Abstract
Given the prevalence of sleep in early development, any satisfactory account of infant brain activity must consider what happens during sleep. Only recently, however, has it become possible to record sleep-related brain activity in newborn rodents. Using such methods in rat pups, it is now clear that sleep, more so than wake, provides a critical context for the processing of sensory input and the expression of functional connectivity throughout the sensorimotor system. In addition, sleep uniquely reveals functional activity in the developing primary motor cortex, which establishes a somatosensory map long before its role in motor control emerges. These findings will inform our understanding of the developmental processes that contribute to the nascent sense of embodiment in human infants.
Keywords: sensory development, REM sleep, myoclonic twitching, somatosensory, sensorimotor integration, brain rhythms, functional connectivity, corollary discharge, embodiment, neurodevelopment disorders
Introduction
How does the infant brain manage to function in the moment in the midst of rapid and transformative developmental change? And what is the significance for the developing brain that the majority of the infant’s day is devoted to sleep? Thanks largely to methods that enable recording brain activity in infant rats as they cycle between sleep and wake [1], the answers to these questions are beginning to emerge. In particular, it is now clear that sleep, especially active (REM) sleep, activates the infant brain in a way that is unique and profound for the nascent sensorimotor system. The self-generated brain activation that characterizes active sleep in the infant is ideally suited to building and refining somatotopic maps, integrating sensory and motor systems, and laying the foundation for the later-emerging, embodied sense of self [2]. These and other insights are increasingly informing investigations of sleep-related neural activity in human infants.
Sleep and brain activity in early development
It is a truism that we sleep most when we are young. At birth in humans, 16 hours of each day are allotted to sleep with this value dropping to eight or fewer hours in adulthood [3]. In addition to the quantity of sleep, its composition also changes. Specifically, preterm human fetuses spend two-thirds or more of their sleep time in active sleep and the remainder in quiet sleep [4,5]. By the time of birth, active sleep has dropped to 50% of total sleep time, which is higher still than the 25% seen in adults and the 15% in the elderly. Similar patterns are observed in other mammalian species [6].
The states of sleep and wake are composed of multiple components that are variable in expression across species and age [6]. In adult mammals, including humans, researchers have relied most heavily on three components—muscle tone, eye movements, and cortical electroencephalogram (EEG)—for distinguishing among wake and the two states of sleep: active sleep and quiet (or non-REM) sleep. Specifically, wake is characterized by high muscle tone, alert eye movements, and desynchronized cortical activity; quiet sleep is characterized by low muscle tone, an absence of eye movements, and synchronized cortical slow-waves; and active sleep is characterized by muscle atonia, rapid eye movements (REMs), and desynchronized cortical activity.
Although newborn animals exhibit clear behavioral hallmarks of sleep and wake, those behaviors often do not map cleanly onto the criteria established in adults [7]. Accordingly, it is necessary to adjust expectations when considering the young of altricial mammals. For example, muscle atonia accompanies sleep in rats at one week of age, but the eyes are immobile beneath eyelids [8]. In preterm human infants, the cortical EEG is characterized not by tonic activation, but rather by long periods of silence interrupted by brief bursts of activity [9–11], with the transition to fully continuous and state-dependent EEG activity emerging over the first several postnatal months [12]. By comparison, in infant rats, the transition to continuous, state-dependent EEG activity occurs toward the end of the second postnatal week [7,13].
The brief bursts of cortical activity that punctuate periods of silence are typically triggered by spontaneous activity in the sensory periphery [13]. For example, in the visual system, peripheral sensory activity arises from spontaneous retinal waves, which provide patterned activation to primary visual cortex (V1) and other structures [14,15]. Although retinal waves occur independently of sleep-wake state, their downstream effects in visual thalamus and cortex are modulated by state [16,17].
In the sensorimotor system, peripheral sensory activity arises from myoclonic twitches, which are brief, jerky, and discrete movements of the limbs, whiskers, and other parts of the body controlled by skeletal muscle [18,19]. Although twitching is a ubiquitous feature of active sleep in infant mammals [20], it is readily observed across the lifespan in a wide diversity of species, such as humans, rats, dogs, cats, ferrets, hedgehogs, and opossums, to name a few (see http://www.twitchsleep.net for videos of twitching in these and other species).
Unlike retinal waves, twitches are a defining feature of active sleep. They are produced most abundantly in early development and are easily distinguished from the high-amplitude and coordinated movements that characterize wake. But also, twitches robustly, reliably, and somatotopically trigger bursts of activity in somatosensory cortex [21] and, indeed, in sensorimotor structures across the neuraxis [2]. Accordingly, as will be reviewed here, neural activity in the developing sensorimotor system is intimately linked to twitches and, therefore, with active sleep.
Neural causes and consequences of myoclonic twitching
Twitches are not like wake behaviors: They do not produce locomotion and are not goal-directed. And because twitches look more like a spasm than an intended output of the nervous system, it is not surprising that they were long considered functionless byproducts—the detritus—of a dreaming brain. However, based largely on research in rats before postnatal day (P) 10, that view is no longer tenable. On the contrary, the unique features of twitches—including their spatiotemporal organization [18], their responsiveness to sensory feedback [22,23], and their unique capacity to activate the infant brain [24,25]—make them a powerful tool for assessing functional activity in the developing sensorimotor system.
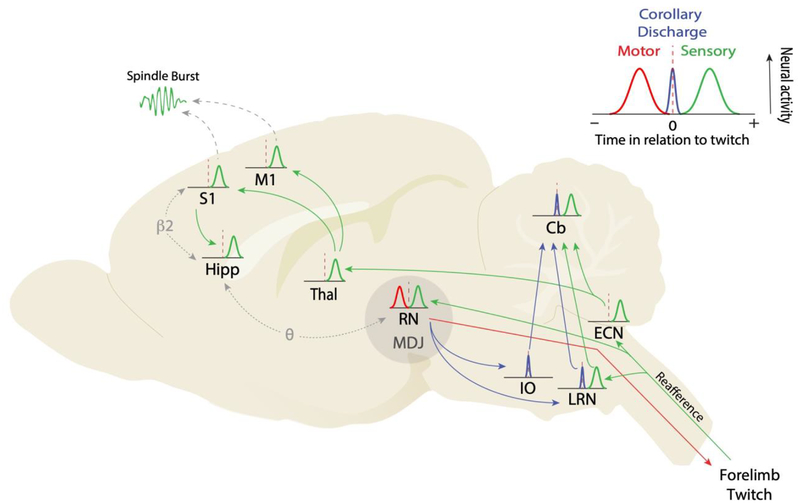
Recent advances in understanding sleep-related processes in infant rats hinged on the development of a method for recording brain activity in head-fixed and unanesthetized rat pups. Vital to this method is the fact that pups will cycle between sleep and wake so long as proper attention is paid to their thermal, hydrational, and nutritional needs [1]. Under such conditions, electrodes can be implanted throughout the infant brain—from cortex to medulla—and even, as recently demonstrated, in the spinal cord [26]. By simultaneously recording limb movements, the neural activity surrounding twitches can be quantified using perievent histograms. As illustrated in the inset in Figure 1, this information can be used to determine, for individual neurons, whether activity reliably precedes twitches (a motor event; red trace) or follows twitches (a sensory event; green trace). Also, when neural activity occurs at the same time as a twitch, that is suggestive of a corollary discharge (CD), that is, a copy of a motor signal that does not itself trigger movement (blue trace) [27].
Figure 1.
The neural causes and consequences of myoclonic twitching in sleeping infant rats. Inset: Illustration of neural activity surrounding a twitch: preceding activity indicative of motor outflow (red), following activity indicative of sensory feedback (i.e., reafference; green), and nearly simultaneous activity indicative of corollary discharge (blue). These icons are used in the main figure below, which is based largely on studies using P8 rats. Main figure: The production of a forelimb twitch begins in midbrain structures, including the red nucleus (RN) and surrounding areas in the mesodiencephalic junction (MDJ). When a forelimb twitch is produced, reafferent signals flow directly to the spinal cord, the external cuneate nucleus (ECN), and the RN. From the ECN, reafferent signals flow to the deep cerebellar nuclei and cerebellar cortex (Cb) as well as the thalamus, arriving next in primary somatosensory (S1) and primary motor (M1) cortex. From S1, reafference flows via the entorhinal cortex to the hippocampus. Coincident with spiking activity in S1 and M1, spindle bursts are detected in the local field potential. Corollary discharges emanating from neurons in and around the RN project separately to the inferior olive (IO) and lateral reticular nucleus (LRN), before projecting to the cerebellum via climbing and mossy fibers, respectively. Finally, the occurrence of twitch-triggered bursts of coherent rhythmic activity between RN and hippocampus in the theta band (θ; 4–7 Hz) and between S1 and hippocampus in the beta2 band (β2; 20–30 Hz) is illustrated. Background image is a sagittal section of an infant rat brain. See text for discussion and citations.
The state of active sleep is largely controlled by structures in the mespontine region, including the laterodorsal tegmental nucleus (LDT) and sublaterodorsal nucleus (SLD) (for review, see [28]). These structures modulate downstream motor nuclei in a state-dependent fashion [29]. One such motor nucleus is the red nucleus (RN), which is part of a group of midbrain motor structures within the mesodiencephalic junction (MDJ) [30]. The RN projects directly to motor neurons in the spinal cord that control the forelimbs and, to a lesser extent, the hindlimbs. Consistent with its motor function and as shown in Figure 1, the activity of RN neurons in P8 rats precedes forelimb twitches, consistent with its motor function [31]; in addition, pharmacological disruption of RN function causes an immediate reduction in twitching. Finally, it must be stressed that forebrain structures, including motor cortex, do not control the production of twitches or, for that matter, any movement through at least the second postnatal week in rats [25,32].
When a forelimb twitch occurs, proprioceptors and tactile receptors are stimulated and sensory feedback signals (reafference) trigger neural activity in the dorsal horn of the spinal cord [26] and other downstream sensorimotor structures. With respect to the forelimb (Figure 1), twitch-related sensory signals can flow directly to neurons in the external cuneate nucleus (ECN; [24]) with a latency of approximately 40 ms. From the ECN, sensory signals are conveyed to the cerebellum and thalamic nuclei. In turn, thalamic nuclei project to primary somatosensory cortex (S1) and primary motor cortex (M1), where reafference from forelimb twitches robustly triggers neural activity within the forelimb regions of those areas [25]. Finally, the hippocampus is also activated after twitches—via cortical projections through entorhinal cortex [33–35].
Twitch-related corollary discharge in the cerebellar system
Twitch-responsive neurons are not restricted to the sensory stream connecting brainstem, thalamus, and cerebral cortex. Both the cerebellar cortex and deep cerebellar nuclei also exhibit twitch-related reafferent activity [36,37]. Some of this activity, however, occurs at latencies that are much shorter than 40 ms and, therefore, cannot be attributed to reafference via the ECN. Instead, this short-latency cerebellar activity suggests the presence of a twitch-related corollary discharge signal. This idea was tested in P8 rats by recording from the inferior olive (IO), which projects exclusively to the cerebellum via climbing fibers. It was found that the IO exhibits a pattern of neural activity with two distinct features: First, IO unit activity increased simultaneously (+10 ms) with the onset of a twitch; and second, IO activity exhibited a rapid onset and offset in relation to each twitch, resulting in a distinctly sharp peak (Figure 1, blue trace) [38].
The source of twitch-related IO activity was traced to neurons within the MDJ that lie immediately adjacent to the RN [38]. Neurons in this region exhibited motor-activity profiles similar to that of the RN and, when electrically stimulated, triggered brief movements of the contralateral forelimb. The sharp IO peak was attributed to the recruitment of local calcium-activated slow potassium (SK) channels: Selective blockade of SK channels broadened the sharp IO peaks such that they mirrored the motor signals emitted by MDJ neurons. Given that the IO does not itself participate in motor control, it was concluded that it conveys a precisely timed, twitch-related corollary discharge signal from the MDJ to the cerebellum [38].
The lateral reticular nucleus (LRN) is yet another precerebellar structure that, like the ECN, provides mossy fiber input to the cerebellum. But unlike the ECN, the LRN processes both motor and sensory input, with descending projections arriving from the RN and ascending projections arriving from the spinal cord [39]. Consistent with these dual inputs, the LRN exhibited twitch-related activity indicative of both corollary discharge and reafference (Figure 1; [38]). Thus, across three precerebellar nuclei, (a) the ECN conveys twitch-related reafference, (b) the IO conveys twitch-related corollary discharge, and (c) the LRN conveys a combination of the two. This diversity of precisely timed and somatotopically organized signals converges on the cerebellum during a sensitive period in its development (e.g., see [40]). Such converging input is critical to the cerebellum’s role in predictive coding and the capacity to distinguish self- from other-generated movements [41,42]. It is not yet known whether twitch-related corollary discharge signals are conveyed to non-cerebellar structures in the sensorimotor system.
Sensory gating of wake-related reafference
Whereas limb twitches are highly effective at triggering a cascade of downstream activity in the somatosensory system, it was repeatedly found that wake movements trigger little or no activity in those same structures [43]. This was true even though wake movements are much more vigorous and intense than twitches, and so their sensory effects should be much greater.
The cause of this mysterious discrepancy in the effects of twitch- and wake-related reafference on downstream sensory processing was traced to the ECN [24]. First, recording from the medullary structure in P8 rats, the robust reafferent activity that followed twitching was absent during wake. To determine whether the ECN was responsible for gating wake-related reafference, recordings were performed before and after local pharmacological disinhibition. As predicted, twitch-related reafference was unaffected by this disinhibition, but wake-related reafference was unmasked. This experiment showed that wake-related reafference is conveyed to the ECN, but it is gated there. The effect is that structures downstream to the ECN, including M1, are similarly deprived of wake-related reafference through at least P8 in rats.
Sensory processing by motor cortex and a key developmental transition
M1 is considered primary motor cortex because of its unambiguous role in adult motor control [44]. Despite many decades of research, however, the nature of M1’s contributions to behavior is still being debated. Indeed, several recent studies in adult rodents are reshaping how we think about the motor functions of M1 [45–47]. These studies emphasize M1’s contributions to the learning—as opposed to execution—of motor tasks and the critical role of sensory input for updating motor commands after an unexpected perturbation.
Whatever the roles of M1 in motor learning and control, they emerge relatively late in development [48]. In rats, this emergence occurs as late as P35 [49], before which movement is controlled by structures within the brainstem. In humans, this late onset dramatically reveals itself in infants who have experienced a perinatal stroke, which is a leading cause of hemiplegic cerebral palsy (CP), the most prevalent childhood motor disability [50,51]. Specifically, the disabling effects of CP are not evident until at least 4–8 months after birth [52], presumably as descending control from M1 begins to emerge.
What does M1 do during this “pre-motor” period of development? To answer this question, consider M1’s functional activity at P8, as illustrated in Figure 1. At this age, M1 robustly responds to twitch-related reafference. Moreover, because it is downstream from the ECN, it does not receive reafference from wake movements. But this state of affairs cannot last: After all, as described above, the sensory consequences of limb movements are critical to adult motor performance and the learning of new motor skills.
By recording from M1 daily between P8 and P12, a key transition was discovered in that structure’s responsiveness to wake-related reafference [25]. Specifically, whereas 90% of M1 neurons responded exclusively to twitches at P8, 80% of M1 neurons responded exclusively to wake movements at P12. This dramatic reversal was attributed to the opening, at P11–12, of the ECN gate to the sensory consequences of wake movements. Interestingly, this “awakening” of M1 occurs at a time marked by substantial improvements in limb control and coordination, including the onset of crawling [53]. Moreover, this transition is not restricted to the sensorimotor domain, as there are similar transitions in visual processing toward the end of the second postnatal week in rats [13].
At P12, when the ECN is responding to wake-related reafference, it continues to respond to twitch-related reafference. In contrast, at P12, only a small proportion of M1 neurons respond to twitch-related reafference. What accounts for this difference in twitch-responsiveness between the two structures? This question was addressed by pharmacologically disinhibiting M1 at P12: By doing so, it was possible to unmask twitch-related responses that resembled those normally observed at P8. This simple experiment suggested that the M1 “awakening” at P12 to wake-related reafference occurs contemporaneously with a second transition in which local inhibitory networks within M1 restrict that structure’s responses to twitches. This notion is consistent with what is known about the role of inhibitory circuits in sharpening and tuning cortical sensory responses [54,55].
Altogether, this research reveals how active sleep and twitches are essential to developing the sensory foundation of M1. This sensory foundation is established long before M1 assumes its motor functionality, thus suggesting that the sensory foundation is critical to M1’s ability to exhibit motor control and learning-related plasticity.
From spikes to oscillations: Sleep, development, and functional connectivity
The focus here thus far has been on neural spiking activity in relation to twitches during active sleep. But there is a second important dimension to neural activity that concerns the local field potential (LFP)—the aggregate, synchronous electrical activity that can be measured using extracellular electrodes implanted within the brain or EEG electrodes at the brain surface [56,57].
Fifteen years ago, researchers discovered that S1 of newborn rats exhibits brief oscillatory events, called spindle bursts (peak frequency: 12–15 Hz), in response to limb movements, including twitches [21]. With that discovery, it was clear that the cerebral cortex of infant rats is not nearly as silent as previously thought. In humans, spindle bursts have long been recognized as a prominent feature of cortical activity in premature infants, often being referred to as delta brushes. It was also recognized that spindle bursts are quite distinct from sleep spindles, which are most closely associated with quiet sleep and first emerge after the first postnatal month [58]. Similarly, in rats, sleep spindles are first detected after the second postnatal week [59], by which time spindle bursts are very difficult to detect [60]. Mechanistically, whereas both spindle bursts and sleep spindles are produced through corticothalamic interactions, the emergence of sleep spindles appears to depend on the development of thalamic inhibition and intrinsic changes in the bursting properties of neurons in the thalamic reticular nucleus [61]. Finally, despite these and other differences, spindle bursts and sleep spindles are both strongly implicated in neural plasticity across the lifespan [62,63].
In addition to S1, twitch-triggered spindle bursts in P8 rats are readily detected in M1 and, as seen with unit activity, are more effectively and reliably triggered by twitches than wake movements [43]. In addition, spindle bursts occur in V1 in response to retinal waves [14], as well as in primary auditory cortex (A1) [64] and prefrontal cortex [65]. In contrast, spindle bursts are not detected in medial dysgranular regions in frontal, parietal, and occipital cortex, although these areas do exhibit other sleep-related oscillations, including 40-Hz gamma bursts [66].
Whereas spindle bursts are detected soon after birth in rodents, hippocampal theta (4–7 Hz) begins to emerge around P8 [67]. Initially, theta occurs in brief bursts immediately after twitches (Figure 1), but then transitions by P12 to a continuous rhythm throughout active sleep [68]. A similar developmental trajectory—from brief twitch-related theta bursts to a continuous rhythm—was found in the RN [68]. Moreover, at P12 the theta rhythms in the RN and hippocampus were highly coherent and were similarly eliminated after pharmacological inhibition of the medial septum. This state-dependent coherence is consistent with the view that the RN and hippocampus form a functional unit in the service of sensorimotor integration [69]. Similar theta-dependent processes may be important for the development of functional connectivity between the hippocampus and prefrontal cortex, with possible implications for our understanding of the developmental origins of schizophrenia and other disorders associated with abnormal connectivity patterns [65,70].
Whereas the hippocampus communicates with the RN and prefrontal cortex in the theta band, there is evidence that it communicates with somatosensory cortex in the beta2 band (20–30 Hz; Figure 1). This was demonstrated in P8 rats using dual recordings in whisker S1 (i.e., barrel cortex) and hippocampus [35]. When pups twitched during active sleep, spindle bursts were triggered in barrel cortex that, as previously reported in V1, span frequencies from 5 to 30 Hz [71]. However, coherence between the two structures was specifically elevated in the beta2 band in the period immediately after a twitch. Moreover, by cutting the infraorbital nerve and thus eliminating reafference from whiskers, coherence between barrel cortex and hippocampus was specifically reduced in the beta2 band. When considered alongside the previous theta findings, the developing hippocampus can exhibit twitch-dependent and band-specific communication with at least two different structures—S1 and RN—at the same time.
Altogether, active sleep provides a critical context for the developmental expression of functional connectivity among cortical and subcortical structures. It follows that prenatal or postnatal environments that disrupt the expression of active sleep can deprive the infant of valuable sensory experience, potentially sending the infant down an atypical developmental path [72].
Implications for human development
There is good reason to believe that sleep-dependent development in the human sensorimotor system parallels that in rat pups. For example, soon after the discovery of somatosensory-driven spindle bursts in infant rats, it was demonstrated that limb twitches trigger similar cortical events in premature human infants at 29–31 weeks postconceptional age [73]. This twitch-related spindle-burst activity persists briefly into the early postnatal period [74].
Given the relatively high levels of sleep in full-term and preterm human infants, one might assume that protecting sleep from unnecessary environmental disturbances would be a central concern of pediatric facilities, especially neonatal intensive care units (NICUs). However, only recently have neonatologists, influenced by pediatric sleep research, reassessed the brightly lit, noisy, and disruptive NICU environments that premature infants routinely encounter [75]. Given that sleep provides a critical context for sensory processing and functional connectivity in the developing nervous system, NICUs should be designed in a way that maximizes opportunities for sleep. Attention to this issue may help to ameliorate some of the numerous long-term negative consequences of premature birth on functional connectivity and other aspects of neurodevelopment [76]. Of particular relevance here is the fact that approximately one-half of all premature infants exhibit a sensory processing disorder [77].
There is, therefore, a compelling need to understand the specific mechanisms by which sleep contributes to sensory development in humans, including the processes by which somatotopic maps develop [78]. A recent upsurge of interest in this area has led investigators to devise a variety of methods for stimulating the limbs of human infants to assess the precision of cortical representations across age [79–83]. But the larger question remains regarding the process by which topographic maps develop in human infants and the role played by sleep in that process.
New approaches are emerging for studying the role of sleep in human sensorimotor development. For example, one recent study focused on the statistical properties of spontaneous head movements during sleep in infants that are at low or high familial risk for autism [84]. In that study, high-risk infants at 1–2 months of age exhibited less diversity in their head movements while asleep than when responding, while awake, to language-related acoustic stimuli. The authors attributed this difference to a less flexible sensorimotor system in the high-risk infants. Such findings suggest that sleep-related movements in human infants provide critical sensory input to the developing nervous system. Unfortunately, in fMRI studies, it is current standard practice to reject (i.e., scrub) data collected during movement due to artifact. As recently noted, this practice must be changed if we are to make progress in understanding the contributions of self-generated movements to sensorimotor development and functional connectivity in humans [85].
Despite remarkable improvements in imaging and recording technology, the tools currently available for investigating state-dependent brain activity in human infants have substantial limitations. Functional magnetic resonance imaging (fMRI) has poor temporal resolution and is performed in a noisy environment that is not conducive to sleep, and electroencephalography (EEG) has poor spatial resolution and provides no information about brain activity below the surface of the cerebral cortex. These and other non-invasive technologies, including magnetoencephalography (MEG), are still unable to match the spatial and temporal resolution provided by those invasive methods (e.g., extracellular neurophysiology) that are used in non-human animals [86]. Therefore, it will be important to seek ways to bridge the gap between what is being discovered about sleep and brain activity in infant rats with what is possible in human infants.
The road to embodiment
Across development, spontaneous neural activity is a necessary contributor to a number of critical processes, including neurogenesis, differentiation, cellular migration, apoptosis, circuit formation, and plasticity (for reviews, see [87,88]). The sleep-related spontaneous activity discussed here—that is, myoclonic twitching—could serve one or more of these functions in different parts of the nervous system and at different developmental periods. Moreover, the ability of twitches to activate sensorimotor structures in topographically precise ways suggests an important role for that behavior in establishing, refining, and maintaining somatotopic maps at multiple levels of the neuraxis. Such maps are the neural instantiation of a body schema, which underlies our capacity to distinguish self from other and provides a foundation for later-developing cognitive and social capacities [78]. In this way, sleep is poised to play a key role in the process of embodiment, providing a context for the brain to interconnect with the body in the ultimate service of adaptive behavior [2].
Footnotes
Conflict of Interest Statement:
Nothing declared
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- [1].Blumberg MS, Sokoloff G, Tiriac A, Del Rio-Bermudez C, A valuable and promising method for recording brain activity in behaving newborn rodents, Dev Psychobiol. 57 (2015) 506–517. doi: 10.1002/dev.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper describes a method for recording neural activity in unanesthetized, head-fixed infant rats.
- [2].Blumberg MS, Dooley JC, Phantom limbs, neuroprosthetics, and the developmental origins of embodiment, Trends Neurosci. 40 (2017) 603–612. doi: 10.1016/j.tins.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Roffwarg HP, Muzio JN, Dement WC, Ontogenetic development of the human sleep-dream cycle, Science. 152 (1966) 604–619. [DOI] [PubMed] [Google Scholar]
- [4].Parmelee A, Wenner W, Akiyama Y, Schultz M, Stern E, Sleep states in premature infants, Dev Med Child Neurol. 9 (1967) 70–77. [DOI] [PubMed] [Google Scholar]
- [5].Kohyama J, Iwakawa Y, Developmental changes in phasic sleep parameters as reflections of the brain-stem maturation polysomnographical examinations of infants, including premature neonates, Electroencephalogr Clin Neurophysiol. 76 (1990) 325–330. [DOI] [PubMed] [Google Scholar]
- [6].Blumberg MS, Rattenborg NC, Decomposing the evolution of sleep: Comparative and developmental approaches, in: Kaas JH (Ed.), Evolution of Nervous Systems, Elsevier, Oxford, 2017: pp. 523–545. doi: 10.1016/B978-0-12-804042-3.00100-7. [DOI] [Google Scholar]
- [7].Blumberg MS, Seelke AMH, The form and function of infant sleep: From muscle to neocortex, in: Blumberg MS, Freeman JH, Robinson SR (Eds.), Oxford Handbook of Developmental Behavioral Neuroscience, Oxford University Press, New York, 2010: pp. 391–423. [Google Scholar]
- [8].Seelke AMH, Karlsson KÆ, Gall AJ, Blumberg MS, Extraocular muscle activity, rapid eye movements and the development of active and quiet sleep, Eur J Neurosci. 22 (2005) 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khazipov R, Luhmann HJ, Early patterns of electrical activity in the developing cerebral cortex of humans and rodents, Trends Neurosci. 29 (2006) 414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- [10].Iyer KK, Roberts JA, Hellström-Westas L, Wikström S, Hansen Pupp I, Ley D, et al. , Cortical burst dynamics predict clinical outcome early in extremely preterm infants, Brain. 138 (2015) 2206–2218. doi: 10.1093/brain/awv129. [DOI] [PubMed] [Google Scholar]
- [11].Dereymaeker A, Pillay K, Vervisch J, De Vos M, Van Huffel S, Jansen K, et al. , Review of sleep-EEG in preterm and term neonates, Early Hum Dev. 113 (2017) 87–103. doi: 10.1016/j.earlhumdev.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jenni OG, Achermann P, Borbély AA, Development of the nocturnal sleep electroencephalogram in human infants, Am J Physiol Regul Integr Comp Physiol. 286 (2004) R528–38. doi: 10.1152/ajpregu.00503.2003. [DOI] [PubMed] [Google Scholar]
- [13].Colonnese MT, Phillips MA, Thalamocortical function in developing sensory circuits, Curr Opin Neurobiol. 52 (2018) 72–79. doi: 10.1016/j.conb.2018.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Review current understanding of the development of thalamocortical circuits, beginning with thalamic interactions with cortical subplate neurons and continuing through the incorporation of inhibitory interneurons.
- [14].Hanganu IL, Ben-Ari Y, Khazipov R, Retinal waves trigger spindle bursts in the neonatal rat visual cortex, J Neurosci. 26 (2006) 6728–6736. doi: 10.1523/JNEUROSCI.0752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ackman JB, Burbridge TJ, Crair MC, Retinal waves coordinate patterned activity throughout the developing visual system, Nature. 490 (2012) 219–225. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Murata Y, Colonnese MT, Thalamus controls development J Neurosci. 38 (2018) 8772–8786. doi: 10.1523/JNEUROSCI.1519-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The authors demonstrate how behavioral state (in this case active wake) modulates thalamocortical activity in the developing visual system of rats. Importantly, they reveal that the modulatory effects of behavioral state are dynamic, with active wake suppressing visual system activity through P12 and augmenting visual activity from P13 onward.
- [17].Mukherjee D, Yonk AJ, Sokoloff G, Blumberg MS, Wakefulness suppresses retinal wave-related neural activity in visual cortex, J Neurophysiol. 118 (2017) 1190–1197. doi: 10.1152/jn.00264.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Blumberg MS, Coleman CM, Gerth AI, McMurray B, Spatiotemporal structure of REM sleep twitching reveals developmental origins of motor synergies, Curr Biol. 23 (2013) 2100–2109. doi: 10.1016/j.cub.2013.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Tiriac A, Uitermarkt BD, Fanning AS, Sokoloff G, Blumberg MS, Rapid whisker movements in sleeping newborn rats, Curr Biol. 22 (2012) 2075–2080. doi: 10.1016/j.cub.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jouvet-Mounier D, Astic L, Lacote D, Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month, Dev Psychobiol. 2 (1970) 216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- [21].Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G, Early motor activity drives spindle bursts in the developing somatosensory cortex, Nature. 432 (2004) 758–761. [DOI] [PubMed] [Google Scholar]
- [22].Blumberg MS, Coleman CM, Sokoloff G, Weiner JA, Fritzsch B, McMurray B, Development of twitching in sleeping infant mice depends on sensory experience, Curr Biol. 25 (2015) 656–662. doi: 10.1016/j.cub.2015.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Petersson P, Waldenström A, Fåhraeus C, Schouenborg J, Spontaneous muscle twitches during sleep guide spinal self-organization, Nature. 424 (2003) 72–75. [DOI] [PubMed] [Google Scholar]
- [24].Tiriac A, Blumberg MS, Gating of reafference in the external cuneate nucleus during self-generated movements in wake but not sleep, eLife. 5 (2016) e18749. doi: 10.7554/eLife.18749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dooley JC, Blumberg MS, Developmental “awakening” of primary motor cortex to the sensory consequences of movement, eLife. y (2018) e41841. doi: 10.7554/eLife.41841.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** The demonstration in P8–12 rats of a rapid state-dependent transition in sensory responsiveness by M1 neurons. The transition is mediated by a change in the gating of wake-related reafference by the external cuneate nucleus as well as local changes in inhibitory processing within M1.
- [26].Inácio AR, Nasretdinov A, Lebedeva J, Khazipov R, Sensory feedback synchronizes motor and sensory neuronal networks in the neonatal rat spinal cord, Nature Comm. 7 (2016) 13060–14. doi: 10.1038/ncomms13060. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Using unanesthetized rats, this paper provides a first-ever description of neural activity in the neonatal spinal cord in relation to sensory feedback from twitches and wake movements.
- [27].Crapse TB, Sommer MA, Corollary discharge across the animal kingdom, Nat Rev Neurosci. 9 (2008) 587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peever J, Fuller PM, The biology of REM sleep, Curr Biol. 27 (2017) R1237–R1248. doi: 10.1016/j.cub.2017.10.026. [DOI] [PubMed] [Google Scholar]; ** A comprehensive review of our current understanding of the mechanisms, proposed functions, and pathologies of REM sleep. It specifically highlights recent research methodologies that reveal the role for REM sleep in learning and memory as well as the circuitry important for REM sleep production that are involved in REM sleep pathologies, including cataplexy.
- [29].Brooks PL, Peever JH, Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia, J Neurosci. 28 (2008) 3535–3545. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Onodera S, Hicks TP, Evolution of the motor system: Why the elephant“s trunk works like a human”s hand, Neuroscientist. (1999). doi: 10.1177/107385849900500411. [DOI] [Google Scholar]
- [31].Sensorimotor processing in the newborn rat red nucleus during active sleep, 35 (2015) 8322–8332. doi: 10.1523/JNEUROSCI.0564-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kreider J, Blumberg MS, Mesopontine contribution to the expression of active “twitch” sleep in decerebrate week-old rats, Brain Res. 872 (2000) 149–159. [DOI] [PubMed] [Google Scholar]
- [33].Valeeva G, Janackova S, Nasretdinov A, Rychkova V, Makarov R, Holmes GL, et al. , Emergence of coordinated activity in the developing entorhinal–hippocampal network, Cereb Cortex. 490 (2018) 219–15. doi: 10.1093/cercor/bhy309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mohns EJ, Blumberg MS, Neocortical activation of the hippocampus during sleep in newborn rats, J Neurosci. 30 (2010) 3438–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Del Rio-Bermudez C, Kim J, Sokoloff G, Blumberg MS, Active sleep promotes coherent oscillatory activity in the cortico-hippocampal system of infant rats, Cereb Cortex. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Del Rio-Bermudez C, Plumeau AM, Sattler NJ, Sokoloff G, Blumberg MS, Spontaneous activity and functional connectivity in the developing cerebellorubral system, J Neurophysiol. 116 (2016) 1316–1327. doi: 10.1152/jn.00461.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sokoloff G, Plumeau AM, Mukherjee D, Blumberg MS, Twitch-related and rhythmic activation of the developing cerebellar cortex, J Neurophys. 114 (2015) 1746–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mukherjee D, Sokoloff G, Blumberg MS, Corollary discharge in precerebellar nuclei of sleeping infant rats, eLife. (2018). doi: 10.7554/eLife.38213.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper demonstrates corollary discharge signals in the precerebellar nuclei of P8 rats. The corollary discharge signal is associated with limb twitches and arises from the red nucleus and adjacent structures.
- [39].Alstermark B, Ekerot C-F, The lateral reticular nucleus: a precerebellar centre providing the cerebellum with overview and integration of motor functions at systems level. A new hypothesis, J Physiol (Lond). 591 (2013) 5453–5458. doi: 10.1113/jphysiol.2013.256669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kano M, Watanabe T, Uesaka N, Watanabe M, Multiple phases of climbing fiber synapse elimination in the developing cerebellum, Cerebellum. 17 (2018) 722–734. doi: 10.1007/s12311-018-0964-z. [DOI] [PubMed] [Google Scholar]
- [41].Huang C-C, Sugino K, Shima Y, Guo C, Bai S, Mensh BD, et al. , Convergence of pontine and proprioceptive streams onto multimodal cerebellar granule cells, eLife. 2 (2013) e00400–e00400. doi: 10.7554/eLife.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Franklin DW, Wolpert DM, Computational mechanisms of sensorimotor control, Neuron. 72 (2011) 425–442. doi: 10.1016/j.neuron.2011.10.006. [DOI] [PubMed] [Google Scholar]
- [43].Tiriac A, Del Rio-Bermudez C, Blumberg MS, Self-generated movements with “unexpected” sensory consequences, Curr Biol. 24 (2014) 2136–2141. doi: 10.1016/j.cub.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Peters AJ, Liu H, Komiyama T, Learning in the rodent motor cortex, Annu Rev Neurosci. 40 (2017) 77–97. doi: 10.1146/annurev-neuro-072116-031407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kawai R, Markman T, Poddar R, Ko R, Fantana AL, Dhawale AK, et al. , Motor cortex is required for learning but not for executing a motor skill, Neuron. 86 (2015) 800–812. doi: 10.1016/j.neuron.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper, and the two that follow, illustrates new directions in understanding the functions of primary motor cortex, including the role of primary motor cortex in learning, not just executing, motor skills.
- [46].Mathis MW, Mathis A, Uchida N, Somatosensory cortex plays an essential role in forelimb motor adaptation in mice, Neuron. 93 (2017) 1493–1503.e6. doi: 10.1016/j.neuron.2017.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Heindorf M, Arber S, Keller GB, Mouse motor cortex coordinates the behavioral response to unpredicted sensory feedback, Neuron. 99 (2018) 1040–1054.e5. doi: 10.1016/j.neuron.2018.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Martin JH, The corticospinal system: From development to motor control, Neuroscientist. 11 (2005) 161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- [49].Young NA, Vuong J, Teskey GC, Development of motor maps in rats and their modulation by experience, J Neurophysiol. 108 (2012) 1309–1317. doi: 10.1152/jn.01045.2011. [DOI] [PubMed] [Google Scholar]
- [50].Raju TNK, Nelson KB, Ferriero D, Lynch JK, Ischemic perinatal stroke: summary of a workshop sponsored by the National Institute of Child Health and Human Development and the National Institute of Neurological Disorders and Stroke, Pediatrics. 120 (2007) 609–616. doi: 10.1542/peds.2007-0336. [DOI] [PubMed] [Google Scholar]
- [51].Lee J, Croen LA, Lindan C, Nash KB, Yoshida CK, Ferriero DM, et al. , Predictors of outcome in perinatal arterial stroke: a population-based study, Annals of Neurology. 58 (2005) 303–308. doi: 10.1002/ana.20557. [DOI] [PubMed] [Google Scholar]
- [52].Golomb MR, MacGregor DL, Domi T, Armstrong DC, McCrindle BW, Mayank S, et al. , Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes, Annals of Neurology. 50 (2001) 163–168. [DOI] [PubMed] [Google Scholar]
- [53].Shriner AM, Drever FR, Metz GA, The development of skilled walking in the rat, Behav Brain Res. 205 (2009) 10–10. doi: 10.1016/j.bbr.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Isaacson JS, Scanziani M, How inhibition shapes cortical activity, Neuron. 72 (2011) 231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wood KC, Blackwell JM, Geffen MN, Cortical inhibitory interneurons control sensory processing, Curr Opin Neurobiol. 46 (2018) 200–207. doi: 10.1016/j.conb.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Buzsáki G, Anastassiou CA, Koch C, The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes, Nat Rev Neurosci. 13 (2012) 407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cohen MX, Where does EEG come from and what does it mean? Trends Neurosci. 40 (2017) 208–218. doi: 10.1016/j.tins.2017.02.004. [DOI] [PubMed] [Google Scholar]
- [58].Ellingson RJ, Development of sleep spindle bursts during the first year of life, Sleep. 5 (1981) 39–46. [PubMed] [Google Scholar]
- [59].Gramsbergen A, The development of the EEG in the rat, Dev Psychobiol. 9 (1976) 501–515. [DOI] [PubMed] [Google Scholar]
- [60].Tiriac A, Blumberg MS, The case of the disappearing spindle burst, Neural Plasticity. (2016) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Murata Y, Colonnese MT, Thalamic inhibitory circuits and network activity development, Brain Res. 1706 (2019) 13–23. doi: 10.1016/j.brainres.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Lindemann C, Ahlbeck J, Neural SB, Hanganu-Opatz IL, Spindle activity orchestrates plasticity during development and sleep, Neural Plasticity. 2016 (2016) 1–14. doi: 10.1155/2016/5787423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Clawson BC, Durkin J, Aton SJ, Form and function of sleep spindles across the lifespan, Neural Plasticity. 2016 (2016) 1–16. doi: 10.1155/2016/6936381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Chipaux M, Colonnese MT, Mauguen A, Fellous L, Mokhtari M, Lezcano O, et al. , Auditory stimuli mimicking ambient sounds drive temporal “delta-brushes” in premature infants, PLoS ONE. 8 (2013) e79028–11. doi: 10.1371/journal.pone.0079028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Brockmann MD, Pöschel B, Cichon N, Hanganu-Opatz IL, Coupled oscillations mediate directed interactions between prefrontal cortex and hippocampus of the neonatal rat, Neuron. 71 (2011) 332–347. doi: 10.1016/j.neuron.2011.05.041. [DOI] [PubMed] [Google Scholar]
- [66].Seelke AMH, Blumberg MS, Developmental appearance and disappearance of cortical events and oscillations in infant rats, Brain Res. 1324 (2010) 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mohns EJ, Blumberg MS, Synchronous bursts of neuronal activity in the developing hippocampus: Modulation by active sleep and association with emerging gamma and theta rhythms, J Neurosci. 28 (2008) 10134–10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Del Rio-Bermudez C, Kim J, Sokoloff G, Blumberg MS, Theta oscillations during active sleep synchronize the developing rubro-hippocampal sensorimotor network, Curr Biol. 27 (2017) 1413–1424. doi: 10.1016/j.cub.2017.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This paper provides evidence in P8 rats of coherent theta activity between the red nucleus, a midbrain structure involved in the production of twitches, and the hippocampus. It emphasizes how active sleep provides a critical context for functional connectivity among developing brain regions that will ultimately form essential neural circuits.
- [69].Dypvik AT, Bland BH, Functional connectivity between the red nucleus and the hippocampus supports the role of hippocampal formation in sensorimotor integration, J Neurophysiol. 92 (2004) 2040–2050. doi: 10.1152/jn.01081.2003. [DOI] [PubMed] [Google Scholar]
- [70].Hartung H, Cichon N, De Feo V, Riemann S, Schildt S, Lindemann C, et al. , From shortage to surge: A developmental switch in hippocampal–prefrontal coupling in a gene–environment model of neuropsychiatric disorders, Cereb Cortex. 26 (2016) 4265–4281. doi: 10.1093/cercor/bhw274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hanganu IL, Staiger J, Ben-Ari Y, Khazipov R, Cholinergic modulation of spindle bursts in the neonatal rat visual cortex in vivo, J Neurosci. 27 (2007) 5694–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Del Rio-Bermudez C, Blumberg MS, Active sleep promotes functional connectivity in developing sensorimotor networks, BioEssays. 304 (2018) 1700234. doi: 10.1002/bies.201700234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R, Rapid cortical oscillations and early motor activity in premature human neonate, Cereb Cortex. 17 (2007) 1582–1594. [DOI] [PubMed] [Google Scholar]
- [74].Whitehead K, Meek J, Fabrizi L, Developmental trajectory of movement-related cortical oscillations during active sleep in a cross-sectional cohort of preterm and full-term human infants, Sci Rep. 8 (2018) 111–8. doi: 10.1038/s41598-018-35850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This paper examines the developmental timeline for the occurrence of alpha-beta oscillations (i.e., spindle bursts) in response to limb movements in human infants (31–42 weeks gestational weeks). Although alpha-beta oscillations decline near term, other aspects of EEG activity appear to persist in response to movement at later ages.
- [75].Lai TT, Bearer CF, Iatrogenic environmental hazards in the neonatal intensive care unit, Clinics in Perinatology. 35 (2008) 163–181. doi: 10.1016/j.clp.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Rogers CE, Lean RE, Wheelock MD, Smyser CD, Aberrant structural and functional connectivity and neurodevelopmental impairment in preterm children, J Neurodev Disord. 10 (2018) 1–13. doi: 10.1186/s11689-018-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ryckman J, Hilton C, Rogers C, Pineda R, Sensory processing disorder in preterm infants during early childhood and relationships to early neurobehavior, Early Hum Dev. 113 (2017) 18–22. doi: 10.1016/j.earlhumdev.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Marshall PJ, Meltzoff AN, Body maps in the infant brain, Trends Cogn Sci. 19 (2015) 499–505. doi: 10.1016/j.tics.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Allievi AG, Arichi T, Tusor N, Kimpton J, Arulkumaran S, Counsell SJ, et al. , Maturation of sensori-motor functional responses in the preterm brain, Cereb Cortex. 26 (2015) 402–413. doi: 10.1093/cercor/bhv203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Rigato S, Ali JB, van Velzen J, Bremner AJ, The neural basis of somatosensory remapping develops in human infancy, Curr Biol. 24 (2014) 1222–1226. doi: 10.1016/j.cub.2014.04.004. [DOI] [PubMed] [Google Scholar]
- [81].Smeds E, Vanhatalo S, Piitulainen H, Bourguignon M, Jousmäki V, Hari R, Corticokinematic coherence as a new marker for somatosensory afference in newborns, Clin Neurophysiol. 128 (2017) 647–655. doi: 10.1016/j.clinph.2017.01.006. [DOI] [PubMed] [Google Scholar]
- [82].Meltzoff AN, Saby JN, Marshall PJ, Neural representations of the body in 60-day-old human infants, Dev Sci. 10 (2018) e12698–8. doi: 10.1111/desc.12698. [DOI] [PubMed] [Google Scholar]
- [83].Whitehead K, Papadelis C, Laudiano-Dray MP, Meek J, Fabrizi L, The emergence of hierarchical somatosensory processing in late prematurity, Cereb Cortex. 26 (2019) 402–16. doi: 10.1093/cercor/bhz030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Denisova K, Zhao G, Inflexible neurobiological signatures precede atypical development in infants at high risk for autism, Sci Rep. 7 (2017) 1–17. doi: 10.1038/s41598-017-09028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Denisova K, Age attenuates noise and increases symmetry of head movements during sleep resting-state fMRI in healthy neonates, infants, and toddlers, Infant Behav Dev. 57 (2019) 101317–10. doi: 10.1016/j.infbeh.2019.03.008. [DOI] [PubMed] [Google Scholar]; ** One of a small number of studies in human infants aimed at understanding the relation between sleep-related movements and brain activity. In this imaging paper, the author calls attention to the problems associated with the standard practice of excluding periods of movement (as artifact) before data analysis, thus removing an important source of information.
- [86].van Gerven M, Farquhar J, Schaefer R, Vlek R, Geuze J, Nijholt A, et al. , The brain-computer interface cycle, J Neural Eng. 6 (2009) 041001. doi: 10.1088/1741-2560/6/4/041001. [DOI] [PubMed] [Google Scholar]
- [87].Luhmann HJ, Sinning A, Yang J-W, Reyes-Puerta V, St ttgen MC, Kirischuk S, et al. , Spontaneous neuronal activity in developing neocortical networks: From single cells to large-scale interactions, Front Neural Circuits. 10 (2016) 166–14. doi: 10.3389/fncir.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kirkby LA, Sack GS, Firl A, Feller MB, A role for correlated spontaneous activity in the assembly of neural circuits, Neuron. 80 (2013) 1129–1144. doi: 10.1016/j.neuron.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]