Abstract
o-Phenylenediamine (OPD) can be readily oxidized by several types of oxidants to generate fluorescent 2,3-diaminophenazine (oxidized OPD, OPDox). The unique fluorescence response process during the oxidation of OPD provides an important model for the design of novel sensors. In recent years, a series of fluorescent and colorimetric sensors have been developed based on the oxidation of OPD. In this review, fluorescent and colorimetric sensors for the detection of metal ions and small organic molecules are discussed. These sensing processes exhibit distinguishable and prominent fluorescent and colorimetric responses. The sensing systems include autocatalytic reactions and using nanomaterials, carbon dots, or fluorophore labeled DNA as reference fluorophore for fluorescent and colorimetric detection.
1. Introduction
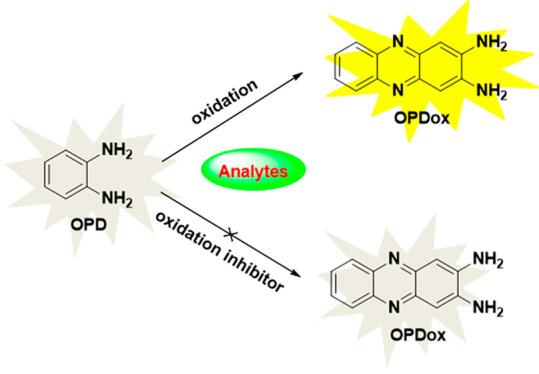
Among various detection techniques, fluorescence detection is one of the most powerful tools for the detection of environmentally and biologically significant analytes in the presence of interfering matrices, exhibiting high sensitivity, excellent selectivity, shorter response times, and low detection limits.1 In addition, fluorescence sensors can be used in intracellular detection, which is useful for understanding numerous interactions in biological systems.2o-Phenylenediamine (OPD), as a precursor used for the fabrication of numerous heterocyclic compounds or polymers, has attracted much attention due to its unique properties. It is noteworthy that OPD can be easily oxidized by various oxidants (Cu2+, Ag+, H2O2, etc.), forming the strongly fluorescent 2,3-diaminophenazine (oxidized OPD, OPDox). Based on this unique fluorescence response, a series of fluorescent sensors have been developed.
In this review, a general overview of the design and application of OPD-based fluorescent sensors is presented. This review mostly focuses on different reporting systems and unique sensing mechanisms between OPD-based fluorescent sensors and analytes, including metal ions, chemical reagents, and biological thiols. These sensing processes exhibit a distinguishable and prominent fluorescent (ON–OFF or OFF–ON) response.
2. OPD-Based Sensors for Metal Ions
Heavy metal ions such as Hg2+, Cu2+, and Ag+ can combine with proteins and enzymes in the human body through an accumulation process, resulting in various metabolic disorders and neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. Thus, the sensitive monitoring of heavy metal ions is vital for waste management, environmental protection, and water safety. Recently, many analytical methods have been established to detect trace levels of heavy metal ions, including atomic absorption/emission spectroscopy, surface-enhanced Raman scattering, inductively coupled plasma mass spectrometry (ICP-MS), electrochemistry, elective electrodes (ISEs), colorimetric detection, fluorescent analysis methods, and so on. In the past decade, some novel detection strategies based on the special response process of the OPD-based fluorescent sensor have been designed and applied for the detection of heavy metal ions.
2.1. Detection of Ag+
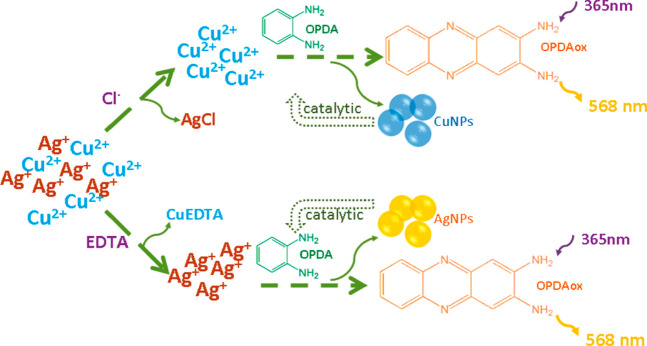
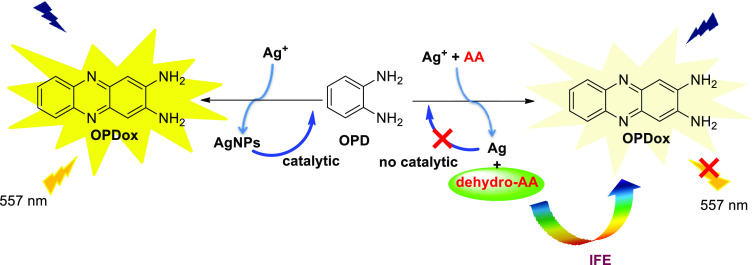
Autocatalytic reactions, employed primarily for the synthesis of nanoparticles (NPs), can be a powerful tool for the OPD-based fluorescence detection of heavy metal ions. Yang et al. reported a novel autocatalytic fluorescence sensor for the selective detection of Cu2+ and Ag+ ions.3 In the presence of Cu2+ and Ag+, nonfluorescent OPD tends to be oxidized to strongly fluorescent OPDox. During the redox reactions, Cu2+ and Ag+ are reduced to zerovalent copper and silver, leading to the formation of the corresponding nanoparticles, which in turn catalyze the redox reaction, resulting in fluorescence enhancement (Scheme 1). The concentrations of Cu2+ and Ag+ can be quantitatively detected by the increasing fluorescence intensity of OPDox. After the addition of Cl– and EDTA·Na2·H2O as the shielding reagents, Cu2+ and Ag+ can be discriminated with detection limits of 2.5 nM and 60 nM, respectively. Moreover, a paper-based test strip was prepared and used as a naked-eye indicator for the rapid determination of Cu2+ and Ag+ in real samples.
Scheme 1. OPD-Based Fluorescence Sensor for Selective Detection of Ag+ and Cu2+.
Reproduced from ref (3) with permission. Copyright 2011 American Chemical Society.
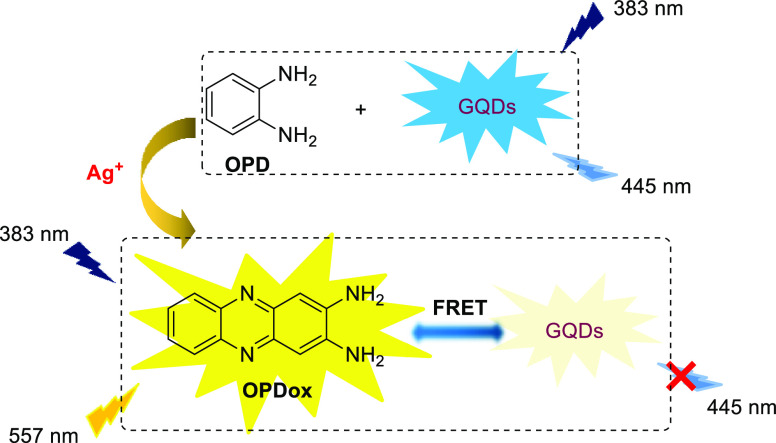
Carbon-based dots such as carbon nanodots (CDs) and graphene quantum dots (GQDs) can be utilized as reference fluorophores owing to their excellent optical absorbance, stability, and biocompatibility. Zhao et al. explored a Ag+ ratiometric fluorescence sensor based on the fluorescent “ON–OFF” strategy of GQDs (Scheme 2).4 The original detection solution exhibits a strong fluorescence emission of GQDs at 445 nm. By adding Ag+, OPD is oxidized to OPDox. This results in the fluorescence quenching of the GQDs via fluorescence resonance energy transfer (FRET) between the GQDs and OPDox were simultaneously accompanied by a new fluorescence emission at 557 nm exhibited by OPDox. The sensor exhibits a distinct ratiometric fluorescence response (F557/F445) to Ag+, with a detection limit as low as 250 nM. Using this methodology, Ag+ ions in tap water and lake water were accurately analyzed. Moreover, as a ratiometric fluoresence assay, this sensor can circumvent complicated interfering factors such as the sensing conditions, probe concentration, and instrument fluctuations that exist in single-intensity-based fluorescence sensors.
Scheme 2. OPD-Based Ratiometric Fluorescence Sensor for Ag+ Detection.
2.2. Detection of Cu2+
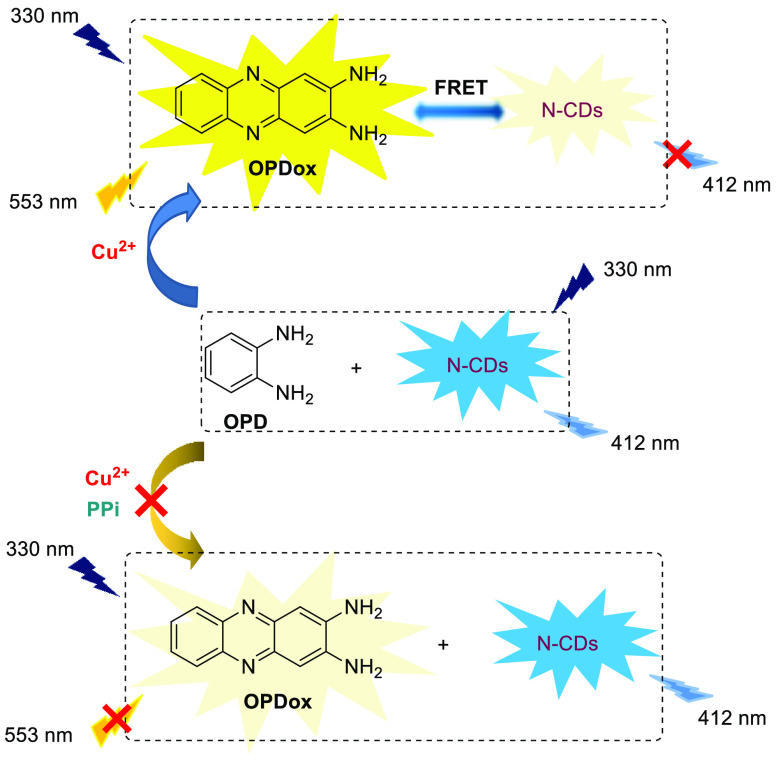
As a derivative of CDs, N-doped carbon dots (N-CDs) can also act as reference fluorophore. On this basis, Zhang et al. designed a ratiometric response of Cu2+ and pyrophosphate ions (PPi) (Scheme 3).5 In this recognition system, OPD is oxidized by Cu2+ to generate OPDox, which emits fluoresent at 553 nm, and cocurrently absorbed on the surface of N-CDs through electrostatic interactions, resulting in the fluorescent quenching of N-CDs at 412 nm. The sensor displays a linear ratiometric fluorescence (F412/F553) response toward Cu2+, with a low detection limit of 23 nM. Excellent anti-interference capability must be mentioned, for not only most common metal ions, but also the more oxidizable Ag+, have a negligible effect on this sensor. Additionally, due to the intense affinity between PPi and Cu2+, the redox reaction is inhibited, thus the fluorescence emission of the N-CDs is recovered when PPi is added. Thus, it can also be applied to a quantitative assay for PPi detection, with detection limits of 0.7 μM.
Scheme 3. OPD-Based Ratiometric Fluorescence Sensor for Ag+ and PPi Detection.
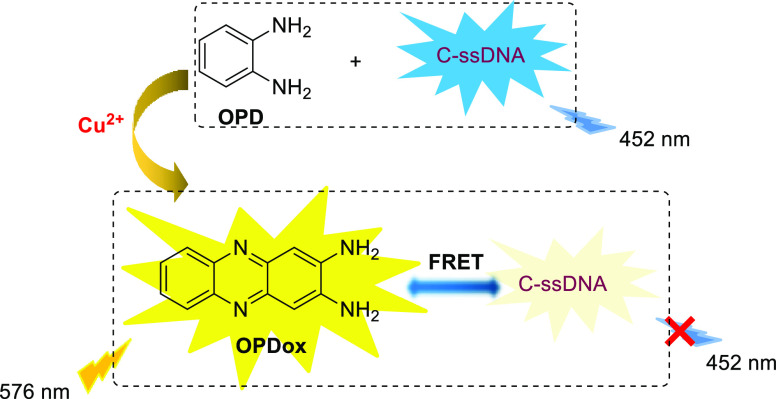
In addition to carbon dots, coumarin-labeled signal-strained DNA(C-ssDNA), which exhibits an emission peak at 452 nm, can also act as a candidate for reference fluorophore in ratiometric fluorometric detection. Recently, a Cu2+ sensor based on C-ssDNA was developed (Scheme 4).6 Similar to the sensing mechanism of the last example, Cu2+-induced oxidation of OPD “turns off” the fluorescence of C-ssDNA due to FRET, which is attributed to the π–π stacking and hydrogen bonding interactions between OPDox and C-ssDNA. The concentrations of Cu2+ are linearly related with the fluorescence intensity ratios between OPDox and C-ssDNA, with the limit of detection (LOD) as low as 4.32 nM, which is superior to the absorbance assay. In addition, by using dye-labeled single-stranded DNA, the sensor provides a reliable and effective method for the in vivo detection of cerebral Cu2+.
Scheme 4. OPD-Based Ratiometric Fluorescence Sensor for Cu2+ Detection.
2.3. Detection of Hg2+
It is of great significance to monitor Hg2+ due to the highly toxic and simple bioaccumulates. Some representative works for the fluorescent detection of Hg2+ have been sumarized,7 and OPD-based sensors in this review may be complementary methods. For example, on the basis of the Ag+ autocatalytic reaction, Dai et al. designed a mercury ion fluorescent sensor by using a fluorescent “ON–OFF” strategy (Scheme 5).8 The oxidation of OPD is accelerated in the presence of Ag+, leading to the fluorescence “turning on”. When Hg2+ is added, it is absorbed on to the surface of the Ag NPs, changing their catalytic activity. This results in the inhibition of the autocatalytic reaction, so that “turns off” the fluorescence intensity. This distinctive inhibition process renders the sensor a prominent selectivity toward mercury among various interfering ions. Meanwhile, this sensor obtains a detection limit down to 8.2 nM and needs neither nanoparticles nor nucleic acids, and exhibits advantages of sensitivity, simplicity, and high cost-efficiency compared with nanomaterial and DNA sensors.
Scheme 5. OPD-Based Fluorescence Sensor for Hg2+ Detection Based on Ag+ Autocatalytic Reaction.
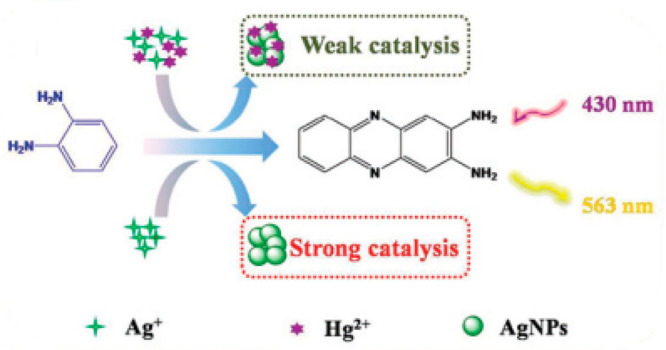
Reproduced from ref (8) with permission. Copyright 2017 The Royal Society of Chemistry.
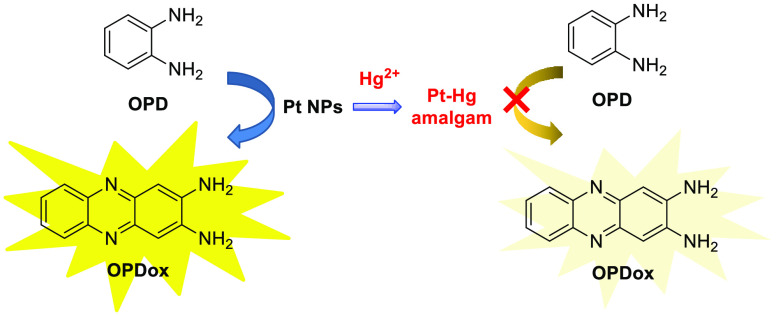
Subsequently, Zhou et al. developed a dual colorimetric and fluorometric assay for mercury ion according to a similar strategy (Scheme 6).9 The recognition mechanism is attributed to a Hg2+-triggered inhibiton of peroxidase-like activity of Pt nanoparticles (Pt NPs), which can catalyze the oxidation of OPD. This impact disturbs the formation of OPDox, resulting in an extremely week fluorescence intensity and fading of the bright yellow color. Using this “ON–OFF” logic gate not only can provide a fluorescence and colorimetric signal sensing system for Hg2+ with ultralow detection limits of 0.14 nM and 0.8 nM, respectively, but also has the potential for the detection of other ions.
Scheme 6. OPD-Based Fluorescence Sensor for Hg2+ Detection Based on PtNPs Catalyzed Oxidation of OPD.
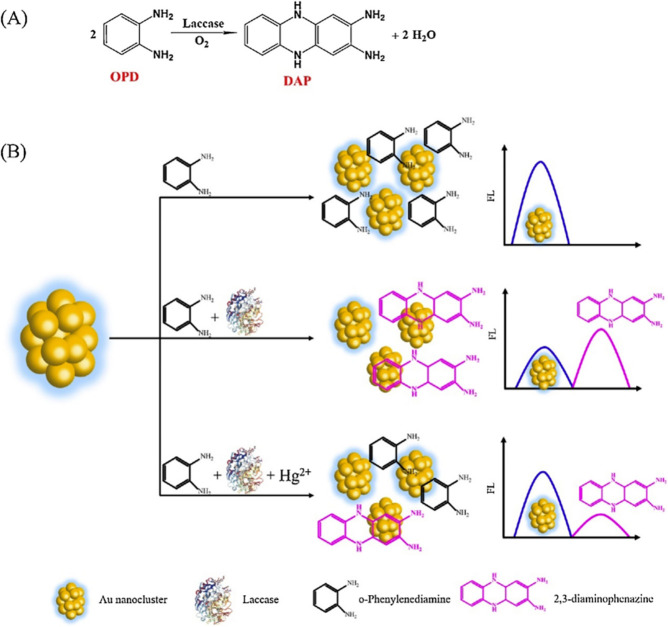
By the use of oxidase mimicking, a novel ratiometric fluorescence assay for the detection of mercury ion was reported (Scheme 7).10 Laccase can catalyze the oxidation of OPD in the present of O2, generating the strongly fluoresent OPDox and leading to the inner filter effect (IFE) induced fluorescence quenching of AuNCs, which is ascribed to the overlap between the emission band of AuNCs and absorption band of OPDox. Whereas Hg2+ could depress the activity of laccase, the enzyme-triggered IFE was inhibited and the fluorescence emission of AuNCs was restored. In this way, the fluorescence intensity ratio between OPDox and AuNCs was dependent on the concentration of Hg2+. As a simple and cost-effective test-strip assay, the ratiometric sensor was successfully utilized to determine mercury ions in tap water and Yangtze river water. It is impressive that the sensor can not only be used for the sensitve detection of Hg2+, but also provide a novel way to assess the activity of laccase.
Scheme 7. Schematic Illustration of (A) the Laccase Catalyzed Oxidation of OPD and (B) OPD Sensing System Based on IFE for Hg2+ Determination.
Reproduced from ref (10) with permission. Copyright 2020 Elsevier.
Yang et al. established an OPD-based colorimetric sensor for the detection of Hg2+, Cu2+, and Ag+ with a gold nanoparticle (GNPs) probe, which possesses unique optical properties related to the localized surface plasmon resonance (LSPR).11 When colloidal GNPs are dispersed in solution in the absence of OPD, a red color is observed. Following the addition of OPD, the color of the reaction solution changes from red to blue due to the aggregation of GNPs via the NH-gold chemistry between the OPD and GNPs. After adding Hg2+, Cu2+, and Ag+, a large amount of OPD is consumed because of the autocatalytic reaction; the NH–gold interactions diminish. As a result, the reaction solution color varies from blue to red (Scheme 8). Thus, any one of the three heavy metal ions can be selectively detected by the “OFF–ON” strategy by employing suitable shielding reagents.
Scheme 8. OPD-Based Colorimetric Sensor for the Detection of Hg2+ (A), Cu2+ (B), and Ag+ (C).
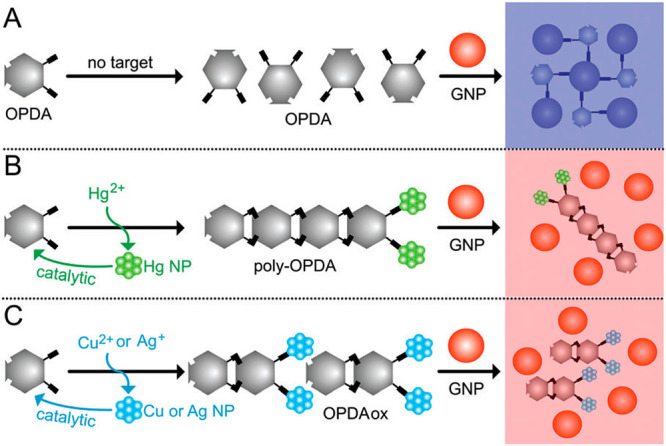
Reproduced from ref (11) with permission. Copyright 2017 The Royal Society of Chemistry.
2.4. Detection of Other Metal Ions
As an important rare earth element in functional materials, cerium might lead to serious diseases such as pulmonary embolism and hepatic artery thrombosis. Due to the strong oxidative ability, Ce4+ exhibits an oxidase-like activity, can oxidize OPD into OPDox in the absence of H2O2. By virtue of this property, a ratiometric fluorescence sensor for Ce4+ was constructed.12 As in the sensing mechanism in ref (24), OPDox not only exhibits strong fluorescent at 562 nm, but also quenches the fluorescent of GQDs at 444 nm via an inner filter effect (IFE). A linear response (F444/F562) to Ce4+ is obtained in the range from 5 to 100 μM, with the LOD of 1 μM. Interestingly, it is found that alendronate sodium (ALDS), which can be oxidized by Ce4+ to prevent the oxidation process of OPD, can be indirectly detected by this sensor under the same ratiometric fluorescent strategy. Furthermore, the new sensor was successfully applied to monitor the real water sample in Weishan Lake under the standard addition method.
3. OPD-Based Sensors for Organic Molecules
It is well-known that a large number of organic molecules such as thiols, purines, ascorbic acid, and amino acid are important components in various physiological functions and pathological conditions. Therefore, the development of highly selective and sensitive detection methods is important for human health. During the past decade, a number of approaches, including high performance liquid chromatography (HPLC), UV–vis absorption spectroscopy, mass spectrometry (MS), and electrochemical analysis, have been established. Among them, fluorescent sensors have attracted considerable attention due to operational simplicity, rapid response time, and high selectivity and sensitivity. In particular, a variety of OPD-based sensors have been designed according to the principle of the fluorescent “ON–OFF” or “OFF–ON” strategies for the detection of organic molecules.
3.1. Detection of Thiols
The fluorescent “ON–OFF” strategy was applied equally to the detection of thiols such as cysteine (Cys), homocysteine (Hcy), and glutathione (GSH), which are vital constituents of proteins and are associated with chronic diseases like arthritis, cardiovascular damage, and HIV/AIDS. Zhu et al. developed a sensitive and simple fluorescence sensor for the determination of biothiols based on the Ag+ triggered oxidation of OPD.13 Interestingly, Li et al. also reported a colorimetric assay that can discriminantly detect GSH over Cys and HCy based on an Ag+-OPD autocatalytic reaction (Scheme 9).14 While GSH, Cys, and HCy can inhibit the oxidative ability of Ag+, GSH has priority over Cys and HCy to reduce the pale-yellow colored OPDox to colorless OPD because of its strong reducibility. Therefore, the color change and UV–vis absorption of the detection solution are more sensitive to variations in GSH concentration, making it an ultrasensitive sensing system that could be visually distinguished by the naked eye. A wide linear relationship is achieved in the range from 2 nM to 1 μM for GSH monitoring, with a detection limit as low as 1.7 nM. More importantly, as a rapid discriminative detection assay, neither nanomaterials nor modification of GSH is needed. Finally, a satisfactory result for the highly selective detection of GSH in plasma and urine samples was achieved by using this protocol.
Scheme 9. OPD-Based Colorimetric Sensor for the Discriminative Detection of GSH.
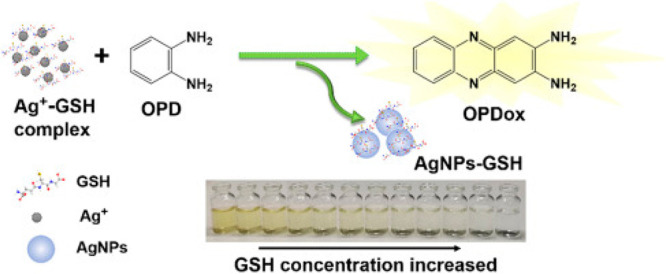
Reproduced from ref (14) with permission. Copyright 2018 Elsevier.
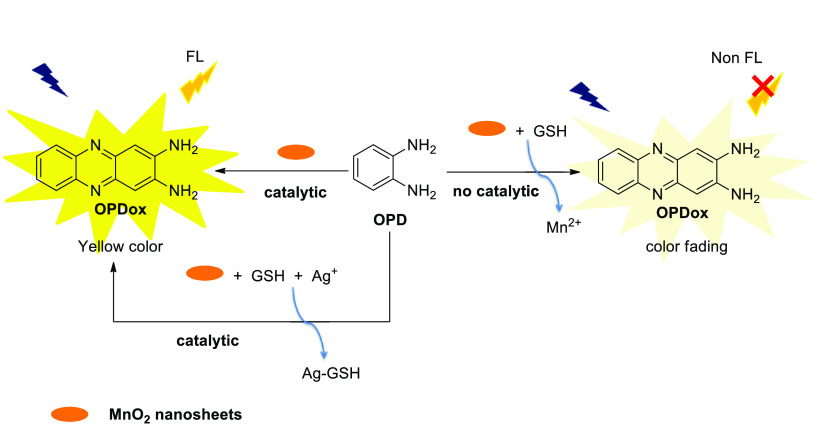
Two-dimensional nanosheets are well-known to possess peroxidase or oxidase activity, which would be alternative catalysts in the oxidation of OPD. Recently, Liu et al. described a colorimetric and fluorescence dual mode assay for GSH and Ag+ detection based on the MnO2 nanosheets, which can catalyze OPD to be oxidized to OPDox with strong fluorescent and yellow color (Scheme 10).15 The sensing mechanism is attributed to the “inhibition and protection” to the oxidase-like property of MnO2 nanosheets. GSH could inhibit the oxidation of OPD since it can easily reduce MnO2 nanosheets into non-enzymatic-like mimic Mn2+, resulting in the fluorescence intensity decrease and color fade. When Ag+ is introduced, it can coordinate with GSH to protect the oxidase-like activity of MnO2 nanosheets, recovering the redox reaction. Fluorometric and colorimetric detection exhibits good selectivity for GSH over other thiols or anions, and high sensitivity with LOD down to 62 nM and 0.94 μM, respectively. Furthermore, due to the facile fabrication of MnO2 nanosheet and excllent anti-interference capability, this sensor is potentially applicable in human serum sample detection and clinical diagnosis.
Scheme 10. OPD-Based Colorimeric and Fluorescence Dual-Readout Sensor for GSH Detection.
Later, by using carbon quantum dots (CQDs) as reference fluorophore, a ratiometric fluorescence sensor for GSH was developed.16 Cu2+-catalyzed oxidation of OPD results in the fluorescent quenching of CQDs due to the FRET between OPDox and CQDs. The coordination effect between GSH and Cu2+ could prevent the oxidative reaction, and thus in turn restore fluorescent emission of CQDs. In addition to the excllent selectivity and sensitivity, it is worth noting that the sensor can be prepared conveniently through “one-pot” hydrothemal synthesis by OPD and citric acid, so it offers a practical assay to the quantitatively detection of GSH in real samples.
3.2. Detection of Purines and Glucose
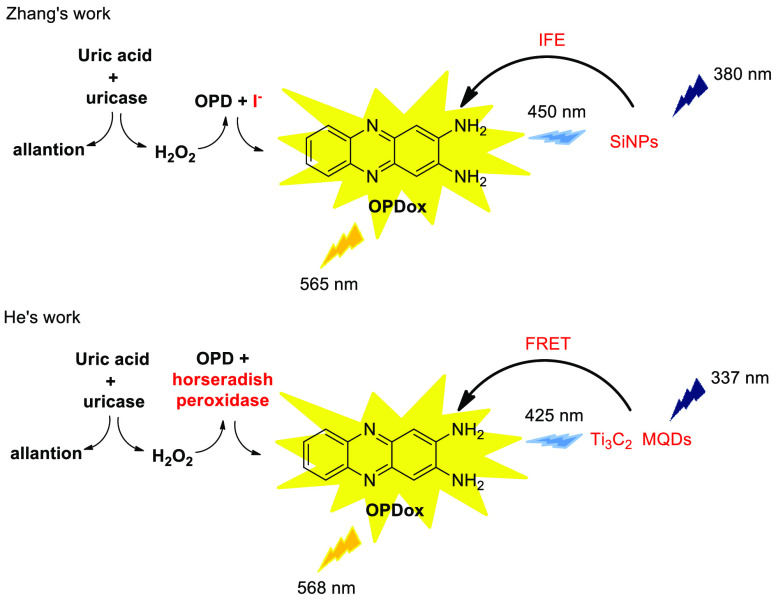
Uric acid (UA), as the final product of purine metabolism in human bodies, is an important biomarker for the clinic diagnosis of diseases such as gout, hypertension, kidney stones, and so on. Since uricase catalyzed oxidation of UA can generate H2O2, which can be an oxidant to OPD, it is possible to sensitively monitoring UA by OPD-based fluorescence sensor.17 In this context, Zhang et al.18 and He et al.19 reported colorimetric and ratiometric fluorescence dual-readout sensors of UA, respectively (Scheme 11). Although the similar sensing mechanism is attributed to the fluorescent as well as color changes between reference fluorophore and OPDox, the sensing systems operate in different ways. In Zhang’s system, the oxidation of OPD was catalyzed by iodide ion, and the fluorescence quenching of silicon nanoparticles, which act as reference fluorophore, is ascribed to inner filtration effect (IFE). However, in He’s system, horseradish peroxidase was utilized to catalyze the redox reaction, and the fluorescent of Ti3C2 MXene quantum dots was quenched due to FRET. Both sensors exhibit a wide linear range and low detection limit, providing “naked-eye” colorimetric and fluoresent chemosensors in the quantitation of UA in real serum samples.
Scheme 11. OPD-Based Fluorescence Sensors for UA Detection.
Following a similar detection strategy, Wang et al. developed a dual signal assay for xanthine, which can also generate H2O2 when catalyzed by xanthine oxidase.20 In this sensing system, Fe and N codoped carbon dots (Fe, N-CDs) possess both peroxidase-like activity and fluoresence and can not only catalyze the oxidation of OPD in the presence of H2O2, but also act as reference fluorophore, of which the fluorescence can be quenched by OPDox via IFE. A good linear relationship ranging from 0 to 40 μM is found between the concentration of xanthine and the fluorescence intensity ratio of OPDox/Fe, N-CDs, with the detection limit down to 0.023 μM. Moreover, excellent anti-interference performance ensures successful detection of human serum and urine.
The sensing mechanism mentioned earlier is capable of detecting glucose because it can be oxidized to H2O2 in the presence of glucose oxidase. Chen et al. reported a ratiometric fluoresence platform for glucose by using ruthenium ion-complexed carbon nitride (Ru–C3N4) nanosheets, which have the same function as Fe and N-CDs in the last example (Scheme 12).21 Several kinds of saccharides, amino acids, and metal ions are demostrated to have negligible effect on the sensor, indicating excellent anti-interference capability. Furthermore, the sensor not only displays a new peroxidase mimic for the oxidation of OPD, but also provides an effective and reliable assay for the determination of glucose.
Scheme 12. OPD-Based Ratiometric Fluorescence Sensor for Glucose Detection.
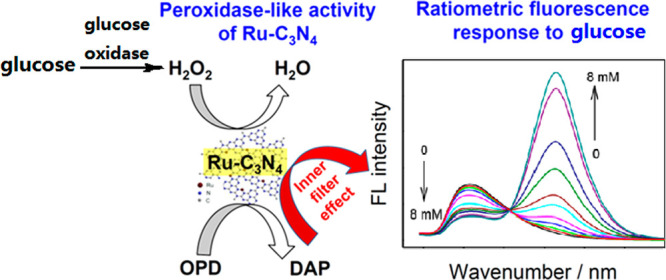
Reproduced from ref (21) with permission. Copyright 2019 American Chemical Society.
3.3. Detection of Ascorbic Acid
Ascorbic acid (AA), which plays an important role as an antioxidant in living systems, possesses a strong reducing ability allowing it to be detected by OPD-based fluorescence sensors via the “ON–OFF” strategy. In virtue of this principle, Zhu et al. established an ultrasensitive fluorescence sensing system for the determination of ascorbic acid in the cerebral system of rats (Scheme 13).22 The Ag+-OPD autocatalytic reaction is inhibited in the presence of ascorbic acid, which is synchronously oxidized into dehydro-ascorbic acid (DHAA) by silver ions. The suppressed redox reaction combined with the IFE between DHAA and OPDox results in the rapidly decreasing fluorescence, in linear response to the concentration of AA. Catecholamines, which coexist with AA in cerebral systems and cause most of the common interference, show negligible influence on detection in this recognition system. Additionally, with merits like high selectivity and accuracy, excellent biocompability, rapid response time (within 10 min), and no required nanomaterials, this sensor provides a facile and real-time detection of AA in biological systems.
Scheme 13. OPD-Based Fluorescence Sensor for Ascorbic Acid Detection Based on Ag+ Autocatalytic Reaction.
Interestingly, recent work described by Zhang et al. provides a novel pathway to detect AA based on the condensation reaction between DHAA and OPD.23 A similar inhibition strategy is employed by adding AA to the Ag+-OPD autocatalytic reaction, leading to the fluorescence quenching of OPDox. Consequently, the dicarbonyl group of as-produced DHAA can easily react with the diamino group of OPD to form 3-(dihydroxyethyl)furo[3,4-b]quinoxaline-1-one (DFQ), which exhibits new fluorescent emission and UV–vis absorbance. Thus, a dual-ratio fluorescent and colorimetric assay is successfully applied by measuring the ratiometric fluorescence (FOPDox/FDFQ) and absorption (AOPDox/ADFQ) toward AA. In spite of a nonoxidation reduction strategy, such an example allows for opening a new gate for designing OPD-based sensor by using the condensation of OPD in the future.
3.4. Detection of Other Organic Molecules
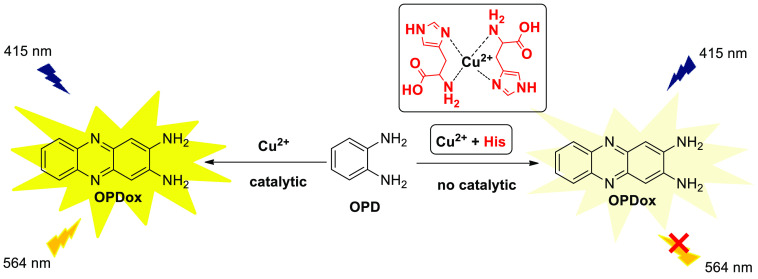
Histidine (His) is a natural α-amino acid containing an imidazole moiety and amino group which are able to coordinate with metal ions to prevent the autocatalytic reaction of OPD. Xu et al. reported a fluorescence recognition system for His detection based on fluorescent “ON–OFF” strategy (Scheme 14).24 The oxidase-like activity of Cu2+ can efficiently catalyze the oxidative reaction of OPD and “turn on” the fluorescence emission at 564 nm. When His is introduced, the redox reaction is suppressed due to the strong interactions between His and Cu2+, resulting in the fluorescence quenching phenomenon. The linear relationship between fluorescence intensity and Cu2+ concentration is obtained ranging from 0.5 to 30 μM, with a LOD of 0.33 μM. In addition, by use of Cu2+ instead of the unstable Cu/CuO nanoparticles as oxidase mimic, this sensor becomes a practical and promising assay for the monitoring of His in human urine samples.
Scheme 14. OPD-Based Fluorescence Sensor for Histidine Detection Based on Cu2+ Autocatalytic Reaction.
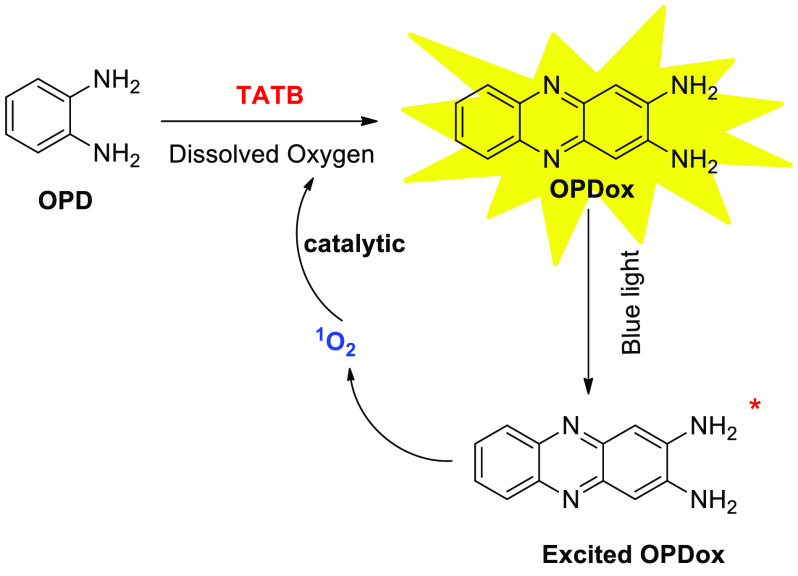
In addition to the detection of the aforementioned biological molecules, the detection of explosives has become increasingly important because of the threat from terrorism. Up to now, numerous studies have been reported concerning the detection of some explosives, including triacetone triperoxide, trinitrotoluene, and pentaerythritol tetranitrate. However, as for some relatively new explosives such as triaminotrinitrobenzene (TATB), detection methods are limited to surface-enhanced Raman scattering (SERS), which requires sophisticated instruments, time-consuming analysis, and complicated operational procedures. Recently, Wang et al. developed a sensitive and facile visual colorimetric assay for the detection of TATB based on the direct blue light induced autocatalytic oxidation of OPD (Scheme 15).25 Under the irradiation of blue light, OPDox is electronically excited and interacts with dissolved oxygen through resonant energy transfer to form a singlet-state oxygen, which possesses high oxidation capability and in turn accelerates the oxidation of OPD. Additionally, TATB can further enhance this photoinduced autocatalytic reaction, because the noncovalent interactions between TATB and OPDox can prevent the π–π stacking of OPDox and eliminate aggregation caused quenching (ACQ). Both effects lead to the UV–vis absorption enhancement, with a linear response to the concentration of TATB. Significantly, instantaneous color variations are observed after the addition of TATB making the straightforward naked-eye monitoring feasible, which would be an exceptional merit compared to traditional detection assays.
Scheme 15. OPD-Based Sensor for Visual Detection of Triaminotrinitrobenzene.
4. Conclusion and Outlook
This review has highlighted recent developments concerning fluorescent and colorimetric sensors based on the oxidation of OPD. The detection mechanisms these works have in common are based on the oxidation of OPD. However, the differences of sensor design that can be found in the majority of sensing systems are based on autocatalytic reactions or fluorescence response strategies, using nanomaterials, carbon dots, or fluorophore-labeled DNA as reference fluorophore for ratiometric fluoromeric detection. In order to facilitate comparison, the main characteristics of the sensors described in this review are listed in Table 1. In general, most of the described OPD-based fluorescence sensors proved to be capable of prominent specificity, sensitivity, and simplicity.
Table 1. Comparison between OPD-Based Sensors Discussed in This Review.
| analyte | detection target | entry | sensor category | reference fluorophore | detection strategy | ref |
|---|---|---|---|---|---|---|
| Heavy metal ions | Ag+, Cu2+ | 1 | Single-intensity-based fluorescence sensor | \ | Fluorescent turn-on | (3) |
| Ag+ | 2 | Ratiometric fluorescence sensor | GQDs | Fluorescent ON–OFF | (4) | |
| Cu2+, PPi | 3 | Ratiometric fluorescence sensor | N-CDs | Fluorescent ON–OFF | (5) | |
| Cu2+ | 4 | Ratiometric fluorescence sensor | C-ssDNA | Fluorescent ON–OFF | (6) | |
| Hg2+ | 5 | Single-intensity-based fluorescence sensor | \ | Fluorescent ON–OFF | (8, 9) | |
| 6 | Ratiometric fluorescence sensor | AuNCs | Fluorescent ON–OFF | (10) | ||
| Hg2+, Cu2+, Ag+ | 7 | Colorimetric sensor | GQDs | Color change of GNPs | (11) | |
| Ce4+ | 8 | Single-intensity-based fluorescence sensor | \ | Fluorescent turn-off | (12) | |
| Organic molecules | GSH, Cys, HCy | 9 | Single-intensity-based fluorescence sensor | \ | Fluorescent turn-off | (13) |
| GSH | 10 | Colorimetic and fluorometric dual signal sensor | \ | Fluorescent and color turn-off | (14, 15) | |
| 11 | Ratiometric fluorescence sensor | CQDs | Fluorescent ON–OFF | (16) | ||
| UA | 12 | Colorimetic and fluorometric dual signal sensor | SiNPs or Ti3C2 MQDs | Fluorescent and color ON–OFF | (18, 19) | |
| Xanthine | 13 | Colorimetic and fluorometric dual signal sensor | Fe, N-CDs | Fluorescent and color ON–OFF | (20) | |
| glucose | 14 | Ratiometric fluorescence sensor | Ru–C3N4 | Fluorescent ON–OFF | (21) | |
| AA | 15 | Single-intensity-based fluorescence sensor | \ | Fluorescent ON–OFF | (22) | |
| 16 | Ratiometric fluorescence sensor | DFQ | Fluorescent turn-on | (23) | ||
| His | 17 | Single-intensity-based fluorescence sensor | \ | Fluorescent ON–OFF | (24) | |
| TATB | 18 | Colorimetric sensor | \ | Color turn-on | (25) |
Nevertheless, there are still a number of problems that must be overcome with regard to the development of OPD-based fluorescence sensors. First, since OPD can be easily oxidized by various oxidants, it is worth improving the anti-interference properties of the sensors, especially in the presence of coexisting oxidants that are commonly found in real-world samples such as sewage waste, serum, urine, and dialysate. Second, as powerful noninvasive analytical tools, water-solubility and biocompatibility of OPD-based fluorescence sensors still need to be improved. Additionally, with the increasing demand of field testing, designing novel portable OPD-based fluorescence sensors and devices, which contain field-operable sensing elements and real-time response, is urgent and important. It is noteworthy that OPD can react with various carbonyl compounds to form fluorescent fused-ring compounds, which can be designed as potential fluorescent sensors. Though recent studies based on the condesation reaction are fragmented, they provide intriguing enlightenment that a new gate for OPD-based fluorescence sensors is opening.
Acknowledgments
Financial support from the Natural Science Foundation of China (21762018 and 21961014), the Science and Technology Project Founded by the Education Department of Jiangxi Province (No. GJJ160668), the program of Qingjiang Excellent Young Talents of Jiangxi University of Science and Technology (JXUSTQJBJ2018003), and the Science and the Technology Innovation Outstanding Young Talents Program of Jiangxi Province (20192BCBL23009) is gratefully acknowledged.
Biographies
Qiuxiang Ye was born in China in 1994 and received his B.S. (2018) at the Jiangxi University of Science and Technology. She is currently undertaking her Master’s degree in organic chemistry at the Jiangxi University of Science and Technology under the supervision of Dr. Jin-Biao Liu. Her research interest lies in the development of organic synthesis and fluorescence chemosensors.
Shangfeng Ren was born in China in 1997 and received his B.S. (2019) at the Zhoukou Normal University. She is currently a postgraduate student in Jiangxi University of Science and Technology under the supervision of Dr. Jin-Biao Liu. Her research interest lies in the development of organic synthesis and fluorescence chemosensors.
Hui Huang was born in 1996 and graduated in 2018 from Jiangxi University of Science and Technology with a Bachelor’s degree.He is currently a postgraduate student in Jiangxi University of Science and Technology and doing research with his postgraduate teacher Kun-Ming Liu.The current research direction is organic synthesis and fluorescence chemosensors.
Gaigai Duan was born in Henan, China, in 1985. She received her PhD in 2017 at the Bayreuth University, Germany. She is currently an Associate Professor at the Nanjing Forestry University. Her research interests mainly focus on synthesis and application of functional polymers, including sensors, catalyst, drug release, energy storage, etc.
Kunming Liu was born in Jiangxi, China, in 1985. He received his PhD in 2015 at the Beijing Normal University. He is currently a lecturer at the Jiangxi University of Science and Technology. His research is focused on design and application of functional organic molecules, including fluorescence sensors, luminescent rare-earth complexes, and rare-earth catalysis.
Jin-Biao Liu was born in Jiangxi, China, in 1986. He completed his PhD in 2013 at the Sun Yat-Sen University. He is currently an Associate Professor at the Jiangxi University of Science and Technology. His research interests include organic synthesis, synthetic methodology, and fluorescence chemosensors.
The authors declare no competing financial interest.
References
- Quang D. T.; Kim J. S. Fluoro- and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 2010, 110, 6280–6301. 10.1021/cr100154p. [DOI] [PubMed] [Google Scholar]
- Du J. J.; Hu M. M.; Fan J. L.; Peng X. J. Fluorescent chemodosimeters using ″mild″ chemical events for the detection of small anions and cations in biological and environmental media. Chem. Soc. Rev. 2012, 41, 4511–4535. 10.1039/c2cs00004k. [DOI] [PubMed] [Google Scholar]
- Yang X.; Wang E. K. A nanoparticle autocatalytic sensor for Ag+, and Cu2+ ions in aqueous solution with high sensitivity and selectivity and its application in test paper. Anal. Chem. 2011, 83, 5005–5011. 10.1021/ac2008465. [DOI] [PubMed] [Google Scholar]
- Zhao X. E.; Lei C. H.; Gao Y.; Gao H.; Zhu S. Y.; Yang X.; You J. M.; Wang H. A ratiometric fluorescent nanosensor for the detection of silver ions using graphene quantum dots. Sens. Actuators, B 2017, 253, 239–246. 10.1016/j.snb.2017.06.086. [DOI] [Google Scholar]
- Zhang W. J.; Liu S. G.; Han L.; Luo H. Q.; Li N. B. A ratiometric fluorescent and colorimetric dual-signal sensing platform based on N-doped carbon dots for selective and sensitive detection of copper (II) and pyrophosphate ion. Sens. Actuators, B 2019, 283, 215–221. 10.1016/j.snb.2018.12.012. [DOI] [Google Scholar]
- Ma S. S.; Zhou Q.-Y.; Mu F.-Y.; Chen Z.-H.; Ding X.-Y.; Zhang M.; Shi G. Y. Ratiometric fluorescence monitoring of cerebral Cu2+ based on coumarin-labeled DNA coupled with the Cu2+-induced oxidation of o-phenylenediamine. Analyst 2017, 142, 3341–3347. 10.1039/C7AN01099K. [DOI] [PubMed] [Google Scholar]
- Kim H. N.; Ren W. X.; Kim J. S.; Yoon J. Y. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. 10.1039/C1CS15245A. [DOI] [PubMed] [Google Scholar]
- Dai H. C.; Ni P. J.; Sun Y. J.; Hu J. T.; Jiang S.; Wang Y. L.; Li Z. Label-free fluorescence detection of mercury ions based on the regulation of the Ag autocatalytic reaction. Analyst 2015, 140, 3616–3622. 10.1039/C4AN02162B. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Ma Z. F. Fluorescent and colorimetric dual detection of mercury (II) by H2O2 oxidation of o-phenylenediamine using Pt nanoparticles as the catalyst. Sens. Actuators, B 2017, 249, 53–58. 10.1016/j.snb.2017.04.076. [DOI] [Google Scholar]
- Li W. J.; Liu D.; Bi X. Y.; You T. Y. Enzyme-triggered inner filter effect on the fluorescence of gold nanoclusters for ratiometric detection of mercury(II) ions via a dual-signal responsive logic. Sens. Actuators, A 2020, 302, 111794–111800. 10.1016/j.sna.2019.111794. [DOI] [Google Scholar]
- Yang J. H.; Zhang Y.; Zhang L.; Wang H. L.; Nie J. F.; Qin Z. X.; Li J.; Xiao W. C. Analyte-triggered autocatalytic amplification combined with gold nanoparticle probes for colorimetric detection of heavy-metal ions. Chem. Commun. 2017, 53, 7477–7480. 10.1039/C7CC02198D. [DOI] [PubMed] [Google Scholar]
- Xia M.; Zhao X. E.; Sun J.; Zheng Z. J.; Zhu S. Y. Graphene quantum dots combined with the oxidase-mimicking activity of Ce4+ for ratiometric fluorescent detection of Ce4+ and alendronate sodium. Sens. Actuators, B 2020, 319, 128321–128328. 10.1016/j.snb.2020.128321. [DOI] [Google Scholar]
- Li R.; Lei C.; Zhao X.-E.; Gao Y.; Gao H.; Zhu S. Y.; Wang H. A label-free fluorimetric detection of biothiols based on the oxidase-like activity of Ag+ ions. Spectrochim. Acta, Part A 2018, 188, 20–25. 10.1016/j.saa.2017.06.056. [DOI] [PubMed] [Google Scholar]
- Li F.; Liu J. C.; Hu Y. T.; Deng N.; He J. B. An ultrasensitive label-free colorimetric assay for glutathione based on Ag+ regulated autocatalytic oxidation of o-phenylenediamine. Talanta 2018, 186, 330–336. 10.1016/j.talanta.2018.04.078. [DOI] [PubMed] [Google Scholar]
- Ma Z. Y.; Wu T. T.; Li P. P.; Liu M. L.; Huang S.; Li H. T.; Zhang Y. Y.; Yao S. Z. A dual (colorimetric and fluorometric) detection scheme for glutathione and silver (I) based on the oxidase mimicking activity of MnO2 nanosheets. Microchim. Acta 2019, 186, 498–507. 10.1007/s00604-019-3613-4. [DOI] [PubMed] [Google Scholar]
- Han Z.; Nan D. Y.; Yang H.; Sun Q. Q.; Pan S.; Liu H.; Hu X. L. Carbon quantum dots based ratiometric fluorescence probe for sensitive and selective detection of Cu2+ and glutathione. Sens. Actuators, B 2019, 298, 126842–126850. 10.1016/j.snb.2019.126842. [DOI] [Google Scholar]
- Chen H. Y.; Lu Q. J.; He K. L.; Liu M. L.; Zhang Y. Y.; Yao S. Z. A cyclic signal amplification strategy to fluorescence and colorimetric dual-readout assay for the detection of H2O2-related analytes and application to colorimetric logic gate. Sens. Actuators, B 2018, 260, 908–917. 10.1016/j.snb.2018.01.085. [DOI] [Google Scholar]
- Wu C. Y.; Zhu L. J.; Lu Q. J.; Li H. T.; Zhang Y. Y.; Yao S. Z. A dual-signal colorimetric and ratiometric fluorescent nanoprobe for enzymatic determination of uric acid by using silicon nanoparticles. Microchim. Acta 2019, 186, 754–761. 10.1007/s00604-019-3862-2. [DOI] [PubMed] [Google Scholar]
- Liu M. W.; He Y.; Zhou J.; Ge Y. L.; Zhou J. G.; Song G. W. A “naked-eye” colorimetric and ratiometric fluorescence probe for uric acid based on Ti3C2 MXene quantum dots. Anal. Chim. Acta 2020, 1103, 134–142. 10.1016/j.aca.2019.12.069. [DOI] [PubMed] [Google Scholar]
- Wang L. Z.; Liu Y.; Yang Z. P.; Wang Y. Y.; Rao H. B.; Yue G. Z.; Wu C. M.; Lu C. F.; Wang X. X. A ratiometric fluorescence and colorimetric dual-mode assay for H2O2 and xanthine based on Fe, N co-doped carbon dots. Dyes Pigm. 2020, 180, 108486–108493. 10.1016/j.dyepig.2020.108486. [DOI] [Google Scholar]
- Deng W. F.; Peng Y.; Yang H.; Tan Y. M.; Ma M.; Xie Q. J.; Chen S. W. Ruthenium Ion-Complexed Carbon Nitride Nanosheets with Peroxidase-like Activity as a Ratiometric Fluorescence Probe for the Detection of Hydrogen Peroxide and Glucose. ACS Appl. Mater. Interfaces 2019, 11, 29072–29077. 10.1021/acsami.9b10715. [DOI] [PubMed] [Google Scholar]
- Zhu S. Y.; Lei C. H.; Gao Y.; Sun J.; Peng H. W.; Gao H.; Zhang R. X.; Wang R.; Zhao X.-E.; Wang H. A simple and label-free fluorescence detection of ascorbic acid in rat brain microdialysates in the presence of catecholamines. New J. Chem. 2018, 42, 3851–3856. 10.1039/C7NJ04574C. [DOI] [Google Scholar]
- Chen H. Y.; Liu Y. L.; Li H. T.; Zhang Y. Y.; Yao S. Z. Non-oxidation reduction strategy for highly selective detection of ascorbic acid with dual-ratio fluorescence and colorimetric signals. Sens. Actuators, B 2019, 281, 983–988. 10.1016/j.snb.2018.11.020. [DOI] [Google Scholar]
- Xu Y.; Wu X.-Q.; Shen J.-S.; Zhang H.-W. Highly selective and sensitive recognition of histidine based on the oxidase-like activity of Cu2+ ions. RSC Adv. 2015, 5, 92114–92120. 10.1039/C5RA17900A. [DOI] [Google Scholar]
- Wang J. H.; Li H.; Cai Y. H.; Wang D. J.; Bian L.; Dong F. Q.; Yu H. L.; He Y. Direct blue light-induced autocatalytic oxidation of o-phenylenediamine for highly sensitive visual detection of triaminotrinitrobenzene. Anal. Chem. 2019, 91, 6155–6161. 10.1021/acs.analchem.9b00759. [DOI] [PubMed] [Google Scholar]