Abstract
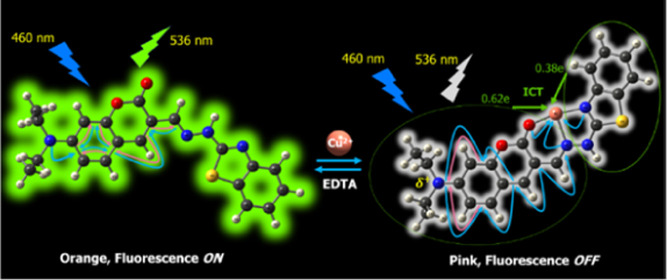
A novel coumarin derivative (5) was synthesized and used as a colorimetric and fluorescent probe for selective detection of Cu2+ ions in the presence of other metal ions, with the detection limits of 5.7 and 4.0 ppb, respectively. Cu2+ ion reacts with probe 5 to form a 1:1 stoichiometry complex, resulting in a remarkable redshift of absorption maximum from 460 to 510 nm, as well as almost completely quenching fluorescence intensity of probe 5 at the wavelength of 536 nm. These changes can be distinctly observed by naked eyes. In addition, the working pH range of probe 5 is wide and suitable for physiological conditions, thus probe 5 may be used for detection of Cu2+ ions in living cells. The stable structures of probe 5 and its 1:1 complex with Cu2+ ion were optimized at the PBE0/6-31+G(d) level of theory. The presence and characteristics of bonds in compounds were studied through atoms in a molecule and natural bond orbital analysis. The formation of the complex led to a strong transfer of electron density from probe 5 as a ligand to Cu2+ ion, resulting in breaking the π-electron conjugated system, which is the cause of fluorescence quenching and color change of 5-Cu2+ complex.
1. Introduction
Copper is both an essential trace element and a toxic substance to plants, animals, and humans.1,2 Copper toxicosis is very rare compared to its deficiency in plants.3 This element exhibits high toxicity to microorganisms such as algae, bacteria, viruses, and aquatic animals.4 Copper is present in almost all tissues of the human body and plays an important role in physiological processes, immunity, and resistance to oxidative stress.5−7 The copper deficiency or copper overload is believed to be related to diseases like Alzheimer’s, Parkinsons, Menkes, Wilsons, and Prion.8−11
There are different methods that can determine Cu2+ ions at ppb levels such as absorption atomic spectrometry (AAS),12,13 inductively coupled plasma mass spectrometry (ICP-MS),14 inductively coupled plasma optical emission spectrometry (ICP-OES),15 and voltammetry.16 However, these methods require expensive equipment, with complicated sample preparation and measurement techniques. Meanwhile, a fluorescence-based analytical method can also determine Cu2+ ions at ppb levels, which is simpler and less expensive than the above methods.17,18 Furthermore, a fluorescence-based analytical method can be used to monitor substances in living cells to detect abnormal cases and causes of diseases.19−21 Therefore, the development of new fluorescent probes for Cu2+ ions has attracted special attention of scientists.22−24 So far, many fluorescent probes for Cu2+ ions have been reported. The development of new fluorescent probes is, however, still being concerned due to the limitations of reported probes such as low sensitivity and selectivity,25,26 a narrow range of pH,20,27−29 working in solutions with a high proportion of organic solvents,26,30 and excitation wavelength in the ultraviolet region.26,28,31,32 These limitations reduce the application of probes to monitor Cu2+ ions in living cells.
In this study, a novel derivative of coumarin, (E)-3-((2-(benzo[d]thiazol-2-yl)hydrazono)methyl)-7-(diethylamino) coumarin (5) that was successfully synthesized could be used as a colorimetric and fluorescent probe for detection of Cu2+ ions in the preeminent operating conditions such as high sensitivity and selectivity, a wide range of pH, and excitation wavelength in the visible region. In addition, quantum chemical calculations have also been used to determine the optimized geometry of 5-Cu2+ complex and shed light on the cause of the changes in fluorescence properties.
2. Results and Discussion
2.1. Synthesis of (E)-3-((2-(Benzo[d]thiazol-2-yl)hydrazono)methyl)-7-(diethylamino)coumarin (5)
Probe 5 was synthesized through four steps as shown in Figure 1. First, 7-diethylamino-3-ethylacetate-coumarin (2) was synthesized from a condensation reaction between 4-diethylaminosalicylaldehyde (1) and diethylmalonate in the presence of triethylamine. Second, 7-diethylamino-coumarin (3) was obtained from the decarboxylation reaction of 2 using concentrated HCl and glacial acetic acid. Third, 7-diethylaminocoumarin-3-aldehyde (4) was obtained from the aldehyde reaction of 3 in the presence of POCl3 and N,N-dimethylformamide (DMF). Finally, probe 5 was obtained from the condensation reaction of 7-diethylaminocoumarin-3-aldehyde (4) with 2-hydrazinobenzothiazole in about 30% overall yield (Supporting Information). The structures of the products 3, 4, 5, and intermediates were confirmed by 1H NMR, 13C NMR, and electrospray ionization mass spectrometry (ESI-MS) analysis (Figures S1–S4, Supporting Information).
Figure 1.
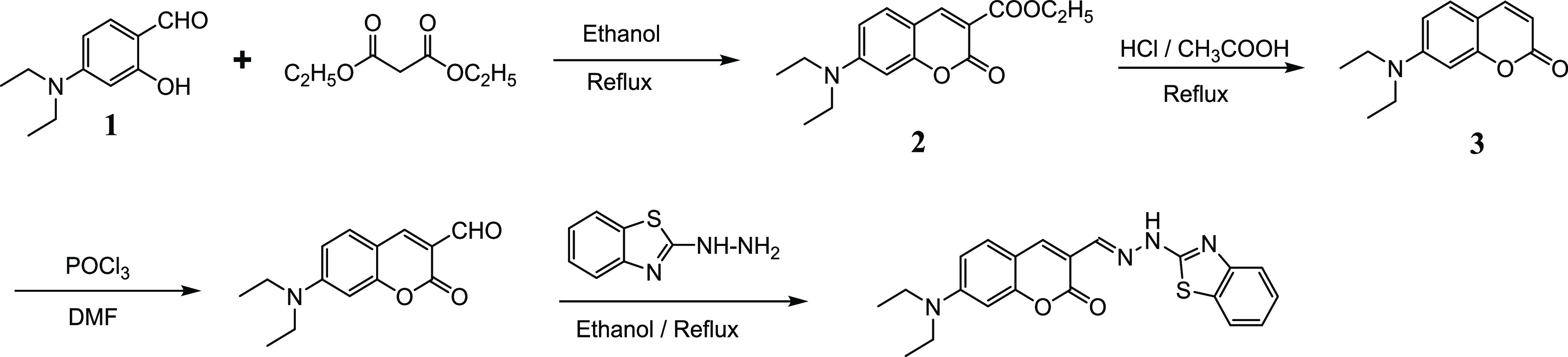
Synthetic pathway for the probe 5.
2.2. Experimental Characterization and Application of Probe 5
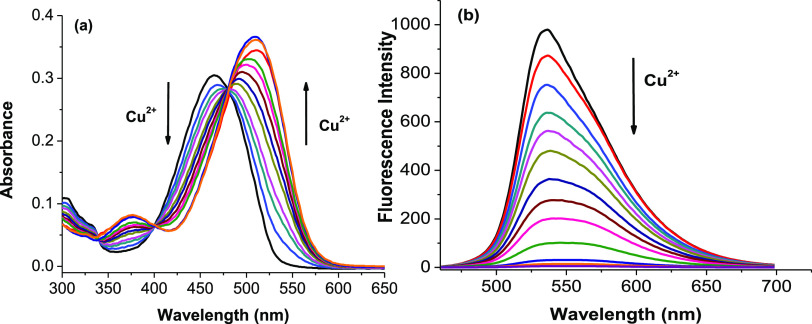
The absorption and fluorescence titration spectra of probe 5 with Cu2+ ions in aqueous solution are presented in Figure 2. It shows that free probe 5 has a characteristic absorption band peaked at the wavelength of 460 nm in aqueous solution. The absorption maximum is progressively redshifted with gradual addition of Cu2+ ions. The absorption maximum at 460 nm is gradually decreased, whereas a new absorption maximum at 510 nm appears. In companion with this, there is a change in the color of the solution from orange to pink. Furthermore, three distinct isosbestic points are observed at 340, 400, and 475 nm. These results indicate that the concentration of the light-absorbing compounds has been converted back and forth in the solution. This is seen as a piece of evidence for the formation of a new compound between probe 5 and Cu2+ ions.33−35
Figure 2.
(a) Absorbance titration spectra of probe 5 (5 μM) in ethanol/4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES, pH 7.4, 1/1, v/v) at 25 °C with gradual addition of 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.5, and 2.0 equiv of Cu2+ ions. (b) Fluorescence titration spectra of probe 5 (5 μM) in ethanol/HEPES (pH 7.4, 1/1, v/v) at 25 °C with gradual addition of 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 1.5, and 2.0 equiv of Cu2+ ions with an excitation of 460 nm.
As shown in Figure 2, free probe 5 has a strong emission band peaked at the wavelength of 536 nm with an excitation wavelength of 460 nm. The fluorescence quantum yield (Φ) of free probe 5 in aqueous solution is determined to be 10.5%, using the fluorescein in 0.1 N NaOH solution as a reference.36 Addition of Cu2+ ions greatly quenches the fluorescence intensity of probe 5, along with a slight redshift in the emission maximum. Upon addition of 1 equiv of Cu2+ ions to the solution of probe 5, the fluorescence intensity is quenched over 95% of its original intensity, along with a shift in the emission maximum from 536 to 545 nm. The fluorescence quantum yield (Φ) of the product between probe 5 and Cu2+ ions in aqueous solution is found to be 0.15% based on the obtained experimental data.
The Job plot in Figure 3a shows a complexation between probe 5 and Cu2+ ions in a molar ratio of 1:1. The stoichiometry is also confirmed by the plot of the fluorescence intensity of probe 5 with different concentrations of Cu2+ ions. Figure 3b shows that the fluorescence intensity is gradually quenched upon an increasing addition of Cu2+ ions. Upon addition of 1 equiv of Cu2+ ions, the fluorescence intensity is quenched to more than 95% and then becomes almost unchanged as the concentration of Cu2+ ions is further increased.
Figure 3.
(a) Job plot of probe 5 and Cu2+ ions. (b) Plot and linear fitting of the fluorescence intensity of probe 5 (5 μM) with different concentrations of Cu2+ ions (excitation wavelength: 460 nm, emission wavelength: 536 nm).
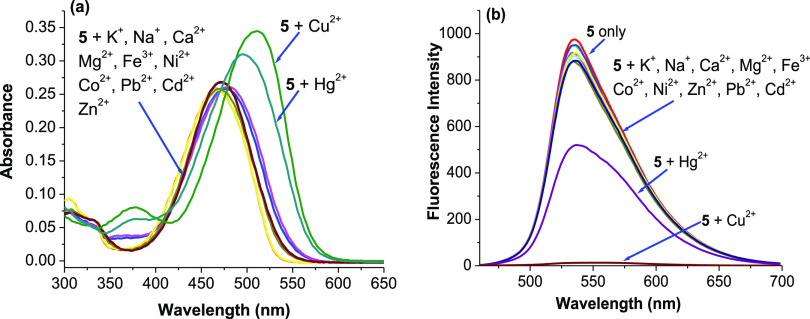
The selectivity of probe 5 toward Cu2+ ions compared to the other metal ions was investigated. Figure 4 gives the variations of absorption and fluorescence spectra of probe 5 caused by miscellaneous ions including Na+, K+, Ca2+, Mg2+, Fe3+, Co2+, Ni2+, Zn2+, Pb2+, Cd2+, Hg2+, and Cu2+ ions. The results show that Cu2+ ions cause a remarkable redshift in absorption maximum from 460 to 510 nm with increased intensity; Hg2+ ions induce a medium change from 460 to 500 nm, whereas other metal ions only produce slight changes in their absorption spectra (Figure 4a). On the other hand, probe 5 shows a strong emissive band peaked at 536 nm. Cu2+ ions can quench the fluorescence of probe 5 to more than 95%, whereas Hg2+ ions can quench fluorescence to about 40%. Other miscellaneous competitive ions do not lead to any significant changes in fluorescence (Figure 4b). The above changes in color and fluorescence can be distinctly observed by naked eyes, and their photos are presented in Figure 5. All these show a remarkably high selectivity of probe 5 for Cu2+ ions over other competitive ions except for Hg2+ ions.
Figure 4.
Absorption spectra (a) and fluorescence spectra (b) of probe 5 (5 μM) in ethanol/HEPES (pH 7.4, 1/1, v/v) at 25 °C upon addition of 1 equiv of Cu2+ ions and other metal ions including Na+, K+, Ca2+, Mg2+, Fe3+, Co2+, Ni2+, Zn2+, Pb2+, Cd2+, and Hg2+ ions (excitation wavelength: 460 nm, emission wavelength: 536 nm).
Figure 5.
Color changes (a) and fluorescence changes (b) of probe 5 with Na+, K+, Ca2+, Mg2+, Fe3+, Co2+, Ni2+, Cu2+, Zn2+, Pb2+, Cd2+, Cu2+, and Hg2+.
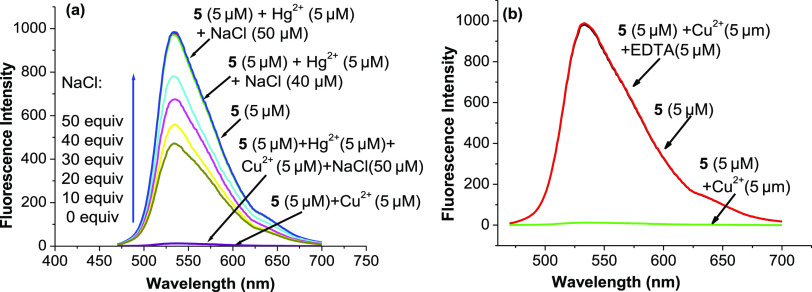
The possibility of using probe 5 to quantify Cu2+ ions in the presence of Hg2+ ions is also investigated. As shown in Figure 6a, the influence of Hg2+ ions on the quantification of Cu2+ ions can be prevented by adding 8–10 equiv of NaCl to the solution containing Hg2+ ions. Furthermore, when 10 equiv of Na4 EDTA is added to the solution of probe 5 containing 1 equiv of Cu2+ ions, the solution returns to strong fluorescence with the same intensity as the solution of free probe 5 (Figure 6b). This result shows that the reaction between Cu2+ ions and probe 5 is reversible. Therefore, probe 5 acts as a chemosensor for Cu2+ ions.
Figure 6.
Fluorescence spectra of probe 5 (5 μM) in ethanol/HEPES (pH 7.4, 1/1, v/v) at 25 °C (a) upon addition of 1 equiv of Hg2+ ions and NaCl (0–50 μM) and (b) upon addition of 1 equiv of Cu2+ ions and EDTA (50 μM) (excitation wavelength: 460 nm).
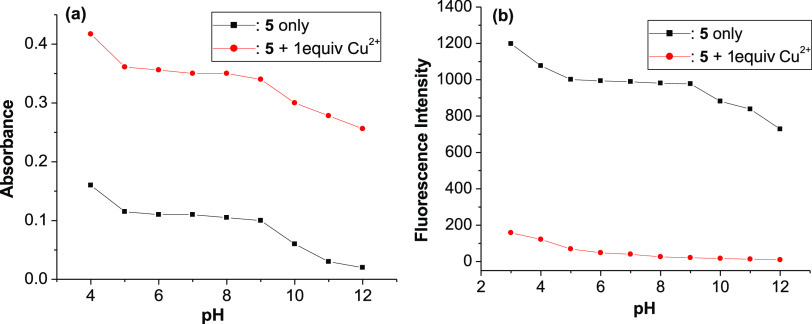
For practical applicability, a proper range of pH values for probe 5 was also evaluated. Figure 7a shows that the absorbance of free probe 5 and of probe 5 in the presence of Cu2+ ions reaches a plateau in the pH range from 5 and 9. Under acidic conditions (pH < 5) or alkaline conditions (pH > 9), the absorbance of these solutions is not stable upon pH changes, it decreases as pH increases. A similar result also comes from the fluorescence spectra with the change of pH conditions, as presented in Figure 7b. These results show that probe 5 can detect Cu2+ ions with a wide pH span, from 5 to 9.
Figure 7.
(a) Absorption intensity at 510 nm and (b) fluorescence intensity at 536 nm of probe 5 (5 μM) in the absence and presence of 1 equiv of Cu2+ ions in ethanol/HEPES (pH 7.4, 1/1, v/v) solution.
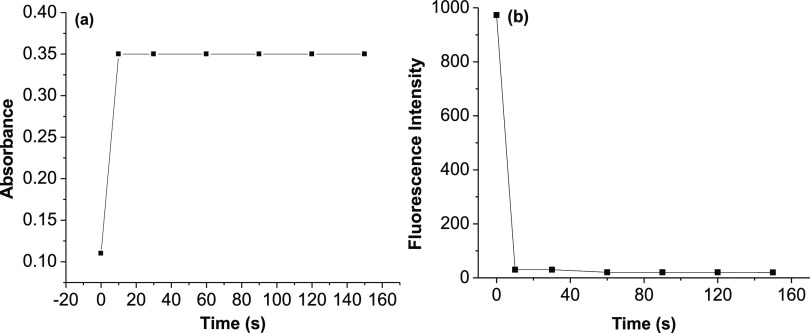
The changes of absorbance and fluorescence intensity of probe 5 (5 μM, in ethanol/HEPES, pH 7.4, 1/1, v/v) after adding 1 equiv of Cu2+ ions with different times are investigated and presented in Figure 8. The results show that the complexation process between probe 5 with Cu2+ ions is very fast. It can reach the equilibrium within several seconds, indicating that probe 5 can be used for real-time detection of Cu2+ ions.
Figure 8.
(a) Changes of absorbance at 510 nm and (b) fluorescence intensity at 536 nm of probe 5 (5 μM) after adding 1 equiv of Cu2+ ions in ethanol/HEPES (pH 7.4, 1/1, v/v) with different times.
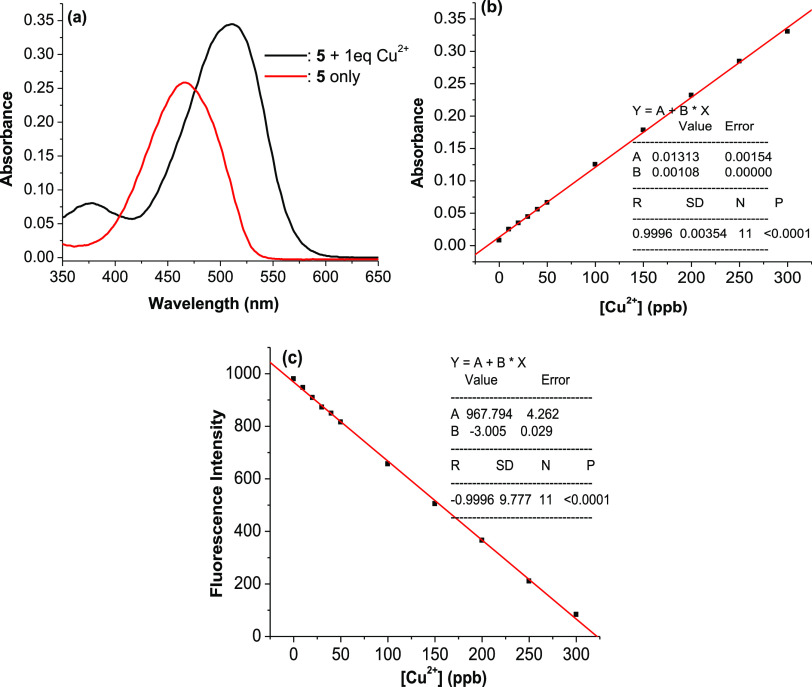
The possibility to use 5 as a chemosensor for quantification of Cu2+ ions by colorimetric and fluorescent methods was investigated. The results obtained from the absorption spectra show that the optimal absorption wavelength for quantification of Cu2+ ions is 525 nm, where the difference between absorbances is the largest, and the absorbance of Cu2+ ions is approximately equal to zero (Figure 9a). Figure 9b,c show that, in the concentration range of Cu2+ ions from 0 to 300 ppb, the linear relationships between the concentration of Cu2+ ions and the absorbance or fluorescence intensity of 5 solution (5 μM) are very good and expressed by the equations that were found from calibration curves: A525 nm = (0.013 ± 0.002) + (0.001 ± 0.000) × [Cu2+] (ppb) or FI536 nm = (967.793 ± 4.262) – (3.004 ± 0.029) × [Cu2+] (ppb). The calculated linear correlation coefficients are 0.9996 and 0.9996, respectively. These results indicate that 5 can be used as a colorimetric or fluorescent chemosensor for quantification of Cu2+ ions. The limit of detection is 5.7 ppb for the colorimetric method and 4.0 ppb for the fluorescent method (Figure S5), much lower than that for the recently published colorimetric and fluorescent sensors.37−40 The method of using probe 5 to quantify Cu2+ ions in the presence or absence of Hg2+ ions has been assessed for accuracy through the repeatability (relative standard deviation, RSD) and recovery rate. The obtained results were reliable (see more details in Supporting Information).
Figure 9.
(a) Absorption spectra of probe 5 (5 μM) and probe 5 (5 μM) + 1 equiv of Cu2+ ions in ethanol/HEPES (pH 7.4, 1/1, v/v). (b) Variation of absorbance at 525 nm of 5 solution (5 μM) in ethanol/HEPES (pH 7.4, 1/1, v/v) versus the concentration of Cu2+ ions (0–300 ppb). (c) Variation of fluorescence intensity at 536 nm of 5 solution (5 μM) in ethanol/HEPES (pH 7.4, 1/1, v/v) versus the concentration of Cu2+ ions (0–300 ppb).
2.3. Investigations on the Structural Properties of Probe 5 and Its Complex with Cu2+ ions
For the optimized geometries of probe 5, the 1:1 complexes between probe 5 and Cu2+ ions were optimized at the PBE0/6-31+G(d) level of theory. The stable structures corresponding to minima on the potential energy surface are shown in Figure 10. All their Cartesian coordinates are presented in Tables S1 and S2 of Supporting Information. The calculated bond lengths, bond angles, and dihedral angles of probe 5 and its 1:1 complex with Cu2+ ions are presented in Table S3 of Supporting Information.
Figure 10.

Stable structures of probe 5 (a) and its 1:1 complex with Cu2+ ions (b) at the PBE0/6-31+G(d) level of theory.
The calculated results show that in free probe 5, most of the atoms are coplanar except for the ones of ethylamino groups. Two chains C(16)–C(15)–C(17)–N(36) and N(36)–N(35)–C(41)–N(34) in free probe 5 are in trans-configurations (Figure 10a). Meanwhile, the 1:1 complexation leads to the changes in configurations of these two chains, being cis-configuration (Figure 10b).
The calculated values of ρ(r) and ∇2(ρ(r)) and the topological properties of 5-Cu2+ complex at the critical points obtained from AIM analysis are presented in Table 1 and Figure 11, respectively. The calculated results confirmed the presence of chemical bonds in each of the O(2)···Cu(49), N(36)···Cu(49), and N(34)···Cu(49) contacts. All values of ∇2(ρ(r)) for these contacts are negative. In addition, the contact distances of atom pairs O(2)···Cu(49), N(36)···Cu(49), and N(34)···Cu(49) are 1.845, 1.952, and 1.898 Å, respectively. They are significantly smaller than the sum of the van der Waals atomic radii of corresponding atom pairs (2.920, 2.950, and 2.950 Å). As a result, these contacts are thought to form covalent bonds. RCPs are found in the center of O(2)···C(16)···C(15)···C(17)···N(36)···Cu(49) and N(36)···N(35)···C(41)···N(34)···Cu(49) atom groups, indicating a cyclic structure present in each group of atoms.
Table 1. Electron density (ρ(r), in au) and the Laplacian (∇2(ρ(r)), in au) at the contact points of atom pairs in 5-Cu2+ complex at the PBE0/6-31+G(d) level of theory.
| bond | ρ(r) | λ1 | λ2 | λ3 | ∇2(ρ(r)) | critical point |
|---|---|---|---|---|---|---|
| O(2)···Cu(49) | 0.1127 | –0.1589 | –0.1573 | 0.9036 | –0.1469 | BCP |
| N(36)···Cu(49) | 0.1032 | –0.1393 | –0.1343 | 0.6041 | –0.0826 | BCP |
| N(34)···Cu(49) | 0.1148 | –0.1525 | –0.1488 | 0.6977 | –0.0991 | BCP |
| O(2)···C(16)···C(15)···C(17)···N(36)···Cu(49) | 0.0146 | –0.0101 | 0.0367 | 0.0526 | –0.0198 | RCP |
| N(36)···N(35)···C(41)···N(34)···Cu(49) | 0.0275 | –0.0229 | 0.0642 | 0.1283 | –0.0424 | RCP |
Figure 11.
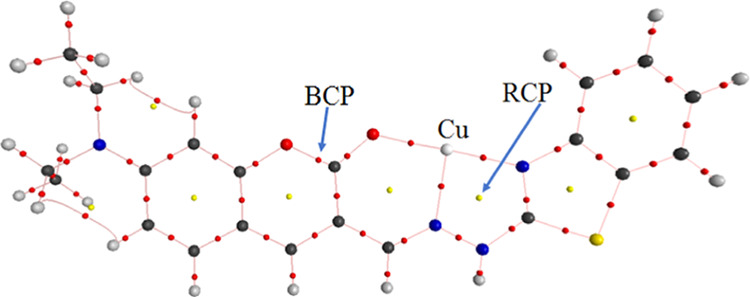
Topological properties of 5-Cu2+ complex at the BCPs (yellow points) and RCPs (red points).
The intermolecular orbital interaction and bond properties are investigated by natural bond orbital (NBO) analysis to explain the fluorescent properties of probe 5 and 5-Cu2+ complex. The calculated results in Table 2 and Figure 11 show that in free probe 5, the presence of π bonds between atom pairs with their significantly large interactive energies (E(2)), including C4–C10, C11–C8, C14–C15, C17–C36, and O2–C16, has led to the formation of a π-electron conjugated system in the first moiety of probe 5 containing the diethylaminocoumarin. This π-electron conjugated system also enhances electron density from the electron pair of the N3 atom by the donor–acceptor interaction, with the interactive energy (E(2)) of 52.50 kcal·mol–1. These intermolecular orbital interaction and bond properties are typical in fluorescent compounds.41−43 These findings are considered as evidence for the fluorescence of probe 5.
Table 2. Significant second-order interaction energies at the PBE0/6-31+G(d) level of theory (kcal·mol–1) between donor and acceptor orbitals in 5 and 5-Cu2+ complex.
| donor NBO (i) | acceptor NBO (j) | E(2) | donor NBO (i) | acceptor NBO (j) | E(2) |
|---|---|---|---|---|---|
| 5 | 5-Cu2+ | ||||
| π(C4–C10) | π*(C7–C9) | 14.80 | π(C7–C9) | π*(N3–C4) | 14.05 |
| π(C4–C10) | π*(C8–C11) | 32.07 | π(C8–C14) | π*(C7–C9) | 12.19 |
| π(C7–C9) | π*(C4–C10) | 24.47 | π(C10–C11) | π*(N3–C4) | 13.15 |
| π(C7–C9) | π*(C8–C11) | 13.91 | π*(N3–C4) | π*(C7–C9) | 48.82 |
| π(C8–C11) | π*(C4–C10) | 17.70 | π*(N3–C4) | π*(C10–C11) | 24.57 |
| π(C8–C11) | π*(C7–C9) | 27.97 | π*(C8–C14) | π*(C10–C11) | 23.42 |
| π(C8–C11) | π*(C14–C15) | 24.83 | LP(O1) | LP*(C16) | 43.31 |
| π(C14–C15) | π*(O2–C16) | 27.96 | LP(O1) | π*(C7–C9) | 14.11 |
| π(C14–C15) | π*(C8–C11) | 13.99 | LP(O2) | LP*(C16) | 64.86 |
| π(C14–C15) | π*(C17–N36) | 19.81 | LP(C15) | π*(C8–C14) | 30.79 |
| LP (O1) | π*(O2–C16) | 40.59 | LP(C15) | π*(C17–N36) | 57.01 |
| LP (O1) | π*(C7–C9) | 33.17 | LP(N35) | π*(C17–N36) | 12.97 |
| LP (O2) | δ*(O1–C16) | 39.11 | LP(O2) | LP*(Cu49) | 13.89 |
| LP (O2) | δ*(C15–C16) | 18.09 | LP(O2) | LP*(Cu49) | 20.19 |
| LP (N3) | π*(C4–C10) | 52.50 | LP(N34) | LP*(Cu49) | 19.52 |
| δ*(O1–C16) | δ*(O1–C9) | 22.77 | LP(N34) | LP*(Cu49) | 23.49 |
| π*(O2–C16) | π*(C14–C15) | 125.95 | LP(N34) | LP*(Cu49) | 11.14 |
| π*(C4–C10) | π*(C8–C11) | 431.68 | LP(N36) | LP*(Cu49) | 14.70 |
| π*(C8–C11) | π*(C14–C15) | 218.22 | LP(N36) | LP*(Cu49) | 29.53 |
| π*(C17–N36) | π*(C14–C15) | 76.60 | π(C37–C39) | LP*(C42) | 20.29 |
| π*(C4–C10) | π*(C7–C9) | 352.98 | π(C37–C39) | π*(N34–C38) | 13.32 |
| π(N34–C41) | π*(C37–C38) | 18.64 | π(C40–C43) | LP*(C42) | 22.65 |
| π(C37–C38) | π*(N34–C41) | 10.85 | π(C40–C43) | π*(N34–C38) | 19.41 |
| π(C37–C38) | π*(C39–C42) | 21.99 | π*(N34–C38) | π*(C37–C39) | 42.65 |
| π(C37–C38) | π*(C40–C43) | 17.54 | π*(N34–C38) | π*(C40–C43) | 29.85 |
| π(C39–C42) | π*(C37–C38) | 19.24 | LP(C41) | π*(N34–C38) | 24.08 |
| π(C39–C42) | π*(C40–C43) | 20.75 | LP*(C42) | π*(C37–C39) | 51.77 |
| π(C40–C43) | π*(C37–C38) | 22.80 | LP*(C42) | π*(C40–C43) | 34.21 |
| π(C40–C43) | π*(C39–C42) | 20.58 | π(C34–C38) | LP*(C41) | 45.19 |
| LP (S33) | π*(N34–C41) | 29.69 | LP(N35) | π*(C17–N36) | 12.97 |
| LP (S33) | π*(C37–C38) | 17.92 | LP(S33) | LP(C41) | 66.00 |
| LP (N34) | δ*(S33–C41) | 19.21 | LP(N35) | LP(C41) | 47.19 |
| LP (N35) | π*(C17–N36) | 33.15 | |||
| LP (N35) | π*(N34–C41) | 53.86 | |||
| π*(N34–C41) | π*(C37–C38) | 119.73 | |||
| π*(C37–C38) | π*(C39–C42) | 197.32 | |||
| π*(C37–C38) | π*(C40–C43) | 158.69 | |||
In 5-Cu2+ complex, the binding between 5 ligand and Cu2+ ion is stabilized by the electron donor–acceptor interactions between electron lone pairs (LP) on the O and N atoms of the ligand and on the Cu atom. The hyperconjugation energies of the LP(2)O2 → LP*(5)Cu49, LP(2)O2 → LP*(6)Cu49, LP(1)N34 → LP*(5)Cu49, LP(1)N34 → LP*(6)Cu49, LP(1)N34 → LP*(7)Cu49, LP(1)N36 → LP*(5)Cu49, and LP(1)N36 → LP*(6)Cu49 in the complex are 13.89, 20.19, 19.52, 23.49, 11.14, 14.70, and 29.53 kcal·mol–1, respectively. These interactions have caused a strong transfer of electron density from 5 ligand to Cu2+ ion. The calculated charge of Cu atom in 5-Cu2+ complex is +1.00e, indicating that 5 ligand becomes deficient in electron density. The charge of the first moiety of 5 in the complex is +0.62e, while the charge of the second moiety of 5 in the complex is +0.38e. The charge values of C17 and N3 in the complex are significantly more positive than those in free 5 (The NBO charges of atoms are presented in Table S4). The deficiency in electron density at C17 and N3 has resulted in breaking the π-electron conjugated system in the first moiety of 5 at the C15 atom. The π bonds between C15–C14 or C15–C17 are not found, while the LP(1)C15 → π*(C8–C14) and LP(1)C15 → π*(C17–N36) interactions are found with the hyperconjugation energies of 30.79 and 57.01 kcal·mol–1, respectively. On the other hand, the calculated results have confirmed the presence of the π(N3–C4) bond and the absence of interactions from the electron lone pairs of the N3 atom in the form of donor–acceptor interaction. These findings shed light on the cause of fluorescence quenching in 5-Cu2+ complex.
3. Conclusions
A novel coumarin derivative was synthesized and could be used as a colorimetric and fluorescent probe for detection of Cu2+ ions with the detection limits of 5.7 and 4.0 ppb, respectively. This probe could operate over a wide range of pH from 5 to 9 and was not affected by the presence of other metal ions, including Na+, K+, Ca2+, Mg2+, Fe3+, Co2+, Ni2+, Zn2+, Pb2+, Cd2+, and Hg2+. The stable structures of probe 5 and its 1:1 complex with Cu2+ ions have been identified at the PBE0/6-31+G(d) level of theory and is confirmed by AIM analysis. The results from NBO analysis showed that the formation of the complex led to a strong transfer of electron density from 5 ligand to Cu2+ ion. As a result, the π-electron conjugated system was broken, resulting in fluorescence quenching and color change in the complex.
4. Materials and Methods
4.1. Instruments
A Shimadzu UV-1800 UV–Vis spectrophotometer was used for the UV–Vis absorption spectra. A Shimadzu RF-5301PC series fluorescence spectrometer was used for the fluorescence spectra. A Varian instrument was used for the 1H NMR and 13C NMR spectra. A Finnigan 4021C instrument and Daltonics flex analysis software were used for the mass spectra.
4.2. Reagents
4-Diethylaminosalicylaldehyde, diethylmalonate, 2-hydrazinobenzothiazole, triethylamine, acetic acid, HEPES, POCl3, HCl, NaOH, and all ions of Na+, K+, Ca2+, Mg2+, Fe3+, Co2+, Ni2+, Hg2+, Zn2+, Pb2+, Cd2+, and Cu2+ as their chloride or perchlorate were purchased from Aldrich Chemical Corporation and used as received. DMF was an HPLC reagent and redistilled prior to use. Ethanol was of HPLC grade without fluorescent impurity. All solvents were purchased from Merck and used as received. Deionized water was used for all experiments.
4.3. Computational Methodology
The optimized geometries of compounds were determined at the PBE0/6-31+G(d) level of theory,44−46 using the Gaussian 09 program package.47,48 The structures and the nature of the bonds in these compounds have also been investigated through the atoms in molecules (AIM) topological analysis,49 using the AIM2000 software.50 The calculated results, including the electron density (ρ(r)), Laplacian ∇2(ρ(r)), the bond critical points (BCPs), and the ring critical points (RCPs), were used to evaluate the stability of bonds, the characteristics of bonds, the presence of bonds, and ring structure.41,51−53 The electronic properties of compounds were investigated by the natural bond orbital (NBO) analysis, using the NBO 3.1 program available in Gaussian 09 package, at the PBE0/6-31+G(d) level of theory. The calculated results, including NBO charge of atoms, the second-order stabilization energy (E(2)) between the donor NBO(i) and the acceptor NBO(j), were used to evaluate interactions and shed light on the fluorescent characteristics of compounds.41,54−57
Acknowledgments
This research was funded by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.06–2017.51 (D.T.Q.).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03097.
Synthetic methods, NMR spectra of synthetic products, and Cartesian coordinates of transition states in all of the studied environments (PDF)
Author Contributions
§ These authors contributed equally to the work
The authors declare no competing financial interest.
Supplementary Material
References
- Willis M. S.; Monaghan S. A.; Miller M. L.; McKenna R. W.; Perkins W. D.; Levinson B. S.; Bhushan V.; Kroft S. H. Zinc-induced copper deficiency: a report of three cases initially recognized on bone marrow examination. Am. J. Clin. Pathol. 2005, 123, 125–131. 10.1309/V6GVYW2QTYD5C5PJ. [DOI] [PubMed] [Google Scholar]
- Robinson N. J.; Winge D. R. Copper metallochaperones. Annu. Rev. Biochem. 2010, 79, 537–562. 10.1146/annurev-biochem-030409-143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevay L. M.Copper as a supplement to iron for hemoglobin building in the rat (Hart et al., 1928). J. Nutr. 1997. [PubMed] [Google Scholar]
- Lawrence C.; Sanders G. E.; Wilson C.. Aquatics. Management of Animal Care and Use Programs in Research, Education, and Testing, 2nd ed.; CRC Press/Taylor & Francis, 2018. [PubMed] [Google Scholar]
- Valko M.; Jomova K.; Rhodes C. J.; Kuča K.; Musílek K. Redox-and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. 10.1007/s00204-015-1579-5. [DOI] [PubMed] [Google Scholar]
- Banci L.; Bertini I.; Ciofi-Baffoni S.; Kozyreva T.; Zovo K.; Palumaa P. Affinity gradients drive copper to cellular destinations. Nature 2010, 465, 645–648. 10.1038/nature09018. [DOI] [PubMed] [Google Scholar]
- Matak P.; Zumerle S.; Mastrogiannaki M.; El Balkhi S.; Delga S.; Mathieu J. R. R.; Canonne-Hergaux F.; Poupon J.; Sharp P. A.; Vaulont S.; Peyssonnaux C. Copper deficiency leads to anemia, duodenal hypoxia, upregulation of HIF-2α and altered expression of iron absorption genes in mice. PLoS One 2013, 8, e59538 10.1371/journal.pone.0059538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood S. Brain–Barrier Regulation, Metal (Cu, Fe) Dyshomeostasis, and Neurodegenerative Disorders in Man and Animals. Inorganics 2019, 7, 108. 10.3390/inorganics7090108. [DOI] [Google Scholar]
- Montes S.; Rivera-mancia S.; Diaz-ruiz A.; Tristan-lopez L.; Camilo R. Review article copper and copper proteins in Parkinson’s disease. Oxid. Med. Cell. Longevity 2014, 2014, 1–15. 10.1155/2014/147251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasmeen S.; Lund K.; De Paepe A.; De Bie S.; Heiberg A.; Silva J.; Martins M.; Skjørringe T.; Møller L. B. Occipital horn syndrome and classical Menkes Syndrome caused by deep intronic mutations, leading to the activation of ATP7A pseudo-exon. Eur. J. Hum. Genet. 2014, 22, 517–521. 10.1038/ejhg.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss K. H.; Runz H.; Noe B.; Gotthardt D. N.; Merle U.; Ferenci P.; Stremmel W.; Füllekrug J. Genetic analysis of BIRC4/XIAP as a putative modifier gene of Wilson disease. J. Inherited Metab. Dis. 2010, 33, 233–240. 10.1007/s10545-010-9123-5. [DOI] [PubMed] [Google Scholar]
- Doner G.; Ege A. Determination of copper, cadmium and lead in seawater and mineral water by flame atomic absorption spectrometry after coprecipitation with aluminum hydroxide. Anal. Chim. Acta 2005, 547, 14–17. 10.1016/j.aca.2005.02.073. [DOI] [Google Scholar]
- Lin T.-W.; Huang S.-D. Direct and simultaneous determination of copper, chromium, aluminum, and manganese in urine with a multielement graphite furnace atomic absorption spectrometer. Anal. Chem. 2001, 73, 4319–4325. 10.1021/ac010319h. [DOI] [PubMed] [Google Scholar]
- Becker J. S.; Matusch A.; Depboylu C.; Dobrowolska J.; Zoriy M. Quantitative imaging of selenium, copper, and zinc in thin sections of biological tissues (Slugs– Genus Arion) measured by laser ablation inductively coupled plasma mass spectrometry. Anal. Chem. 2007, 79, 6074–6080. 10.1021/ac0700528. [DOI] [PubMed] [Google Scholar]
- Otero-Romaní J.; Moreda-Piñeiro A.; Bermejo-Barrera P.; Martin-Esteban A. Inductively coupled plasma–optical emission spectrometry/mass spectrometry for the determination of Cu, Ni, Pb and Zn in seawater after ionic imprinted polymer based solid phase extraction. Talanta 2009, 79, 723–729. 10.1016/j.talanta.2009.04.066. [DOI] [PubMed] [Google Scholar]
- Buffle J.; Tercier-Waeber M.-L. Voltammetric environmental trace-metal analysis and speciation: from laboratory to in situ measurements. TrAC, Trends Anal. Chem. 2005, 24, 172–191. 10.1016/j.trac.2004.11.013. [DOI] [Google Scholar]
- Aragay G.; Pons J.; Merkoçi A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. 10.1021/cr100383r. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Min X.; Zhang X.; Zhang F.; Lu S.; Xu L.-P.; Lou X.; Xia F.; Zhang X.; Wang S. AIE-based superwettable microchips for evaporation and aggregation induced fluorescence enhancement biosensing. Biosens. Bioelectron. 2018, 111, 124–130. 10.1016/j.bios.2018.04.011. [DOI] [PubMed] [Google Scholar]
- Zhou M.; Wang X.; Huang K.; Huang Y.; Hu S.; Zeng W. A fast, highly selective and sensitive dansyl-based fluorescent sensor for copper (II) ions and its imaging application in living cells. Tetrahedron Lett. 2017, 58, 991–994. 10.1016/j.tetlet.2017.01.090. [DOI] [Google Scholar]
- Wei M.; Zhang Y.; Li H.; Yao S. A new “on–off” fluorescent probe for the selective detection of copper ions in living cells. Anal. Methods 2017, 9, 3956–3961. 10.1039/C7AY01097D. [DOI] [Google Scholar]
- Quang D. T.; Kim J. S. Fluoro-and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem. Rev. 2010, 110, 6280–6301. 10.1021/cr100154p. [DOI] [PubMed] [Google Scholar]
- Yin C.; Li J.; Huo F. Cu2+ biological imaging probes based on different sensing mechanisms. Curr. Med. Chem. 2019, 26, 3958–4002. 10.2174/0929867324666170428110724. [DOI] [PubMed] [Google Scholar]
- Wang Z.-G.; Ding X.-J.; Huang Y.-Y.; Yan X.-J.; Ding B.; Li Q.-Z.; Xie C.-Z.; Xu J.-Y. The development of coumarin Schiff base system applied as highly selective fluorescent/colorimetric probes for Cu2+ and tumor biomarker glutathione detection. Dyes Pigm. 2020, 175, 108156 10.1016/j.dyepig.2019.108156. [DOI] [Google Scholar]
- Zhang Y.; Li L.; Wang J.; Jia L.; Yang R.; Guo X. A 4, 5-quinolimide-based fluorescent sensor for sequential detection of Cu2+ and cysteine in water and living cells with application in a memorized device. Spectrochim. Acta, Part A 2020, 230, 118030 10.1016/j.saa.2020.118030. [DOI] [PubMed] [Google Scholar]
- Baslak C.; Kursunlu A. N. A naked-eye fluorescent sensor for copper (II) ions based on a naphthalene conjugate Bodipy dye. Photochem. Photobiol. Sci. 2018, 17, 1091–1097. 10.1039/C8PP00137E. [DOI] [PubMed] [Google Scholar]
- Jung J.; Jo J.; Dinescu A. Rapid turn-on fluorescence detection of copper (II): Aromatic substituent effects on the response rate. Org. Process Res. Dev. 2017, 21, 1689–1693. 10.1021/acs.oprd.7b00269. [DOI] [Google Scholar]
- Xu Z.; Deng P.; Li J.; Tang S. Fluorescent ion-imprinted sensor for selective and sensitive detection of copper (II) ions. Sens. Actuators, B 2018, 255, 2095–2104. 10.1016/j.snb.2017.09.007. [DOI] [Google Scholar]
- Guan M.; Xu C.; Ma J.; Yang T.; Liu J.; Feng G. A Conjugated Polymer Fluorescent Sensor for Continuous Identification of Copper (II) and Pyrophosphate in Blood Serum and Synovial Fluid. Anal. Sci. 2019, 35, 625–630. 10.2116/analsci.18P576. [DOI] [PubMed] [Google Scholar]
- Kim H. J.; Quang D. T.; Hong J.; Kang G.; Ham S.; Kim J. S. Ratiometry of monomer/excimer emissions of dipyrenyl calix [4] arene in aqueous media. Tetrahedron 2007, 63, 10788–10792. 10.1016/j.tet.2007.05.130. [DOI] [Google Scholar]
- Fu Y.; Pang X.-X.; Wang Z.-Q.; Chai Q.; Ye F. A highly sensitive and selective fluorescent probe for determination of Cu (II) and application in live cell imaging. Spectrochim. Acta, Part A 2019, 208, 198–205. 10.1016/j.saa.2018.10.005. [DOI] [PubMed] [Google Scholar]
- Zhang B.; Liu H.; Wu F.; Hao G.; Chen Y.; Tan C.; Tan Y.; Jiang Y. A dual-response quinoline-based fluorescent sensor for the detection of Copper (II) and Iron (III) ions in aqueous medium. Sens. Actuators, B 2017, 243, 765–774. 10.1016/j.snb.2016.12.067. [DOI] [Google Scholar]
- Kim J. S.; Quang D. T. Calixarene-derived fluorescent probes. Chem. Rev. 2007, 107, 3780–3799. 10.1021/cr068046j. [DOI] [PubMed] [Google Scholar]
- Geissler P. L. Temperature dependence of inhomogeneous broadening: On the meaning of isosbestic points. J. Am. Chem. Soc. 2005, 127, 14930–14935. 10.1021/ja0545214. [DOI] [PubMed] [Google Scholar]
- Suzuki T.; Yamamoto R.; Higuchi H.; Hirota E.; Ohkita M.; Tsuji T. Electrochiroptical response of a hexaarylethane derivative with a helical π-skeleton: Drastic UV–Vis and CD spectral changes upon electrolysis of 4′, 5′-dibromodispiro [xanthene-9, 9′(9′ H, 10′ H)-phenanthrene-10′, 9 ″-xanthene]. J. Chem. Soc., Perkin Trans. 2 2002, 1937–1942. 10.1039/B204515J. [DOI] [Google Scholar]
- Kondo S.-i.; Nagamine M.; Karasawa S.; Ishihara M.; Unno M.; Yano Y. Anion recognition by 2, 2′-binaphthalene derivatives bearing urea and thiourea groups at 8-and 8′-positions by UV–vis and fluorescence spectroscopies. Tetrahedron 2011, 67, 943–950. 10.1016/j.tet.2010.12.004. [DOI] [Google Scholar]
- More K. N.; Lim T.-H.; Kang J.; Yun H.; Yee S.-T.; Chang D.-J. Asymmetric and Reduced Xanthene Fluorophores: Synthesis, Photochemical Properties, and Application to Activatable Fluorescent Probes for Detection of Nitroreductase. Molecules 2019, 24, 3206. 10.3390/molecules24173206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi A.; Ghasemi Z. A simple pyrimidine based colorimetric and fluorescent chemosensor for sequential detection of copper (II) and cyanide ions and its application in real samples. Spectrochim. Acta, Part A 2020, 228, 117730 10.1016/j.saa.2019.117730. [DOI] [PubMed] [Google Scholar]
- Kumar A.; Chae P. S.; Kumar S. A dual-responsive anthrapyridone-triazole-based probe for selective detection of Ni2+ and Cu2+: A mimetic system for molecular logic gates based on color change. Dyes Pigm. 2020, 174, 108092 10.1016/j.dyepig.2019.108092. [DOI] [Google Scholar]
- Xie H.-F.; Yu C.-J.; Huang Y.-L.; Xu H.; Zhang Q.-L.; Sun X.-H.; Feng X.; Redshaw C. A turn-off fluorescent probe for the detection of Cu 2+ based on a tetraphenylethylene-functionalized salicylaldehyde Schiff-base. Mater. Chem. Front. 2020, 4, 1500–1506. 10.1039/C9QM00759H. [DOI] [Google Scholar]
- Dong W.; Wang R.; Gong X.; Liang W.; Fan L.; Song S.; Dong C. A ratiometric and far-red fluorescence “off-on” sensor for sequential determination of copper (II) and L-histidine based on FRET system between N-acetyl-L-cysteine-capped AuNCs and N, S, P co-doped carbon dots. Microchim. Acta 2020, 187, 299 10.1007/s00604-020-04242-6. [DOI] [PubMed] [Google Scholar]
- Hien N. K.; Bao N. C.; Nhung N. T. A.; Trung N. T.; Nam P. C.; Duong T.; Kim J. S.; Quang D. T. A highly sensitive fluorescent chemosensor for simultaneous determination of Ag (I), Hg (II), and Cu (II) ions: Design, synthesis, characterization and application. Dyes Pigm. 2015, 116, 89–96. 10.1016/j.dyepig.2015.01.014. [DOI] [Google Scholar]
- Valeur B. Principles of steady-state and time-resolved fluorometric techniques. Mol. Fluorescence: Princ. Appl. 2001, 155–199. [Google Scholar]
- Mahar J.; Shabir G.; Channar P. A.; Saeed A.; Belfield K. D.; Irfan M.; Ul-Hamid A. Donor-Pi-Acceptor Fluorene Conjugates, Based on Chalcone and Pyrimidine Derivatives: an Insight into Structure-Property Relationship, Photophysical and Electrochemical Properties. J. Fluoresc. 2020, 30, 419–426. 10.1007/s10895-020-02516-z. [DOI] [PubMed] [Google Scholar]
- Adamo C.; Scuseria G. E.; Barone V. Accurate excitation energies from time-dependent density functional theory: Assessing the PBE0 model. J. Chem. Phys. 1999, 111, 2889–2899. 10.1063/1.479571. [DOI] [Google Scholar]
- Ali A.; Rafiq M. I.; Zhang Z.; Cao J.; Geng R.; Zhou B.; Tang W. TD-DFT benchmark for UV–visible spectra of fused-ring electron acceptors using global and range-separated hybrids. Phys. Chem. Chem. Phys. 2020, 22, 7864–7874. 10.1039/D0CP00060D. [DOI] [PubMed] [Google Scholar]
- Coskun D.; Jerome S. V.; Friesner R. A. Evaluation of the performance of the B3LYP, PBE0, and M06 DFT functionals, and DBLOC-corrected versions, in the calculation of redox potentials and spin splittings for transition metal containing systems. J. Chem. Theory Comput. 2016, 12, 1121–1128. 10.1021/acs.jctc.5b00782. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R.; Sreejalekshmi K. Computational Design, Synthesis, and Structure Property Evaluation of 1, 3-Thiazole-Based Color-Tunable Multi-heterocyclic Small Organic Fluorophores as Multifunctional Molecular Materials. J. Org. Chem. 2018, 83, 3453–3466. 10.1021/acs.joc.7b02978. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; J. Bloino G. Z.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; P J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Keith T.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas O.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford CT, 2009. [Google Scholar]
- Bader R. F. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. 10.1021/cr00005a013. [DOI] [Google Scholar]
- Wojtulewski S.; Grabowski S. J. DFT and AIM studies on two-ring resonance assisted hydrogen bonds. J. Mol. Struct: THEOCHEM 2003, 621, 285–291. 10.1016/S0166-1280(02)00608-5. [DOI] [Google Scholar]
- Koch U.; Popelier P. L. Characterization of CHO hydrogen bonds on the basis of the charge density. J. Phys. Chem. A. 1995, 99, 9747–9754. 10.1021/j100024a016. [DOI] [Google Scholar]
- Bader R. F. W.Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, 1990. [Google Scholar]
- Nhan D. T.; Hien N. K.; Van Duc H.; Nhung N. T. A.; Trung N. T.; Van D. U.; Shin W. S.; Kim J. S.; Quang D. T. A hemicyanine complex for the detection of thiol biomolecules by fluorescence. Dyes Pigm. 2016, 131, 301–306. 10.1016/j.dyepig.2016.03.050. [DOI] [Google Scholar]
- André J.-M.Exploring Aspects of Computational Chemistry: Concepts and Exercises 2 Volumes; Presses universitaires de Namur, 1997. [Google Scholar]
- Reed A. E.; Weinhold F. Natural localized molecular orbitals. J. Chem. Phys. 1985, 83, 1736–1740. 10.1063/1.449360. [DOI] [Google Scholar]
- Reed A. E.; Weinstock R. B.; Weinhold F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. 10.1063/1.449486. [DOI] [Google Scholar]
- Osman O. I. DFT study of the structure, reactivity, natural bond orbital and hyperpolarizability of thiazole azo dyes. Int. J. Mol. Sci. 2017, 18, 239. 10.3390/ijms18020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.