Abstract
Immunotoxins are antibody–toxin fusion proteins directed to kill cancer cells displaying specific target antigens on their surface. Remarkably, immunotoxins directed to CD22 on hairy cell leukemia have produced complete remissions in approximately 60% of patients enrolled in phase I/II trials. For reasons that are not yet clear, 40% of patients responded less well. In addition, patients with other CD22-positive malignancies have not yet achieved complete remissions. In trying to understand ‘resistance’ to immunotoxin therapy, a number of challenging issues have been raised. These include insufficient dosing, the production of neutralizing anti-immunotoxin antibodies, poor access to malignant cells, and resistance to toxin killing. In designing immunotoxins, we employ truncated Pseudomonas exotoxin, which enzymatically inactivates protein synthesis and produces cell death in sensitive cells. To begin to address toxin resistance we have explored combination therapy with the BH3-only mimetic, ABT-737. Our results indicate that immunotoxin–ABT combinations often exhibit greater killing activity than either compound alone and in some instances overcome resistance. Expression of high levels of prosurvival Bcl-2 proteins may contribute to toxin resistance.
Keywords: apoptosis, ABT-737, BH3-only, Pseudomonas exotoxin
Introduction
Immunotoxins are antibody–toxin chimeric proteins targeted to kill cancer cells [1]. Because native antibodies, even when they bind cancer cells, are rarely cytotoxic, modifications are needed to increase potency. One strategy is to attach a bacterial toxin to the antibody, combining the binding specificity of the antibody with the cell-killing action of the toxin. Recombinant immunotoxins, composed of antibody fragments (Fv) joined to domains II and III of Pseudomonas exotoxin (PE), have shown great promise for the treatment of hematologic malignancies, especially hairy cell leukemia (see Kreitman et al., this issue), but have exhibited less activity against cancers derived from epithelial cells. PE-derived immunotoxins are cytotoxic because they gain access to the cell cytosol and inhibit protein synthesis via the enzymatic adenosine diphosphate (ADP)-ribosylation of elongation factor 2 (EF2). While the mechanism of toxin-mediated cell death is still under investigation, some features have been reported. Du et al. have reported Bak-dependent apoptosis [2], and several groups have noted that inhibition of protein synthesis results in the loss of the prosurvival protein, Mcl-1, potentially mediating apoptosis via this route [3].
Despite their potency, immunotoxins produce complete remissions infrequently when administered as single agents. Accordingly, various strategies have been proposed to increase effectiveness. Immunotoxins constructed with bacterial toxins are frequently immunogenic, prompting strategies to eliminate major antigenic epitopes [4]. Reducing immunogenicity would allow more cycles of treatment. In solid tumor masses, immunotoxin delivery may be restricted because of tumor architecture and the presence of high concentrations of soluble target antigen. To address this, various combination therapies have been proposed to ‘break up’ tumor architecture, lowering the concentration of blocking antigen and delivering more immunotoxin [5]. Another possible reason for immunotoxin resistance relates to anti-apoptotic strategies frequently employed by tumor cells to overcome chemotherapy-mediated death. With regard to the latter, high levels of prosurvival proteins such as Bcl-2, Bcl-xl, and Mcl-1 contribute to resistance. Because immunotoxin action frequently results in the loss of Mcl-1 (Mcl-1 is a short-lived protein and is not replenished when protein synthesis is shut down), we speculate that high levels of Bcl-2 or Bcl-xl could result in less than complete immunotoxin-mediated killing [6]. We are beginning to explore this concern.
Results and discussion
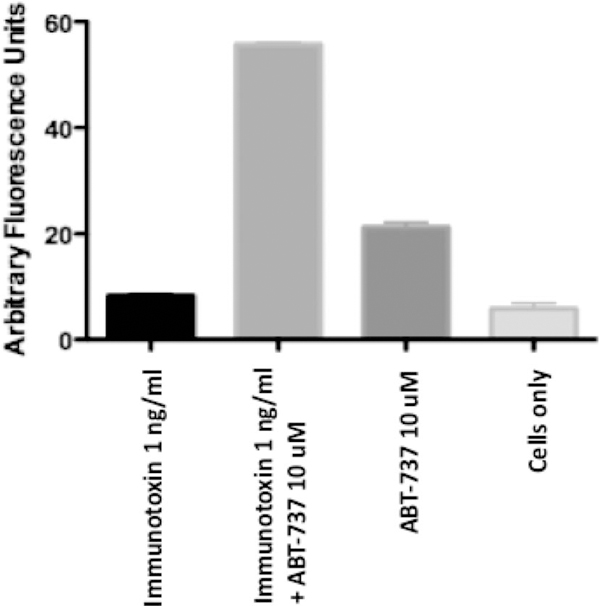
One way to combat a high expression level of prosurvival proteins is the use of a BH3-only mimetic. To appreciate this approach, elements of the apoptosis machinery need to be considered. Activation of the proapoptotic proteins, Bax and Bak, depends on the action of BH3-only proteins, which are up-regulated in response to stress or cell injury. BH3-only proteins can activate Bax/Bak directly or they can trigger apoptosis via the neutralization of the prosurvival proteins, Bcl-2, Bcl-xl, Mcl-1. Therefore, BH3 mimetics were developed as potential standalone antitumor agents or as sensitizers for chemotherapy, thus eliminating resistance to apoptosis. ABT-737, one such agent, was shown to have strong binding affinity for Bcl-2 and -xl but little or none for Mcl-1 [7]. Because immunotoxins result in the loss of Mcl-1 via inhibition of protein synthesis, it seemed worth investigating whether ABT-737 could sensitize cells to immunotoxin action. Therefore, experiments were conducted whereby cells were treated with immunotoxin alone, ABT-737 alone, or immunotoxin plus ABT-737 [6]. We have completed this kind of experiment with several cell lines, including immunotoxin resistant cells, and frequently see a result qualitatively similar to that shown in Figure 1, where immunotoxin–ABT-737 combinations act synergistically to kill cells via apoptosis. Using caspase 3 activation as a measure of apoptosis, we note that DLD1 cells were not readily killed by either immunotoxin or ABT-737 alone. However, the combination showed extensive apoptotic cell death (Figure 1, and reported more extensively in reference [6]). Further, it should be noted that we have been able to duplicate most of our findings obtained with ABT-737 with the related clinical compound, ABT-263 (data not shown).
Figure 1.
Immunotoxin resistance is overcome by combination treatment with ABT-737. DLD1 cells were treated with an immunotoxin (48 h) directed to the human transferrin receptor either alone or in combination with ABT-737. At 1 ng/mL there was no evidence of immunotoxin-mediated apoptosis as measured by activation of caspase 3. When the immunotoxin was added in combination with ABT-737, there was activation of caspase 3, indicating cell death via apoptosis. In data not shown, PARP [poly(ADP-ribose) polymerase] cleavage was also evident, but only in cells treated with the combination of immunotoxin and ABT-737. ABT-737 was not toxic for DLD1 cells.
In conclusion, our results support using combination treatments whereby immunotoxins are administered along with agents that overcome resistance. These adjunct agents will likely be chosen from compounds that either reduce antibody responses, increase tumor ‘break up,’ or sensitize cells to apoptosis. While laboratory experiments point the way for such development, concepts will have to be evaluated and treatments proven in the clinic.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article at www.informahealthcare.com/lal.
References
- 1.Pastan I, Hassan R, FitzGerald DJ, Kreitman RJ. Immunotoxin treatment of cancer. Annu Rev Med 2007;58:221–237. [DOI] [PubMed] [Google Scholar]
- 2.Du X, Youle RJ, FitzGerald DJ, Pastan I. Pseudomonas exotoxin A-mediated apoptosis is Bak dependent and preceded by the degradation of Mcl-1. Mol Cell Biol 2010;30: 3444–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson Y, Juell S, Fodstad Ø. Downregulation of theantiapoptotic MCL-1 protein and apoptosis in MA-11 breast cancer cells induced by an anti-epidermal growth factor receptor-Pseudomonas exotoxin a immunotoxin. Int J Cancer 2004;112:475–483. [DOI] [PubMed] [Google Scholar]
- 4.Onda M, Beers R, Xiang L, Nagata S, Wang QC, Pastan I. An immunotoxin with greatly reduced immunogenicity by identification and removal of B cell epitopes. Proc Natl Acad Sci USA 2008;105:11311–13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y, Xiang L, Hassan R, Pastan I. Immunotoxin and taxol synergy results from a decrease in shed mesothelin levels in the extracellular space of tumors. Proc Natl Acad Sci USA 2007;104:17099–17104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traini R, Ben-Josef G, Pastrana DV, et al. ABT-737 overcomes resistance to immunotoxin-mediated apoptosis and enhances the delivery of pseudomonas exotoxin-based proteins to the cell cytosol. Mol Cancer Ther 2010;9:2007–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 2005;435:677–681. [DOI] [PubMed] [Google Scholar]