp hair loss
- Severity of Alopecia Tool (SALT) Score The SALT I visual aid8 first published in 1999 and updated in 2004,26 significantly helps one to visualize the amount of terminal hair loss in each of 4 quadrants of the scalp and then upon summing of these, generates the total percentage of scalp hair loss or SALT score (Fig 1). The SALT score captures the total area of the scalp bereft of any terminal hair.
- The SALT II visual aid, which was first published in 201629 with subsequent minor modification (Fig 2), is core to calculating the ALODEX score and tracking specifics of the hair loss over time. The ALODEX score combines both extent and hair density into an overall percentage of scalp hair loss. Although it is possible to calculate by paper scoring, an iPAD app makes it possible to score all the 1% areas quickly and generate electronically the total ALODEX score. An example of both SALT and ALODEX grading of scalp hair loss for a representative patient with AA is given in Fig 3.
- Extent of scalp hair loss (percentage of scalp surface area that has any degree of hair loss)
Table III.
Methods of assessment for potential use in alopecia areata AA, Alopecia areata; ALODEX, Alopecia Density and Extent; BL, baseline; IL, intralesional; LAD, lesional area and density; SALT, Severity of Alopecia Tool.
| Method | Measures | Study use |
Clinic use |
Comments |
|---|---|---|---|---|
| SALT score | % scalp baldness | X | X | By visualization, no way of documenting specific areas of hair loss |
| ALODEX Score | % scalp baldness | X | X | Requires use of iPAD app or too time consuming |
| ALODEX Tool | % scalp baldness, % scalp with any hair loss, areas of hair loss | X | X | Requires use of iPAD app. Documents areas of hair loss and density in each |
| AA Progressive Index | % severity of hair loss | X | Must also do hair pull and dermoscopic examinations and record findings | |
| Half-head assessment | % hair loss treated vs untreated scalp | X | Topical medications only. Best for >50% loss at BL | |
| Lesional size of target areas | Area of several target areas | X | Best for local treatment (such as topical steroids) to selected areas. May be difficult to select margins of hair loss | |
| LAD score | Combines area and density of several target areas | X | Best for local treatment (such as topical steroids) to selected areas. May be difficult to select margins of hair loss | |
| Hair pigmentation | Pigmented vs unpigmented | X | Interest only until proved to be of prognostic significance | |
| Vellus hair | Vellus only vs terminal hair | X | Interest only until proved to be prognostic significance | |
| Miscellaneous signs of hair loss | Hair pull, exclamation point hairs. dermoscopic findings typical of AA | X | Interest only, although signifies active hair loss if present | |
| Patient assessment of extent of hair loss | Either categorization by % hair loss or So-S5 AA categories | X | X | Useful for understanding patient expectations |
| Quality of life assessments | Several that can be utilized | X | Determines impact of hair loss on patient’s life | |
| Photographic documentation | Standardized photos of scalp hair | X | X | Corroborates response to therapy |
Fig 1.
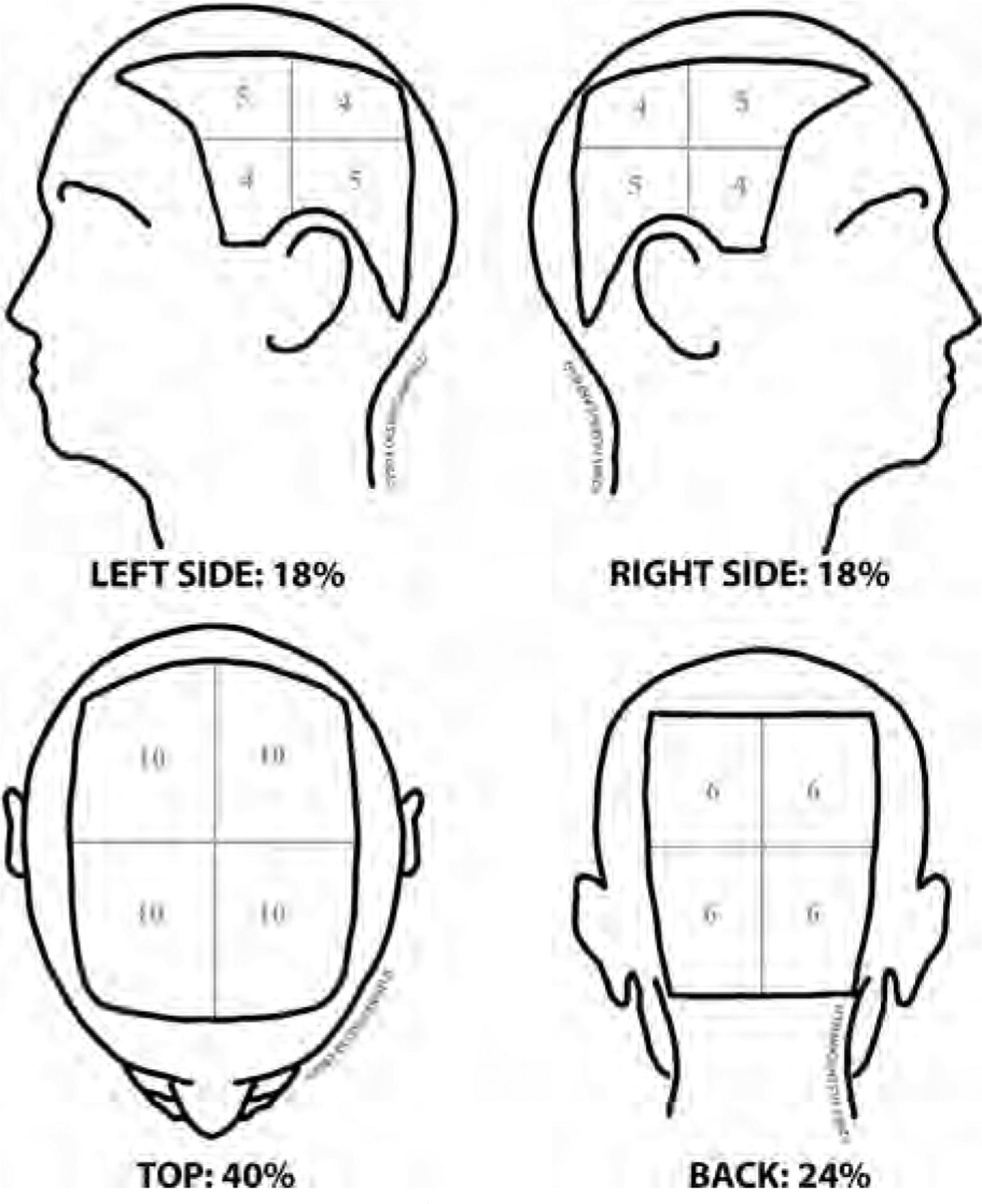
SALT I visual aid26 and computation of SALT score. The percentage terminal hair loss on the top of the scalp is determined and multiplied by 40/100, the percentage of hair loss in the back of the scalp is determined and multiplied by 24/100, the percentage of hair loss on the left side of the scalp is determined and multiplied by 18/100, and the percentage of hair loss on the right side of the scalp is determined and multiplied by 18/100, with A plus B plus C plus D equal to the SALT score.
Fig 2.
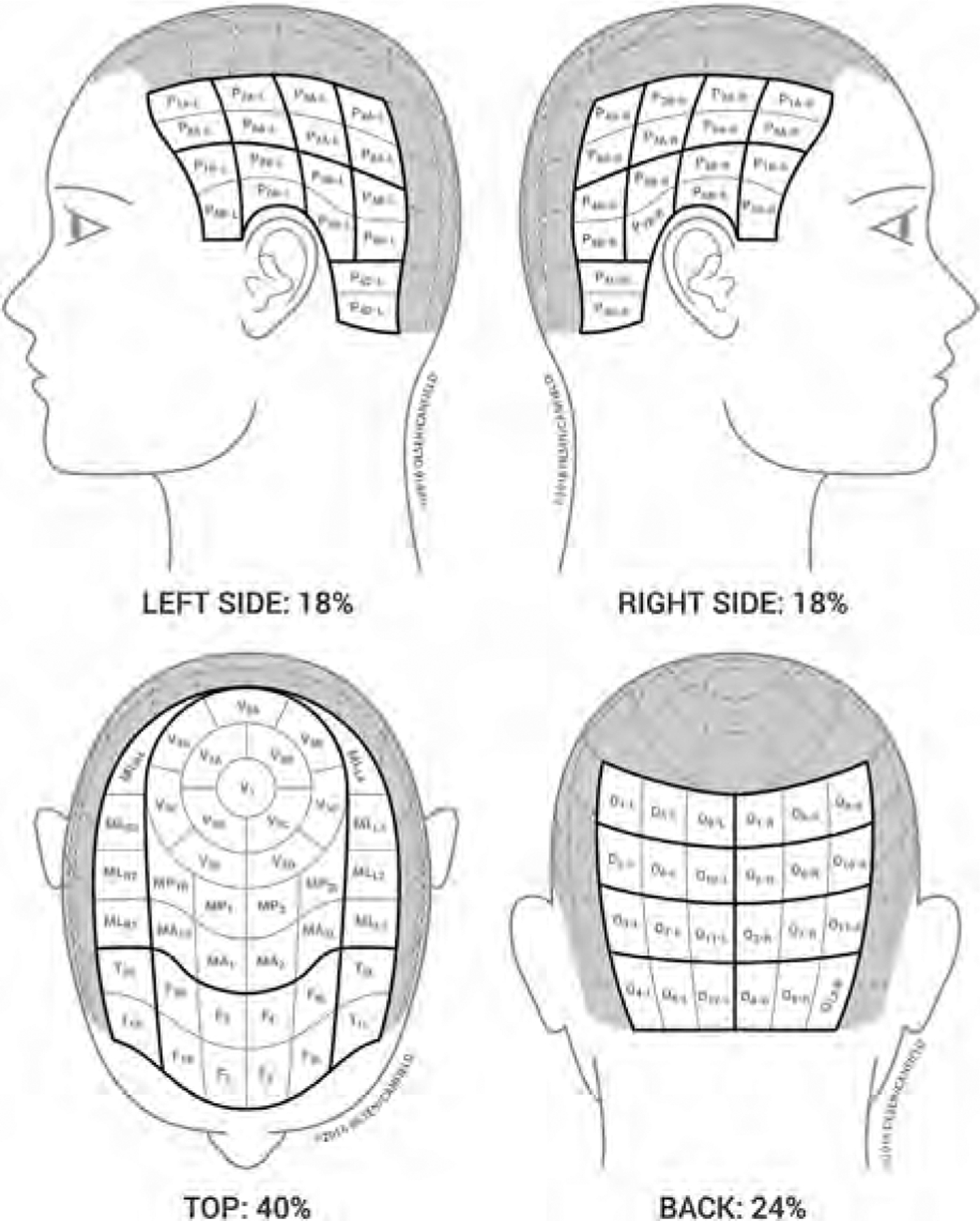
Modified SALT II Visual Aid29 and computation of ALODEX score density is assigned for each 1% of scalp area. The density scale of 0 to 10 that is utilized here is related to percentage of terminal hair loss, where 100% hair loss is equal to 10, 90% is equal to 9, 80% is equal to 8, 70% is equal to 7, 60% is equal to 6, 50% is equal to 5, 40% is equal to 4, 30% is equal to 3, 20% is equal to 2, 10% is equal to 1 and no hair loss is equal to 0. The density assignments in each of the 1% scalp areas in a given quadrant are added together and divided by the maximum grade of hair loss (10) to give the percent hair loss for that quadrant. The score in each quadrant is then added together to give the ALODEX score.
Fig 3.
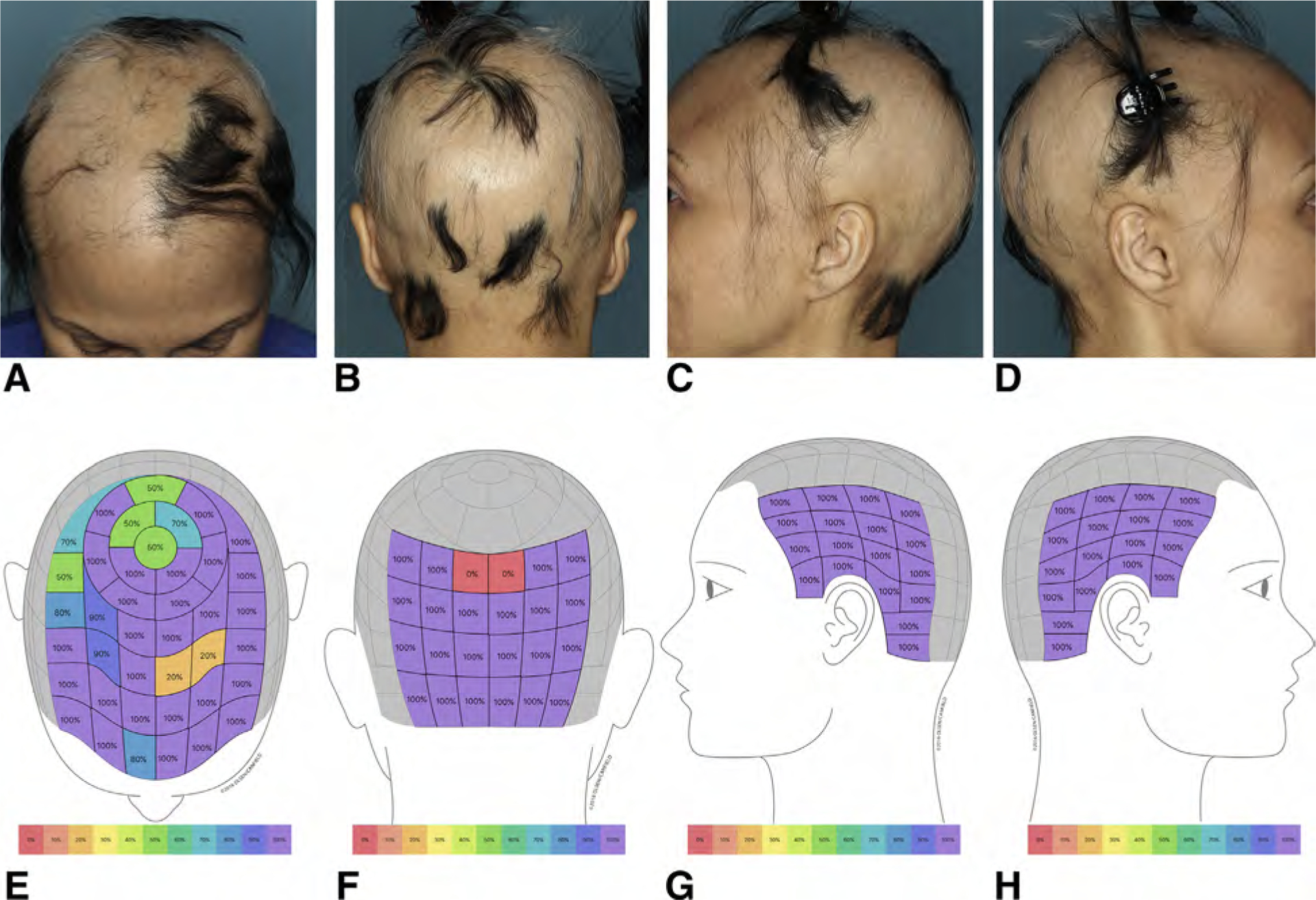
Determination of ALODEX score in a representative patient: 28% (A) plus 22% (B) plus 18% (C) plus 18% (D) hair loss equals 86% scalp hair loss or the ALODEX score. Note that the scattered terminal hairs in a given area have been counted as 100% loss because that is the closest estimated percentage density when the current density scale is used. The SALT score for the same patient is 28% (E) plus 22% (F) plus 17% (G) plus 17% (H) equals 84% total scalp hair loss.
The ALODEX methodology (modified SALT II diagram plus iPAD app documentation of the density of each 1% area of the scalp) can also independently determine the extent of hair loss, not just the percent of scalp with baldness. This may have added utility for quantifying the amount of topical medication applied per cm2 and could potentially be an independent prognostic factor. The ALODEX methodology also allows (1) tracking of density in target bald patches or regions of hair loss as the hair loss progresses or improves over time and (2) confirmation of patterns of hair loss (for example, ophiasis).
Alopecia Areata Progressive Index30
Introduced in 2016, this method includes determining the percent scalp hair loss per quadrant by using the SALT I visual aid, multiplying this number by a hair loss activity score, and then summing the products of each quadrant. This activity score is based on (1) hair pull and (2) dermoscopic findings of exclamation point hairs, broken hairs, and black dots in a representative area of each quadrant.
-
Half-head assessment
This method is often used to assess the response of chemical sensitizers or topical agents in patients who have extensive AA by determining the amount of hair loss on the treated versus untreated sides of the scalp before treatment and at follow-up visits.31 One could use a modification of the SALT or ALODEX score to assess hair loss on each side of the scalp. If hair growth occurs only on the treated side, it is presumed to be entirely related to the agent applied. However, if there is hair growth on both sides of the scalp, explanations include spontaneous remission, systemic absorption by the topical agents, diffusion of the agent to the untreated side of the scalp, and inadvertent or purposeful application by the patient of agent to both sides of the scalp.
- Lesional assessment. This has utility primarily for patches of hair loss that are treated with topical or intralesional agents.
- Lesional size. Designation of 1 to 3 target areas of hair loss, preferably the largest and those that show active progressive hair loss by hair pull or presence of exclamation point hairs and determination of area covered by each and collectively (Fig 4).
- The Lesional Area and Density (LAD) score. This combines a density score (using a 100-point density scale compared with normal) with the area of each target lesion (Fig 4). If multiple target areas are utilized, the sum of all individual LAD scores is the overall LAD score.
- Adjuvant measurements
- Hair pigmentation. The percentage of natural color (nondyed) or white hair should be noted at the beginning and end of treatment.
- Vellus hair. Given that the Scalp, Body, and Nail (SBN) classification, SALT score, and the ALODEX score only grade terminal hair, vellus hair would not typically be noted. However, the percentage scalp coverage of vellus hair could be determined by SALT or ALODEX score as a potential prognostic indicator.
- Activity of hair loss. As already noted, evaluation for both exclamation point hairs and a gentle hair pull at the periphery of several patches of hair loss will help to determine active progressive hair loss. In active patches, dermoscopy shows black dots, broken hairs of various lengths, and exclamation mark hairs.
- Patient assessment
Fig 4.
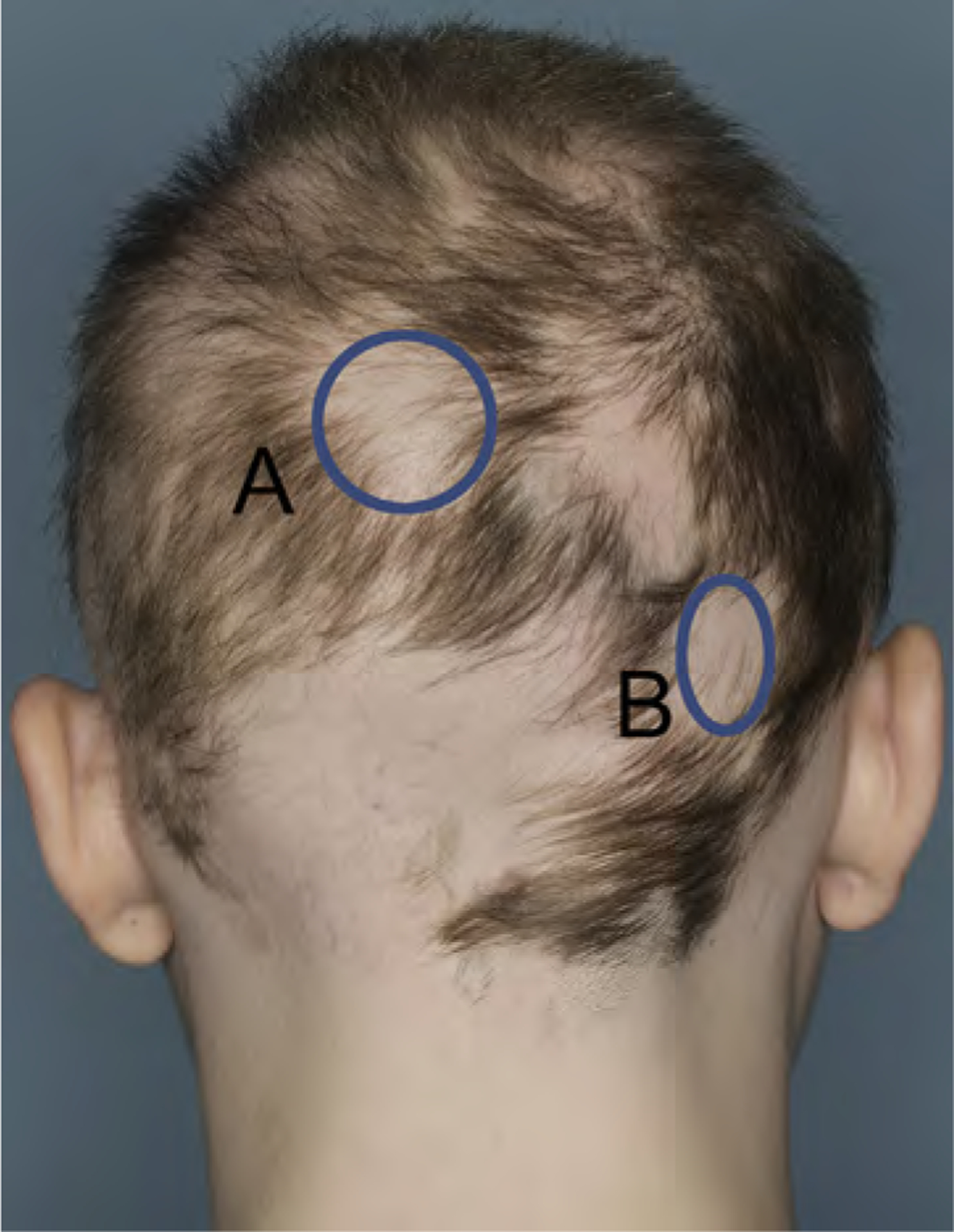
Lesional area and senstivity (LAD) score calculation. If the LAD score is used for determination of the response to a given treatment, for example, in the assessment of response to intralesional steroids, it is best if the area(s) of alopecia chosen to track is clearly demarcated from the surrounding hair. If the margins of the areas are instead less clear, as in the areas in Fig 4 marked A and B, then one should consider marking the edges of the alopecic patch directly on the scalp and taking a representative photograph so that there is a record of which margins were used at the initial assessment. The area of each patch is determined by multiplying the long axis by its perpendicular axis. If the patch of alopecia is totally circular, πr2 may be used instead. This method will require determining ways of identifying these areas of alopecia on subsequent visits (landmarks, photographs, and/or tattoos). Target area A is a 4-cm diameter circular patch of alopecia with an area of πr2 = 3.1416 × (4/2)2 = 12.57 cm2. The density of this area equals 90% hair loss. The area x density = 12.57 × 90/100 which is a LAD score of 11.31. Target area B is a × 3 2.5-cm patch of alopecia with an area of 7.5 cm2. The density of this area equals 98% hair loss. The area X density = 7.5 × 98/100 which is a LAD score of 7.35. The overall LAD score equals A + B = 11.31 + 7.35 = 18.66.CAPSULE
What patients find meaningful in terms of response will be related to the severity of hair loss at baseline (BL) and whether the remaining alopecia is able to be camouflaged. Some options for quantifiable assessment methods by patients include the following:
Classification of hair loss. We recommend that patients perform self-assessment of their hair loss by using the scalp (S) terminal hair loss categorization (ie, S0-S5). Performing this exercise with the patient will enable investigators/clinicians to determine how extensive patients believe their hair loss to be and facilitate further discussion and education about their AA. A more explicit query would be the percentage overall hair loss that the patient believes he or she has, which will be able to be correlated with the SALT or ALODEX scores.
- Quality of life. There are several quality of life instruments available for skin disorders or hair loss that could be modified for use in AA.32–36
-
Role of photographs. Given the dynamic nature of hair loss in AA and the typical time interval between clinic visits, it is important to have photographic documentation of the hair loss at least at the start of treatment. Photographic views of the top, sides, and back of the scalp and the face with hair pulled back to expose the frontal hair line, eyebrows, and eyelashes are best for documentation. However, if patients have multiple patches of hair loss, one may need to part the hair in multiple areas to unmask the different areas of hair loss and take additional photographs of each area of hair loss in a serial manner. Standard photographs to document extent of hair loss cannot be taken in patients with attached hair pieces.We also recommend that for patients being followed in the clinic, physicians consider also taking photographs of the hair loss with the patient’s cell phone (if this option is available). This allows the patient to have a record of his or her own hair loss and allows sharing of subsequent photographs by the patient with the physician without concerns of Health Insurance Portability and Accountability Act violation.
-
V. RECOMMENDED RESPONSE SCORES
Global. Percent change from BL in SALT or ALODEX score.
- Lesional (Fig 4)
- Percent change from BL of target area size.
- Percent change from BL in LAD score.
Patient generated. Using a simple scale for change in hair growth such as +1 to +3 (slightly, moderately, or greatly increased), with 0 equal to no change and −1 to −3 equal to slightly, moderately, or greatly decreased,37 may help to correlate patient appreciation of changes in hair growth with the physician-generated SALT or ALODEX scores.
VI. END POINTS
-
Primary end point. Physician assessment of change in hair growth from BL.
Although the goal of treatment is to return the patient’s scalp hair to that present before the current AA episode, the hair density and any male or female pattern hair loss present before the AA episode cannot be documented. Because of this, determination of 100% regrowth by SALT or ALODEX scoring is not feasible; instead, a 50% improvement in SALT or ALODEX score, ideally with diffuse scalp coverage, is a reasonable target for efficacy of a treatment for AA. In addition, spontaneous remission in AA has to be considered in assessing efficacy; this can be relatively high in patchy AA38 and has been documented to be as high as 8% over a 3-month period in extensive AA (>50% scalp hair loss).39
Time for regrowth should be taken into account in determining efficacy. Often, topical medications such as corticosteroids or anthralin take 3 to 4 months to achieve obvious hair growth, and when regrowth does occur, it will likely not be uniform in all areas treated but will be steady with continued use. Patients treated with intralesional steroids can usually expect some hair growth in about 4 to 6 weeks if effective, although the hair growth may be patchy in the areas of injections secondary to uneven distribution of the injected medication or the variable responsiveness of individual follicles to this treatment modality. Systemic medications such as corticosteroids or other immunomodulators usually show more diffuse hair growth beginning at 4 to 6 weeks. To determine the efficacy of any new agent for AA, it is recommended that at least a 12 week treatment/observation period be utilized.
Secondary end points. Patient assessment of moderately increased hair growth may be the equivalent of a physician-assessed 50% change from BL, but further data on this issue are needed. A certain level of change in quality of life instrument could also be considered a secondary end point if validated.
VII. CONCLUSIONS
We realize that most practitioners will not be able to collect all the information we recommend at each visit on all patients with AA. However, we do recommend obtaining some very basic information that could assist in determining which treatments work best for certain subtypes of AA and in association with which prognostic factors. The collection of standardized outcomes data by large numbers of dermatologists, along with data generated by clinical trials, should help establish best treatment practices for this challenging condition.
Supplementary Material
CAPSULE SUMMARY.
Currently, the only standardized method for assessment of alopecia areata is the Severity of Alopecia Tool score.
This article offers recommendations for standardized assessment and response criteria in patients with alopecia areata.
These methods will facilitate direct comparison of alopecia areata treatment outcome in both clinical practice and clinical trials.
Acknowledgments
Supported in part by the Duke University Hair Disorders Research and Treatment Center and the Leirion Foundation.
Abbreviations used:
- AA
alopecia areata
- ALODEX
Alopecia Density and Extent
- BL
baseline
- LAD
lesional alopecia and density S: scalp
- SALT
Severity of Alopecia Tool
Footnotes
Disclosure: Dr Olsen reports serving as a consultant for Incyte, Concert, Kerastem, Lilly, and Cassiopeia; serving as a consultant for and having another relationship with Allergan; serving on advisory boards for Aclaris and Samumed; and having another relationship with UpToDate. Dr Roberts reports serving as an investigator for Incyte, Concert, Allergan, Samumed, and Theradome. Dr Tosti reports serving on an advisory board and as an investigator for Incyte; serving on advisory boards for Kythera and Aclaris; serving as a consultant for P&G, DS Laboratories, and Polichem; and having other relationships with Karger, Taylor & Francis, and Springer Verlag. Dr Shapiro reports serving as a consultant for Biologics, J&J, Incyte, and Merck; serving as a consultant for and being a stockholder of Eirion; being a stockholder of and creating intellectual property with Replicel Life Sciences, Inc; serving on advisory boards for Applied Biology, Aclaris, and Samumed; and serving as an investigator for Allergan and Regenlab. Dr McMichael reports serving as a consultant for Aclaris, J&J, Covance, eResearch Technology, Guthey Renker, Intraderm, Merck and Co, and Pfizer; being an investigator and advisory board member for Allergan; serving as a consultant and investigator for Galderma, P&G, Samumed, and Incyte; creating intellectual property with Informa Healthcare; serving as an investigator for Cassiopea; and having another relationship with UpToDate. Dr Mirmirani reports serving as a consultant for Cassiopeia and Samumed, being an investigator for Concert, and having another relationship with UpToDate. Dr Bergfeld reports serving as a consultant for Concert, Cassiopeia, Aclaris, P&G, and Pfizer; serving as a consultant and investigator for Allergan; serving as an advisory board member for J&J, Samumed, Kythera, and Incyte; and having another relationship with UpToDate. Dr Callender reports serving as a consultant and investigator for Aclaris, Allergan, Avon, and Gallderma; serving as a consultant for Cassiopeia, Intraderm, L’Oreal, Pfizer, Promius, Samumed, Sensus Healthcare, and Unilever; serving as an investigator for Nevance; and having another relationship with UpToDate. Dr Washenik reports serving as a consultant for Cassiopeia, Aclaris, Allergan, and J&J; serving on an advisory board for Follica and Kythera; serving as a consultant and investigator for Kerastem; serving as a medical monitor for Theradome; being a stockholder of Bosley Medical Group; and creating intellectual property with Aderans. Dr Cotsarelis reports serving as a consultant for Cassiopeia and Lilly, serving as a consultant for J&J, serving as an investigator for Allergan, creating intellectual property with the University of Pennsylvania, and serving as an advisory board member for Follica. Dr Hordinsky reports serving as a consultant for Biologics Inc, P&G, and Pfizer; serving as a consultant and investigator for Concert; serving as an investigator for Allergan; serving as an advisory board member and investigator for Incyte; serving as an advisory board member for Aclaris; and having other relationships with Informa Healthcare, McGraw-Hill, and UpToDate. Drs Sperling and Whiting have no conflicts of interest to disclose.
REFERENCES
- 1.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton J. Incidence of alopecia areata in Olmsted County, Minnesota, 1975–1989. Mayo Clin Proc. 1995;70:628–633. [DOI] [PubMed] [Google Scholar]
- 2.Mirzoyev SA, Schrum AG, Davis MD, Torgerson RR. Lifetime incidence risk of alopecia areata estimated at 2.1% by Rochester Epidemiology Project, 1990–2009. J Invest Dermatol. 2014;134:1141–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringworm Aldersmith H. and Areata Alopecia. Their Pathology, Diagnosis and Treatment. London, United Kingdom: H. K. Lewis; 1897. [Google Scholar]
- 4.Van Scott EJ. Evaluation of disturbed hair growth in alopecia areata and other alopecias. Ann N Y Acad Sci. 1959;83:480–490. [DOI] [PubMed] [Google Scholar]
- 5.Olsen EA. Clinical tools for assessing hair loss In: Olsen EA, ed. Disorders of Hair Loss: Diagnosis and Treatment. New York, NY: McGraw-Hill; 2003. [Google Scholar]
- 6.Pirmez R, Pineiro-Maceira J, Sodre CT. Exclamation marks and other trichoscopic signs of chemotherapy induced alopecia. Australas J Dermatol. 2013;54:129–132. [DOI] [PubMed] [Google Scholar]
- 7.Miteva M, Tosti A. Hair and scalp dermoscopy. J Am Acad Dermatol. 2012;67:1040–1048. [DOI] [PubMed] [Google Scholar]
- 8.Olsen EA, Hordinsky M, McDonald-Hull S, et al. Alopecia areata investigative guidelines. J Am Acad Dermatol. 1999;40:242–246. [DOI] [PubMed] [Google Scholar]
- 9.Walker SA, Rothman S. Alopecia areata. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950; 14:403–413. [DOI] [PubMed] [Google Scholar]
- 10.Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55: 438–441. [DOI] [PubMed] [Google Scholar]
- 11.De Waard-van der Spek FB, Oranje AP, De Raeymaecker DM, Peereboom-Wynia JD. Juvenile versus maturity-onsopecia areata—a comparative retrospective clinical study. Clin Exp Dermatol. 1989;14:429–433. [DOI] [PubMed] [Google Scholar]
- 12.Colombe BW, Price VH, Khory EL, Garovoy MR, Lou CD. HLA class II antigen associations help to define two types of alopecia areata. J Am Acad Dermatol. 1995;33:757–764. [PubMed] [Google Scholar]
- 13.Wiseman M, Shapiro J, MacDonald N, Lui H. Predictive model for immunotherapy of alopecia areata with diphencyprone. Arch Dermatol. 2001;137:1063–1068. [PubMed] [Google Scholar]
- 14.Uchiyama M, Egusa C, Hobo A, Irisawa R, Yamazaki M, Tsuboi R. Multivariate analysis of prognostic factors in patients with rapidly progressive alopecia areata. J Am Acad Dermatol. 2012;67:1163–1173. [DOI] [PubMed] [Google Scholar]
- 15.van der Steen PH, van Baar HM, Happle R, Boezeman JB, Perret CM. Prognostic factors in the treatment of alopecia areata with diphenylcyclopropenone. J Am Acad Dermatol. 1991;24(2 Pt 1):227–230. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald Hull SP, Wood ML, Hutchinson PE, Sladden M, Messenger AG. Guidelines for the management of alopecia areata. Br J Dermatol. 2003;149:692–699. [DOI] [PubMed] [Google Scholar]
- 17.Olsen EA. Investigative guidelines for alopecia areata. Dermatol Ther. 2011;24:311–319. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda T A new classification of alopecia areata. Dermatologica. 1965;131:421–445. [DOI] [PubMed] [Google Scholar]
- 19.Anderson I Alopecia areata: a clinical study. Br Med J. 1950;2: 1250–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freyschmidt-Paul P, Happle R, McElwee KJ, Hoffmann R. Alopecia areata: treatment of today and tomorrow. J Investig Dermatol Symp Proc. 2003;8:12–17. [DOI] [PubMed] [Google Scholar]
- 21.Weise K, Kretzschmar L, Jonh SM, Hamm H. Topical immunotherapy in alopecia areata: anamnestic and clinical criteria of prognostic significance. Dermatology. 1996;192:129–133. [DOI] [PubMed] [Google Scholar]
- 22.Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nelson DA, Spielvogel RL. Alopecia areata. Int J Dermatol. 1985;24:26–34. [DOI] [PubMed] [Google Scholar]
- 24.Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467–1473. [PubMed] [Google Scholar]
- 25.Olsen EA. Topical and systemic corticosteroids in alopecia areata. Australas J Dermatol. 1997;38:20. [Google Scholar]
- 26.Olsen EA, Hordinsky MK, Price VH, et al. Alopecia areata investigational assessment guidelines—art II. J Am Acad Dermatol. 2004;51:440–447. [DOI] [PubMed] [Google Scholar]
- 27.Whiting DA. Histopathologic features of alopecia areata: a new look. Arch Dermatol. 2003;139:1555–1559. [DOI] [PubMed] [Google Scholar]
- 28.Olsen EA, Green C, Hordinsky M, et al. Alopecia Density and Extent Score (ALODEX): a new method for assessing severity of hair loss in alopecia areata. In press.
- 29.Olsen EA, Canfield D. SALT II: a new take on the Severity of Alopecia Tool (SALT) for determining percentage scalp hair loss. J Am Acad Dermatol. 2016;75:1268–1270. [DOI] [PubMed] [Google Scholar]
- 30.Jang YH, Moon SY, Lee WJ, et al. Alopecia areata progression index, a scoring system for evaluating overall hair loss activity in alopecia areata patients with pigmented hair: a development and reliability assessment. Dermatology. 2016;232:143–149. [DOI] [PubMed] [Google Scholar]
- 31.van der Steen PHM, Happle R. Topical immunotherapy of alopecia areata. What, how, and why? Dermatol Clin. 1993;11:619–622. [PubMed] [Google Scholar]
- 32.Dolte KS, Girman CJ, Hartmaier S, Roberts J, Bergfeld W, Waldstreicher J. Development of a health-related quality of life questionnaire for women with androgenetic alopecia. Clin Exp Dermatol. 2000;25:637–642. [DOI] [PubMed] [Google Scholar]
- 33.Chren M-M, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin disease. J Cutan Med Surg. 2001;5:105–110. [DOI] [PubMed] [Google Scholar]
- 34.Picardi A, Abeni D, Pasquini P. Assessing psychological distress in patients with skin diseases: reliability, validity and factor structure of the GHQ-12. J Eur Acad Dermatol Venerol. 2001;15:410–417. [DOI] [PubMed] [Google Scholar]
- 35.Williamson D, Gonzalez M, Finlay AY. The effect of hair loss on quality of life. J Eur Acad Dermatol Venerol. 2001;15:137–139. [DOI] [PubMed] [Google Scholar]
- 36.Mendoza TR, Osei JS, Shi Q, Duvic A. Development of the alopecia areata symptom impact scale. J Investig Dermatol Symp Proc. 2013;16:S51–S52. [DOI] [PubMed] [Google Scholar]
- 37.Olsen EA, Whiting DA, Savin R, et al. Global photographic assessment of men age 18 to 60 years with male pattern hair loss receiving finasteride 1 mg or placebo. J Am Acad Dermatol. 2012;67:379–386. [DOI] [PubMed] [Google Scholar]
- 38.Messenger AG, McKillop J, Farrant P, McDonagh AJ, Sladden M. British Association of Dermatologists’ guidelines for the management of alopecia areata 2012. Br J Dermatol. 2012;166:916–926. [DOI] [PubMed] [Google Scholar]
- 39.Price VH, Hordinsky MK, Olsen, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol. 2008;58:395–402. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


