Abstract
Much attention has been recently paid to the design of sustainable processes for the production of functional food additives based on renewable resources. Thus, methods for incorporation of green techniques in treatment of undeveloped biomass, resulting in value-added bioproducts, are in great demand. We focus here on the biological activity and chemical properties of Erigeron canadensis (horseweed) functional food fiber, which can be strongly affected by the extraction procedure employed. In the present contribution, we report on an attempt to introduce a sustainable and energy-efficient ultrasound-assisted extraction process, followed by a multistep purification procedure, resulting in a macromolecular plant-derived anticoagulant agent. The most efficient ultrasound-assisted process was determined by optimization through the response surface methodology I-optimal design (24). A comparison with the conventional procedure for retrieval of horseweed biomacromolecules revealed that the optimized ultrasound-assisted extraction was more sustainable, with the cumulative energy demand being 38% lower (12.2 MJ), 6.6 times reduced water consumption (3.5 L), and 1.2 times shorter (41 h) total processing time. Moreover, the optimal ultrasound-assisted extraction process-purified food fiber turned out to be a better anticoagulant agent by 57%, compared to a conventional product, and was a more selective indirect inhibitor of the human Xa coagulation factor.
Introduction
The conventional methodology for separation of valuable plant bioproducts,1 like dietary fiber, frequently employs high-temperature solid–liquid extraction, which relies on the use of water-based systems, such as a dilute alkaline or acidic environment.2 Any conventional extraction process from a natural source, unfortunately, is typically time-consuming and requires great amounts of solvents and energy, which highly increases the operating costs.3 In 2012, Chemat et al.4 proposed the concept of “green extraction of natural products on the basis of green chemistry and green engineering”. This approach focused on reduced energy consumption, utilization of renewable natural resources and alternative solvents, and ensuring safe and high-quality bioproducts. Hence, there is a need for new and sustainable technological processes, which are currently becoming the most important approaches in the treatment of bioresource materials.4 Far-reaching industrial advancement goals have been set, after establishment of the REACH directive (Registration, Evaluation, Authorization and Restriction of Chemicals) by the European Union,5 which noticeably helps to eradicate nonsustainable development. In addition, facing the problem of increasing emission of CO2 and energy prices, the emerging extraction technologies must be developed to attain the legal requirements on emissions, safety, and cost reduction.4 The best available technology (BAT) introduced in the commercial sector makes it necessary to focus on employing processes with sustainable expenditure of raw materials and reagents, tending to reduce water and energy consumption.6
For that reason, novel technologies that utilize mainly physical factors have been proposed. Examples of them are ultrasound-assisted extraction (UAE),7 microwave-assisted extraction,8 and subcritical and supercritical water or CO2 extraction,9 as well as utilization of pulsed electric fields10 or steam explosion techniques.11 They all focus on process intensification and cost reduction, while they maintain safety and low impact on the environment, frequently, by minimizing waste formation and using water recirculation.12 However, the chemical composition of the treated biomass and potential biological activity of the isolated bioproducts play key roles in selecting the appropriate extraction technique. The main goal is to preserve those properties, since, for example, the main components of dietary fiber are especially vulnerable to damage during extraction.2
Use of ultrasound turned out to be especially valuable in the isolation of bioactive compounds from plant biomass.13,14 The cavitation forces of ultrasound generate shockwaves resulting in immediate fragmentation or disruption of plant cell walls, thus effectively enhancing mass transfer, accelerating the diffusion rate of the biomolecules into the solvent, and increasing the yield of the extracted material.15 Moreover, when compared to the other techniques employing physical factors, UAE has advantages such as reproducibility, lower maintenance costs, easier temperature control, shorter processing time, reduced energy and water consumption, and lowered waste formation.13,15 The properties of ultrasound enable feasible adaptation to the industrial scale.16 A good example of possible upscaling is the industrial ultrasonic reactor with 850 L working capacity, designed by a Romanian research group (EU Copernicus Programme, Romanian Academy) for large-scale solvent extraction of medicinal plants. The introduced reactor operates at Plafar factory, one of the biggest producers of herbal extracts on the Romanian market.17 However, academic and industrial research studies focus mainly on presenting the positive effect of ultrasound-assisted extraction when compared to conventional methods. Important aspects that should be studied simultaneously are physical mechanisms occurring on the surface of the complex plant matrix, i.e., erosion, sonoporation, shear forces, fragmentation, detexturation, and others. Such an approach to extend the knowledge to sonochemical processes was proposed by Chemat et al.18
In the modern lifestyle, functional dietary fiber is frequently undervalued, and the lack of it in human diet may lead to a range of civilization diseases, such as obesity, cardiovascular diseases, diabetes, and many others.19 Moreover, the research literature shows that those risks could be lowered by incorporating different types (soluble or insoluble) and components of functional food fiber (FF) (lignin, pectin, hemicellulose, and a variety of associated phytochemicals).20 Those findings, as well as a tendency to follow a healthy lifestyle, have resulted in rising consumer awareness about keeping a balanced diet. As a result, the global functional FF market value surpassed 5.0 billion USD in 2019, and it is believed that the compound annual growth rate for the period 2020–2026 will exceed 12%, with the projected global value in 2026 at approximately 12 billion USD.21 Thus, the demand for production and the search for new sources of dietary FF will constantly grow.
Canadian horseweed (Erigeron canadensis L.) is a popular, very invasive one-year plant of the Asteraceae family, which is spread mainly in the northern climate zone.22 Canadian horseweed is recognized as a hard-to-eradicate weed, frequently invading croplands.23 Moreover, horseweed is able to reduce crop efficiency by competing for resources due to its height (100–150 cm) and by releasing toxic substances able to inhibit growth of other plants. That is why horseweed is commonly generated as a waste in crop cultivation.24 However, it may be treated as an undeveloped biomass that can yield functional FF and many beneficial phytopharmaceuticals, since the infusions of horseweed are well known in traditional medicine as diuretic, antifungal, toning, and astringent agents. Moreover, polyphenolic glycoconjugates from E. canadensis exhibit effective anticoagulant properties.25
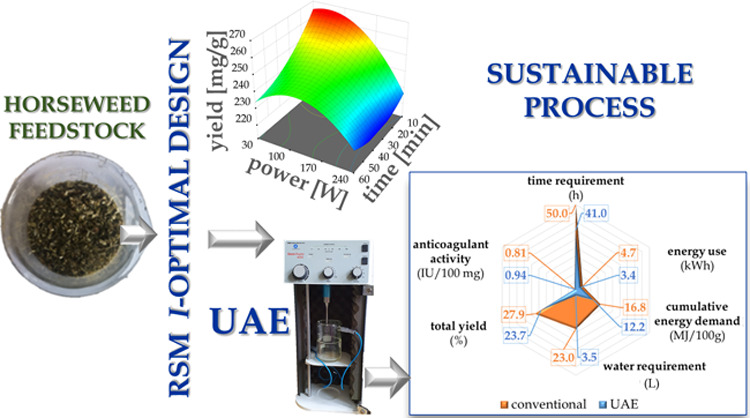
The work reported here extends our studies on new methodologies and techniques regarding biopharmaceuticals and new biocompatible drug nanocarriers.25−29 In the present contribution, horseweed has been selected as a source of plant-originated functional food fiber. Moreover, its easy availability and the fact that this weed is a common issue in agriculture draw attention to the need for development of this valuable bioresource. The presented studies follow the current necessity for developing cost-effective bioprocessing technologies with a low environmental impact. An important issue in this context is providing an example of the downstream bioprocessing of agricultural waste, resulting in a concept of value-added bio-based products. The proposed process was developed based on the response surface methodology (RSM) I-optimal design and a series of experimental runs, which determined the optimal conditions for the ultrasound-assisted extraction procedure, ensuring reduced time and cost of operation, thereby attaining green and sustainable development by visibly lowering energy and water consumption.
Results and Discussion
Conventional Extraction of Functional Dietary Fiber vs UAE
According to our previous studies on plant compounds as inhibitors of the human blood clotting process, the best conditions that allow crude plant extracts to be obtained that are rich in such bioproducts are a mild alkaline environment, especially aqueous 0.1 mol/L NaOH.25 Appropriate selection of the extraction solvent is an efficient way to isolate a weakly soluble FF, i.e., a polyphenolic matrix combined with polysaccharide. Low-concentration alkaline extraction, due to the presence of hydroxide ions, allows breaking of the hydrogen bonding connecting hemicellulose and cellulose,30 without degradation of other valuable components of the plant cell wall. Polysaccharide fragments of the food fiber are solubilized, thus increasing the diffusion rate to the extraction solvent.31
For that reason, conditions of the isolation procedure from plant bioresources affect the properties of the final product. Moreover, the initial extraction process resulting in a raw bioproduct is considered as the crucial stage; hence, it was chosen as a target of the modification. The conventional method of horseweed FF separation includes 6 h of extraction procedure under boiling conditions using a power-consuming, reflux system.25 According to the concept of sustainable development,1 the biomass treatment stage of the whole isolation process of functional FF was modified by employing ultrasound-assisted extraction.
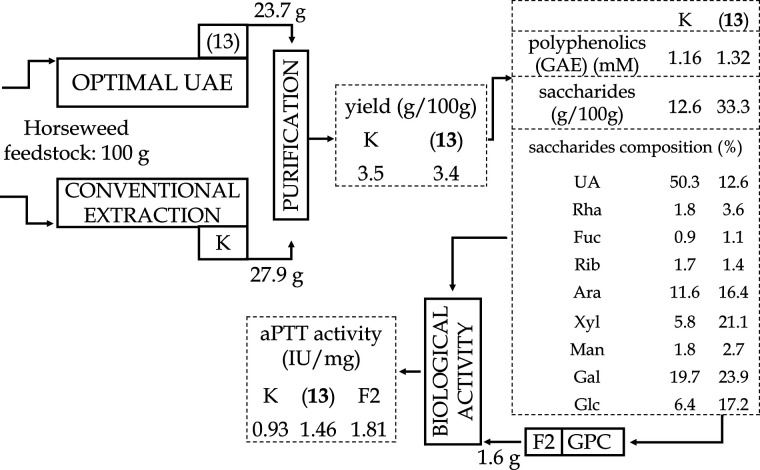
The conventional extraction that followed the maceration prep-stage resulted in a crude dietary fiber isolate (K′), which was characterized for the basic parameters, i.e., extraction yield (27.9% of the plant dry mass), pH of the crude product (8.7), and the anticoagulant activity (0.081 IU/mg), using the activated partial thromboplastin time in vitro test (aPTT). The conventionally obtained raw extract served as a reference substance for further studies of the UAE process.
The UAE procedure described in this paper introduced a major change for the extraction process; i.e., it was performed at the ambient temperature, i.e., 25 °C, which was maintained by water cooling of the glass reactor. To further optimize the UAE procedure and to determine the influence of the process parameters on the quality of the crude bioproduct, different combinations of time and power of applied ultrasound were employed. This resulted in 16 different crude FF isolates (numbered (1′)–(16′) and annotated with apostrophe). Afterward, the obtained raw bioproducts were subjected to the same characterization as the conventionally obtained raw product K′. The relation between time and power parameters of the applied ultrasound, yield, pH value, and anticoagulant activity is presented in Figure 1. In each of the conducted UAE processes, the raw products were obtained with similar efficiency (Figure 1), i.e., 220.0–264.0 mg/g (wt of dry plant mass). However, it can be observed that with the increase of time and power of the ultrasound employed, the yield decreased. Nevertheless, each of the obtained crude bioproducts gave comparable yields, with 10–60 min of total processing time, in comparison to the conventional isolate K′, which required much longer extraction, i.e., 6 h to yield 279.0 mg/g (dried mass).
Figure 1.
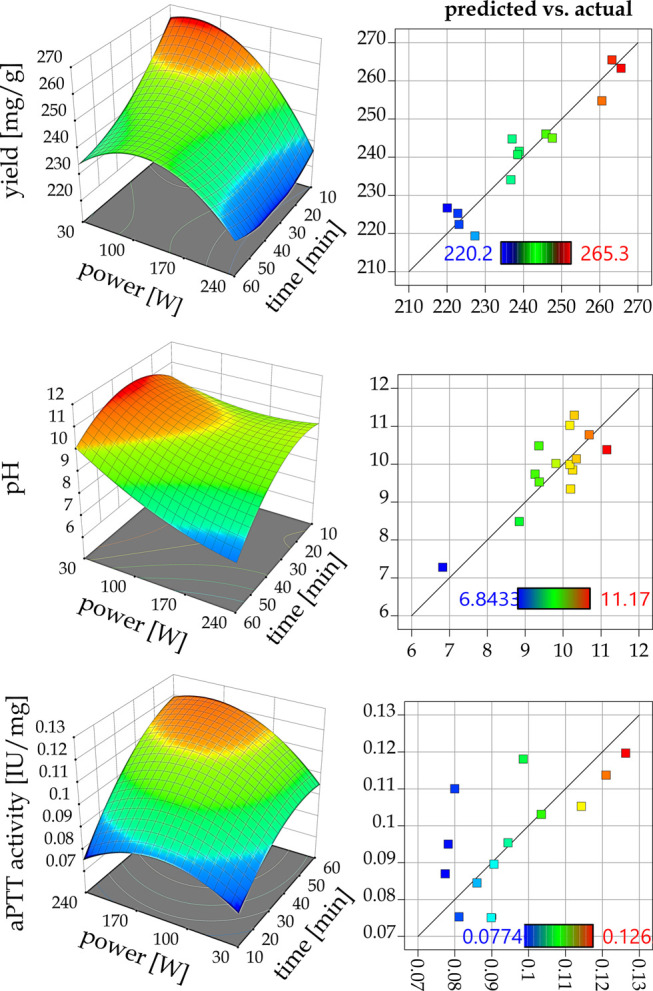
Graphical representation of the 24I-optimal design response surfaces for the dependent variables Y1 = yield, Y2 = pH, and Y3 = aPTT activity vs independent variables (time, power) and actual model fitting.
The pH value of the isolates oscillated between 11.7 and 6.8. A general decreasing trend was observed with the increase of time and power of ultrasound (Figure 1). Finally, the determined anticoagulant activity for the isolates (1′)–(16′) showed the opposite trend when compared to the extraction yield; i.e., with the increase of time and power of UAE, the activity also increased (Figure 1). Thus, 10 min of sonication time at 120 W was not enough, which is represented by isolate (3′) with the lowest activity observed (0.077 IU/mg), whereas the highest activity at maximum power and time of extraction (60 min, 240 W) was observed for the isolate (16′) (0.126 IU/mg). Moreover, almost all of the isolates obtained gave better or similar activity when compared to the conventional raw bioproduct K′ (0.081 IU/mg). The relation between the ratios of saccharides to polyphenols is a key element affecting the anticoagulant properties of the horseweed FF, which will be described in detail further in the section on chemical characterization. The results related to the characterization of raw extracts are also crucial for studying the main effects of the UAE process parameters on the response factors, where only extracts with good activity will be subjected to further analysis as described in the following section devoted to RSM optimization.
RSM Optimization of the UAE Process and Further Purification of the Range of Raw FF
The response surface methodology makes a good compromise when it is necessary to determine the influence of the independent variables on the response factors, especially in the complex cases where these variables may additionally exist in multiple correlations, which might bring various constraints when fitting an optimization model.32Table S1 in the Supporting Information (SI) presents the main assumptions of the 24I-optimal model utilized in the present contribution, i.e., coding of the independent variables with the corresponding value levels. Table 1 shows the results of all of the performed experimental runs with the actual values of the dependent variables—yield (Y1), pH (Y2), and aPTT activity (Y3)—taken as response factors.
Table 1. Experimental Matrix with Experimental Runs and Corresponding Actual Values of the Independent and Dependent Variablesa.
| experimental
design |
independent
variables |
dependent
variables |
||||
|---|---|---|---|---|---|---|
| number of experimental run | symbol of extract | A time (min) | B power (W) | Y1: yield (mg/g) | Y2: pH | Y3: activity in aPTT test (IU/mg) |
| 1 | (11′) | 40 | 120 | 245.9 | 10.2 | 0.121 |
| 2 | (3′) | 10 | 120 | 265.3 | 9.3 | 0.077 |
| 3 | (11′) | 40 | 120 | 245.9 | 10.2 | 0.121 |
| 4 | (15′) | 60 | 120 | 247.3 | 8.8 | 0.099 |
| 5 | (3′) | 10 | 120 | 265.3 | 9.3 | 0.077 |
| 6 | (11′) | 40 | 120 | 245.9 | 10.2 | 0.121 |
| 7 | (4′) | 10 | 240 | 220.2 | 10.2 | 0.090 |
| 8 | (12′) | 40 | 240 | 227.3 | 10.2 | 0.080 |
| 9 | (16′) | 60 | 240 | 222.6 | 6.8 | 0.126 |
| 10 | (13′) | 60 | 30 | 236.7 | 10.3 | 0.094 |
| 11 | (5′) | 20 | 30 | 260.5 | 10.2 | 0.086 |
| 12 | (10′) | 40 | 60 | 237.1 | 10.8 | 0.104 |
| 13 | (14′) | 60 | 60 | 238.3 | 9.4 | 0.115 |
| 14 | (10′) | 40 | 60 | 237.1 | 10.7 | 0.104 |
| 15 | (11′) | 40 | 120 | 245.9 | 10.2 | 0.121 |
| 16 | (11′) | 40 | 120 | 245.9 | 10.2 | 0.121 |
| 17 | (3′) | 10 | 120 | 265.3 | 9.3 | 0.077 |
| 18 | (11′) | 40 | 120 | 245.9 | 10.2 | 0.121 |
| 19 | (8′) | 20 | 240 | 223.2 | 9.8 | 0.090 |
| 20 | (8′) | 20 | 240 | 223.2 | 9.8 | 0.091 |
| 21 | (16′) | 60 | 240 | 222.6 | 6.8 | 0.126 |
| 22 | (1′) | 10 | 30 | 263.5 | 11.2 | 0.081 |
| 23 | (1′) | 10 | 30 | 263.5 | 11.2 | 0.081 |
| 24 | (13′) | 60 | 30 | 236.7 | 10.3 | 0.094 |
| 25 | (10′) | 40 | 30 | 238.9 | 10.3 | 0.078 |
The ultimate goal of the optimization was to maximize the anticoagulant activity of the raw FF extracts while maintaining the yield of UAE products at a level comparable to that obtained conventionally. In this study, the resulting experimental data were fitted by multiple regression analysis into a quadratic polynomial optimization model. This resulted in formulation of empirical equations representing the response surface for the dependent variables Y1–Y3. The predicted response factor values were obtained by the restricted maximal likelihood (REML) analysis of variance (ANOVA) with Kenward–Roger p-values, supporting the statistical significance of the analyzed experimental data,33 which can be seen in Table 2.
Based on the presented analysis of variance, it was possible to state the significance of optimization model assumptions and thus determine the actual influence of the independent variables on the response factors. Considering Y1, the existing interactions between process parameters (A: time, B: power), i.e., A, B, AB, A2, and B2, turned out to be statistically significant (p < 0.05) and had a direct influence on Y1. In the case of pH values of the raw extracts, the ANOVA test gave similar results, showing an analogous effect of power and time on the Y2 response factor Table 2. Finally, the third response factor, Y3, was only affected by interactions of A and B2 variables (p < 0.05). Moreover, experimental results presented by Zhang et al.34 showed that, in the case of the UAE process, parameters such as sonication power, time effect, and simple interaction between those variables mainly affected the quality and extraction yield of functional FF. Thus, based on the discussed results, literature data, and the response surfaces presented in Figure 1, the most desirable raw extracts with the appropriate anticoagulant activity and acceptable yield were obtained at 60 min of total processing time and within the sonication power range of 30–240 W. Therefore, extracts (13′) (parameter combination A4B1), (14′) (A4B2), (15′) (A4B3), and (16′) (A4B4), which exhibited a response surface within the acceptable area of dependent variables (Figure 1), were subjected, together with the conventionally obtained product K′, to a multistep purification procedure.
Table 2. ANOVA Results for the I-Optimal Design Quadratic Model for Dependent Variables of UAE Raw Horseweed Food Fibera.
| source | term df | error df | F-value | p-value |
|---|---|---|---|---|
| Dependent Variable: Yield | ||||
| whole-plot | 2 | 5.19 | 76.35 | 0.0001 |
| b (power) | 1 | 4.78 | 77.20 | 0.0004 |
| b2 (power2) | 1 | 5.74 | 43.45 | 0.0007 |
| subplot | 3 | 14.63 | 42.67 | <0.0001 |
| A (time) | 1 | 15.58 | 59.63 | <0.0001 |
| Ab (time × power) | 1 | 14.08 | 34.18 | <0.0001 |
| A2 (time2) | 1 | 13.99 | 14.87 | 0.0017 |
| S.D. = 4.18, mean = 242.80, R2 = 0.9490, adj. R2 = 0.9235 | ||||
| mathematical correlation between the response factor and independent variables: yield = 246.1 – 8.105 × A – 11.94 × b + 7.293 × Ab + 6.556 × A2 – 14.85 × b2 | ||||
| Dependent Variable: pH | ||||
| whole-plot | 2 | 19.00 | 17.73 | <0.0001 |
| b (power) | 1 | 19.00 | 33.98 | <0.0001 |
| b2 (power2) | 1 | 19.00 | 3.43 | 0.0796 |
| subplot | 3 | 19.00 | 15.94 | <0.0001 |
| A (time) | 1 | 19.00 | 23.45 | 0.0001 |
| Ab (time × power) | 1 | 19.00 | 10.67 | 0.0041 |
| A2 (time2) | 1 | 19.00 | 19.59 | 0.0003 |
| S.D. = 0.5253, mean = 9.83, R2 = 0.08052, adj. R2 = 0.7078 | ||||
| mathematical correlation between the response factor and independent variables: pH = 10.04 – 0.7078 × A – 0.8477 × b – 0.5907 × Ab – 1.073 × A2 + 0.4444 × b2 | ||||
| Dependent Variable: aPTT Activity | ||||
| whole-plot | 2 | 19.00 | 3.90 | 0.0380 |
| b (power) | 1 | 19.00 | 3.44 | 0.0791 |
| b2 (power2) | 1 | 19.00 | 5.20 | 0.0344 |
| subplot | 3 | 19.00 | 9.43 | 0.0005 |
| A (time) | 1 | 19.00 | 24.49 | <0.0001 |
| Ab (time × power) | 1 | 19.00 | 2.17 | 0.1568 |
| A2 (time2) | 1 | 1900 | 2.28 | 0.1471 |
| S.D. = 0.0118, mean = 0.0999, R2 = 0.6637, adj. R2 = 0.4956 | ||||
| mathematical correlation between the response factor and independent variables: IU = 0.1120 + 0.01623 × A + 0.006055 × b + 0.005982 × Ab – 0.008222 × A2 – 0.01227 × b2 | ||||
The obtained coefficients of determination were as follows: R2 = 0.949, Radjusted2 = 0.924 for the yield values; R2 = 0.805, Radjusted2 = 0.708 for the pH of the raw extracts; and R2 = 0.664, Radjusted2 = 0.496 for the anticoagulant activity assessed by the aPTT test.
Refinement techniques are widely utilized due to the fact that usually a raw plant isolate obtained after an extraction procedure does not meet requirements for its practical use. One of those requirements, besides the appropriate chemical character, is the physical properties of the bioproduct, which highly affect the biological activity and potential value in use. Especially from the point of view of biopolymers, it might be the appropriate level of homogeneity or range of molecular weights.35 Despite the fact that additional purification will generate additional cost of the whole procedure, it is necessary to ensure a final bioproduct with satisfactory purity and bioactivity.35
A multistep purification process was performed and resulted in five pure FF isolates from the horseweed, i.e., a conventional reference bioproduct K and four from the UAE procedure selected based on the optimization results—(13), (14), (15), and (16), yielded from 100 g of biomass feedstock, respectively, with 3.5, 3.4, 3.0, 4.7, and 4.8% (w/w) efficiency. All of the obtained purified FF isolates were verified by their anticoagulant ability to choose one suitable optimal UAE bioproduct. The results are presented in Figure 2A. The evaluation revealed that the best UAE-purified product was (13) with increased activity by 15.5 times when compared to the raw isolate (13′) (from 0.094 to 1.46 IU/mg). Additionally, it acted 1.6 times better than the FF retrieved in the conventional procedure (K, 0.93 IU/mg). Thus, (13) was selected as the optimal UAE product and was subjected to further qualitative and quantitative evaluation due to both its overall yield and increased bioactivity.
Figure 2.
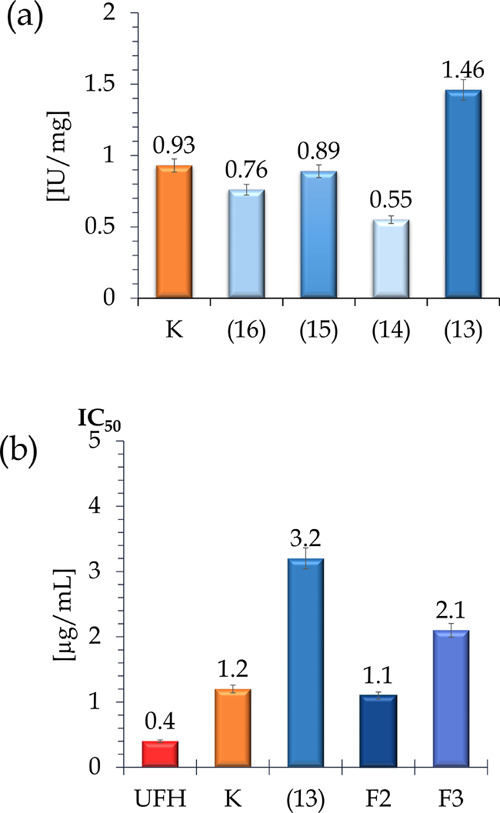
Comparison of the aPTT activity of the conventional process bioproduct vs selected ultrasound-assisted process bioproducts (a); comparison of the inhibitory activity toward the Xa factor of the reference compound unfractionated heparin (UFH), conventional process bioproduct, and UAE optimal isolate (13) and its separated active fractions (b).
Energy Consumption of the Conventional vs UAE Horseweed FF Extraction Procedures
The demand for naturally based functional food production in the food industry has constantly been increasing over recent years; thus, there is a growing trend of improvements of extraction procedures utilizing clean and green technologies.36 Research literature reports that ultrasound assisted techniques are considered to be effective in isolation of macromolecules with specific biological activity from herbal sources.37 Traditional extraction techniques of natural compounds, such as functional FF, require a prolonged time and, in many cases, higher temperature. However, this may result in structural changes or even degradation of some of the acquired valuable bioproducts.37 Therefore, employment of the ultrasound-assisted extraction can result in an added value to the isolation of dietary fiber.
An important element that should be considered is the economic aspect of the proposed pretreatment of biomass. Thus, time and water requirements as well as energy consumption and FF quality were taken into account in the present study. The analyzed factors are given in Figure 3. The treated feedstock of biomass that was subjected for the analyses was 100 g of dried horseweed. Based on the results presented in Table S2A,B (Supporting Information), it can be seen that the ultrasound-assisted extraction procedure (30 W and 60 min) for the optimal product (13′) was more energy efficient (12.2 MJ) than the conventional one (16.8 MJ), with the cumulative energy demand (CED) factor being 38% lower. According to the energy consumption and average electricity gross prices for a household consumer (0.2292 €/kWh, based on Eurostat), the proposed UAE optimal process will allow reduction of the cost by 41%/100 g of feedstock approximately. With other reductions achieved, the total processing time (41 h) and the water requirement (3.5 L) were 1.2 and 6.6 times lower, respectively, when contrasted with the conventional extraction procedure (50 h, 23 L). However, the raw food fiber K′ was yielded from the treated 100 g of horseweed with 4.2% higher efficiency (27.9 g) than the optimal UAE product (13′) (23.7 g). Nevertheless, the final factor describing the quality of the obtained bioproduct, i.e., the anticoagulant activity, turned out to be higher in the case of (13′) UAE raw extract by 16.1% than in K′ obtained by conventional extraction. The optimized UAE proposed in the present contribution may give rise to a functional FF production process from undeveloped horseweed biomass in a sustainable manner with possible reduction of the operational cost.
Figure 3.
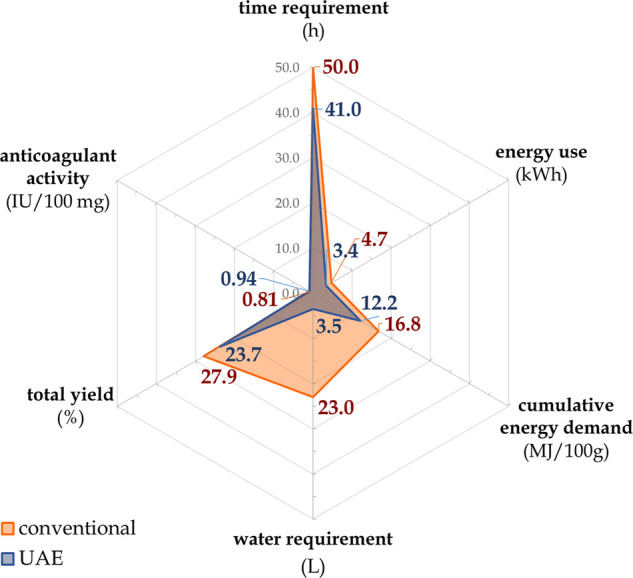
Sustainability comparison of the conventional extraction vs ultrasound-assisted extraction (UAE) optimized procedure.
Chemical Description of the Obtained Horseweed FF Isolates
Chemical composition of the conventionally obtained product K and the optimal UAE one (13), with polyphenolic–polysaccharide nature, was as expected (see Figure 4).25 Both FF products showed the presence of phenolics rich in free hydroxyl groups, i.e., 1.16 ± 0.02 and 1.32 ± 0.01 mM gallic acid equivalent (GAE) per 1 g of dry mass of K and (13), respectively. The total saccharide concentration and composition, however, differed substantially. The carbohydrate content is 12.6 ± 0.5% in K, while it is 33.3 ± 0.7% in (13) (w/w). The uronic acid (UA) fraction in K is 50.3 ± 0.9% of the total carbohydrates, while in (13), it is only 12.6 ± 0.2%. This may suggest that the conventional product contains mainly a pectic food fiber,30 while (13) is a more neutral one.38 In addition, the monosaccharide composition is different; the xylose and glucose contents are very low in the K product and relatively high in (13), i.e., 21.1 ± 1.8 and 17.2 ± 0.9%, respectively. Another noticeable difference is the presence of arabinose and galactose with concentrations of 16.4 ± 1.1 and 23.9 ± 2.0%, respectively, in the UAE product, which suggests a structure having an arabinogalactan or hemicellulose backbone with arabinoxylan branches, characteristic of plant-derived FF.41 In contrast, the domination of arabinose (11.6 ± 0.4%) and galactose (19.7 ± 1.5%) units suggests a pectin character for the polysaccharide part in K.30
Figure 4.
Comparison of the mass balance of conventional and optimal ultrasound-assisted extraction (UAE), followed by purification and biochemical characterization.
To determine the degree of homogeneity and to perform molecular mass assessment, the optimal UAE (13) bioproduct was analyzed by the gel permeation chromatography (GPC) technique and compared to the conventionally obtained FF K (see Text S2 and Figure S1, Supporting Information). The optimal product (13) FF was composed of three independent fractions, i.e., F1, F2, and F3 (shown in Figure S1b, Supporting Information), with estimated molecular mass of 129.0, 5.3, and 3.0 × 103 (g/mol) and recovery levels of 20.4, 44.9, and 35.0%, respectively. Moreover, overlapping profiles of both phenolics and saccharides revealed the conjugated form39 of the analyzed macromolecules, which may be additionally supported by the results obtained from Fourier transform infrared (FT-IR) spectra (see Text S3 and Figure S2, Supporting Information). However, relatively low molecular weight of the received fractions (F2–F3) and their smaller number when compared to the conventionally obtained product K may be connected with the specificity of employing assisted extraction utilizing ultrasound, which might have a degradative effect15,18 on the structure of the optimal UAE (13) bioproduct, resulting in isolation of smaller polysaccharide fragments. In addition, the obtained characteristic IR frequencies supported the statement, which was mentioned beforehand, that isolated FF (13) consisted of different plant cell wall elements,40 either arabinogalactan or hemicellulose chains.38 Nevertheless, the polyphenolic–polysaccharide conjugate character was retained for (13), resulting in attractive anticoagulant activity, especially after separation of fractions with lower mass, which is discussed in the following section.
Anticoagulant Activity of the Optimized Horseweed FF and its Fractions
The purified E. canadensis FF isolates obtained by the conventional process25 and from the modified one utilizing UAE (13) have been subjected to measurements of anticoagulant activity. It is important to verify if the UAE bioproduct and its fractions are attractive anticoagulants with the same selective mechanism of action on coagulation pathways (Figure 2B) as K. The anticoagulant activity of the optimally obtained product (13) examined using an aPTT in vitro test was 1.46 IU/mg, being almost 1.6-fold higher than the activity of the FF obtained in the conventional process, i.e., 0.93 IU/mg. Of the three fractions of (13), F2 turned out to be the best inhibitor of the coagulation process (1.81 IU/mg) with its activity 1.2-fold higher than that of (13) and almost two times higher than that of the K isolate. Thereafter, the inhibition ability magnitude was assessed toward factor Xa and factor IIa, both of which could be mediated by antithrombin (AT), as well as toward factor IIa mediated by heparin cofactor II (HC II) for (13) and its fractions. The results shown in Figure 2B revealed that both the conventionally derived K bioproduct and (13) along with its fractions contain selective indirect inhibitors of factor Xa. The value of half maximal inhibitory concentration (IC50) for product K was moderately better (1.2 μg/mL) than that for the optimal product (13) (IC50 = 3.2 μg/mL), being respectively three and eight times weaker in their action than the reference substance, unfractionated heparin (UFH) (IC50 = 0.4 μg/mL). In addition, from the point of view of indirect inhibition of factor IIa, mediated by either AT or HC II, bioproduct K showed no activity, being 500 times weaker than UFH (IC50 = 0.1 μg/mL, AT-IIa), and minor activity, being 90 times weaker than DS (IC50 = 0.7 μg/mL, HC II-IIa), respectively. The optimal isolate (13) was more selective and showed no activity in the AT-IIa mechanism. However, some minor activity was detected in the HC II-IIa mechanism, where (13) was 120 times weaker than the reference substance, dermatan sulfate (DS). Nevertheless, separated fraction F2 from (13) was found to be the best nondirect inhibitor of factor Xa among all of the products examined, being 2.75 times weaker in its action (IC50 = 1.1 μg/mL) than the reference substance UFH while showing no action in AT-IIa and HC II-IIa at all. In conclusion, horseweed FF obtained from optimized UAE might be a source of a valuable food additive with potent anticoagulant activity, whose properties might be augmented with additional purification steps.
Conclusions
Introduction of novel and sustainable green extraction processes aims to decrease the environmental burden and biomass treatment costs while increasing the product quality and efficacy. Horseweed (E. canadensis) as a source of common cropland waste can be transformed in a sustainable manner into valuable dietary food fiber, leading to valorization of biomass, which has limited use nowadays. Thus, an ultrasound-assisted extraction process may provide an efficient procedure for plant biomass treatment. The most efficient UAE was determined by optimization through the RSM I-optimal design (24), i.e., 60 min of extraction process with 30 W of ultrasonication. When followed by a multistep purification, utilization of the UAE procedure gave an excellent option for isolation of E. canadensis functional food fiber, with more selective anticoagulant activity as compared to the conventionally obtained bioproduct. The green extraction process presented in this contribution might be further employed for recovery of value-added biocompounds from different renewable resources and evaluated for a possible scale-up application.
Experimental Section
Plant Materials and Chemicals
The dry, minced flowering parts of E. canadensis were obtained from the Central European market (F-DENTAL Hodonin s.r.o, Czech Republic). Voucher specimen No. 019361 was deposited in the Botanical Garden of the University of Wrocław (Wrocław, Poland), while the plant identity was certified by Prof. Kramer and Dr. Mularczyk from the University of Wrocław (Wrocław, Poland). Reagents for the anticoagulant activity assay (aPTT test), i.e., human pooled blood plasma reagent (universal MDA reference plasma, solvent/detergent type, for control and calibration purposes, approved by FDA), aPTT HS (TriniCLOT), and CaCl2 (TriniCLOT 0.025 M calcium chloride) were purchased from Trinity Biotech, Ireland. Chromogenic substrates S-2765 and S-2238 were provided by Chromogenix. The sixth International Standard of UFH was obtained from the National Institute of Biological Standards, U.K. Sodium salts of heparin (≥180 USP IU/mg), dermatan sulfate (purity ≥ 90%), heparin cofactor II (purity ≥ 90%), and other chemicals of analytical grade were obtained from Sigma-Aldrich, Germany.
Isolation of E. canadensis Crude Extracts
Conventional Extraction
The conventional procedure of extraction was adopted with slight modifications from one of our previous contributions25 and is presented in the Supporting Information (see Text S1).
Ultrasound-Assisted Extraction Procedure
Briefly, 100 g of feedstock of dry, minced flowering parts (average particle size 1.6 mm ± 0.6, humidity of the plant material 10% ± 1) of E. canadensis was placed in a glass reactor and thoroughly suspended in 1200 mL of 0.1 mol/L aqueous NaOH (solvent to dry mass ratio 12:1) and then subjected to maceration for 24 h in ambient conditions. Afterward, the water-cooled glass reactor with the mixture was placed into an Omni-Ruptor 4000 (Omni International Inc., Kennesaw) ultrasound homogenizer (20 kHz) equipped with a large titanium processing tip (i.d. 25 mm), which was immersed at 50% depth of the solution, and UAE was conducted at the selected process parameters. The constant temperature of extraction was maintained at 25 °C by a thermostated water circulating pump, being monitored during and after the experiment. No significant (i.e., within reasonable error of ±1 °C) change of temperature was observed for any sample. Solid plant residues were filtered off, and the resulting supernatant was centrifuged at 1850g for 20 min. The obtained solution was then neutralized with 1.0 mol/L HCl or 1.0 mol/L NaOH to pH 7 and concentrated to a smaller volume under reduced pressure to produce a raw aqueous plant extract. The separate runs of the UAE procedure were done each time at different combinations of ultrasound power (30, 60, 120, or 240 W) and extraction time (10, 20, 40, or 60 min). Energy density (ED, J/cm3), i.e., the energy input per volume unit, transmitted from the ultrasound processing tip to the extraction mixture was determined from the following equation41
| 1 |
for each combination of the UAE process parameters, and the data are presented in Table S1.
UAE Optimization by the Response Surface Methodology
For the optimization purpose of the ultrasound-assisted extraction procedure, the response surface methodology (RSM) with the 24I-optimal design was used, where two independent four-level variables were considered, i.e., time of the extraction process (A: 10, 20, 40, or 60 min) and power of the ultrasound (B: 30, 60, 120, or 240 W). The dependent variables, by means of the response factors, were the total output of the UAE process (mg/g of dry plant material), pH of the raw aqueous extracts, and their anticoagulant activity assessed by an aPTT test (IU/mg). Meanwhile, the constant parameters were the solvent/dry plant mass ratio (12:1) and the temperature of the process (25 °C). With the aid of RSM and Design Expert software (ver. 11.0.5.0, Stat-Ease, Inc.), the impact of the independent variables on the response factors was investigated. The experimental design matrix included 13 separate runs with different combinations of the independent variable levels, while six of them were repeated in a randomized way giving 25 experimental runs in total (Table 1). A series of raw extracts were obtained with regard to the design matrix. The obtained experimental data were examined by multivariate ANOVA tests. Generated estimative equations allowed interpretation of the correlation between experimental design variables. The final prediction of the optimal UAE conditions resulted from fitting the obtained results to an empirical regression model
| 2 |
where Y is the corresponding response factor of the UAE process and β0 is an intercept, while βi, βij, and βii are the linear interaction and squared coefficients of the model terms, respectively. Xi and Xj represent the coded values of the two independent variables (time and power of UAE).
Multistep Purification of the Raw E. canadensis Extracts
The procedure adopted was a previously reported one with minor changes.25 Briefly, the purification of the conventional and selected UAE procedure raw aqueous concentrates was performed by extraction with hexane (1:1, v/v, 6 h, 69 °C), followed by extraction with diethyl ether (1:1, v/v, 6 h, 34 °C). Thereafter, the aqueous solution was concentrated under reduced pressure to a pastelike form and leached in 4000 mL of ethanol (25 °C). The residue precipitated in alcohol was dialyzed through a membrane (molecular weight cutoff, MWCO, >12 000 g mol–1) against distilled water (5000 mL). Finally, the aqueous retentate was evaporated to dry mass to give the E. canadensis bioproduct.
Energy Consumption of Raw Food Fiber Extraction
For each stage of the extraction procedure with application of the required device over a period of time T (s), either in the conventional procedure or in the UAE procedure of E. canadensis FF isolation, an energy consumption parameter Wi (MJ) was calculated by the following equation42
| 3 |
where U is the voltage (V) and I is the current (A).
The CED (WCED, MJ) required for treatment of 100 g of dry plant raw material was estimated according to the equation
| 4 |
Chemical Analysis
The influence of the UAE process of the separation of E. canadensis functional FF on the overall chemical properties was analyzed using a variety of colorimetric and spectroscopic techniques, described previously.26 All colorimetric assays were performed with a SPECTROStar UV–vis spectrophotometer (BMG LABTECH, Germany). The contents of saccharides, uronic acids, and total phenolics were estimated by the phenol–sulfuric, m-hydroxybiphenyl, and Folin–Ciocalteu assay, respectively.25 The monosaccharide composition was determined by the gas chromatography-mass spectrometry (GC-MS) technique (Trace GC Ultra chromatograph with an ion trap detector ITQ 700, Thermo Scientific, equipped with an Agilent HP-88 column (0.25 mm × 30 m), where the mixture of the neutral monosaccharides was obtained from the corresponding bioproducts after hydrolysis with TFA (trifluoroacetic acid, 2 mol/L), 120 °C, 5 h) and derived to obtain the volatile alditol acetate forms, prior to the analysis (see Text S4, Supporting Information).25 The separation of the horseweed FF obtained through the optimized UAE procedure was conducted with the GPC technique on a glass column (15 mm × 1500 mm) packed with a Sephacryl 300 HR gel (Sigma-Aldrich, Germany) with aqueous 0.1 mol/L NaOH eluent, followed by determination of the molecular weights of the obtained fractions.
Anticoagulant Assays
The anticoagulant performance of the E. canadensis raw aqueous extracts as well as purified functional FF was determined using a well-established diagnostic test protocol,43 i.e., aPTT test, performed on an Option 2 Plus (BioMérieux SCC Europe, Poland) coagulometer. The mechanism of action of the purified horseweed functional FF was determined through AT-mediated inhibition of factors IIa and Xa compared to the UFH standard;44 for the latter, the activities were IC50 = 0.4 μg/mL and IC50 = 0.1 μg/mL. Moreover, the inhibitory activity of the obtained food fiber toward factor IIa mediated by HC II was also verified, where DS, with IC50 = 0.7 μg/mL activity, was used as a reference.45 All three experiments, i.e., AT-IIa, AT-Xa, and HC II-IIa, were performed using a SPECTROstar Nano UV–vis microplate reader (BMG LABTECH, Germany), where the inhibitory activity of the obtained food fiber was expressed as IC50 and compared to reference compounds (UFH, DS).
Statistical Analysis
All the experimental data obtained were statistically analyzed by StatSoft Statistica software (13.3, TIBCO Software), where the mean differences were examined by one-way ANOVA with a significance level of p < 0.05.
Glossary
Abbreviations
- UAE
ultrasound-assisted extraction
- FF
food fiber
- aPTT
activated partial thromboplastin time
- SI
supporting information
- REML
restricted maximal likelihood
- ANOVA
analysis of variance
- CED
cumulative energy demand
- GAE
galic acid equivalent
- UA
uronic acids
- GPC
gel permeation chromatography
- FT-IR
Fourier transform infrared
- AT
antithrombin
- HC II
heparin cofactor II
- IC50
half maximal inhibitory concentration
- UFH
unfractionated heparin
- DS
dermatan sulfate
- ED
energy density
- GC-MS
gas chromatography-mass spectrometry
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c02181.
Variables corresponding to the I-optimal design; conventional extraction procedure; economical metrics of the extraction processes; GPC analysis; and FT-IR analysis (PDF)
This research was supported by Wrocław University of Science and Technology, Wrocław, Poland (internal grant no. 049U/0047/19), and was partly supported by the Slovak Grant Agency VEGA (grant no. 2/0051/18). Part of analyses was made on the instruments purchased through the Project “WroVasc—Integrated Cardiovascular Center”, co-financed by the European Regional Development Fund, within the Innovative Economy Operational Program, 2007–2013, realized at the Regional Specialist Hospital, Research and Development Center in Wroclaw, “European Funds—for the development of innovative economy”. The authors thank Prof. Krystyna Kromer and Dr. Magdalena Mularczyk (Wrocław University, Wrocław, Poland) for plant material identification.
The authors declare no competing financial interest.
Supplementary Material
References
- Rodríguez-Padrón D.; Zhao D.; Garín Ortega R. N.; Len C.; Balu A. M.; García A.; Luque R. Characterization and Antioxidant Activity of Microwave-Extracted Phenolic Compounds from Biomass Residues. ACS Sustainable Chem. Eng. 2020, 8, 1513–1519. 10.1021/acssuschemeng.9b06002. [DOI] [Google Scholar]
- Praveen M. A.; Parvathy K. R. K.; Balasubramanian P.; Jayabalan R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends Food Sci. Technol. 2019, 92, 46–64. 10.1016/j.tifs.2019.08.011. [DOI] [Google Scholar]
- Sucheta N. N. M.; Yadav S. K. Extraction of pectin from black carrot pomace using intermittent microwave, ultrasound and conventional heating: Kinetics, characterization and process economics. Food Hydrocolloids 2020, 102, 105592 10.1016/j.foodhyd.2019.105592. [DOI] [Google Scholar]
- Chemat F.; Vian M. A.; Fabiano-Tixier A.-S.; Nutrizio M.; Jambrak A. R.; Munekata P. E. S.; Lorenzo J. M.; Barba F. J.; Binello A.; Cravotto G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. 10.1039/C9GC03878G. [DOI] [Google Scholar]
- REGULATION (EC) No 1907/2006 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 18 December 2006 concerning the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) establishing a European Chemicals Agency.
- Huybrechts D.; Derden A.; Van den Abeele L.; Vander Aa S.; Smets T. Best available techniques and the value chain perspective. J. Cleaner Prod. 2018, 174, 847–856. 10.1016/j.jclepro.2017.10.346. [DOI] [Google Scholar]
- Fu X.; Belwal T.; Cravotto G.; Luo Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020, 60, 104726 10.1016/j.ultsonch.2019.104726. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Lei Z.; Zhao M.; Wu C.; Wang L.; Xu Y. Microwave-assisted extraction of an acidic polysaccharide from Ribes nigrum L.: Structural characteristics and biological activities. Ind. Crops Prod. 2020, 147, 112249 10.1016/j.indcrop.2020.112249. [DOI] [Google Scholar]
- Essien S. O.; Young B.; Baroutian S. Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials. Trends Food Sci. Technol. 2020, 97, 156–169. 10.1016/j.tifs.2020.01.014. [DOI] [Google Scholar]
- Pashazadeh B.; Elhamirad A. H.; Hajnajari H.; Sharayei P.; Armin M. Optimization of the pulsed electric field -assisted extraction of functional compounds from cinnamon. Biocatal. Agric. Biotechnol. 2020, 23, 101461 10.1016/j.bcab.2019.101461. [DOI] [Google Scholar]
- Chadni M.; Grimi N.; Bals O.; Ziegler-Devin I.; Brosse N. Steam explosion process for the selective extraction of hemicelluloses polymers from spruce sawdust. Ind. Crops Prod. 2019, 141, 111757 10.1016/j.indcrop.2019.111757. [DOI] [Google Scholar]
- Moreira S. A.; Alexandre E. M. C.; Pintado M.; Saraiva J. A. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Res. Int. 2019, 115, 177–190. 10.1016/j.foodres.2018.08.046. [DOI] [PubMed] [Google Scholar]
- Molina G. A.; Gonzalez-Fuentes F.; Loske A. M.; Fernandez F.; Estevez M. Shock wave-assisted extraction of phenolic acids and flavonoids from Eysenhardtia polystachya heartwood: A novel method and its comparison with conventional methodologies. Ultrason. Sonochem. 2020, 61, 104809 10.1016/j.ultsonch.2019.104809. [DOI] [PubMed] [Google Scholar]
- Moczkowska M.; Karp S.; Niu Y.; Kurek M. A. Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed – A physicochemical approach. Food Hydrocolloids 2019, 90, 105–112. 10.1016/j.foodhyd.2018.12.018. [DOI] [Google Scholar]
- Sicaire A.-G.; Vian M. A.; Fine F.; Carre P.; Tostain S.; Chemat F. Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrason. Sonochem. 2016, 31, 319–329. 10.1016/j.ultsonch.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Belwal T.; Chemat F.; Venskutonis P. R.; Cravotto G.; Jaiswal D. K.; Bhatt I. D.; Devkota H. P.; Luo Z. Recent advances in scaling-up of non-conventional extraction techniques: Learning from successes and failures. TrAC, Trends Anal. Chem. 2020, 127, 115895 10.1016/j.trac.2020.115895. [DOI] [Google Scholar]
- Vinatoru M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. 10.1016/S1350-4177(01)00071-2. [DOI] [PubMed] [Google Scholar]
- Khadhraoui B.; Turk M.; Fabiano-Tixier A. S.; Petitcolas E.; Robinet P.; Imbert R.; El Maâtaoui M.; Chemat F. Histo-cytochemistry and scanning electron microscopy for studying spatial and temporal extraction of metabolites induced by ultrasound. Towards chain detexturation mechanism. Ultrason. Sonochem. 2018, 42, 482–492. 10.1016/j.ultsonch.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Cassidy Y. M.; McSorley E. M.; Allsopp P. J. Effect of soluble dietary fibre on postprandial blood glucose response and its potential as a functional food ingredient. J. Funct. Foods 2018, 46, 423–439. 10.1016/j.jff.2018.05.019. [DOI] [Google Scholar]
- Patel S.Anti-Obesity and Anti-Diabetes Foods: High Fibre Diets. In Encyclopedia of Food Chemistry, Melton L.; Shahidi F.; Varelis P., Eds.; Elsevier Inc.: Amsterdam, 2019; pp 248–252. [Google Scholar]
- Ahuja K.; Rawat A.. GlobalDietary Fibers Market Size By Product (Soluble [Inulin, Pectin, Polydextrose,Beta-Glucan, Fructooligosaccharides (FOS), Galactooligosaccharides (GOS), CornFiber], Insoluble [Cellulose, Hemicellulose, Chitin & Chitosan, Lignin,Fiber/Bran, Resistant Starch]), By Source (Cereals & Grains, Fruits & Vegetables), By Application (Food [Bakery, Breakfast Cereals & Snacks,Confectionery, Dairy, Meat Products, Infant Food], Beverages, Pharmaceutical,Companion Animal Nutrition) Industry Analysis Report, Regional Outlook, Growth Potential, Price Trends, Competitive Market Share & Forecast, 2020 – 2026, Report ID: GMI2331; GlobalMarket Insights Inc., 2020.
- Beres Z. T.; Ernst E. E.; Ackley B. A.; Loux M. M.; Owen M. D. K.; Snow A. A. High levels of glyphosate resistance in Conyza canadensis from agricultural and non-agricultural sites in Ohio and Iowa. Sci. Rep. 2018, 8, 10483 10.1038/s41598-018-28163-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurdjević L.; Mitrović M.; Gajić G.; Jarić S.; Kostić O.; Oberan L.; Pavlović P. An allelopathic investigation of the domination of the introduced invasive Conyza canadensis L.. Flora Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 921–927. 10.1016/j.flora.2011.06.001. [DOI] [Google Scholar]
- Yan H.; Feng L.; Zhao Y.; Feng L.; Zhu C.; Qu Y.; Wang H. Predicting the potential distribution of an invasive species, Erigeron canadensis L., in China with a maximum entropy model. Global Ecol. Conserv. 2020, 21, e00822 10.1016/j.gecco.2019.e00822. [DOI] [Google Scholar]
- Pawlaczyk I.; Czerchawski L.; Kuliczkowski W.; Karolko B.; Pilecki W.; Witkiewicz W.; Gancarz R. Anticoagulant and anti-platelet activity of polyphenolic-polysaccharide preparation isolated from the medicinal plant Erigeron canadensis L. Thromb. Res. 2011, 127, 328–340. 10.1016/j.thromres.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Pawlaczyk-Graja I.; Balicki S.; Ziewiecki R.; Capek P.; Matulova M.; Wilk K. A. New isolation proces for bioactive food fiber from wild strawberry leaf. Biochem. Eng. J. 2020, 161, 107639 10.1016/j.bej.2020.107639. [DOI] [Google Scholar]
- Tsirigotis-Maniecka M.; Pawlaczyk-Graja I.; Ziewiecki R.; Balicki S.; Matulova M.; Capek P.; Czechowski F.; Gancarz R. The polyphenolic-polysaccharide complex of Agrimonia eupatoria L. as an indirect thrombin inhibitor - isolation and chemical characterization. Int. J. Biol. Macromol. 2019, 125, 124–132. 10.1016/j.ijbiomac.2018.12.017. [DOI] [PubMed] [Google Scholar]
- Wawrzyńczyk D.; Bazylińska U.; Lamch Ł.; Kulbacka J.; Szewczyk A.; Bednarkiewicz A.; Wilk K. A.; Samoć M. Förster Resonance Energy Transfer – activated processes in smart nanotheranostics fabricated in a sustainable manner. ChemSusChem 2019, 12, 706–719. 10.1002/cssc.201801441. [DOI] [PubMed] [Google Scholar]
- Lamch Ł.; Pucek A.; Kulbacka J.; Chudy M.; Jastrzębska E.; Tokarska K.; Bułka M.; Brzózka Z.; Wilk K. A. Recent progress in the engineering of multifunctional colloidal nanoparticles for enhanced photodynamic therapy and bioimaging. Adv. Colloid Interface Sci. 2018, 261, 62–81. 10.1016/j.cis.2018.09.002. [DOI] [PubMed] [Google Scholar]
- Broxterman S. E.; Schols H. A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydr. Polym. 2018, 192, 263–272. 10.1016/j.carbpol.2018.03.070. [DOI] [PubMed] [Google Scholar]
- de Freitas C.; Carmona E.; Brienzo M. Xylooligosaccharides production process from lignocellulosic biomass and bioactive effects. Bioact. Carbohydr. Diet. Fibre 2019, 18, 100184 10.1016/j.bcdf.2019.100184. [DOI] [Google Scholar]
- Myers R. H.; Montgomery D. C.; Anderson-Cook C. M.. Response Surface Methodology: Process and Product Optimization Using Designed Experiments, 4th ed.; Wiley Inc: New York, 2016. [Google Scholar]
- Luke S. G. Evaluating significance in linear mixed-effects models in R. Behav. Res. Methods 2017, 49, 1494–1502. 10.3758/s13428-016-0809-y. [DOI] [PubMed] [Google Scholar]
- Zhang W.; Zeng G.; Pan Y.; Chen W.; Huang W.; Chen W.; Li Y. Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr. Polym. 2017, 172, 102–112. 10.1016/j.carbpol.2017.05.030. [DOI] [PubMed] [Google Scholar]
- Rostagno M. A.; Prado J. M.. Natural Product Extraction: Principles and Applications. In Green Chemistry Series; RSC Publishing: Cambridge, 2013. [Google Scholar]
- Islam M. K.; Wang H.; Rehman S.; Dong C.; Hsu H.-Y.; Lin C. S. K.; Leu S.-Y. Sustainability metrics of pretreatment processes in a waste derived lignocellulosic biomass biorefinery. Bioresour. Technol. 2020, 298, 122558 10.1016/j.biortech.2019.122558. [DOI] [PubMed] [Google Scholar]
- Ojha K. S.; Aznar R.; O’Donnell C.; Tiwari B. K. Ultrasound technology for extraction of biologically active molecules from plant, animal and marine sources. TrAC, Trends Anal. Chem. 2020, 122, 115663 10.1016/j.trac.2019.115663. [DOI] [Google Scholar]
- Lampugnani E. R.; Khan G. A.; Somssich M.; Persson S. Building a plant cell wall at a glance. J. Cell Sci. 2018, 131, jcs207373 10.1242/jcs.207373. [DOI] [PubMed] [Google Scholar]
- Pantophlet A. J.; Wopereis S.; Eelderink C.; Vonk R. J.; Stroeve J. H.; Bijlsma S.; van Stee L.; Bobeldijk I.; Priebe M. G. Metabolic Profiling Reveals Differences in Plasma Concentrations of Arabinose and Xylose after Consumption of Fiber-Rich Pasta and Wheat Bread with Differential Rates of Systemic Appearance of Exogenous Glucose in Healthy Men. J. Nutr. 2017, 147, 152–160. 10.3945/jn.116.237404. [DOI] [PubMed] [Google Scholar]
- Zhou C.; Yu X.; Zhang Y.; He R.; Ma H. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis Udea. Carbohydr. Polym. 2012, 87, 2046–2051. 10.1016/j.carbpol.2011.10.026. [DOI] [Google Scholar]
- Monteiro S. H. M. C.; Silva E. K.; Guimarães J. T.; Freitas M. Q.; Meireles M. A. A.; Cruz A. G. High-intensity ultrasound energy density: How different modes of application influence the quality parameters of a dairy beverage. Ultrason. Sonochem. 2020, 63, 104928 10.1016/j.ultsonch.2019.104928. [DOI] [PubMed] [Google Scholar]
- Ju T.; Deng Y.; Xi J. Optimization of circulating extraction of polysaccharides from Gracilaria lemaneiformis using pulsed electrical discharge. ACS Sustainable Chem. Eng. 2019, 7, 3593–3601. 10.1021/acssuschemeng.8b06183. [DOI] [Google Scholar]
- Ignjatovic V.Activated Partial Thromboplastin Time. In Methods in Molecular Biology Monagle P., Ed.; Humana Press: Totowa, NJ, 2013; Vol. 992, pp 111–120. [DOI] [PubMed] [Google Scholar]
- Martinez C.; Savadogo A.; Agu C.; Anger P. Reproducibility of the anti-Factor Xa and anti-Factor IIa assays applied to enoxaparin solution. J. Pharm. Biomed. Anal. 2013, 81–82, 138–145. 10.1016/j.jpba.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Wu M.; Wen D.; Gao N.; Xiao C.; Yang L.; Xu L.; Lian W.; Peng W.; Jiang J.; Zhao J. Anticoagulant and antithrombotic evaluation of native fucosylated chondroitin sulfates and their derivatives as selective inhibitors of intrinsic factor Xase. Eur. J. Med. Chem. 2015, 92, 257–269. 10.1016/j.ejmech.2014.12.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.