Abstract
Recently, lignin utilization in advanced applications has gained much interest. Due to the limitation of the current use of standard lignin, lignin particles have recently gained attention in overcoming the challenge. In this work, the spherical lignin particles obtained from organosolv lignin (OL) were prepared using the dialysis method with tetrahydrofuran (THF) or ethanol as the solvent. From the result, it was found that the types of lignin and solvent, initial lignin concentration, and stirring rate strongly affected the size of fabricated particles. Moreover, the preparation of lignin particles using THF as a solvent showed more uniform and symmetric spherical lignin particles. The stability of the particle dispersion was examined under various pH conditions. Moreover, lignin samples were also introduced into the poly(vinyl alcohol) (PVA) for the production of ultraviolet (UV)-blocking composite film. Mechanical and optical properties of composite film were also observed. As a result, the prepared lignin–PVA composite film showed great ultraviolet (UV) protective potential in both UVA and UVB regions.
1. Introduction
Lignin, asthe second most abundant component in biomass, is considered one of the main available renewable resources. Because of its phenolic hydroxyl and aromatic rich structure, lignin has gained attention in many new applications. Many researchers use lignin as an “additive” in chemicals or materials for industrial composites and admixtures. In the composite material, “lignin” has been added into polymers to improve their physical and chemical properties.1−3 Due to its antioxidant and antidegradation properties, not only the materials but also the addition of lignin in various type of commercial products including cement, paint, ink, dye, dispersant, etc. has recently gained interest.4,5 As the admixture, lignin has also been used in the generation of several synthetic polymeric units.6,7 Besides, the production of several advanced materials including resins, adhesives, and carbon fibers from lignin have also gained interest.8−12
However, the current use of lignin despite various potential applications is still limited due to its high complexity and nonuniform chemical structure.13,14 The structure of lignin can vary depending on various factors, including the origin of the lignin and the pretreatment processes. To overcome the problem, one possibility is to down-size the lignin into a submicron scale where the properties of lignin can be different from those of the natural lignin of the same composition.15 Several preparation methods of lignin particles have been studied in several research articles, including sonication, thermal carbonization, and dialysis method. For the sonication method, Gilca et al. used the ultrasonication method to modify lignin into lignin nanoparticles.16 Gonugunta et al. introduced the lignin-based carbon nanoparticles prepared by thermal carbonization of the freeze-dried lignin solution.17 Frangville et al. proposed the preparation of lignin nanoparticles using the dialysis method with ethylene glycol. As a result, the final lignin particles have an irregular shape, which may become a limitation in many applications if the spherical shape of the particle is required.18 For the spherical lignin particles, Lievonen et al. also prepared the lignin nanoparticles by dialysis method but using tetrahydrofuran (THF) instead of ethylene glycol.19 Wang et al. fabricated spherical enzymatic hydrolysis lignin-based nanoparticles by solution self-assembly in organic–aqueous solvent mixtures where dissolution and aggregation were observed under various organic–aqueous combination.20 Due to their unique properties, lignin particles have the potential to be used in multiple applications including biocide, surfactant, reinforcement, carrier, antioxidant, ultraviolet (UV)-blocker, etc. Because of the high content of UV-absorbing functional groups in its macromolecular structure and aromatic skeleton, lignin is considered as a natural UV blocker.21,22 Qian et al. suggested that the lignin with a higher number of methoxy groups has a larger UV transmittance rate in both UVA and UVB regions.23 Various investigations have suggested that the phenolic hydroxyl group of the lignin could scavenge free radicals, which promotes antioxidant and antimicrobial properties of the lignin.24−26
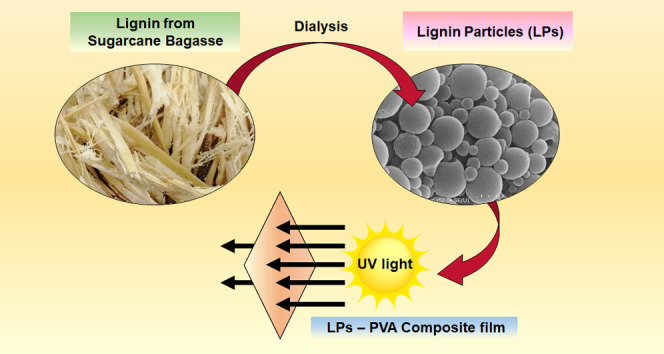
In this research, for the utilization of lignin, the lignin–poly(vinyl alcohol) (PVA) composite films were prepared. Two different technical lignins, commercial-grade organosolv lignin (OL) and synthesized sugarcane bagasse organosolv lignin (BL), were used with alkali lignin and compared. It is noted that the organosolv process is an effective procedure to isolate lignin from biomass since it offers high selectivity and purity of lignin products. Previously, several organic solvents including alcohols (e.g., methanol and ethanol), esters, and ketones have been studied for organosolv fractionation of different lignocellulosic materials.27,28 Recently, we have proposed a clean fractionation process, which can efficiently extract high-purity lignin (up to 89%) from local biomass, i.e., sugarcane bagasse, using a ternary mixture of water/ethanol/ethyl acetate in the presence of formic acid.29 The extracted lignin from this fractionation process can be used as a lignin source for several conversion processes, e.g., depolymerization to biobased valuable chemicals. The organosolv lignin particles (OLPs) and sugarcane bagasse organosolv lignin particles (BLPs) were fabricated using the dialysis process where THF and ethanol were used as solvents. Then, the lignin sample was incorporated into poly(vinyl alcohol) (PVA) for the production of composite films via a simple solvent-casting method. The UV-blocking efficiency and mechanical strength of the final composite film were evaluated (Scheme 1).
Scheme 1. Formation of Lignin Particle–PVA and Lignin–PVA Composite Films.
2. Results and Discussion
2.1. Synthesis and Characterization of Lignin Particles
2.1.1. Effect of Solvent on Particle Properties
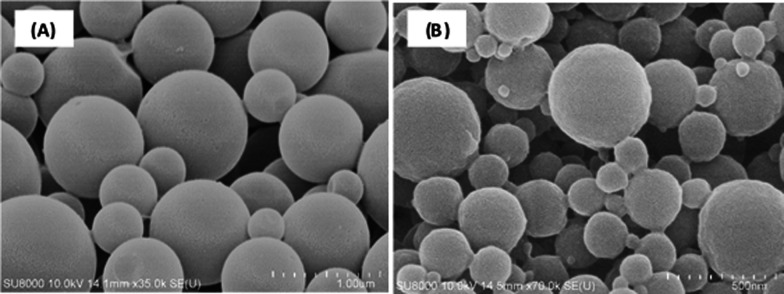
The difference in the chemical structures of lignin was dependent on various factors, including the type of wood, pretreatment process conditions, and separation methods. These differences affect the solubility of lignin and potentially the applicability of various particle fabrication methods. The lignin used in this study is organosolv lignin (OL), which can be well dissolved in THF and ethanol. Therefore, the lignin dispersion was prepared by solvent exchange via dialysis. The type of the solvent used for the solvent-exchange dialysis was observed to affect the final particle size. As shown in Figure 1, a more uniform and symmetric spherical lignin particles were obtained from the THF system compared with the final particle from the ethanol system owing to the polarity of the solvent. Less polarity of the THF resulted in it being more suitable for solvent-exchange interaction with water and might be an advantage for particle formation. In addition, negligible amounts of solvents (THF and ethanol) were detected in the final particle dispersions using GC–MS, indicating that they were removed during the preparation processes (data not shown).
Figure 1.
Scanning electron microscopy (SEM) image of lignin particles prepared from organosolv lignin dissolved in (A) THF and (B) ethanol using the dialysis method with rotational speeds of 300 rpm and initial lignin concentration of 10 g/L.
2.1.2. Effect of Initial Lignin Concentration on Lignin Particle Size
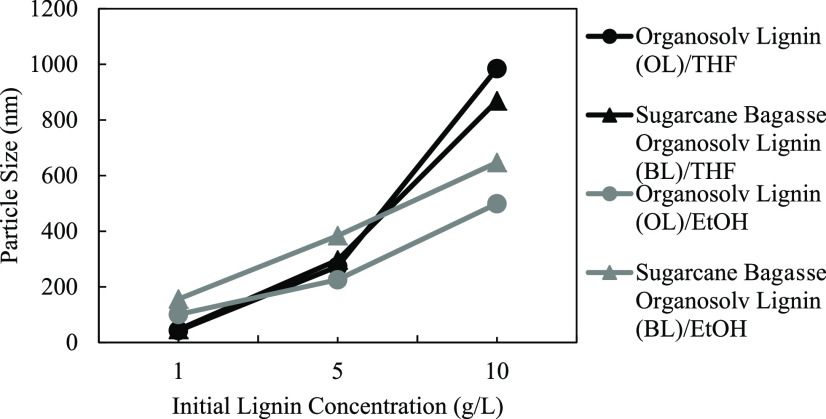
The influence of the initial lignin concentration on the lignin particulate diameter is shown in Figure 2. The results showed that the smallest average particle diameter was observed at an initial lignin concentration of 1 g/L. On the other hand, the largest particle size was observed at a concentration of 10 g/L for all conditions. In the dialysis process, the lignin particles may probably form via the nucleation growth mechanism as THF (or ethanol) was exchanged with deionized (DI) water. The increase in particle size with increasing initial lignin concentration could be due to the high amount of available lignin present in the system for the growth of particulate lignin. A similar trend could also be observed in various polymerization processes.30,31 On the other hand, the lower probability of particle formation at low initial lignin concentration could lead to the small size of fabricated particles. The same trend was observed both in THF and ethanol systems. It can also be seen from Figure 2 that the initial lignin concentration shows a much more significant effect on particle size in the THF system. The results can explain that the less polarity of THF made it more suitable for solvent-exchange interaction during dialysis compared with ethanol.
Figure 2.
Effect of the initial lignin concentration (1, 5, and 10 g/L) on lignin particle size using rotational speed of 300 rpm and THF as the solvent.
2.1.3. Effect of Dialysis Rotational Speed on the Lignin Particle Size
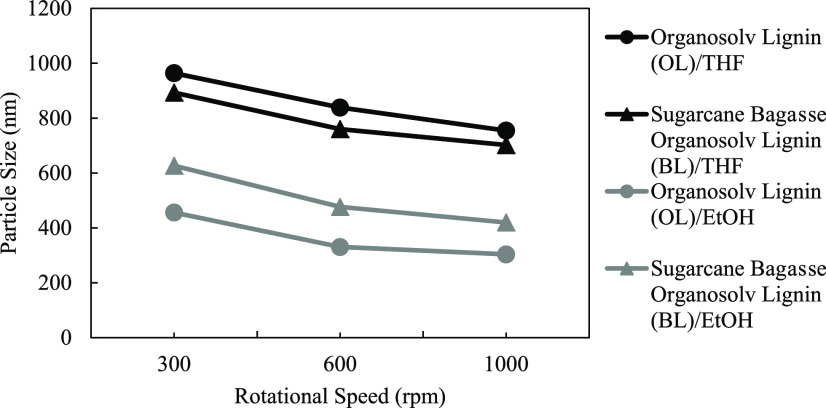
The effect of the stirring rate on the lignin particle size was observed at 300, 600, and 1000 rpm with a fixed initial lignin concentration of 10 g/L. Figure 3 shows that an increase in the stirring rate resulted in a decrease in the average size of lignin particles for both organosolv lignin (OL) and sugarcane bagasse organosolv lignin (BL). The same trend was observed with both THF and ethanol conditions. The results can be explained wherein the higher stirring rate conditions cause a greater shear force that leads to a smaller size of lignin particle.32
Figure 3.
Effect of dialysis rotational speed (300, 600, and 1000 rpm) on the lignin particle size using an initial lignin concentration of 10 g/L and THF as the solvent.
2.1.4. Effect of Time and pH on Lignin Particle Stability
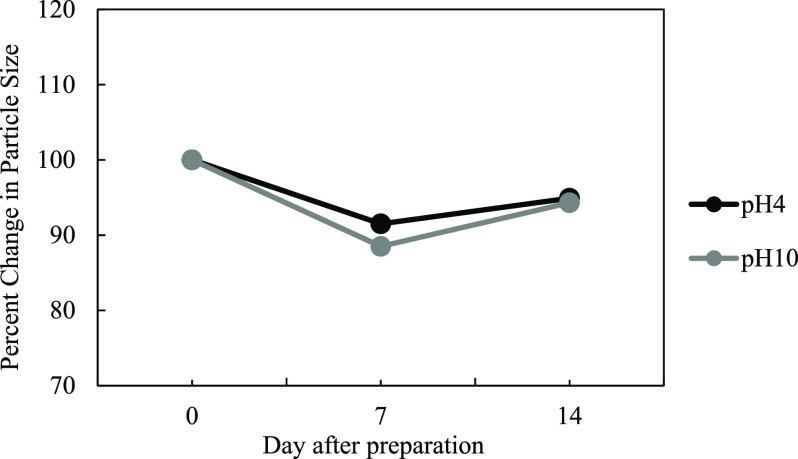
For the use of lignin particles in various fields, including biomedical, food additive, biocide, etc., the study of the stability of particle dispersion over time and pH values is required. An electrical double-layer repulsion force was formed in the dispersion, which helps to promote the stability of the particulate lignin. In this study, the stability of lignin was observed at pH 4 and 10 for 14 days using a particle with an average diameter of 500 nm. As shown in Figure 4, the prepared lignin dispersion is quite stable in both conditions, where no significant change is observed over 14 days of the experiment. Moreover, as shown in Figure 5, the negative ζ-potential is observed for both of the conditions (approximately −50 mV). It was reported that the hydroxyl ion adsorption on the particle surface in the lignin dispersion resulted in the negative charge observed.19 The small decrease in the ζ-potential value for both of the conditions (pH 4 and 10) was observed over a period of time. From all the above results, it can be concluded that the lignin dispersion was relatively stable and insensitive to the pH values (4 and 10), which correlate well with the results reported in previous studies.19,33
Figure 4.
Effect of pH on the average lignin particle size.
Figure 5.
Effect of pH on the ζ-potential of lignin particles.
2.2. Fabrication and Characterization of Lignin–PVA Composite Film
2.2.1. Mechanical Performance
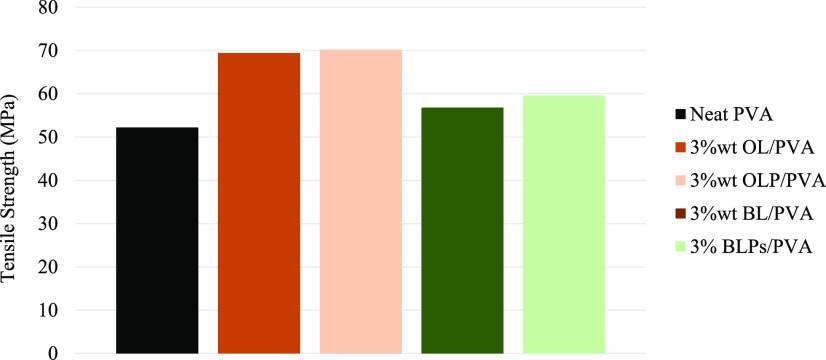
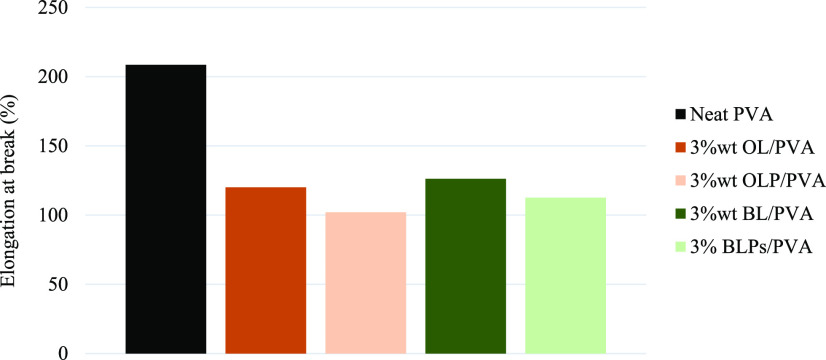
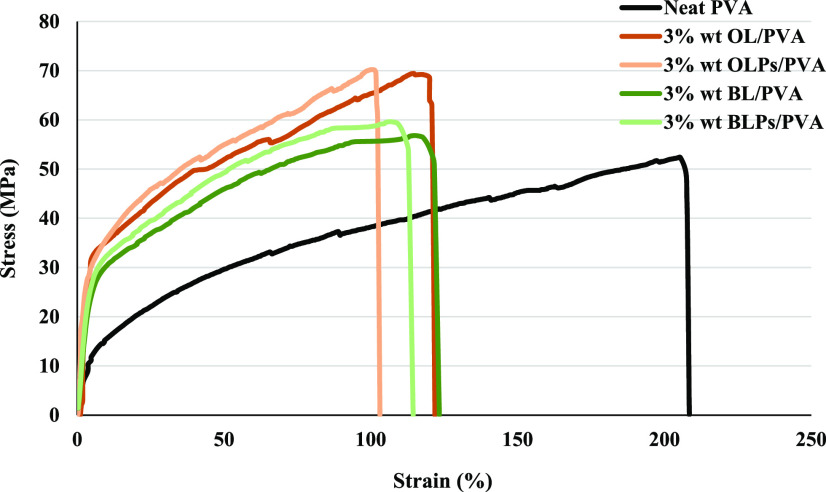
In Figure 6, it can be seen that the lignin–PVA composite film had a higher tensile strength compared with the neat PVA for both organosolv lignins. A slightly higher tensile strength in the lignin particle–PVA film was observed compared with that in the lignin–PVA film. The results can be explained by the good dispersion state and the increased surface area of the particulate lignin that potentially strengthen their compatibility with the polymer matrix, resulting in better mechanical performance.34,35 Moreover, the increasing lignin concentration resulted in a higher tensile strength of the composite film (data not shown). As shown in Figure 7, both the lignin–PVA and the lignin particle–PVA show lower elongations at break compared with the neat PVA. The presence of lignin particles seems to decrease the elongation at the break of the composite film. The experiment results showed that a higher lignin content caused lower elongation at the break of the film (data not shown) (Figure 8). 8
Figure 6.
Comparison of the tensile strength of neat PVA, lignin–PVA composite film, and lignin particle–PVA composite film.
Figure 7.
Comparison of the elongation at the break of neat PVA, lignin–PVA composite film, and lignin particle–PVA composite film.
Figure 8.
Stress–strain curves of the PVA and the composite films.
2.2.2. UV-absorbance Properties
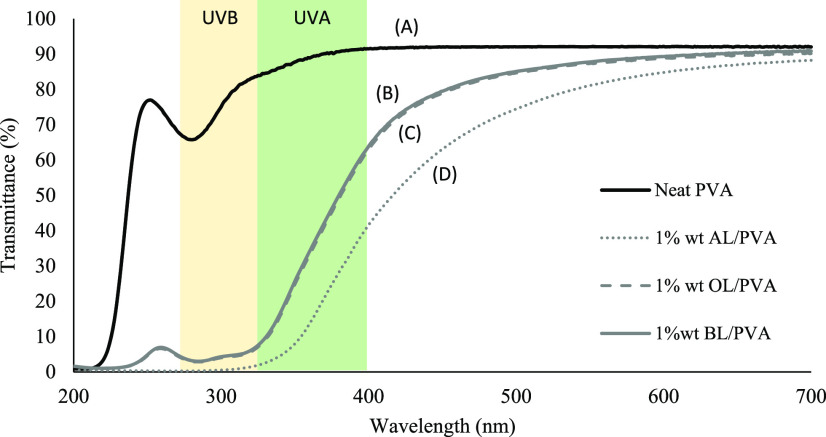
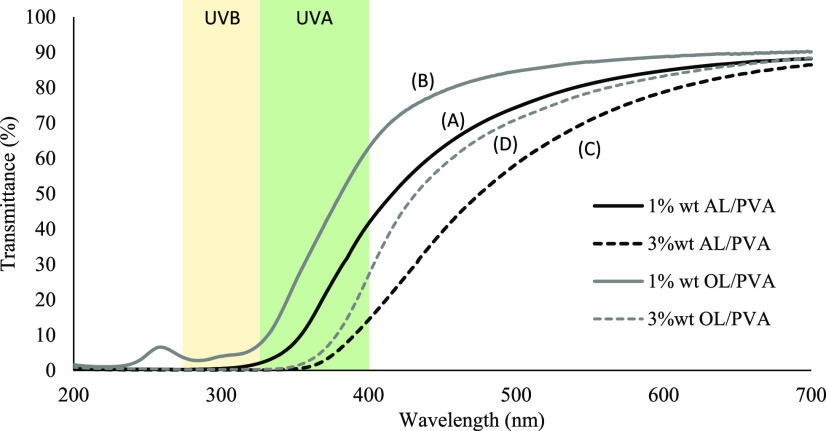
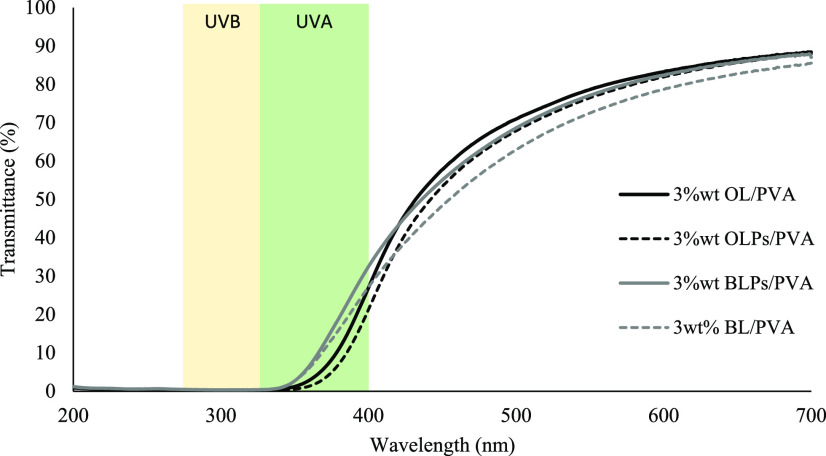
As shown in Figure 9, when all of the fabricated composite films are exposed to UV–vis light at 200–700 nm wavelength, the prepared lignin–PVA film is efficient in blocking the UV light of 280–400 nm wavelength, including both UVA and UVB regions, compared with the neat PVA sample. The obtained results can suggest that the blocking capacity could be due to the high amount of UV-absorbing functional groups such as phenolic, ketone, and other chromophores in lignin.36,37 The results also showed that the blocking efficiency of the alkali lignin was much higher than that of organosolv lignin in the PVA composite film with the same concentration. The increase in lignin content exhibits high UV absorbance in the UVB region for both alkali and organosolv lignin, as shown in Figure 10. Moreover, the presence of the lignin particle also leads to higher UV blocking efficiency, as shown in Figure 11. All of the results showed that the addition of particulate lignin could provide an efficient UV-blocking ability for the PVA composite film.19,34
Figure 9.
Comparison of the UV–vis light transmittance spectra of (A) neat PVA, (B) 1 wt % alkali lignin–PVA, (C) 1 wt % organosolv lignin–PVA, and (D) 1 wt % sugarcane bagasse organosolv lignin–PVA composite film.
Figure 10.
Comparison of the UV–vis light transmittance spectra of (A) 1 wt % alkali lignin–PVA, (B) 1 wt % organosolv lignin–PVA, (C) 3 wt % alkali lignin–PVA, and (D) 1 wt % organosolv lignin–PVA composite film.
Figure 11.
Comparison of the UV–vis light transmittance spectra between lignin–PVA and lignin particle–PVA composite film at 3 wt %.
2.2.3. Interaction between Lignin and PVA Matrix
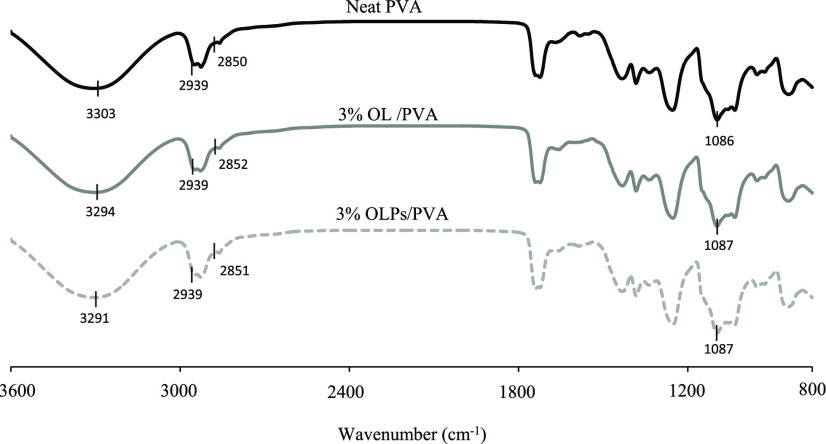
According to the previous studies, the surfaces of the lignin particles were mainly composed of hydrophilic hydroxyl groups, which have a strong ability to interact with the hydroxyl groups in the PVA matrix.38,39 Due to the Fourier transform infrared (FTIR) spectroscopy analysis, the neat PVA showed an absorption band at 3303.33 cm–1 in the hydroxyl stretching region. Otherwise, this band shifts to a lower wavenumber region upon mixing with lignin at 3294.73 and 3291.93 cm–1 for OL/PVA and OLPs/PVA, respectively. The results indicated the presence of the hydrogen bond between the lignin and the PVA matrix, which suggested the formation of a hydrogen bond between the hydroxyl groups of the lignin and the PVA matrix. This formation of a new hydrogen bond between the lignin and the polymer matrix has been confirmed by several studies.40−43 The small bands at 2939.92 cm–1 (neat PVA), 2939.60 cm–1 (OL/PVA), and 2939.01 cm–1 (OLPs/PVA) are assigned to the characteristic bands of CH2 stretching vibration, while 2850.92 cm–1 (neat PVA), 2852.03 cm–1 (OL/PVA), and 2851.42 cm–1 (OLPs/PVA) are related to the stretching vibrations of the C–H. The bands observed at 1086.90 cm–1 (neat PVA), 1087.64 cm–1 (OL/PVA), and 1087.57 cm–1 (OLPs/PVA) were recognized as C–O stretching vibration in which similar results were also reported (Figure 12).40,44
Figure 12.
FTIR spectra of the neat PVA and their composite films with 3 wt % organosolv lignin and organosolv lignin particles.
3. Conclusions
In this study, the spherical lignin particle was prepared from organosolv lignin (OL) and sugarcane bagasse organosolv lignin (BL) using the dialysis method where THF and ethanol were used as solvents. The size of the final particles varied due to several factors, including initial lignin concentration, stirring rate, and type of solvent. The result showed that the fabricated lignin–PVA and lignin particle–PVA showed an improvement in the tensile strength and the UV absorbance compared with a neat PVA sample in which it can be suggested that lignin is an excellent candidate for producing advance UV-protection composite material.
4. Experimental Section
4.1. Materials
The materials used in this study included commercial-grade alkali lignin (AL) (Sigma Aldrich), commercial-grade organosolv lignin (OL) (Chemical Point UG, Germany), and sugarcane bagasse (PTT Global Chemicals PCL, Thailand). The chemicals included tetrahydrofuran (THF) (99.8%) (Ajax) and ethanol (95%) (Emsure). Deionized water was received from the Faculty of Engineering, Mahidol University. Dialysis bag (MWCO 6000–8000) was purchased from Membrane Filtration Product, Inc.
4.2. Preparation of Sugarcane Bagasse Organosolv Lignin29
The developed fractionation process was used to extract lignin from bagasse. Ten grams of bagasse was introduced into 100 mL of the single-phase organic solvent mixture containing water/ethanol/ethyl acetate in the ratio of 2:1:1 in the presence of formic acid. To adjust the pressure to 20 bar, nitrogen gas was then introduced into the reactor. The reaction was performed at 160 °C for 30 min. To stop the reaction, the reactor was then quenched in a water bath. To separate the cellulose, the solid cellulose-enriched fraction was filtrated and washed with water/ethyl acetate mixture solution (2:1 v/v). For the phase separation of the aqueous phase, water was added until phase separation was obtained followed by stirring and placing at 30 °C for 20 min. The separated organic phase was dried at 105 °C to obtain isolated lignin. Important characteristics of isolated lignin were reported elsewhere.29
4.3. Preparation of Lignin Particles
Lignin was dissolved in either THF or ethanol at various concentrations of 1, 5, and 10 g/L and transferred to a dialysis bag (Spectra/Por 1 Standard RC Dry Dialysis Tubing, 6–8 kDa, Spectrum Labs) before being submerged in deionized water (DI water). The dialysis process was carried out approximately 24 h using a certain stirring rate (300, 600, and 1000 rpm) in a fume hood where the formation of particulate lignin was observed. After the dialysis process, the residual amount of THF (or ethanol) in the solution was detected using gas chromatography–mass spectrometry (GC–MS). The effect of initial lignin concentration on the lignin particle size was confirmed by repeating the experiments. The mean particle size and the stability of the fabricated lignin particles were measured using the Malvern Zetasizer Nano-ZS90 instrument. In this study, the stability of the dispersion was observed under various pH conditions, including pH values of 4 and 10. All of the experimental conditions were repeated to confirm the reproducibility. Also, the shape and surface morphology of the fabricated particle were examined using the scanning electron microscopy (SEM) images taken using JEOL JSM-5600 SEM with the freeze-dried sample.
4.4. Preparation and Characterization of Lignin Particle–PVA Composite Films
The simple solvent-casing method was used as the preparation method for the lignin–PVA composite films. The PVA was dissolved in deionized water (DI water) with vigorous stirring at a rotational speed of 600 rpm at 90 °C for 3 h. The required amount of lignin (or lignin particles) and PVA solution was mixed to prepare 1 and 3% w/w lignin–PVA composite films. Then, the homogeneous mixture was transferred into the glass plate. For the evaporation processes, the prepared sample was dried for 24 h at room temperature and 40 °C in an oven for another 24 h. The fabricated composite film was uniform with an average thickness of about 50 μm. For optical properties, the UV–vis spectrometer was used to observe the UV-shielding performance and transparency of the composite films. Mechanical properties, including tensile strength and elongation at break, were examined using Universal Testing Machine (UTM). Repeated measurements were done to confirm the reproductivity, and the averaged results were presented. Moreover, the Fourier transform infrared (FTIR) spectra were also recorded to observe the interaction between lignin (or lignin particle) and the PVA matrix.
Acknowledgments
This research project is supported by the Mahidol University.
The authors declare no competing financial interest.
References
- Sun Y.; Yang L.; Lu X.; He C. Biodegradable and renewable poly(lactide)–lignin composites: synthesis, interface and toughening mechanism. J. Mater. Chem. A 2015, 3, 3699–3709. 10.1039/C4TA05991C. [DOI] [Google Scholar]
- Chung Y.-L.; Olsson J.; Li R.; Frank C.; Waymouth R.; Billington S.; Sattely E. A Renewable Lignin-PLA Copolymer and Application in Biobased Composites. ACS Sustainable Chem. Eng. 2013, 1, 1231–1238. 10.1021/sc4000835. [DOI] [Google Scholar]
- Al Mamun A.; Nikousaleh M. A.; Feldmann M.; Rüppel A.; Sauer V.; Kleinhans S.; Heim H.-P.. Lignin Reinforcement in Bioplastic Composites. In Lignin in Polymer Composites; Faruk O.; Sain M., Eds.; William Andrew Publishing, 2016; pp 153–165. [Google Scholar]
- Wells T.; Kosa M.; Ragauskas A. Polymerization of Kraft lignin via ultrasonication for high-molecular- weight applications. Ultrason. Sonochem. 2013, 20, 1463–1469. 10.1016/j.ultsonch.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Belgacem M. N.; Blayo A.; Gandini A. Organosolv lignin as a filler in inks, varnishes and paints. Ind. Crops Prod. 2003, 18, 145–153. 10.1016/S0926-6690(03)00042-6. [DOI] [Google Scholar]
- Samal S. K.; Fernandes E. G.; Corti A.; Chiellini E. Bio-based Polyethylene–Lignin Composites Containing a Pro-oxidant/Pro-degradant Additive: Preparation and Characterization. J. Polym. Environ. 2014, 22, 58–68. 10.1007/s10924-013-0620-0. [DOI] [Google Scholar]
- Windeisen E.; Wegener G.. Lignin as Building Unit for Polymers. In Polymer Science: A Comprehensive Reference, 1st ed.; Elsevier, 2012; Vol. 10, pp 255–265. [Google Scholar]
- Bengtsson A.; Bengtsson J.; Sedin M.; Sjöholm E. Carbon Fibers from Lignin-Cellulose Precursors: Effect of Stabilization Conditions. ACS Sustainable Chem. Eng. 2019, 7, 8440–8448. 10.1021/acssuschemeng.9b00108. [DOI] [Google Scholar]
- Dai Z.; Shi X.; Liu H.; Li H.; Han Y.; Zhou J. High-strength lignin-based carbon fibers via a low-energy method. RSC Adv. 2018, 8, 1218–1224. 10.1039/C7RA10821D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffar S.; Fan M. Lignin in straw and its applications as an adhesive. Int. J. Adhes. Adhes. 2014, 48, 92–101. 10.1016/j.ijadhadh.2013.09.001. [DOI] [Google Scholar]
- Kadla J. F.; Kubo S.; Venditti R. A.; Gilbert R. D.; Compere A. L.; Griffith W. Lignin-based carbon fibers for composite fiber applications. Carbon 2002, 40, 2913–2920. 10.1016/S0008-6223(02)00248-8. [DOI] [Google Scholar]
- Khan T. A.; Lee J.-H.; Kim H.-J.. Lignin-Based Adhesives and Coatings. In Lignocellulose for Future Bioeconomy; Ariffin H.; Sapuan S. M.; Hassan M. A., Eds.; Elsevier, 2019; pp 153–206. [Google Scholar]
- FitzPatrick M.; Champagne P.; Cunningham M. F.; Whitney R. A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. 10.1016/j.biortech.2010.06.125. [DOI] [PubMed] [Google Scholar]
- Vishtal A. G.; Kraslawski A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar]
- Hussain F.; Hojjati M.; Okamoto M.; Gorga R. E. Review article: Polymer-matrix Nanocomposites, Processing, Manufacturing, and Application: An Overview. J. Compos. Mater. 2006, 40, 1511–1575. 10.1177/0021998306067321. [DOI] [Google Scholar]
- Gilca I. A.; Popa V. I.; Crestini C. Obtaining lignin nanoparticles by sonication. Ultrason. Sonochem. 2015, 23, 369–375. 10.1016/j.ultsonch.2014.08.021. [DOI] [PubMed] [Google Scholar]
- Gonugunta P.; Vivekanandhan S.; Mohanty A.; Misra M. A Study on Synthesis and Characterization of Biobased Carbon Nanoparticles from Lignin. World J. Nano Sci. Eng. 2012, 2, 148–153. 10.4236/wjnse.2012.23019. [DOI] [Google Scholar]
- Frangville C.; Rutkevičius M.; Richter A. P.; Velev O. D.; Stoyanov S. D.; Paunov V. N. Fabrication of Environmentally Biodegradable Lignin Nanoparticles. ChemPhysChem 2012, 13, 4235–4243. 10.1002/cphc.201200537. [DOI] [PubMed] [Google Scholar]
- Lievonen M.; Valle-Delgado J. J.; Mattinen M.-L.; Hult E.-L.; Lintinen K.; Kostiainen M. A.; Paananen A.; Szilvay G. R.; Setälä H.; Österberg M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. 10.1039/C5GC01436K. [DOI] [Google Scholar]
- Wang J.; Qian Y.; Li L.; Qiu X.. Atomic Force Microscopy and Molecular Dynamics Simulations for Study of Lignin Solution Self-Assembly Mechanisms in Organic–Aqueous Solvent Mixtures ChemSusChem 2020, 10.1002/cssc.201903132. [DOI] [PubMed]
- Qian Y.; Qiu X.; Zhu S. Lignin: a nature-inspired sun blocker for broad-spectrum sunscreens. Green Chem. 2015, 17, 320–324. 10.1039/C4GC01333F. [DOI] [Google Scholar]
- Wu Y.; Qian Y.; Lou H.; Yang D.; Qiu X. Enhancing the Broad-Spectrum Adsorption of Lignin through Methoxyl Activation, Grafting Modification, and Reverse Self-Assembly. ACS Sustainable Chem. Eng. 2019, 7, 15966–15973. 10.1021/acssuschemeng.9b02317. [DOI] [Google Scholar]
- Qian Y.; Qiu X.; Zhu S. Sunscreen Performance of Lignin from Different Technical Resources and Their General Synergistic Effect with Synthetic Sunscreens. ACS Sustainable Chem. Eng. 2016, 4, 4029–4035. 10.1021/acssuschemeng.6b00934. [DOI] [Google Scholar]
- Yang W.; Fortunati E.; Dominici F.; Giovanale G.; Mazzaglia A.; Balestra G. M.; Kenny J. M.; Puglia D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. 10.1016/j.ijbiomac.2016.04.068. [DOI] [PubMed] [Google Scholar]
- Klein S. E.; Rumpf J.; Alzagameem A.; Rehahn M.; Schulze M. Antioxidant activity of unmodified kraft and organosolv lignins to be used as sustainable components for polyurethane coatings. J. Coat. Technol. Res. 2019, 16, 1543–1552. 10.1007/s11998-019-00201-w. [DOI] [Google Scholar]
- Chen K.; Qiu X.; Yang D.; Qian Y.. Amino acid-functionalized polyampholytes as natural broad-spectrum antimicrobial agents for high-efficient personal protection Green Chem. 2020, 10.1039/D0GC01505A. [DOI]
- Wildschut J.; Smit A. T.; Reith J. H.; Huijgen W. J. Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour. Technol. 2013, 135, 58–66. 10.1016/j.biortech.2012.10.050. [DOI] [PubMed] [Google Scholar]
- Boeriu C. G.; Fitigau F.; Gosselink R. J. A.; Frissen A. E.; Stoutjesdijk J. H.; Peter F. Fractionation of five technical lignins by selective extraction in green solvents and characterization of isolated fractions. Ind. Crops Prod. 2014, 62, 481–490. 10.1016/j.indcrop.2014.09.019. [DOI] [Google Scholar]
- Suriyachai N.; Champreda V.; Kraikul N.; Techanan W.; Laosiripojana N. Fractionation of lignocellulosic biopolymers from sugarcane bagasse using formic acid-catalyzed organosolv process. 3 Biotech. 2018, 8, 221. 10.1007/s13205-018-1244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F.; Yang X.; Huang W. Synthesis of Narrow or Monodisperse Poly(divinylbenzene) Microspheres by Distillation–Precipitation Polymerization. Macromolecules 2004, 37, 9746–9752. 10.1021/ma048566l. [DOI] [Google Scholar]
- Dziomkina N. V.; Hempenius M. A.; Vancso G. J. Synthesis of cationic core-shell latex particles. Eur. Polym. J. 2006, 42, 81–91. 10.1016/j.eurpolymj.2005.07.015. [DOI] [Google Scholar]
- Mateovic T.; Kriznar B.; Bogataj M.; Mrhar A. The influence of stirring rate on biopharmaceutical properties of Eudragit RS microspheres. J. Microencapsulation 2002, 19, 29–36. 10.1080/02652040010055289. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y.; Katoh Y.; Ohshima H. Colloidal stability of aqueous polymeric dispersions: Effect of pH and salt concentration. Colloids Surf. 2005, 42, 53–58. 10.1016/j.colsurfb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Yang W.; Mazzaglia A.; Puglia D.; Fortunati E.; Balestra G. M.; Kenny J. M.; Owczarek J. S.; Torre L.; Kozanecki M. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. 10.1016/j.indcrop.2016.09.061. [DOI] [Google Scholar]
- Zhao W.; Simmons B.; Singh S.; Ragauskas A.; Cheng G. From lignin association to nano-/micro-particle preparation: extracting higher value of lignin. Green Chem. 2016, 18, 5693–5700. 10.1039/C6GC01813K. [DOI] [Google Scholar]
- Barsberg S.; Elder T.; Felby C. Lignin–Quinone Interactions: Implications for Optical Properties of Lignin. Chem. Mater. 2003, 15, 649–655. 10.1021/cm021162s. [DOI] [Google Scholar]
- Lanzalunga O.; Bietti M. Photo- and radiation chemical induced degradation of lignin model compounds. J. Photochem. Photobiol., B 2000, 56, 85–108. 10.1016/S1011-1344(00)00054-3. [DOI] [PubMed] [Google Scholar]
- Qian Y.; Deng Y.; Qiu X.; Li H.; Yang D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. 10.1039/c3gc42131g. [DOI] [Google Scholar]
- Tian D.; Hu J.; Bao J.; Chandra R. P.; Saddler J. N.; Lu C. Lignin valorization: lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10, 192 10.1186/s13068-017-0876-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbag I.; Mohamed Saleh S. Studies on the formation of intermolecular interactions and structural characterization of polyvinyl alcohol/lignin film. Int. J. Environ. Stud. 2016, 73, 226–235. 10.1080/00207233.2016.1143700. [DOI] [Google Scholar]
- Kubo S.; Kadla J. F. The Formation of Strong Intermolecular Interactions in Immiscible Blends of Poly(vinyl alcohol) (PVA) and Lignin. Biomacromolecules 2003, 4, 561–567. 10.1021/bm025727p. [DOI] [PubMed] [Google Scholar]
- Su L.; Xing Z.; Wang D.; Xu G.; Ren S.; Fang G. Mechanical Properties Research and Structural Characterization of Alkali Lignin/Poly(vinyl alcohol) Reaction Films. BioResources 2013, 8, 3532–3543. 10.15376/biores.8.3.3532-3543. [DOI] [Google Scholar]
- Xu G.; Ren S.; Wang D.; Sun L.; Fang G. Fabrication and Properties of Alkaline Lignin/Poly (Vinyl Alcohol) Blend Membranes. BioResources 2013, 8, 2510–2520. 10.15376/biores.8.2.2510-2520. [DOI] [Google Scholar]
- Reis E. F. d.; Campos F. S.; Lage A. P.; Leite R. C.; Heneine L. G.; Vasconcelos W. L.; Lobato Z. I. P.; Mansur H. S. Synthesis and characterization of poly (vinyl alcohol) hydrogels and hybrids for rMPB70 protein adsorption. Mater. Res. 2006, 9, 185–191. 10.1590/S1516-14392006000200014. [DOI] [Google Scholar]