Abstract
Introduction:
Tobacco use during pregnancy is the most modifiable risk factor associated with poor pregnancy outcomes. Self-reported tobacco use has been demonstrated to have high misclassification rates. The aims were to examine misclassification rates of perinatal tobacco use during each trimester of pregnancy and 8 weeks postpartum, and to evaluate characteristics associated with misclassification of tobacco use status.
Methods:
This is secondary analysis of a prospective, multicenter trial of pregnant women, and it includes participants who were biochemically identified as tobacco users during their first trimester (N = 103). Each trimester and once postpartum, tobacco use was assessed via self-report and validated using a cutoff of 100 ng/mL for urine cotinine via NicAlert test strips to indicate current use. Those who self-reported as nonusers but were identified as users via urine cotinine were considered misclassified; misclassification rates were determined for each time period. Logistic regression assessed maternal factors associated with misclassification status.
Results:
Misclassification rates declined from 35.0% at first trimester to 31.9% and 26.6% at the second and third; the postpartum rate was 30.4%. These rates did not differ significantly from each other at the 0.05 level. Race/ethnicity was associated with misclassification status; white/non-Hispanic women were 87% less likely to be misclassified (p < .001).
Conclusion:
Misclassification of prenatal smoking status decreases as pregnancy progresses, though the observed rate change was not significant. Minority women may be at particular risk for non-disclosure of tobacco use. Biochemical validation should be considered when assessing perinatal tobacco use via self-report, given high misclassification rates throughout the perinatal period.
Implications:
These results demonstrate that regardless of trimester, more than one-quarter of tobacco-using pregnant women may not disclose tobacco use throughout pregnancy and early postpartum. Although the rate of misclassification decreased from first to third trimester and then increased in the immediate postpartum, these changes in misclassification rates were not significant. Minority groups may be at particular risk of misclassification compared with white/non-Hispanic women. Biochemical validation is warranted throughout pregnancy to encourage cessation as tobacco use is one of the most easily-modified risk factors for poor birth outcomes.
Introduction
Prenatal tobacco use is the most modifiable risk factor associated with poor pregnancy outcomes, including impaired placental attachment and function, delayed fetal lung and brain development, miscarriage, preterm birth, and low birth weight.1 This remains a major public health concern: 12% of women in the US report smoking during pregnancy,2 and prenatal smoking rates in many countries exceed 20%–30%.3
Due to social pressures and the unacceptability of smoking during pregnancy, many women misrepresent their smoking status.4,5 Self-reported nonsmoking status or quitting has been associated with misclassification rates among pregnant women, typically ranging from 10% to 25%,6–10 though lower11,12 and higher4 rates have been reported. Unreliable self-reporting of tobacco use among pregnant women can have negative health consequences for both the mother and child. A healthcare provider’s ability to appropriately intervene is diminished if the mother’s true smoking status remains unknown.
Perez-Stable et al.13 found that serum cotinine values are accurate measures of smoking status among women and can be a useful in validating self-reported smoking status. Cotinine is the main metabolite of nicotine and is detectable in human fluids, such as urine, saliva, and blood, and is considered the most accurate measurement of active and passive smoke exposure among pregnant women.14 Although cotinine is metabolized at a faster rate during pregnancy, its half-life remains approximately 12–18 hours, making it a reliable measurement of daily nicotine consumption.15–18
Several published analyses of cotinine levels used to validate self-reported smoking data exist, but few have examined this relationship among pregnant women at multiple times during pregnancy/postpartum. The purpose of this study was to examine misclassification rates of tobacco use during each trimester of pregnancy and early postpartum, and to evaluate personal characteristics associated with women who misclassify their smoking status in early pregnancy. We hypothesized that third trimester self-report would be the most reliable measure of prenatal smoking status.
Methods
Design and Sample
This longitudinal study was a secondary analysis of a larger prospective trial of pregnant and postpartum women recruited between 2008 and 2013 from three prenatal clinics in Central and Western Kentucky and Central Virginia. This sample included all pregnant women from the parent study who were biochemically validated as tobacco users via urine cotinine level in their first trimester (N = 103). The subsequent assessments were done once in each remaining trimester and during the first 8 weeks postpartum.
Procedures
The study was approved by the medical Institutional Review Boards in all three sites. Women were approached to participate at their initial, first-trimester appointment. Potential participants were screened to exclude any pre-existing diabetes, heart disease, a medical history of HIV, bacterial vaginosis, sexually transmitted infections, chronic conditions with implications for immune function or any autoimmune disease. For women who met eligibly and verbalized willingness to participate, informed written consent was obtained. Participants completed the surveys via iPad or on paper and were given modest compensation at each of the four data collection points. On average, the survey took 20 minutes, and nearly all completed the iPad version. Participants also provided a midstream urine sample of 25 cc or more into in a disposable container. The urine samples were analyzed for cotinine level within 4 hours based on manufacturer guidelines.19 Urine was discarded after analysis.
Measures
Personal Characteristics
Demographics included age (in years), race/ethnicity (with five options, including Hispanic), education (with seven options), and household income (with nine options, ranging from “$4999 or less” to “$50 000 or more”). Other personal factors included whether the woman was partnered, if the pregnancy was planned (yes/no), and how many hours she was exposed to other people’s tobacco smoke indoors at home. Age, income, and planned pregnancy were used in their original format. Given the small numbers in each racial/ethnic minority, this variable was categorized as “white/non-Hispanic” or “Other.” A binary variable was created to indicate whether the woman was at least a high school graduate.
Participants indicated their marital/living status with seven possible options; those who chose “living with partner” or “married” were coded as “partnered,” while other responses were coded as “non-partnered.” Exposure to secondhand smoke (SHS) in the home was coded as “no” if the reported number of hours exposed in the home was zero and as “yes” for any positive number of hours.
Self-reported Tobacco Use
In each of the four surveys, participants were asked “Do you currently smoke cigarettes or use smokeless tobacco (loose leaf, dip, chew, and snuff) even just once in a while?” Those who answered “no” or “have never smoked or used tobacco products” were coded as nonusers; those who indicated “yes” were coded as tobacco users.
Biochemical Validation of Tobacco Use
Urine samples from each longitudinal assessment were tested using NicAlert strips. NicAlert, is a valid, cost effective and commercial immunochromatographic assay that uses cutoff limits of urine cotinine levels to validate smoking status in pregnant women.19–22 A score of 3 or higher on this test is indicative of a urine cotinine value of 100 ng/mL or greater; this cutoff was used to determine tobacco use. NicAlert cutoffs for smoking validation are consistent with previous reported urine cotinine ranges.23,24 NicAlert cotinine assessment also correlates well with more complex laboratory tests using high performance liquid chromatography.23
Data Analysis
Descriptive statistics, including means and standard deviations or frequency distributions, were used to summarize the personal characteristics and tobacco use indicators. Tobacco use misclassification rate is defined as the ratio of those who reported they were not using cigarettes or smokeless tobacco but had above-threshold urine cotinine level relative to all with a positive cotinine assay. These rates and corresponding 95% confidence limits were used to assess changes in disclosure of tobacco use over the perinatal period. Logistic regression assessed personal factors associated with misclassification status in the first trimester. The Hosmer–Lemeshow test determined model fit and variance inflation factors gauged the presence of multicollinearity. Data analysis was done using SAS, with an alpha level of 0.05.
Results
The average age of participants was 24.9 (SD = 4.8). Most were white/non-Hispanic (70.6%), and had at least a high school education (74.5%). The majority had an annual household income of less than $15 000 (54.6%) and most were partnered (62.8%). Most women indicated their pregnancy was unplanned (73.2%) and the majority reported that they were exposed to SHS in the home (68.0%).
The number of biochemically validated tobacco users at each evaluation ranged from 103 in the first trimester to 72, 64, and 46 for the second, third, and postpartum assessments, respectively. Of the initial 103, 25 were omitted from one or more subsequent misclassification rate calculations because their urine cotinine values were below the threshold for indication of tobacco product use (whether they self-identified as a user or nonuser). The rest of the missing participants were lost to follow-up; the largest loss of this type was between the third trimester and postpartum.
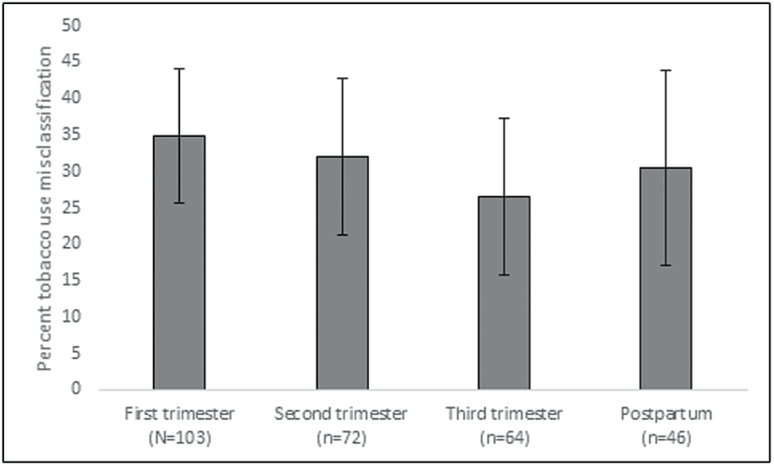
The misclassification rate, namely the rate of self-identifying as a nonuser while having a NicAlert value corresponding to a urine cotinine level of 100 or greater, varied over time from high of 35.0% at the first trimester to a low of 26.6% at the third trimester (seeFigure 1); misclassification rates at second trimester and postpartum were 31.9% and 30.4%, respectively. While the trend was a decrease in under-reporting until the birth, followed by an increase postpartum, misclassification rates did not differ significantly over time, as evidenced by the overlapping confidence intervals. There were also some women with a mismatch between self-reported tobacco use status and urine cotinine in the opposite direction, namely self-identification as a tobacco user and a NicAlert value corresponding to a cotinine level of less than 100. The rate of this type of mismatch was very low at each trimester of the perinatal period (in particular, 3.6%, 4.0%, 0.4% and 1.7% in the first, second, third trimesters and postpartum, respectively).
Figure 1.
Misclassification rates for each trimester and postpartum assessment, with 95% confidence intervals.
The logistic model to examine factors related misclassification (ie, having a NicAlert test corresponding to cotinine of 100 or above while self-identifying as nonuser) was significant overall (χ2 = 19.0,p = .008; seeTable 1). The only personal factor in the model that predicted first-trimester misclassification was race/ethnicity. Compared to other racial/ethnic groups, white/non-Hispanic women were 87% less likely misclassify themselves as nonusers. This model fit the data well, with a nonsignificant Hosmer–Lemeshow test (χ2 = 7.6,p = .47), and the variance inflation factors were less than 1.5, suggesting multicollinearity did not influence regression parameters.
Table 1.
Logistic Regression Modeling the Likelihood of Misclassifying First Trimester Tobacco Use Status (n = 94)a
| OR | 95% CI forOR | p | |
|---|---|---|---|
| Age | 0.91 | 0.81–1.02 | .11 |
| Race/ethnicity | |||
| White/non-Hispanic | 0.13 | 0.04–0.42 | <.001 |
| Other | 1.00 | ||
| Education | |||
| Less than high school | 0.85 | 0.25–2.84 | .79 |
| At least high school/GED | 1.00 | ||
| Total household income | 1.20 | 0.97–1.49 | .097 |
| Partnered status | |||
| Partnered | 1.79 | 0.55–5.88 | .34 |
| Non-partnered | 1.00 | ||
| Planned pregnancy | |||
| Yes | 1.15 | 0.38–3.50 | .80 |
| No | 1.00 | ||
| SHS exposure in the home | |||
| Yes | 0.44 | 0.14–1.34 | .15 |
| No | 1.00 | ||
CI = confidence interval;OR = odds ratio; SHS = secondhand smoke.
aWhile the number of missing values for each of the demographic and personal variables was limited, nine participants were excluded from this model due to missing one or more of these.
Discussion
Biochemical validation of tobacco use should be considered in the prenatal period since misclassification rates are high throughout pregnancy. Confirmed identification of tobacco use by health practitioners will help ensure that patients are appropriately counseled about the adverse risks associated with prenatal tobacco use and provided cessation information. The findings from this study are similar to prior research that identified between 20% and 25% of pregnant women who reported a nonsmoking or quit status but who had cotinine measures above the minimum threshold for tobacco use.6–9
The rate of tobacco use misclassification among pregnant women is typically higher than in the general population. In a population-based study, Vartianinen and colleagues25 determined that 2.7% to 5.2% of female participants falsely reported that they had not used tobacco in the last month, conflicting with their serum cotinine levels. The rate of misclassification was higher if they indicated they had ever used tobacco. In the general population aged 17 and above,26 the misclassification rate of those who self-reported as nonsmokers was 1.4%.
The higher misclassification rate among pregnant women may be related to the stigma associated with smoking during pregnancy. Wigginton and Lee27 found that pregnant women who smoked were much more likely to be labelled as “unhealthy” and “bad influences” compared to their nonpregnant and nonsmoking counterparts. Another study28 found that pregnant women who smoke were more likely to deny smoking to health professionals due to social pressure. Low socioeconomic status may have influenced the high misclassification rate in this sample, given three-quarters of participants had household incomes of less than $25 000. Webb and colleagues4 determined a 73% misclassification rate among low-income pregnant women self-reporting as nonsmokers.
Consistent with prior research,29 this study determined that women of minority race/ethnicity may be at particular risk for underreporting tobacco use during pregnancy. The discrepancy between racial/ethnic groups in rate of misclassification of tobacco use status among females in the general population has been reported. Wells and colleagues30 found that the misclassification rate among women was relatively low overall, but the rate among occasional smokers was lower for those of majority race/ethnicity (6.0%) compared with minority participants (15.3%). The higher propensity of minorities to underreport tobacco use while pregnant suggests that the poorer pregnancy and birth outcomes among tobacco users may affect minority populations disproportionately.
The primary limitation of this study is the attrition from the first trimester to the postpartum period, either due to drop out or to cessation of tobacco use during pregnancy. This is consistent with other longitudinal studies of this population, where attrition of up to 54% has been reported.31 Further, maternal self-reported smoking during pregnancy is a predictor of higher attrition independent of whether the woman was able to be contacted or not,32 underscoring challenges to sample retention in this at-risk population. The accuracy of self-reported tobacco use may have been increased if we had given the participants more options for reporting their use. For example, a prior study of smoking during pregnancy also provided an option indicating the level of tobacco use had been “cut down.”33 An additional limitation is lack of assessment of other tobacco products in addition to cigarettes and smokeless tobacco; a portion of the misclassified tobacco users may have been using types of tobacco not specifically identified in the self-reported use question. Related to this, we did not assess whether any of the participants were using Nicotine replacement therapy; this has the potential for false positives via the NicAlert assay. The cutoff for urine cotinine is relatively high at 100 ng/mL; this may have incorrectly labeled some light tobacco users as nonusers. This concern is mitigated by the observation that this high cutoff made it less likely that those who were only exposed to SHS but not actively using tobacco would be labeled as users; the cutoff of 100 ng/mL is higher than has been used previously in this population.34 Metabolism of nicotine and cotinine in pregnant is increased while the half-life is decreased when compared to nonpregnant women.15 Although this concept is increasingly important in Nicotine replacement therapy dosing, limited evidence exists limiting validation of perinatal smoking status using preset cotinine limits. Finally, these participants were recruited from an area of the country with high smoking rates, even among pregnant women, so the findings may not be widely generalizable.
This is the first identified study to assess tobacco use misclassification throughout pregnancy and the early postpartum period using repeated biochemical validation assessments. The results suggest that self-report of current tobacco use may be lowest among minority women. In addition, given the consistently high rates of undisclosed use of tobacco throughout pregnancy, biochemical validation is warranted at each trimester. Future research in this area should be focused on the development of new approaches to discuss and intervene related to perinatal tobacco use, including strategies that encourage cessation while reducing social stigma for those still using tobacco products.
Funding
National Institutes for Health Building Interdisciplinary Research Careers in Women’s Health (BIRCWH K12DA14040), Center for Biomedical Research Excellence (COBRE: 5P20GM103538); also supported by the University of Kentucky Clinical and Translational Research Center (KL2RR033171), and the University of Kentucky Office of Undergraduate Research Summer Research and Creativity Fellowship Grant.
Declaration of Interests
None declared.
References
- 1. Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM.Infant morbidity and mortality attributable to prenatal smoking in the U.S.Am J Prev Med.2010;39(1):45–52. [DOI] [PubMed] [Google Scholar]
- 2. Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy—Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000–2010.MMWR Surveill Summ.2013;62(6):1–19. [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Report on the Global Tobacco Epidemic, 2009: Implementing Smoke-Free Environments. Geneva, Switzerland: World Health Organization; 2013: 8–11. [Google Scholar]
- 4. Webb DA, Boyd NR, Messina D, Windsor RA.The discrepancy between self-reported smoking status and urine continine levels among women enrolled in prenatal care at four publicly funded clinical sites.J Public Health Manag Pract.2003;9(4):322–325. [DOI] [PubMed] [Google Scholar]
- 5. Lawrence T, Aveyard P, Croghan E.What happens to women’s self-reported cigarette consumption and urinary cotinine levels in pregnancy? Addiction.2003;98(9):1315–1320. [DOI] [PubMed] [Google Scholar]
- 6. Pärna K, Rahu M, Youngman LD, Rahu K, Nygård-Kibur M, Koupil I.Self-reported and serum cotinine-validated smoking in pregnant women in Estonia.Matern Child Health J.2005;9(4):385–392. [DOI] [PubMed] [Google Scholar]
- 7. George L, Granath F, Johansson AL, Cnattingius S.Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy.Acta Obstet Gynecol Scand.2006;85(11):1331–1337. [DOI] [PubMed] [Google Scholar]
- 8. England LJ, Grauman A, Qian C, et al. Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes.Nicotine Tob Res.2007;9(10):1005–1013. [DOI] [PubMed] [Google Scholar]
- 9. Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J.Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study.BMJ.2009;339:b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tong VT, Althabe F, Aleman A, et al. Accuracy of self-reported smoking cessation during pregnancy.Acta Obstet. Gynecol. Scand.2015;94(1):106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindqvist R, Lendahls L, Tollbom O, Aberg H, Håkansson A.Smoking during pregnancy: comparison of self-reports and cotinine levels in 496 women.Acta Obstet Gynecol Scand.2002;81(3):240–244. [DOI] [PubMed] [Google Scholar]
- 12. Mattsson K, Källén K, Rignell-Hydbom A, et al. Cotinine validation of self-reported smoking during pregnancy in the Swedish Medical Birth Register.Nicotine Tob Res.2016;18(1):79–83. [DOI] [PubMed] [Google Scholar]
- 13. Pérez-Stable EJ, Benowitz NL, Marín G.Is serum cotinine a better measure of cigarette smoking than self-report? Prev Med.1995;24(2):171–179. [DOI] [PubMed] [Google Scholar]
- 14. Markovic N, Ness RB, Cefilli D, Grisso JA, Stahmer S, Shaw LM.Substance use measures among women in early pregnancy.Am J Obstet Gynecol.2000;183(3):627–632. [DOI] [PubMed] [Google Scholar]
- 15. Dempsey D, Jacob P, Benowitz NL.Accelerated metabolism of nicotine and cotinine in pregnant smokers.J Pharm Exp Ther.2002;301(2):594–598. [DOI] [PubMed] [Google Scholar]
- 16. Russell T, Crawford M, Woodby L.Measurements for active cigarette smoke exposure in prevalence and cessation studies: why simply asking pregnant women isn’t enough.Nicotine Tob Res.2004;6(suppl 2):S141–S151. [DOI] [PubMed] [Google Scholar]
- 17. Vaz LR, Coleman T, Cooper S, Aveyard P, Leonardi-Bee J.The nicotine metabolite ratio in pregnancy measured by trans-3′-hydroxycotinine to cotinine ratio: characteristics and relationship with smoking cessation.Nicotine Tob Res.2015;17(11):1318–1323. [DOI] [PubMed] [Google Scholar]
- 18. Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., IIINicotine metabolite ratio as a predictor of cigarette consumption.Nicotine Tob Res.2003;5(5):621–624. [DOI] [PubMed] [Google Scholar]
- 19. NicAlert.Expressing the results as cotinine concentration ranges2007www.nicalert.net/nicalert/er.html Accessed May 2, 2007.
- 20. Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA.A randomized trial of computer-delivered brief intervention and low-intensity contingency management for smoking during pregnancy.Nicotine Tob Res.2012;14(3):351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaalema DE, Higgins ST, Bradstreet MP, Heil SH, Bernstein IM.Using NicAlert strips to verify smoking status among pregnant cigarette smokers.Drug Alcohol Depend.2011;119(1–2):130–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ashford KB, Hahn E, Hall L, Rayens MK, Noland M, Collins R.Measuring prenatal secondhand smoke exposure in mother–baby couplets.Nicotine Tob Res.2010;12(2):127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernert JT, Harmon TL, Sosnoff CS, McGuffey JE.TECHNICAL NOTE: use of cotinine immunoassay test strips for preclassifying urine samples from smokers and nonsmokers prior to analysis by LCMSMS.J Anal Toxicol.2005;29(8):814–818. [DOI] [PubMed] [Google Scholar]
- 24. Higgins ST, Heil SH, Badger GJ, et al. Biochemical verification of smoking status in pregnant and recently postpartum women.Exp Clin Psychopharmacol.2007;15(1):58–66. [DOI] [PubMed] [Google Scholar]
- 25. Vartiainen E, Seppälä T, Lillsunde P, Puska P.Validation of self reported smoking by serum cotinine measurement in a community-based study.J Epidemiol Community Health.2002;56(3):167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caraballo RS, Giovino GA, Pechacek TF, Mowery PD.Factors associated with discrepancies between self-reports on cigarette smoking and measured serum cotinine levels among persons aged 17 years or older: Third National Health and Nutrition Examination Survey, 1988-1994.Am J Epidemiol.2001;153(8):807–814. [DOI] [PubMed] [Google Scholar]
- 27. Wigginton B, Lee C.Stigma and hostility towards pregnant smokers: does individuating information reduce the effect? Psychol Health.2013;28(8):862–873. [DOI] [PubMed] [Google Scholar]
- 28. Bull L, Burke R, Walsh S, Whitehead E.Social attitudes towards smoking in pregnancy in East Surrey: a qualitative study of smokers, former smokers and non-smokers.J Neonatal Nurs.2007;13(3):100–106. [Google Scholar]
- 29. Tabb KM, Huang H, Menezes PR, Azevedo e Silva G, Chan YF, Faisal-Cury A.Ethnic differences in tobacco use during pregnancy: findings from a primary care sample in São Paulo, Brazil.Ethn Health.2015;20(2):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wells AJ, English PB, Posner SF, Wagenknecht LE, Perez-Stable EJ.Misclassification rates for current smokers misclassified as nonsmokers.Am J Public Health.1998;88(10):1503–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. CDC. Dual Use of Tobacco Products. Overviews of Diseases/Conditions2016www.cdc.gov/tobacco/campaign/tips/diseases/dual-tobacco-use.html Accessed October 03, 2016.
- 32. Ng S-K, Scott R, Scuffham PA.Contactable non-responders show different characteristics compared to lost to follow-up participants: insights from an Australian longitudinal birth cohort study.Matern Child Health J.2016;20(7):1472– 1484. [DOI] [PubMed] [Google Scholar]
- 33. Mullen PD, Carbonari JP, Tabak ER, Glenday MC.Improving disclosure of smoking by pregnant women.Am J Obstet Gynecol.1991;165(2):409–413. [DOI] [PubMed] [Google Scholar]
- 34. Aurrekoetxea JJ, Murcia M, Rebagliato M, et al. Determinants of self-reported smoking and misclassification during pregnancy, and analysis of optimal cut-off points for urinary cotinine: a cross-sectional study.BMJ open.2013;3(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]