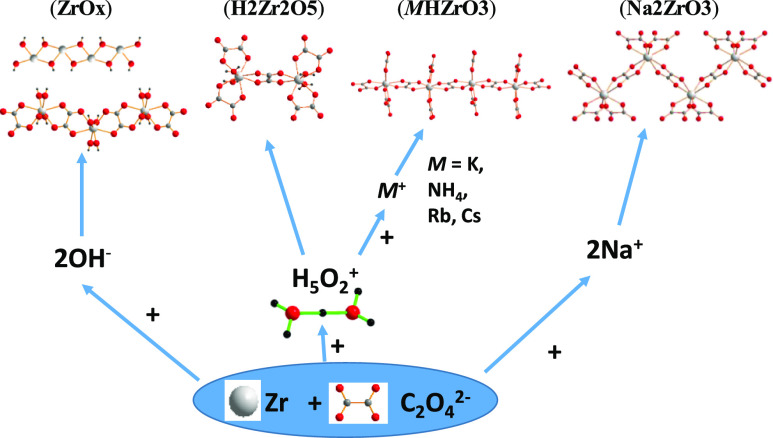
Abstract
Crystallized powder of dihydroxide zirconium oxalate Zr(OH)2(C2O4) (ZrOx) was obtained by precipitation and the structure determined from powder X-ray data. The three-dimensional (3D) framework observed in (ZrOx) results from the interconnection of zirconium hydroxide chains 1∞[Zr(OH)2]2+ and zirconium oxalate chains 1∞[{Zr(C2O4)}2+]. Single crystals of (H11O5)2[Zr2(C2O4)5(H2O)4] (H2Zr2O5) were obtained by evaporation. The structure contains dimeric anions [Zr2(C2O4)5(H2O)4]2– connected through hydrogen bonds to hydroxonium ions (H11O5)+ to create a 3D supramolecular framework. The addition of ammonium or alkali nitrate led to the formation of single crystals of Na2[Zr(C2O4)3]·2H2O (Na2ZrOx3), M(H7O3)[Zr(C2O4)3]·H2O, M = K (KHZrOx3), M = NH4 (NH4HZrOx3), M(H5O2)0.5(H9O4)0.5[Zr(C2O4)3], M = Rb (RbHZrOx3), and M = Cs (CsHZrOx3). For the five compounds, the structure contains ribbons 1∞[{ZrOx3}2–] formed by entities Zr(C2O4)4 sharing two oxalates. In (Na2ZrOx3), the shared oxalates are in cis positions and the chain 1∞[Zr–Ox] is stepped with a Zr–Zr–Zr angle of 98.27(1)°. In the other compounds, the shared oxalates are in trans positions and the chains 1∞[Zr–Ox] are corrugated with Zr–Zr–Zr angles in the range 140.34(1)–141.07(1)°. In the compounds (MHZrOx3), the cohesion between the ribbons is ensured by the alkaline or ammonium cations and the hydroxonium ions (H7O3)+ for M = K, NH4, (H5O2)+, and (H9O4)+ for M = Rb and Cs. During the thermal decomposition of the alkaline-free zirconium oxalates (ZrOx), (H2Zr2Ox5), and (NH4HZrOx3), the formed amorphous zirconia is accompanied by carbon; the oxidation of carbon at about 540 °C to carbon dioxide is concomitant with the crystallization of the stabilized tetragonal zirconia.
Introduction
Zirconium-based oxalates play an important role in various domains. In chemistry, for example, the calcination of zirconium oxalate gels or molecular oxalate precursors was used for the preparation of nanosized powders,1 minispheres,2,3 or porous4 stabilized zirconium dioxide; a precursor of tetragonal yttria-stabilized zirconia (YSZ) was prepared by the oxalate method in ethanol solution;5,6 the calcination of barium zirconium oxalate produced pure BaZrO3 used as an inert crucible material for melt processing of YBCO superconductors7 or as nanopowders to induce artificial pinning centers in YBCO;8 the calcination of lead zirconium oxalate produced antiferroelectric lead zirconate PbZrO3.9 In catalysis, the calcination of a Cu/Zr coprecipitate by a NaOH–Na2C2O4 mixture solution produced a highly active and stable Cu/ZrO2 catalyst for the partial oxidation of methanol to hydrogen for proton-exchange membrane fuel cell application.10 In biochemistry, zirconium oxalate increases the thermal and enzymatic stability of type I collagen.11,12
These potential applications have attracted many solid-state chemists to study the synthesis and the structure of many double or triple zirconium oxalates with monovalent and divalent cations. Almost all well-characterized double zirconium oxalates contain the tetrahedral anionic Zr(C2O4)44– unit, the charge being compensated by divalent cations in M2Zr(C2O4)4·nH2O compounds with M = Ba, Sr,13 Ca,13,14 Pb,15 Cd,16 and (H2en)CdZr(C2O4)4·nH2O (en = ethylene diamine)17 or by one divalent and two monovalent cations in M′2MZr(C2O4)4·4.4nH2O compounds with M = Cd, M′ = Na,18 K,19 NH4,17 and M = Mn, M′ = K20 or by four monovalent cations in M′4Zr(C2O4)4·nH2O compounds obtained with M′ = Na21,22 and K.23,24 Hybrid supramolecular architectures have been created by the assemblage of the Zr(C2O4)44– units through organic dications with N–H donor functions25,26 or through H-bonds involving ditopic benzimidazolium or bisimidazoliumbenzene monocations.27,28
Astonishingly, little is known about single zirconium oxalates. The first accessible report of the study on zirconium oxalate is from Venable and Baskerville in 1896.29 The authors did not succeed in the preparation of the neutral oxalate Zr(C2O4)2 but obtained by precipitation the basic oxalate noted Zr(C2O4)2·Zr(OH)4 and by crystallization the acid oxalate, Zr(C2O4)2·H2C2O4·8H2O. They also prepared double acid oxalates with sodium, Zr(C2O4)2·3Na2C2O4·H2C2O4·5H2O, and potassium, [Zr(C2O4)2]2·(K2C2O4)2·H2C2O4·8H20. The precipitation of the basic oxalate (Zr(C2O4)(OH)2) under acidic conditions was confirmed by Kobayashi et al. and the amorphous hydroxide Zr(OH)4 precipitates under neutral conditions.30,31 In 1931, Gable reported the preparation of anhydrous oxalate Zr(C2O4)2 by precipitation from a methanol solution.32 In fact, the obtained compound was later identified as Zr(C2O4)2·2CH3OH and the tetrahydrate Zr(C2O4)2·4H2O was also prepared from methanol solution but without precautions to exclude humidity.33 However, except the structure of a compound formulated Zr(OH)2(C2O4)·H2O,34 no other structure determination of simple zirconium oxalate has been reported.
In this paper, we report the structures of the basic oxalate Zr(OH)2(C2O4) determined from powder X-ray diffraction pattern and of the acidic oxalate (H11O5)2[Zr2(C2O4)5(H2O)4] determined from single-crystal X-ray data. The introduction of a monovalent cation (Na+, K+, NH4+, Rb+, Cs+) in the reagent solution gives a series of zirconium oxalates with three different structures determined from single-crystal X-ray data and built on zirconium-oxalate chains.
In general, the decomposition of oxalates, whether they are of transition metals, lanthanides, or even actinides are the subject of numerous studies motivated by their role as precursors of oxides obtained at relatively low temperature giving them good sintering properties. Owing to the large panel of applications of stabilized zirconia (electrolytes and electrodes for solid oxide fuel cell,35 catalysts,36 structural ceramics, and bioceramics,.37..), the thermal decompositions of the alkali-free oxalates Zr(OH)2(C2O4), (H11O5)2[Zr2(C2O4)5(H2O)4], and NH4(H7O3)[Zr(C2O4)3]·H2O which can lead to tetragonal (or cubic) stabilized zirconia have been studied and are reported herein.
Results and Discussion
Structure of the Dihydroxooxalatozirconate Zr(OH)2(C2O4) (ZrOx)
Crystal data and structure refinement parameters are reported in Table 1 for the basic oxalate Zr(OH)2(C2O4) (ZrOx) and the acidic one (H11O5)2[Zr2(C2O4)5(H2O)4] (H2Zr2Ox5).
Table 1. Crystal Data and Structure Refinement Parameters for Zr(OH)2(C2O4) (ZrOx) and (H11O5)2[Zr2(C2O4)5(H2O)4] (H2Zr2Ox5).
| ZrOx Zr(OH)2(C2O4) | H2Zr2Ox5 (H11O5)2[Zr2(C2O4)5(H2O)4] | |
|---|---|---|
| CCDC number | 2011396 | 2011397 |
| empirical formula | C2O6H2Zr1 | C10O34H30Zr2 |
| formula weight | 213.3 | 876.78 |
| temperature/K | 293 | 296 |
| crystal system | Monoclinic | triclinic |
| space group | C2/c | P1̅ |
| a/Å | 12.813(4) | 6.9269(3) |
| b/Å | 5.8882(14) | 10.4427(6) |
| c/Å | 6.7088(13) | 10.6447(6) |
| α/deg | 90 | 78.421(2) |
| β/deg | 118.25(2) | 86.155(2) |
| γ/deg | 90 | 89.945(2) |
| volume/Å3 | 445.9(2) | 752.56(7) |
| Z | 4 | 1 |
| ρcalc g/cm3 | 3.177 | 1.935 |
| μ/mm–1 | 20.01 | 0.821 |
| F(000) | 408 | 442 |
| radiation | Cu Kα | Mo Kα |
| 2Θ range for data collection/deg | 5–110 (stepsize 0.02) | 3.90–51.88 |
| index ranges | –8 ≤ h ≤ 8 | |
| –12 ≤ k ≤ 12 | ||
| –13 ≤ l ≤ 13 | ||
| reflections collected | 19,393 | |
| independent reflections | 2914 [Rint = 0.0389, Rsigma = 0.0581] | |
| data/restraints/parameters | 5614/39/9 | 2914/15/253 |
| goodness-of-fit on F2 | 4.45 | 1.045 |
| final R indexes [I ≥ 2σ (I)] | R1 = 0.0417 wR2 = 0.0600 | R1 = 0.0282 wR2 = 0.0529 |
| final R indexes [all data] | R1 = 0.0417 wR2 = 0.0600 | R1 = 0.0378 wR2 = 0.0566 |
| largest diff. peak/hole/e·Å–3 | 1.01/–1.82 | 0.55/–0.40 |
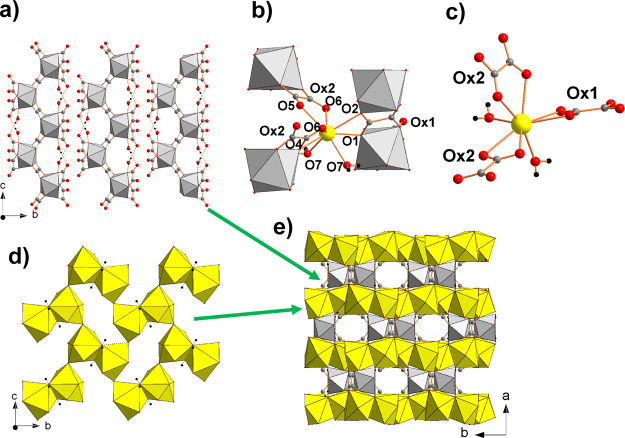
The structure of Zr(OH)2(C2O4) contains one symmetrically independent cation Zr4+ located on a twofold axis parallel to b, one centrosymmetric oxalate ion and one symmetrically independent hydroxide group. The zirconium atom is coordinated by two bidentate oxalate ions and four hydroxide ions forming a {Zr(C2O4)2(OH)4} entity (Figure 1a) with a distorted trigonal pyramidal overall environment (TPY) considering the hydroxide oxygen atoms and the middle of the C–C bond (approximately the center of gravity of the ligands) as the apices of the polyhedron (Figure 1b) and a square antiprism ZrO8 polyhedron (Figure 1c) according to the criteria developed by Haigh38 to distinguish the eight-coordinated structures. The {Zr(C2O4)2(OH)4}4– entities share the two oxalates acting as bis-bidentate (μ2-oxalates) and the OH groups to build a three-dimensional (3D) framework (Figure 1d) that can be deconstructed in two intersecting ribbons. It is first instructional to consider the connection of the {Zr(C2O4)2(OH)4}4– entities by OH–OH bridges to form a monodimensional ribbon 1∞[{Zr(C2O4)2(OH)2}2–] that extends infinitely down the [0 0 1] direction (Figure 1e), these ribbons being further connected by sharing μ2-oxalates. The chains 1∞[Zr(OH)2]2+ present in the ribbons have been observed in various zirconium di-hydroxide salts such as nitrates,39−41 sulfates,42,43 and chromates44−46 and other M4+ hydroxide salts with M = Hf, Th, U such as sulfates.47−50 The second ribbon 1∞[{Zr(C2O4)}2+] resulting from the oxalates’ sharing between {Zr(C2O4)2(OH)4}4– entities (Figure 1f) has not been reported up today. The Zr–OH distances within the ZrO8 polyhedron (2.16(4) and 2.13(4) Å), the OH–OH bridge distance (2.48(5) Å), the Zr–Zr distance (3.511(5) Å), and the Zr–Zr–Zr (145.7(5)°) angle are comparable to the distances observed in other zirconium di-hydroxide salts with eight-coordinated zirconium (Table S1). The other Zr–O distances within the ZrO8 polyhedron involving oxygen from oxalate ligands are longer (2.33(5) and 2.39(4) Å). These distances are in agreement with those calculated in the hydroxide oxalate anion [(ZrOH(C2O4)3)2]6– built from two crystallographic independent [ZrOH(C2O4)3] entities connected by two bridging OH groups observed in the complex Na6[ZrOH(C2O4)3]2·7H2O.51 The average Zr–O value (2.25 Å) is in agreement with the sum of the ionic radii (rZr4+ + rOH– = 2.16 Å).52 The bond-valence sum for Zr calculated using Brese and O’Keeffe data53 with b = 0.37 Å is 3.48 vu.
Figure 1.
In (ZrOx), the zirconium atom is coordinated by four OH and two bidentate oxalates (a) with a trigonal pyramidal overall environment (M is the middle of the C–C bond) (b) giving a square antiprism ZrO8 polyhedron (c) and a (3D) framework (d) that can be deconstructed in two intersecting ribbons 1∞[{Zr(OH)2}2+] (e) and 1∞[{Zr(C2O4)}2+] (f).
The structure of a compound Zr(OH)2(C2O4)·0.5H2O determined from single-crystal X-ray data has been reported;34 the refined structure perfectly matches that of SrC2O4·2H2O54
Structure of (H11O5)2[Zr2(C2O4)5(H2O)4] (H2Zr2Ox5)
The structure of (H11O5)2[Zr2(C2O4)5(H2O)4] (H2Zr2Ox5) contains one site for zirconium and three independent oxalate ions. Two of them, denoted Ox2 and Ox3, are noncentrosymmetric and one, Ox1, is centrosymmetric. The zirconium atom is coordinated by three bidentate oxalate ions and two water molecules forming a [Zr(C2O4)3(H2O)2] entity (Figure 2a) with a quasiregular trigonal bipyramidal overall environment (TBPY) (Figure 2b) and a dicapped 4,4′-trigonal prism ZrO8 (Figure 2c). The Zr–O distances are on the range 2.147(2)–2.294(2) Å (average 2.196 Å). The bond-valence sum for Zr is 3.90 vu. Such an entity has been observed as an isolated ion [Zr(C2O4)3(H2O)2]2– in the series MZr(C2O4)3(H2O)2·nH2O (M = Pb, Cd, Hg).55 In (H2Zr2Ox5) two [Zr(C2O4)3(H2O)2] entities share the oxalate Ox1 acting as bis-bidentate (μ2 oxalate) to build a centrosymmetric di-metallic ion [Zr2(C2O4)5(H2O)4]2– (Figure 2d). Between these ions, the supplementary oxygen atoms O13 to O17 are linked by hydrogen bonds to form a finite chain with general formula (H11O5)+ (Figure 2e) that can be considered as a supramolecular ionic aggregate as discussed by Bernal and Watkins.56 Within this chain, the distance between O13 and O14 atoms is short (2.435(4) Å) and the H proton is shared between the two water molecules and located close to midway between O13 and O14 joining, creating a Zundel ion (H5O2)+. The (H11O5)+ entity can be written as H2O···(H5O2)+···(H2O)···(H2O) and constitutes a third isomer of the hydroxonium ion (H11O5)+.56 The chains are linked at their extremities to two zirconium through coordinated O1 and O2 atoms and to five other [Zr2(C2O4)5(H2O)4]2– ions through hydrogen bonds involving oxygen oxalate ions O5, O7, O9, O10, O11, and O12 (Figure 3e) to create a 3D supramolecular framework (Figure 2f). The characteristics of the hydrogen bonds in the hydroxonium ion (H11O5)+ and between (H11O5)+ and di-metallic ion [Zr2(C2O4)5(H2O)4]2– are reported in Table S2.
Figure 2.
Zirconium atom in (H11O5)2[Zr2(C2O4)5(H2O)4] (H2Zr2Ox5) is coordinated by three bidentate oxalate ions and two water molecules (a) giving a trigonal bipyramid as overall environment (Mi is the middle of the C–C bond of Oxi, i = 1–3) (b) and a distorted 4-4-di-capped trigonal prism ZrO8 (c). Two [Zr(C2O4)3(H2O)3] are linked by Ox1 oxalate to form a dimeric unit [Zr2(C2O4)5(H2O)4] (d). The dimers are hydrogen-bonded to (H11O5)+ ions through the coordinated water molecules and oxalate oxygen atoms (the bonds within the (H11O5)+ ion are in green) (e) to build a 3D framework (f).
Figure 3.
[Zr(C2O4)4] structural building unit, the overall polyhedron around Zr, the bicapped 4-4′ trigonal environment of Zr, and the zirconium-oxalate ribbons in (a) (Na2ZrOx3), (b) (KHZrOx3) and (NH4HZrOx3), and (c) (RbHZrOx3) and (CsHZrOx3). (× green) = inversion center. Mi is the middle of the C–C bond of Oxi (i = 1–4).
Zirconium Ammonium or Alkali Metal Double Oxalates Na2[Zr(C2O4)3]·2H2O (Na2ZrOx3), M(H7O3)[Zr(C2O4)3]·H2O (M = K, KHZrOx3; M = NH4, NH4HZrOx3), M(H5O2)0.5(H9O4)0.5 [Zr(C2O4)3] (M = Rb, RbHZrOx3: M = Cs, CsHZrOx3)
1∞[Zr–Ox] Chains
The structure of (Na2ZrOx3) was determined in the space group C2/c; (KHZrOx3) and (NH4HZrOx3) are isostructural and the structures determined in the space group P21/c, (RbHZrOx3) and (CsHZrOx3) are isostructural and the structures determined in the space group P1̅. Crystal data and structure refinement parameters are reported in Table 2. In the five compounds, the zirconium atom is coordinated by eight oxygen atoms forming a bicapped 4-4′ trigonal prism and belonging to four bidentate oxalate ions that create a [Zr(C2O4)4] entity (Figure 3) with an overall geometry between square and tetrahedron. Its description as a pseudosquare rather than a distorted tetrahedron is more appropriate (the sides of the pseudosquare are between 4.249(1) and 4.620(8) Å, the diagonals between 5.518(4) and 5.587(1) Å, four M–Zr–M angles are between 92.52(1) and 104.1(1)°, and two others between 139.54(1) and 143.98(1)° (Table S3). The use of a deformed square geometry as the global environment of Zr also facilitates the description of the geometries of the ribbons formed by connecting the entities [Zr(C2O4)4]. For the five compounds, the Zr–O distances vary in a narrow range (2.112(2)–2.317(1) Å) (Table S4) with an average of 2.207 Å. The bond valence sums are between 3.78 and 3.85 vu.
Table 2. Crystal Data and Structure Refinement Parameters for Na2[Zr(C2O4)3]·2H2O (Na2ZrOx3), K(H7O3)[Zr(C2O4)3]·H2O (KHZrOx3), (NH4) (H7O3)[Zr(C2O4)3]·H2O ((NH4HZrOx3)), Rb(H5O2)0.5(H9O4)0.5[Zr(C2O4)3] (RbHZrOx3), and Cs(H5O2)0.5(H9O4)0.5[Zr(C2O4)3] (CsHZrOx3).
| NaZrOx3 Na2[Zr(C2O4)3]·2H2O | KHZrOx3 K(H7O3)[Zr(C2O4)3]·H2O | NH4HZrOx3 (NH4)(H7O3)[Zr(C2O4)3]·H2O | RbHZrOx3 Rb(H5O2)0.5(H9O4)0.5[Zr(C2O4)3] | CsHZrOx3 Cs(H5O2)0.5(H9O4)0.5[Zr(C2O4)3] | |
|---|---|---|---|---|---|
| CCDC number | 2011398 | 2011399 | 2011400 | 2011401 | 2011402 |
| empirical formula | Na2ZrC6O14H4 | KZrC6O16H9 | ZrC6O16NH13 | RbZrC6O15H7 | CsZrC6O15H7 |
| formula weight | 437.29 | 467.45 | 446.39 | 495.81 | 540.73 |
| temperature/K | 296 | 296 | 296 | 296 | 296 |
| crystal system | monoclinic | monoclinic | monoclinic | triclinic | triclinic |
| space group | C2/c | P21/c | P21/c | P1̅ | P1̅ |
| a/Å | 13.8646(9) | 9.2166(9) | 9.3411(7) | 7.7027(5) | 7.7318(4) |
| b/Å | 10.4241(6) | 11.169(1) | 11.1701(8) | 8.9788(6) | 9.1847(5) |
| c/Å | 8.9290(5) | 14.094(2) | 14.2537(9) | 10.6518(7) | 10.6352(5) |
| α/deg | 90 | 90 | 90 | 79.179(3) | 78.861(2) |
| β/deg | 95.743(3) | 107.718(5) | 107.699(2) | 72.476(4) | 72.333(2) |
| γ/deg | 90 | 90 | 90 | 76.975(3) | 77.713(2) |
| volume/Å3 | 1284.00(13) | 1382.0(2) | 1416.85(17) | 678.70(8) | 696.41(6) |
| Z | 4 | 4 | 4 | 2 | 2 |
| ρcalc g/cm3 | 2.262 | 2.247 | 2.093 | 2.426 | 2.579 |
| μ/mm–1 | 1.006 | 1.191 | 0.871 | 4.459 | 3.449 |
| F(000) | 856 | 928 | 896 | 480 | 511 |
| radiation | Mo Kα (λ = 0.71073) | ||||
| 2Θ range for data collection/deg | 4.90–59.36 | 4.64–53.06 | 4.72–67.34 | 4.69–46.00 | 5.60–68.52 |
| index ranges | –17 ≤ h ≤ 18 | –11 ≤ h ≤ 11 | –14 ≤ h ≤ 14 | –8 ≤ h ≤ 8 | –12 ≤ h ≤ 12 |
| –14 ≤ k ≤ 14 | –13 ≤ k ≤ 14 | –17 ≤ k ≤ 17 | –9 ≤ k ≤ 9 | –14 ≤ k ≤ 14 | |
| –11 ≤ l ≤ 12 | –16 ≤ l ≤ 17 | –21 ≤ l ≤ 22 | –11 ≤ l ≤ 11 | –16 ≤ l ≤ 16 | |
| reflections collected | 11,377 | 21,953 | 58,123 | 14,524 | 37,161 |
| independent reflections | 1722 [Rint = 0.0385, Rsigma = 0.0440] | 2868 [Rint = 0.0389, Rsigma = 0057] | 5638 [Rint = 0.0219, Rsigma = 0.0446] | 1886 [Rint = 0.0411, Rsigma = 0.0655] | 5777 [Rint = 0.0322, Rsigma = 0.0463] |
| data/restraints/parameters | 1722/2/111 | 2868/9/244 | 5638/13/256 | 1886/2/214 | 5777/2/214 |
| goodness-of-fit on F2 | 1.068 | 1.060 | 1.043 | 1.026 | 0.973 |
| final R indexes [I ≥ 2σ (I)] | R1 = 0.0286 | R1 = 0.0291 | R1 = 0.0248 | R1 = 0.0367 | R1 = 0.0377 |
| wR2 = 0.0534 | wR2 = 0.0593 | wR2 = 0.0583 | wR2 = 0.0900 | wR2 = 0.0900 | |
| final R indexes [all data] | R1 = 0.0368 | R1 = 0.0422 | R1 = 0.0340 | R1 = 0.0524 | R1 = 0.0486 |
| wR2 = 0.0572 | wR2 = 0.0640 | wR2 = 0.0624 | wR2 = 0.0986 | wR2 = 0.1020 | |
| largest diff. peak/hole/e Å–3 | 0.77/–0.38 | 0.42/–0.50 | 0.70/–0.43 | 0.90/–0.99 | 2.34/–3.59 |
In (Na2ZrOx3), the entities [Zr(C2O4)4] are connected by two centrosymmetric Ox1 oxalate ions acting as bis-bidentate and directed in a cis configuration toward the extremities of the side M1–M1 of the pseudosquare, giving a stepped chain 1∞[Zr–Ox1] with an Ox1–Zr–Ox1 = Zr–Zr–Zr angle of 98.27(1)°. The Zr–Zr distance is 5.903(1) Å. The water molecules strengthen the intraribbon cohesion by the formation of hydrogen bonds with oxygen atoms of the oxalates Ox2 (Figure S1) giving a ribbon 1∞[{ZrOx3(H2O)2}2–]. A similar ribbon 1∞[{ZrOx3}2–] has been observed in K2[Zr(C2O4)3]·H2C2O4·H2O57 with Zr–Zr = 5,975(1) Å and Zr–Zr–Zr = 96.85(1)°.
In (MHZrOx3), M = K and NH4; the entities [Zr(C2O4)4] are connected by two noncentrosymmetric Ox1 oxalate ions related by the 21 axis and directed in a trans configuration toward a diagonal of the pseudosquare. The bis-bidentate character of the two Ox1 oxalate ions leads to the formation a zig-zag chain 1∞[Zr–Ox1] with a Ox1–Zr–Ox1 = Zr–Zr–Zr angle of 141.07(1) and 140.98(1)° for M = K and NH4, respectively. The Zr–Zr distance is 5.923(1) and 5.926(1) Å for M = K and NH4, respectively.
In (MHZrOx3), M = Rb and Cs; the entities [Zr(C2O4)4] are connected by two centrosymmetric crystallographic independent oxalate ions Ox2 and Ox3 directed in a trans configuration toward the diagonal of the pseudosquare. The oxalate ions Ox2 and Ox3 are both bis-bidentate. Overall, it gives a zig-zag chain 1∞[Zr–Ox2–Zr–Ox3] with a Ox2–Zr–Ox3 = Zr–Zr–Zr angle of 140.83(2) and 140.34(1)° for M = Rb and Cs, respectively. The Zr–Zr distances are 5.885(15) and 5.905 (15) Å for Zr connected by Ox2 and Ox3, respectively, for M = Rb and 5.888(8) and 5.900(8) Å for M = Cs.
The ribbons are very similar for the four (MHZrOx3) compounds, the main difference being the symmetry of the chains with the presence of a 21 axis along the chain for M = K and NH4 and inversion centers at the middle of the C–C bonds of connecting oxalates for M = Rb and Cs (Figure 3).
Several actinide oxalates with formulae M4[An(C2O4)4]·nH2O (An = Th, U, Np, Pu) are built of metal-oxalate chains 1∞[An–Ox] very similar to that in (MHZrOx3), M = Rb and Cs. However, because of the largest ionic radius for AnIV compared to ZrIV, AnIV ions tend to adopt a 9 or 10 coordination. In M4[An(C2O4)4]·nH2O, the actinides atoms are coordinated by five bidentate oxalates and the ribbons are formulated 1∞[An(C2O4)4]4–. According to the geometry of the overall environment of the metal atom (square pyramid or trigonal bipyramid), the chains are corrugated with An–An–An angles close to 160° or quasilinear with a An–An–An angle of 178.215(2)°) in (NH4)4Th(C2O4)4·4H2O.58
Interchain Cohesion
Na2[Zr(C2O4)3]·2H2O (Na2ZrOx3)
The 1∞[ZrOx3(H2O)2] ribbons are arranged in layers parallel to the plane (1 0 0) (Figure 4a). The ribbons are held together by the charge-balancing Na+ ions (Figure 4b), which are surrounded by eight oxygen atoms at distances between 2.413 and 2.709 Å, six belonging to three oxalate ligands (one Ox1 and two Ox2) and two belonging to water molecules (Figure 4c). Two NaO8 polyhedra are connected by a H2O–H2O bridge to form a dimer. These dimers are connected by apex to give a layer parallel to the plane (1 0 0) (Figure 4d). The structure can therefore be described as a succession of Zr layers and Na layers in the direction [1 0 0] (Figure 4e).
Figure 4.
Arrangement of 1∞[ZrOx3(H2O)2] ribbons in layers parallel to the plane (1 0 0) (a) and their connection by Na+ ions (b) that are coordinated by three oxalate ions and two water molecules (c) to form layers (d). Zirconium and sodium layers are pillared in the [1 0 0] direction (e).
M(H7O3)[Zr(C2O4)3]·H2O (M = K, (KHZrOx3); M = NH4, (NH4HZrOx3)
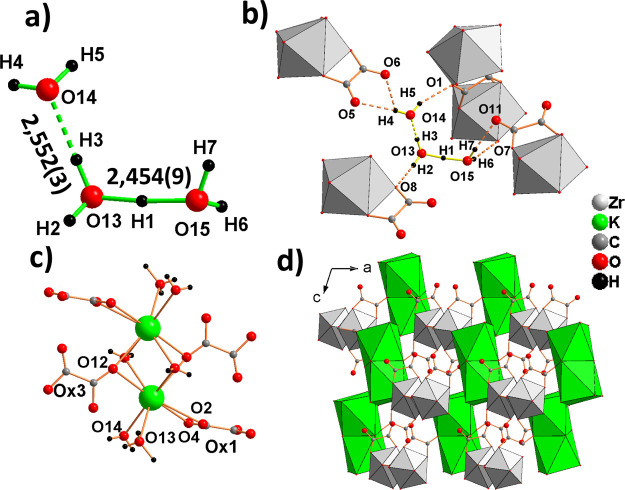
The negative charge of the zirconium-oxalate ribbon 1∞[{ZrOx3}2–] is balanced by the hydroxonium ion (H7O3)+ and the M+ ion (M+ = K+ and NH4+). The (H7O3)+ ion presents the geometry of the isomer 2, (H5O2)+(H2O) (Figure 5a), described by Bernal and Watkins.56 As in the hydroxonium ion (H11O5)+ present in (H2Zr2Ox5), the O–O distance in the (H5O2)+ ion is short (2.454(9) for M = K and 2.458(4) Å for M = NH4) and the H atom approximately at the midway between O and O joining. The ion (H7O3)+ is hydrogen-bonded to oxygen atoms of oxalate ligands of four ribbons (Figure 5b). The M atom is coordinated by eight oxygen atoms forming a distorted square antiprism MO8, four oxygen atoms are oxygen oxalates, two water oxygens, and two pertain to two different (H7O3)+ ions. Two MO8 polyhedra share a square base to form a dimer M2O12 (Figure 5c). K+ and NH4+ occupy the same position and have similar environments; the increase of the M–O distances with average values 2.884 and 2.975 Å for M = K+ and NH4+, respectively (Table S5), are in agreement with the increase of the ionic radius (rK+ = 1.5152 and rNH4+ = 1.66 Å59 for C.N. = 8). The bond valence sum calculated with the value of the bond valence parameters is given by Brese and O’Keeffe53 for K+ (r0 = 2.13) and by Garcia-Rodriguez et al.60 for NH4+ (r0 = 2.219) and the universal value of B = 0.37 is 1.06 vu for the two cations. These results are in accordance with an ammonium cation behaving like an alkaline cation; however, N–H···O bondings are present (Figure S2), the three shorter N–O bonds are established with nonoxalate oxygens (two water oxygens an one oxygen of a (H7O3)+ ion) and are hydrogen bonds involving H10, H13, and H11. The fourth hydrogen atom H12 of the ammonium group is on the line joining the N atom and the midpoint between the two acceptor atoms O4 and O12 and established a bifurcated hydrogen bond as commonly observed for a five-coordinated ammonium ion.59 The water molecules that belong to the MO8 entities also participate in the cohesion of the 3D arrangement of 1∞[{ZrOx3}2–] ribbons and M2O12 dimers (Figure 5d) owing to the establishment of hydrogen bonds with oxygen oxalates. The characteristics of the hydrogen bonds in the hydroxonium ions (H7O3)+, between (H7O3)+ or H2O and 1∞[{ZrOx3}2–] ribbons, are reported in Table S6.
Figure 5.
Hydroxonium ion (H7O3)+ (a) is hydrogen-bonded to four 1∞[{ZrOx3}2–] ribbons (b). Dimers M2O12 (c), ribbons 1∞[{ZrOx3}2–], and hydroxonium ions (H7O3)+ (not represented for clarity) form a supramolecular 3D arrangement (d).
M(H5O2)0.5(H9O4)0.5[Zr(C2O4)3] (M = Rb, (RbHZrOx3); M = Cs, (Cs4HZrOx3)
The charge neutrality is provided by M+ cations and hydroxonium ions (H5O2)+ and (H9O4)+. Two O13 atoms linked by an inversion center are distant from 2.445(5) Å and the common hydrogen located on the Fourier synthesis at the inversion center constitutes a symmetrical Zundel ion (H5O2)+ (Figure 6a). Two O15 atoms linked by an inversion center are distant from 2.445(5) Å and are hydrogen-bonded to water oxygen, creating a symmetric hydroxonium ion (H9O4)+ (Figure 6b). The hydroxonium ions (H5O2)+ and (H9O4)+ connect the ribbons 1∞[{ZrOx3}2–] via hydrogen bonds with oxalates Ox1 for (H5O2)+ (Figure 6c) and oxalates Ox1 and Ox4 for (H9O4)+ (Figure 6d). The M atom is coordinated by eight oxygen atoms, six oxygen atoms belonging to six different oxalate ions, one from the Zundel ion (H5O2)+, and one from the (H5O2)+ part of the (H9O4)+ ion (Figure 6e). Two MO8 polyhedra share an edge O11–O11 to form a dimer M2O14. Dimers participate in the cohesion of the structure (Figure 6f).
Figure 6.
In (MHZrOx3), M = Rb and Cs, the (H5O2)+ (a) and (H9O4)+ (b) ions connect the ribbons 1∞[{ZrOx3}2–] via hydrogen bonds involving Ox1 oxygens (c) and Ox1 and Ox4 oxygens (d), respectively. The MO8 polyhedra share an edge to form dimers M2O14 (e) that connect the ribbons 1∞[{ZrOx3}2–] (f).
Thermal Decomposition of the Alkaline-Free Zirconium Oxalates (ZrOx), (H2Zr2Ox5), and (NH4HZrOx3)
The coupled TG–DTA–MS and high-temperature X-ray diffraction (HTXRD) analyses for (ZrOx), (H2Zr2Ox5), and (NH4HZrOx3) are reported in Figures S3–S5, respectively.
The HTXRD of (ZrOx) shows that Zr(OH)2(C2O4) is stable up to 250 °C, above which the compound is amorphous. On the DTA, the endothermic peak at about 320 °C with evolving of H2O and the exothermic peak at about 340 °C with evolving of CO2 correspond to the nearly concomitant decomposition of hydroxide and oxalate ions, giving amorphous ZrO2.
For (H2Zr2Ox5), the four first endothermic peaks observed on the DTA between 80 and 170 °C and accompanied by departures of H2O are due to the decomposition of the hydroxonium ion (H11O5)+ and the dehydration of the compound with variation of the X-ray pattern and further destruction of the structure. The endothermic peak and the departure of H2O and CO2 at about 200 °C correspond to the decomposition of oxalate ions and the formation of an instable intermediate that is possibly a carbonate Zr(CO3)2 (observed mass loss of 53.6%, calculated mass loss of 51.8%). The exothermic peak at about 330 °C with evolving of CO2 corresponds to the decomposition of the carbonate, giving amorphous ZrO2.
For (NH4HZrOx3), the endothermic peaks on the DTA at about 80, 100, and 120 °C correspond to the decomposition of hydroxonium and ammonium ions and that at 200 °C corresponds to the decomposition of one oxalate ion to give an intermediate that could be an oxalato-carbonate of formulae H2Zr(C2O4)1.5(CO3)1.5 (theoretical and experimental weight loss of 32.5 and 31.0%, respectively). The two exothermic peaks at about 300 °C correspond to the decomposition of oxalate and carbonate ions to give amorphous ZrO2.
For the three compounds, the black color of the powder observed during the plateau on the TG between about 350 and 540 °C is due to the presence of carbon produced during the disproportionation of carbon monoxide. The presence of carbon has already been observed during thermal decomposition in air of some zirconium oxalates.13,61 At about 560 °C, carbon dioxide is released because of the oxidation of carbon and ZrO2 crystallizes. The HTXRDs of the three compounds reveal that tetragonal-ZrO2 (t-ZrO2) is first obtained at low temperature, about 550, 425, and 400 °C, respectively. Monoclinic-ZrO2 (m-ZrO2) appears only above 725 °C. As it is well known, zirconia is monoclinic at ambient temperature and transforms into t-ZrO2 at 1205 °C and to cubic fluorite structure (c-ZrO2) at 2377 °C.62 Tetragonal and cubic forms can be stabilized at ambient temperature by creation of oxygen vacancies, essentially by substitution of Zr4+ by trivalent or divalent cations (Y3+, Ca2+...). Tetragonal pure zirconia has been obtained at ambient temperature by precipitation and calcination, the stabilization of the high temperature tetragonal phase being attributed to a crystallite size effect.63 During the preparation by the oxalate method in ethanol solution of a precursor of YSZ powder, Gongyi et al.5 obtained, among many amorphous phases, a crystallized compound (sample no. 25) with X-ray pattern and TG/DTA behavior close to that of Zr(OH)2(C2O4). Note that a black nonstoichiometric stabilized tetragonal zirconia was obtained by heating amorphous zirconia in an oxygen-free atmosphere.64 Many mechanisms of tetragonal phase stabilization in pure ZrO2 have been reported.65,66 During the decomposition under air of the three zirconium oxalates (ZrOx3), (H2Zr2Ox5), and (NH4HZrOx3), the crystallization of the t-ZrO2 occurs simultaneously with the oxidation of residual carbon and the stabilization of t-ZrO2 could be due to the energy generated by this combustion.
Conclusions
In all the zirconium oxalates structurally characterized, the coordination number of Zr4+ is 8 and the more common environment is formed by four bidentate oxalates giving isolated ions Zr(C2O4)44–. In many metal complexes, the oxalate ion acts as bis-bidentate, creating di-metallic ions or chains. A di-metallic ion [Zr2(C2O4)7]6– has been described in K6[{Zr(C2O4)3}2(μ-C2O4)]·4H2O;67 in (H2Zr2Ox5), one oxalate ion is replaced by two water molecules in the coordination of Zr and a di-metallic ion [Zr2(C2O4)5(H2O)4]2– is obtained. In compounds (Na2ZrOx3) and (MHZr(Ox)3), M = K, NH4, Rb, and Cs, chains 1∞[{ZrOx}] are formed with an isomer cis and stepped chains in (Na2ZrOx3) and an isomer trans and zig-zag chains in (MHZr(Ox)3). Similar zig-zag chains 1∞[{ZrOx}] are formed in (ZrOx) but the two nonshared oxalates are replaced by two pairs of hydroxide ions, bridging the chains to form a 3D framework. The acidic compounds contain the Zundel ion (H5O2)+ hydrogen-bonded to water oxygens to form hydroxonium ions (H7O3)+ in (MHZrOx3), M = K, NH4, and (H9O4)+ in (MHZrOx3) and M = Rb, Cs, and (H11O5)+ in (H2Zr2Ox5). The formation of various hydroxonium ions does not seem to result from the conditions of synthesis; the pH and the composition of the solutions being very similar, the nature and the size of the countercation seem to be the predominant factors; they indeed orient the geometry of the zirconium oxalate anion and the nature and geometry of the hydroxonium ion formed adapt to it to create a 3D supramolecular structure. By varying the synthesis conditions, other structural arrangements and hydroxonium ions are expected.
Owing to the industrial importance of stabilized pure zirconia, the formation of stabilized tetragonal zirconia during thermal decomposition of the alkaline-free zirconium oxalates is of interest. The study of the thermal decomposition of the numerous double zirconium oxalates characterized up to day is an interesting issue to investigate the role of the oxidation of residual carbon on the final oxides.
The potassium zirconium oxalate K4[Zr(C2O4)4]·5H2O has been recently used as a precursor for the preparation of perovskite, pyrochlore, and Nasicon-type materials.24 The alkali zirconium oxalates described here are possible candidates for this application.
Experimental Section
Synthesis
Zirconium oxynitrate (ZrO(NO3)2) was purchased from Aldrich, Alfa Aesar, and Acros Organics. The X-ray diffraction patterns of the three products (Figure S6) correspond in fact to hydrated basic zirconium nitrates Zr(OH)2(NO3)2·xH2O whose structures differ according to x.39−41 The product from Acros Organics, with the formula Zr(OH)2(NO3)·4.7H2O, was chosen for synthesis.
Zr(OH)2(C2O4) (ZrOx)
Dihydroxide zirconium oxalate was obtained by slow evaporation of a basic zirconium nitrate and oxalic acid solution. Zr(OH)2(NO3)2·4.7H2O (1 g, 3 mmol) was dissolved under stirring in 200 mL of nitric acid 0.05 M at 50 °C. An oxalic acid solution 0.5 M was added (12.5 mL), giving a pH of about 1.3. The evaporation of half the solution at 50 °C produced a microcrystalline powder (Figure 7) of the basic oxalate.
Figure 7.
Morphology of the dihydroxide zirconium oxalate (ZrOx) observed with a HITACHI S400 scanning electron microscope.
(H11O5)2[Zr2(C2O4)5(H2O)4] (H2Zr2Ox5)
A solution obtained by dissolution of 0.25 mmol (0.0821 g) of Zr(OH)2(NO3)2·4.7H2O in 4 mL of water was added with 9 mL of ethanol and further with 1.25 mL (0.625 mmol) of a solution of oxalic acid 0.5 M; the pH of the obtained solution is about 1.5. After evaporation at room temperature during about 1 week, well-formed crystals of (H2Zr2Ox5) were obtained.
Na2[Zr(C2O4)3]·2H2O (Na2ZrOx3), K(H7O3)[Zr(C2O4)3]·H2O (KHZrOx3), (NH4)(H7O3)[Zr(C2O4)3]·H2O (NH4HZrOx3), Rb(H5O2)0.5(H9O4)0.5[Zr(C2O4)3] (RbHZrOx3), and Cs(H5O2)0.5(H9O4)0.5[Zr(C2O4)3] (CsHZrOx3)
The different compounds were obtained by evaporation of a solution containing zirconium nitrate, monovalent cations, and oxalic acid. A solution obtained by dissolution of 0.25 mmol (0.0821 g) of Zr(OH)2(NO3)2·4.7H2O in 4 mL of water was added with 0.25 mmol of MNO3 (0.021, 0.025, 0.020, 0.037, 0.049 g for M = Na, K, NH4, Rb, and Cs, respectively) and 9 mL of ethanol. After addition of 1.25 mL (0.625 mmol) of a solution of oxalic acid 0.5 M giving solutions with pH between 1.2 and 1.8, the evaporation at room temperature during about 1 week allowed the formation of well-formed crystals of (Na2ZrOx3), (KHZrOx3), (NH4HZrOx3), (RbHZrOx3), and CsHZrOx3), respectively.
Powder X-ray Diffraction and Structure Determination
For the structure determination of ZrOx, powder X-ray diffraction data were recorded on a conventional D8 θ/θ ADVANCE Bruker laboratory diffractometer, using Cu Kα radiation (1.5618 Å). The powder X-ray diffraction data were measured with a 2θ range of 5–120° and a step size of 0.02°. The powder pattern indexing was performed using DICVOL0468 giving a monoclinic unit cell with parameters a = 12.813(4) Å, b = 5.8882(14) Å, c = 6.7088(13) Å, and β = 118.25(2)°. The complete crystal structure was obtained by the charge-flipping method using SUPERFLIP69 and then refined in the C2/c space group with JANA200670 giving Rp and Rwp factors of 3.36 and 5.01, respectively. Soft constraints have been introduced on the common isotropic displacement parameter for O atoms. As is often the case for inorganic structure refinements, even from single crystal data, H atoms have been introduced using geometric constraints on distances and an isotropic displacement equal to 1.2 that of O atoms. The diffraction measurement parameters and refinement results are summarized in Table 1 and a comparison between the observed and calculated X-ray powder patterns obtained from the Rietveld refinement is shown in Figure S7.
The single-crystal diffraction intensities for each of the compounds were measured on a Bruker X8 CCD 4K diffractometer using Mo Kα radiation (0.71073 Å) with an optical fiber as a collimator. The intensities were extracted from the collected frames using the Bruker program SAINT V7.53a.71 The structure resolutions and refinements were performed with the SHELX software72 with the WINGX interface.73 The heavy atoms were located using the direct methods, while the remaining atoms were found from successive Fourier map analyses. The atomic positions for all atoms and the anisotropic displacement parameters for non-H atoms were included in the last cycles of refinement. The isotropic displacement parameters of the H atoms retrieved from difference Fourier maps were taken with coefficients being 1.2 times larger than the respective equivalent isotropic displacement parameters of parent O or N atoms.
Thermal Analysis
Thermogravimetric (TG) analysis and differential thermal analysis (DTA) were carried out with a SETARAM 92 thermal-1600 instrument at a heating at 1 °C·min–1 using platinum crucibles, up to 800 °C, under air with heating at 1 °C·min–1. This instrument was coupled with an evolved gas analyzer Pfeiffer Omnistar Mass Spectrometer.
HTXRD experiments were performed under dynamic air (5 L·h–1) with an Anton Paar HTK1200N of a D8 Bruker diffractometer (θ–θ mode, Cu Kα radiation) equipped with an Anton Paar HTK 1200 high-temperature chamber and a Vantec1 linear position sensitive detector. Several diagrams were recorded every 25 °C between ambient temperature and 800 °C, in the range of 10–80° (2θ), with a step of 0.02° and heating at 0.08 °C·min–1.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03224.
Hydrogen bonds in Na2ZrOx3; environment of NH4+ in (NH4HZrOx3); TG/DTA/MSA analyses and HTXRD patterns of ZrOx, H2Zr2Ox5, and NH4HZrOx3; X-ray powder patterns of zirconium nitrates; X-ray powder pattern from Rietveld refinement of ZrOx; comparison of the chains 1∞[M(OH)2]n+ (M = Zr, Hf, Th, U); hydrogen bonds in H2Zr2Ox5; characteristics of the overall environment of Zr in Na2Zr2O7 and MHZrOx3; Zr–O distances and valence bond sum; M–O distances and bond valences in MHZrOx3 (M = K, NH4); and hydrogen bonds in KHZrOx3 and NH4HZrOx3 (PDF)
Crystallographic data of ZrOx (CIF)
Accession Codes
CCDC 2011396–2011402 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- Vasylkiv O. O.; Sakka Y.; Skorokhod V. V. Features of Preparing Nano-Size Powders of Tetragonal Zirconium Dioxide Stabilized with Yttrium. Powder Metall. Met. Ceram. 2005, 44, 228–239. 10.1007/s11106-005-0086-2. [DOI] [Google Scholar]
- Judes J.; Kamaraj V. Sol-gel preparation and characterization of ceria stabilized zirconia minispheres. J. Sol-Gel Sci. Technol. 2009, 49, 159–165. 10.1007/s10971-008-1853-6. [DOI] [Google Scholar]
- Judes J.; Kamaraj V. Study on process development and property evaluation of sol-gel derived magnesia stabilized zirconia minispheres. Mater. Sci.-Pol. 2014, 32, 145–156. 10.2478/s13536-013-0190-9. [DOI] [Google Scholar]
- Kumar C.; Manohar P. Conductivity and dielectric properties of sol-gel derived porous zirconia. Ionics 2007, 13, 333–335. 10.1007/s11581-007-0119-6. [DOI] [Google Scholar]
- Gongyi G.; Fang N. Z.; Yuli C. Crystallizability and sinterability of a precursor prepared by oxalate method in ethanol solution. J. Mater. Sci. 1991, 26, 3511–3516. 10.1007/bf00557139. [DOI] [Google Scholar]
- Gongyi G.; Yuli C. Effect of Preparation Methods and Condition of Precursors on the Phase Composition of Yttria-Stabilized Zirconia Powders. J. Am. Ceram. Soc. 1992, 75, 1294–1296. 10.1111/j.1151-2916.1992.tb05576.x. [DOI] [Google Scholar]
- Kirby N. M.; Van Riessen A.; Buckley C. E.; Wittorff V. W. Oxalate-precursor processing for high quality BaZrO3. J. Mater. Sci. 2005, 40, 97–106. 10.1007/s10853-005-5692-3. [DOI] [Google Scholar]
- Ciontea L.; Celentano G.; Augieri A.; Ristoiu T.; Suciu R.; Gabor M. S.; Rufoloni A.; Vannozzi A.; Galluzzi V.; Petrisor T. Chemically processed BaZrO3nanopowders as artificial pinning centres. J. Phys.: Conf. Ser. 2008, 97, 012289. 10.1088/1742-6596/97/1/012289. [DOI] [Google Scholar]
- Deshpande A. S.; Khollam Y. B.; Patil A. J.; Deshpande S. B.; Potdar H. S.; Date S. K. Improved chemical route for quantitative precipitation of lead zirconyl oxalate (PZO) leading to lead zirconate (PZ) powders. Mater. Lett. 2001, 51, 161–171. 10.1016/s0167-577x(01)00284-1. [DOI] [Google Scholar]
- Chen H.; Yin A.; Guo X.; Dai W.-L.; Fan K.-N. Sodium Hydroxide-Sodium Oxalate-Assisted Co-Precipitation of Highly Active and Stable Cu/ZrO2 Catalyst in the Partial Oxidation of Methanol to Hydrogen. Catal. Lett. 2009, 131, 632–642. 10.1007/s10562-009-0008-x. [DOI] [Google Scholar]
- Fathima N. N.; Balaraman M.; Rao J. R.; Nair B. U. Effect of zirconium(IV) complexes on the thermal and enzymatic stability of type I collagen. J. Inorg. Biochem. 2003, 95, 47–54. 10.1016/s0162-0134(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Haroun M. A.; Khirstova P. K.; Gasmelseed G. A.; Covington A. D. Influence of oxazolidines and zirconium oxalate crosslinkers on the hydrothermal, enzymatic, and thermo mechanical stability of type 1 collagen fiber. Thermochim. Acta 2009, 484, 4–10. 10.1016/j.tca.2008.11.008. [DOI] [Google Scholar]
- Chapelet-Arab B.; Nowogrocki G.; Abraham F.; Grandjean S. New alkaline earth-zirconium oxalates M2Zr(C2O4)4·nH2O (M=Ba, Sr, Ca) synthesis, crystal structure and thermal behavior. J. Solid State Chem. 2004, 177, 4269–4281. 10.1016/j.jssc.2004.06.053. [DOI] [Google Scholar]
- Audebrand N.; Jeanneau E.; Bataille T.; Raite S.; Louër D. A family of microporous mixed oxalates with isotypic-framework structures based on eight-coordinate metals. Solid State Sci. 2004, 6, 579–591. 10.1016/j.solidstatesciences.2004.03.007. [DOI] [Google Scholar]
- Boudaren C.; Auffrédic J. P.; Louër M.; Louër D. Synthesis, Structure Determination from Powder Diffraction Data, and Thermal Behavior of Lead Zirconium Oxalate Hydrate Pb2Zr(C2O4)4·nH2O (3 < n < 9). Chem. Mater. 2000, 12, 2324–2333. 10.1021/cm001063j. [DOI] [Google Scholar]
- Jeanneau E.; Audebrand N.; Auffrédic J.-P.; Louër D. Crystal structure, thermal behaviour and zeolitic properties of Cd2Zr(C2O4)4·(4+n)H2O. J. Mater. Chem. 2001, 11, 2545–2552. 10.1039/b103711k. [DOI] [Google Scholar]
- Jeanneau E.; Audebrand N.; Louër D. New Open-Framework Ammonium and Amine Cadmium Zirconium Oxalates with Helical Structures. Chem. Mater. 2002, 14, 1187–1194. 10.1021/cm0112136. [DOI] [Google Scholar]
- Jeanneau E.; Audebrand N.; Floch M. L.; Bureau B.; Louër D. New ternary cadmium–zirconium–sodium oxalate with an open framework: crystal structure, solid-state NMR spectroscopy and thermal behavior. J. Solid State Chem. 2003, 170, 330–338. 10.1016/s0022-4596(02)00100-7. [DOI] [Google Scholar]
- Jeanneau E.; Audebrand N.; Louër D. Synthesis, crystal structure and thermal behaviour of CdZrK2(C2O4)4·8H2O. J. Mater. Chem. 2002, 12, 2383–2389. 10.1039/b202463m. [DOI] [Google Scholar]
- Imaz I.; Bravic G.; Sutter J.-P. Structural and zeolitic features of a series of heterometallic supramolecular porous architectures based on tetrahedral {M(C2O4)4}4– primary building units. Dalton Trans. 2005, 2681–2687. 10.1039/b503964a. [DOI] [PubMed] [Google Scholar]
- Hoard J. L.; Glen G. L.; Silverton J. V. The configuration of Zr(C2O4)4-4 and the stereochemistry of discrete eight-coordination. J. Am. Chem. Soc. 1961, 83, 4293–4295. 10.1021/ja01481a050. [DOI] [Google Scholar]
- Glen G. L.; Silverton J. V.; Hoard J. L. Stereochemistry of Discrete Eight-Coordination. III. Tetrasodium Tetrakisoxalatozirconate(IV) Trihydrate. Inorg. Chem. 1963, 2, 250–255. 10.1021/ic50006a003. [DOI] [Google Scholar]
- Kojić-Prodić B.; Rużić-Toroš Z.; Šljukić M. Structure of Tetrapotassium Tetrakis(oxalato)zirconate(IV) Pentahydrate. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 1978, 34, 2001–2002. 10.1107/s0567740878007189. [DOI] [Google Scholar]
- Malathi M.; Vaishnavi K.; Ravi G.; Sunku M.; Vithal M. Potassium zirconium oxalate a new precursor for the preparation of perovskite, pyrochlore and Nasicon type materials. J. Solid State Chem. 2019, 276, 133–138. 10.1016/j.jssc.2019.04.038. [DOI] [Google Scholar]
- Imaz I.; Thillet A.; Sutter J.-P. Charge-Assisted Hydrogen-Bonded Assemblage of an Anionic {M(C2O4)4}4- Building Unit and Organic Cations: A Versatile Approach to Hybrid Supramolecular Architectures. Cryst. Growth Des. 2007, 7, 1753–1761. 10.1021/cg060905+. [DOI] [Google Scholar]
- Thétiot F.; Duhayon C.; Venkatakrishnan T. S.; Sutter J.-P. Modular Assembling of [Zr(C2O4)4]4- and [DabcoH2]2+ Units in Supramolecular Hybrid Architectures Including an Open Framework with Reversible Sorption Properties (Dabco) 1,4-Diazabicyclo[2. 2. 2]octane). Cryst. Growth Des. 2008, 8, 1870–1877. 10.1021/cg700971n. [DOI] [Google Scholar]
- Mouchaham G.; Roques N.; Imaz I.; Duhayon C.; Sutter J.-P. Driving the Assembling of Zirconium Tetraoxalate Metallotectons and Benzimidazolium Cations: From Three Dimensional Hydrogen-Bonded Compact Architectures to Open-Frameworks. Cryst. Growth Des. 2010, 10, 4906–4919. 10.1021/cg1009785. [DOI] [Google Scholar]
- Mouchaham G.; Roques N.; Kaiba A.; Guionneau P.; Sutter J.-P. Tubular crystals growth for a nanoporous hydrogen-bonded metal-organic framework. CrystEngComm 2010, 12, 3496–3498. 10.1039/c0ce00256a. [DOI] [Google Scholar]
- Venable F. P.; Baskerville C. The Oxalates of Zirconium. J. Am. Chem. Soc. 1897, 19, 12–18. 10.1021/ja02075a003. [DOI] [Google Scholar]
- Kobayashi T.; Sasaki T.; Takagi I.; Moriyama H. Zirconium Solubility in Ternary Aqueous System of Zr(IV)-OH-Carboxylates. J. Nucl. Sci. Technol. 2009, 46, 142–148. 10.1080/18811248.2007.9711515. [DOI] [Google Scholar]
- Kobayashi T.; Sasaki T.; Takagi I.; Moriyama H. Solid phase precipitates in (Zr,Th)-OH-(oxalate, malonate) ternary aqueous system. Radiochim. Acta 2009, 97, 237–241. 10.1524/ract.2009.1603. [DOI] [Google Scholar]
- Gable H. S. Zirconium. II. Zirconium oxalate and Diphenyldinitrogen Zirconium. J. Am. Chem. Soc. 1931, 53, 1276–1278. 10.1021/ja01355a013. [DOI] [Google Scholar]
- Clearfield A.; Baskerville C. Some observations on zirconium oxalates. J. Inorg. Nucl. Chem. 1959, 11, 169–170. 10.1016/0022-1902(59)80066-x. [DOI] [Google Scholar]
- Hamdouni M.; Walha S.; Kabadou A.; Duhayon C.; Sutter J.-P. Synthesis and Crystal Structures of Various Phases of the Microporous Three-Dimensional Coordination Polymer [Zr(OH)2(C2O4)]n. Cryst. Growth Des. 2013, 13, 5100–5106. 10.1021/cg401286a. [DOI] [Google Scholar]
- Zakaria Z.; Awang Mat Z.; Abu Hassan S. H.; Boon Kar Y. A review of solid oxide fuel cell component fabrication methods toward lowering temperature. Int. J. Energy Res. 2020, 44, 594–611. 10.1002/er.4907. [DOI] [Google Scholar]
- Han Y.; Zhu J. Surface Science Studies on the Zirconia-Based Model Catalysts. Top. Catal. 2013, 56, 1525–1541. 10.1007/s11244-013-0156-5. [DOI] [Google Scholar]
- Kontonasaki E.; Giasimakopoulos P.; Rigos A. E. Strength and aging resistance of monolithic zirconia: an update to current knowledge. Jpn. Dent. Sci. Rev. 2020, 56, 1–23. 10.1016/j.jdsr.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigh C. W. A new simple criterion for distinguishing the types of structures in eight-coordinate complexes: the pattern of bond angle. Polyhedron 1995, 14, 2871–2878. 10.1016/0277-5387(95)00213-c. [DOI] [Google Scholar]
- Bénard P.; Louër D. The Layer Structure of Zr(OH)3NO3 Determined ab initio using Conventional Monochromatic X-Ray Powder Diffraction. J. Phys. Chem. Solids 1995, 56, 1345–1352. 10.1016/0022-3697(95)00068-2. [DOI] [Google Scholar]
- Bénard P.; Louër M.; Louër D. Crystal Structure Determination of Zr(OH)2(NO3)2.4·7H2O from X-Ray Powder Diffraction Data. J. Solid State Chem. 1991, 94, 27–35. 10.1016/0022-4596(91)90217-6. [DOI] [Google Scholar]
- Bénard-Rocherullé P.; Rius J.; Louër D. Structural Analysis of Zirconium Hydroxide Nitrate Monohydrates by X-Ray Powder Diffraction. J. Solid State Chem. 1997, 128, 295–304. 10.1006/jssc.1996.7226. [DOI] [Google Scholar]
- Hansson M.; Solbakk J.; Lindström B.; Enzell C. R.; Enzell C. R.; Swahn C.-G. The Crystal Structure of Zr(OH)2SO4H2O. Acta Chem. Scand. 1973, 27, 2614–2622. 10.3891/acta.chem.scand.27-2614. [DOI] [Google Scholar]
- McWhan D. B.; Lundgren G. The Crystal Structure of Zr2(OH)2(SO4)3(H2O)4. Inorg. Chem. 1966, 5, 284–289. 10.1021/ic50036a027. [DOI] [Google Scholar]
- Mark W.; Stomberg R.; Krogh-Moe J.; Songstad J.; Pilotti Å. The 0D-Structure of Zr(OH)2CrO4. Acta Chem. Scand. 1972, 26, 3744–3756. 10.3891/acta.chem.scand.26-3744. [DOI] [Google Scholar]
- Casari B.; Langer V. Structural Study of Cerium Chromates. Acta Crystallogr., Sect. C: Struct. Chem. 2000, 56, s424. 10.1107/s0108270100000421. [DOI] [Google Scholar]
- Mark W.; Lyssandtræ N.; Maartmann-Moe K.; Tysseland M.; Korppi-Tommola J.; Swahn C.-G. The Crystal Structure of Zr4(OH)6(CrO4)5(H2O)2. Acta Chem. Scand. 1973, 27, 177–190. 10.3891/acta.chem.scand.27-0177. [DOI] [Google Scholar]
- Hansson M.; Brattås L.; Kjekshus A.; Enzell C. R.; Enzell C. R.; Swahn C.-G. The crystal structure of Hf(OH)2SO4. Acta Chem. Scand. 1973, 27, 2455–2462. 10.3891/acta.chem.scand.27-2455. [DOI] [Google Scholar]
- Lundgren C. The crystal structure of Th(OH)2SO4. Ark. Kemi 1950, 2, 535–549. [Google Scholar]
- Knope K. E.; Wilson R. E.; Skanthakumar S.; Soderholm L. Synthesis and characterization of thorium(IV) sulfates. Inorg. Chem. 2011, 50, 8621–8629. 10.1021/ic201175u. [DOI] [PubMed] [Google Scholar]
- Lundgren C. The crystal structure of U(OH)2SO4. Ark. Kemi 1952, 4, 421–428. [Google Scholar]
- Morris S.; Almond M. J.; Cardin C. J.; Drew M. G. B.; Rice D. A.; Zubavichus Y. The anions [(ZrOH(CO3)3)2]6- and [(ZrOH(C2O4)3)2]6- : single crystal X-ray diffraction studies of the complexes guanidinium zirconium carbonate [(C(NH2)3)3ZrOH(CO3)3·H2O]2 and sodium zirconium oxalate Na6(ZrOH(C2O4)3)2·7H2O. Polyhedron 1998, 17, 2301–2307. 10.1016/s0277-5387(98)00001-1. [DOI] [Google Scholar]
- Shannon R. D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr. 1976, 32, 751–767. 10.1107/s0567739476001551. [DOI] [Google Scholar]
- Brese N. E.; O’Keeffe M. Bond-Valence Parameters for Solids. Acta Crystallogr., Sect. B: Struct. Sci. 1991, 47, 192–197. 10.1107/s0108768190011041. [DOI] [Google Scholar]
- Sterling C. Crystal-structure of Tetragonal Strontium Oxalate. Nature 1965, 205, 588–589. 10.1038/205588a0. [DOI] [Google Scholar]
- Gavilan E.; Audebrand N.; Jeanneau E. A new series of mixed oxalates MM’(C2O4)3(H2O)3·nH2O (M = Cd, Hg, Pb; M’ = Zr, Hf) based on eight-fold coordinated metals: Synthesis, crystal structure from single-crystal and powder diffraction data and thermal behavior. Solid State Sci. 2007, 9, 985–999. 10.1016/j.solidstatesciences.2007.07.024. [DOI] [Google Scholar]
- Bernal I.; Watkins S. F. Molecular and supramolecular ionic aggregates HxOyzin organic and organometallic crystalline hydrates. Acta Crystallogr., Sect. C: Struct. Chem. 2014, 70, 566–574. 10.1107/s2053229614009826. [DOI] [PubMed] [Google Scholar]
- Baggio R.; Garland M. T.; Perec M. Preparation and X-ray Crystal Structure of the Polymeric Zirconium(IV) Oxalate Complex [K2{Zr(C2O4)3}·H2C2O4·H2O]n. Inorg. Chem. 1997, 36, 737–739. 10.1021/ic960987z. [DOI] [PubMed] [Google Scholar]
- Blanchard F.; Rivenet M.; Vigier N.; Hablot I.; Grandjean S.; Abraham F. Solid State Chemistry of Ten-Fold Coordinate Thorium(IV) Complexes with Oxalates in the Presence of Ammonium and Hydrazinium Ions. Cryst. Growth Des. 2018, 18, 4593–4601. 10.1021/acs.cgd.8b00565. [DOI] [Google Scholar]
- Khan A. A.; Baur W. H. Salt Hydrates. VIII. The Crystal Structures of Sodium Ammonium Orthochromate Dihydrate and Magnesium Diammonium Bis(Hydrogen Orthophosphate) Tetrahydrate and a Discussion of the Ammonium Ion. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 1972, 28, 683–693. 10.1107/s0567740872003024. [DOI] [Google Scholar]
- García-Rodríguez L.; Rute-Pérez Á.; Piñero J. R.; González-Silgo C. Bond-valence parameters for ammonium-anion interactions. Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem. 2000, 56, 565–569. 10.1107/s0108768100002615. [DOI] [PubMed] [Google Scholar]
- Reddy V. B.; Mehrotra P. N. Preparation, Characterisation and Thermal Decomposition of Barium Zirconyl Oxalate. Thermochim. Acta 1979, 31, 31–37. 10.1016/0040-6031(79)80004-0. [DOI] [Google Scholar]
- Kisi E. H.; Howard C. J. Crystal Structures of Zirconia Phases and their Inter-Relation. Key Eng. Mater. 1998, 153–154, 1–36. 10.4028/www.scientific.net/kem.153-154.1. [DOI] [Google Scholar]
- Garvie R. C. The Occurrence of Metastable Tetragonal Zirconia as a Crystallite Size Effect. J. Phys. Chem. 1965, 69, 1238–1243. 10.1021/j100888a024. [DOI] [Google Scholar]
- Li Vage J.; Doi K.; Mazieres C. Nature and Thermal Evolution of Amorphous Hvdrated Zirconium Oxide. J. Am. Ceram. Soc. 1968, 51, 349–353. 10.1111/j.1151-2916.1968.tb15952.x. [DOI] [Google Scholar]
- Štefanić G.; Štefanić I. I.; Musić S. Influence of the synthesis conditions on the properties of hydrous zirconia and the stability of low-temperature t-ZrO2. Mater. Chem. Phys. 2000, 65, 197–207. 10.1016/s0254-0584(00)00247-9. [DOI] [Google Scholar]
- Shukla S.; Seal S. Mechanisms of room temperature metastable tetragonal phase stabilisation in zirconia. Int. Mater. Rev. 2005, 50, 45–64. 10.1179/174328005x14267. [DOI] [Google Scholar]
- Baggio R.; Garland M. T.; Perec M. Binuclear Zirconium(IV) Oxalate Complex with a μ-Oxalate Coordination Mode. Crystal Structure of K6[{Zr(C2O4)3}2(μ-C2O4)]·4H2O. Inorg. Chem. 1997, 36, 3198–3200. 10.1021/ic9613826. [DOI] [PubMed] [Google Scholar]
- Boultif A.; Louër D. Powder pattern indexing with the dichotomy method. J. Appl. Crystallogr. 2004, 37, 724–731. 10.1107/s0021889804014876. [DOI] [Google Scholar]
- Palatinus L.; Chapuis G. SUPERFLIP- a computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. 10.1107/s0021889807029238. [DOI] [Google Scholar]
- Petricek V.; Dusek M.; Palatinus L.. Jana2006. Structure Determination Software Programs; Institute of Physics: Praha, Czech Republic, 2006.
- SAINT Plus Version 7.53a, Bruker Analytical X-Ray Systems: Madison, WI, 2008.
- Sheldrick G. M. A short history ofSHELX. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. 10.1107/s0108767307043930. [DOI] [PubMed] [Google Scholar]
- Farrugia L. J. WinGXsuite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. 10.1107/s0021889899006020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.