Summary
Context
The 68Ga-labelled somatostatin analogues (68Ga-DOTA-SSAs) is becoming popular as an important diagnostic tool in neuroendocrine tumours as evidenced by a growing number of reports detailing institutional experience with various DOTA peptides. However, only few prospective studies have compared 68Ga-DOTA-SSAs and somatostatin receptor scintigraphy (SRS) in gastroenteropancreatic neuroendocrine tumours (GEP-NETs) and pulmonary neuroendocrine tumours.
Objective
The aim of our prospective study was to perform head-to-head comparison between 68 Ga-DOTATATE PET/CT and standard imaging work-up (SI) that included multiphasic CT, liver MRI and SRS using single photon emission computed tomography.
Design
In this prospective study, the patients were enrolled only if they met any of the following inclusion criteria: (i) initial staging of a NETs without distant metastases on SI or neuroendocrine tumour with unknown primary on SI; (ii) restaging of NETs that could be treated by focused therapeutic interventions; (iii) elevated serum neuroendocrine hormones or peptides. The exclusion criteria was grade 3 GEP-NETs.
Results
Thirty-two patients were enrolled in the study. Eleven patients (6 pancreas, 4 ileum, 1 duodenal) were included for initial evaluation and staging of NETs, 8 patients (5 pancreas, 1 ileal, 1 lung, 1 duodenal gastrinoma) for restaging, and 13 patients for elevated serum neuroendocrine biomarkers (5 ectopic Cushing’s syndrome, 5 organic hypoglycaemia, 1 patient each with elevated vasoactive inhibitory peptide, chromogranin A and neuron-specific enolase). 68Ga-DOTATATE PET/CT detected more primary tumours than SRS (15/18 vs 10/18: P = .074). The missed tumours on 68Ga-DOTATATE PET/CT were located in the lung in two cases and duodenum in one case. For other anatomical regions (nodal and distant metastasis), no statistical difference was observed between imaging modalities using 68Ga-DOTATATE PET/CT and SRS. Overall, 68Ga-DOTATATE PET/CT+CT+MRI detected 31/33 of the involved regions (including primaries) (29 and 22 for 68Ga-DOTATATE and SRS, respectively).
Conclusion
Our study shows that 68Ga-DOTATATE PET/CT detected similar number of sites than combination of SRS, liver MRI and thoraco-abdominopelvic CT on region-based analysis. 68Ga-DOTATATE PET/CT missed half of primary lung carcinoids with ectopic Cushing’s syndrome.
Keywords: biomarker, gallium radiopharmaceuticals, neuroendocrine tumour, positron emission tomography, somatostatin
1 |. INTRODUCTION
Gastroenteropancreatic and pulmonary neuroendocrine tumours (NETs) are a heterogeneous group of neoplasms that arise from endoderm-derived cells. These tumours can synthesize and secrete hormones or other substances and are characterized by a wide spectrum of clinical manifestations. Despite their low incidence, their prevalence can be high due to their slow-growing pattern and prolonged survival.1 These tumours may be diagnostically challenging since primary or secondary lesions can be millimetric in size. Furthemore, it is known that anatomic imaging can fail to locate these extremely small tumours, especially those located in the midgut.2 The role of imaging is to provide the most comprehensive and precise evaluation at initial staging and re-staging of NETs. To achieve this goal, imaging work-up can rely on various imaging techniques depending upon clinical presentations suggestive of the primary tumour location as well as its pathologic grade suggesting its future behaviour, including the development of metastatic disease.
The functional imaging protocol for evaluation of these patients has historically relied on somatostatin receptor scintigraphy (SRS). However, the sensitivity of SRS may be suboptimal, especially for NETs of midgut origin. Novel PET radiotracers like 18F-fluorodopa (18F-FDOPA) and 68Ga-labelled somatostatin analogues (68Ga-DOTA-SSAs) appear promising. 18F-FDOPA PET/CT performed better than SRS and CT of midgut NETs but may have suboptimal sensitivity in some pancreatic NETs.2 Several studies have shown that 68Ga-DOTA-SSAs like 68Ga-DOTATATE,3–7 68Ga-DOTATOC8–11 and 68Ga-DOTANOC12 have a high detection rate and a high impact on patient management, especially in NETs with unknown primary or in potential candidates for curative surgery or requiring liver transplantation.
Although these results have given a great impetus for switching from single-photon emission computed tomography (SPECT/CT) to positron emission tomography-computed tomography (PET/CT), only few prospective studies have been published6 and most included patients had widespread metastatic disease.
Thus, the aim of our prospective study was to perform head-to-head comparison between 68Ga-DOTATATE and standard imaging work-up (SI), including SRS with SPECT/CT, multiphasic CT and liver MRI.
2 |. METHODS
2.1 |. Eligibility criteria
The inclusion criteria were:
age ≥ 18
One of the following situations: (i) initial staging of a gastroenteropancreatic neuroendocrine tumour (GEP-NET) or pulmonary neuroendocrine tumour without distant metastases on SI or neuroendocrine tumour with unknown primary on SI; (ii) restaging of GEP-NET or pulmonary NET that could be treated by focused therapeutic interventions (i.e. surgical approaches like resection of primary with curative intent, total or subtotal organectomy, interventional radiology procedures, radiotherapy); (iii) elevated serum neuroendocrine hormones or peptides.
-
Standard imaging (SI) work-up performed <3 months prior to 68Ga-DOTATATE PET/CT, including multiphasic thoraco-abdomino-pelvic CT scan, somatostatin receptor scintigraphy (SRS), and liver MRI.
The exclusion criteria were:
pregnant or breast-feeding women,
non-GEP or pulmonary NET (ie paraganglioma/pheochromocy-toma, medullary thyroid cancer),
grade 3 GEP-NET.
2.2 |. Study design and goals
The present study is a prospective, open-label single centre study. All patients were evaluated by SRS, multiphasic thoraco-abdominopelvic CT scan, liver MRI and 68Ga-DOTATATE PET/CT. During the study period, PET/CT and SI examinations were interpreted in standard clinical fashion with knowledge of the patients’ clinical context and available imaging studies. The study was approved by the local ethics committee (Comité de ClinicalTrials.gov des Personnes Sud-Méditerranée II) and the French drug and device Regulation Agency (ANSM). The study has been registered at ClinicalTrials.gov (identifier: NCT02150408). Informed consent was obtained from all individual participants included in the study. All patients gave their signed informed consent for participation. The specific goals of the study were to determine the value of 68Ga-DOTATATE PET/CT in the detection of primary NETs, lymph nodes (LNs) and distant metastases that were not picked up by other imaging modalities that might change clinical course and management of NETs.
2.3 |. 68Ga-DOTATATE preparation
The whole 68Ga-DOTATATE preparation process was automated through an EluSynthGa-68 synthesis module (Iason) placed in a shielded hot cell under class-A/ISO4.8 conditions in our radiopharmacy unit.
Fifty μg of DOTATATE lyophilisate (Iason) was dissolved in water for injection and 1.14M acetate buffer was added to the peptide solution. Prior to synthesis, this solution was transferred into a module reactor. 68Ga chloride was eluted from a 68Ga/68Ge generator (Galliapharm®, Eckert&Ziegler). The resulting 68Ga chloride solution was prepurified onto a preconditioned SCX cartridge (Bond-Elut SCX, Agilent) and eluted into the reaction vial with the DOTA peptide.
The labelling reaction occured at 95°C, pH 4.5 for 8 minutes. After the labelling, the raw product was loaded onto a RP tC18 cartridge (Wat03605, Waters). The product was washed with water for injection (5 mL twice).
Desorption of the product from the reversed phase cartridge was achieved by washing with 2 mL aqueous 30% ethanol solution into the collection vial, which contained 10 mL 0.9% sodium chloride for injection. This solution was then sterile-filtered through a 0.22 μm membrane filter (Millex-GV 33 mm PVDF 0.22 μm, EMD Millipore) and collected in a sterile product vial. Quality controls included organoleptic controls, activity measurements, physical half-life measurement, bubble-test of the sterilizing filter, pH assessment, radiochemical purity assessed by HPLC in a water/acetonitrile gradient and radionuclidic purity by ɣ-radioactivity-TLC-scanner. The solution was also checked for endotoxins and microbiological safety.
2.4 |. SPECT/CT protocol and reconstruction parameters
[111In]In-pentetreotide (OCTREOSCAN®, Curium) was prepared according to the manufacturer’s instructions. After reconstitution and labelling, quality control included organoleptic controls, activity measurements, pH assessment, radiochemical purity may be confirmed using a ɣ-radioactivity-TLC-scanner.
Somatostatin receptor scintigraphy in all the patients was performed 4 and 24 hours following an intravenous injection of 140–220 MBq of 111In-Pentetreotide using a large field of view double-headed gamma camera (SymbiaT6®, Siemens) equipped with a medium energy collimator. Patients without diarrhea took a laxative the day before imaging to ensure a bowel cleaning to reduce interfering background radioactivity by intestinal content.
The images acquired consisted of a whole body planar in anterior and posterior views with a speed of 8 cm/min (1024 × 256 matrix) and SPECT/CT at 24 hours centred on the abdomen or the thorax depending on the clinical situation, with a optional additional SPECT/CT centred on positive uptake foci detected on the whole-body scan (WBS). SPECT was first acquired always followed by CT and the images, reconstructed with the iterative method (OSEM), were fused with those of CT using a dedicated software package.
2.5 |. PET/CT protocol and reconstruction parameters
Images were acquired using a Siemens 3D tomograph (Biograph 16 TruePoint). A low-dose noncontrast CT was performed for PET/CT protocols with the following parameters: 110 kV, 100 mA, 24.7 second; pitch 1. PET data was reconstructed with a 6 mm Full Width at Half Maximum (FWHM) filter in a pixel matrix (168 × 168 for whole body (WB) acquisition/256×256 for craniocervical scanning (CS)) using 3D OSEM with eight subsets and four iterations. Correction attenuation was performed with CT scan and a Gaussian filter smoothing (6 mm for WB/4 mm for CS). Image acquisition started at 45 minutes after injection of 68Ga-DOTATATE (mean ± SD: 2.4 ± 0.5 MBq/kg, median 2.4 MBq/kg). The acquisition consisted in a whole body (WB) acquisition from the top of head to mid thigh (3 minutes/bed position, arms above the head).
2.6 |. Image analysis and quantification of PET/CT examinations
CT, PET (corrected) and fusion images were displayed for visual analysis on a syngo.via workstation with image fusion software (Siemens Medical Solutions, Knoxville, Tennessee). PET/CT were interpreted by experienced nuclear medicine physicians. Each focus of increased extra physiologic radiotracer uptake was recorded and interpreted according to the particular case.
2.7 |. Computed tomography
Multiphasic contrast-enhanced thoraco-abdominopelvic computed scans (CT) consisted of three helical scans obtained in an automated, predetermined and timed sequence schedule, including precontrast abdomino-pelvic CT scan, arterial thoraco-abdominal CT and portal abdomino-pelvic CT.
2.8 |. Liver MR imaging
Liver MR included T1-weighted in-phase and out-of-phase, T2-weighted fat saturated, diffusion-weighted and T1-weighted fat saturated post-gadolinium images.
2.9 |. Gold standard
Histology was considered as the gold standard for the diagnosis of NETs. In cases where no surgical resection was performed, the diagnosis of NETs was made by reaching a consensus between experienced clinicians, radiologists nuclear medicine physicians based on detailed clinical examination, positive biochemistry as well as results of different imaging modalities and regular follow ups of patients. The number of positive regions corresponded to the maximum number of positive regions (after consensus) depicted by all imaging modalities.
2.10 |. Statistical analysis
Sensitivities were calculated for patients and for involved anatomical regions. The differences in sensitivity between the imaging procedures were compared using the chi2 test and Fisher’s exact test when n was <5, according to Hawass et al13
Statistical analysis was performed using IBM SPSS Statistics version 20 (IBM SPSS Inc., Chicago, IL, USA). For all tests, a two-sided P value <.05 was considered statistically significant.
3 |. RESULTS
3.1 |. Patients and tumours characteristics
Thirty-two patients (19 females, 13 males; aged 22–80) were included in the study. Patients were included for initial staging of a GEP-NETs in 11 cases (6 pancreas, 4 ileum, 1 genu superius), for restaging in 8 cases (5 pancreas, 1 ileal, 1 lung, 1 duodenal gastrinoma) and for elevated serum neuroendocrine biomarkers in 13 cases in patients without previous diagnosis of NETs (5 ectopic adrenocorticotropic hormone (ACTH) secretion, 5 organic hypoglycaemia, 1 vasoactive intestinal peptide (VIP), 1 chromogranin A (CgA), and 1 neuron-specific enolase (NSE). None of the patients were treated by somatostatin analogues at the time of 68Ga-DOTATATE PET/CT study.
3.2 |. Initial staging of NETs or screening for primary tumour of NET origin
68Ga-DOTATATE PET/CT detected 10/11 primary tumours (3 midgut, 1 ileocaecal, 6 pancreatic NETs), confirmed by pathology in 8 and by follow-up in the remaining 2 cases (Figures 1 and 2, Table S1). In one case with negative imaging findings including 68Ga-DOTATATE PET/CT, the diagnostic of millimetric primary duodenal NET (genu superius) was performed via endoscopy. Four out of eleven patients had locoregional lymph node metastases, all detected by 68Ga-DOTATATE PET/CT (3 pathologically confirmed, 1 diagnosed based on followup imaging). 68Ga-DOTATATE was found to be superior to SI in nodal staging in one case. Only one patient had distant liver metastasis which were detected by 68Ga-DOTATATE PET/CT and anatomical imaging but remained occult for SRS. In additional cases, SI found falsely positive findings: one case with follicular nodular hyperplasia on CT and MRI; one case with both pancreatic foci of uptake on SRS and an ileocaecal valve thickness on CT.
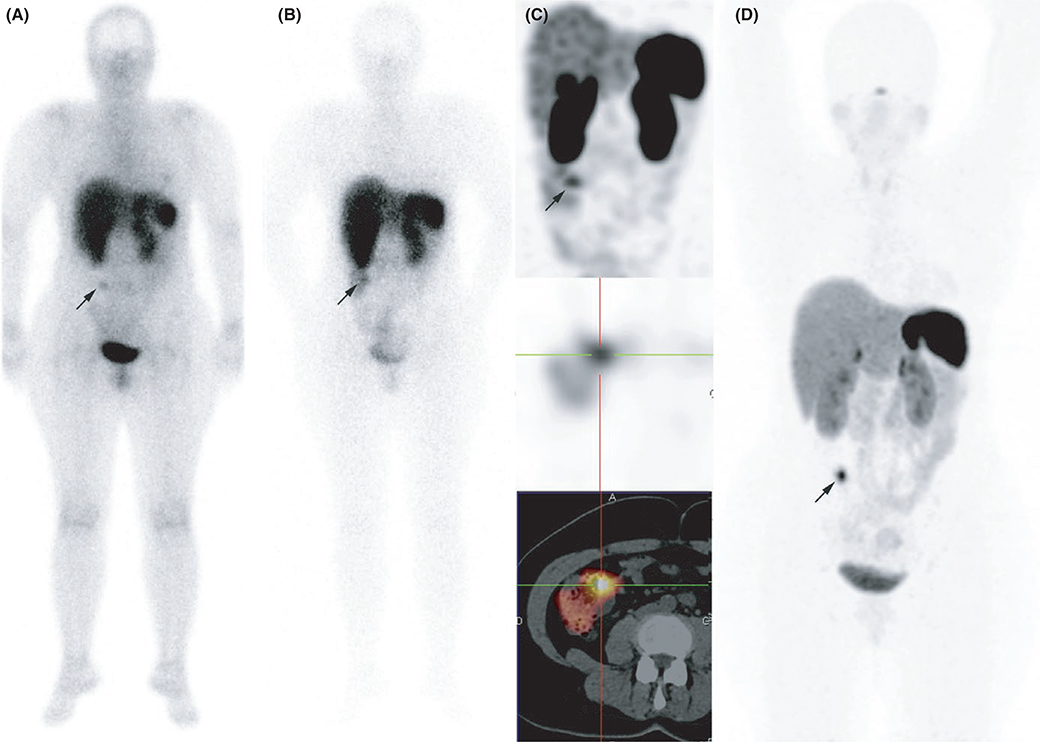
FIGURE 1.
Ileal neuroendocrine tumours (NET). Somatostatin receptor scintigraphy A, 4 h anterior whole-body planar scan, B, 24 h anterior whole-body planar scan, C, abdominal single-photon emission computed tomography (SPECT) MIP, C, attenuation corrected axial SPECT image, D, axial fused SPECT/CT image and 68Ga-DOTATATE PET/CT (attenuation-corrected axial PET image) were concordant for a NET located in the last ileal loop
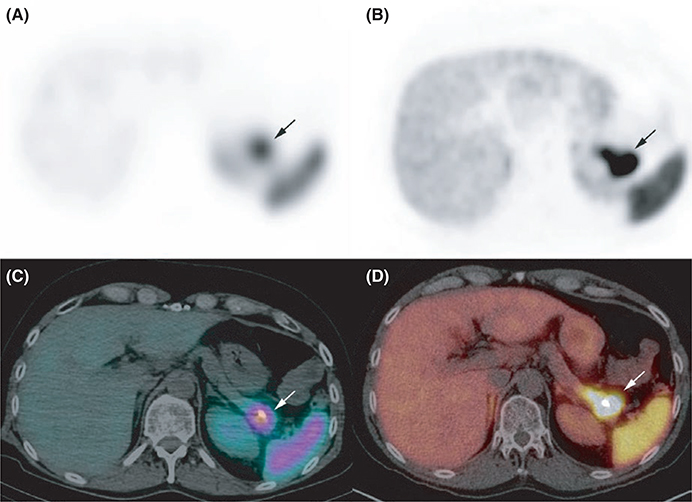
FIGURE 2.
Pancreatic neuroendocrine tumours (NET). Somatostatin receptor scintigraphy A, attenuation corrected axial single-photon emission computed tomography (SPECT) image, C: axial SPECT/CT fusion image and 68Ga-DOTATATE PET/CT B, attenuation-corrected axial PET image, C, axial fused PET/CT image were concordant for a pancreatic NET. The area of uptake was superior on 68Ga-DOTATATE PET/CT, a finding which was in agreement with the final diagnosis of a 40 mm NET
3.3 |. Restaging of NETs
Four out of eight patients had distant metastases (liver, n = 4 and bone, n = 1). 68Ga-DOTATATE PET/CT was concordant to SI in three cases and detected additional lymph node (LN) in one patient. In one patient with a single rib metastasis, the lesion was only detected by somatostatin receptor imaging (SRS and 68Ga-DOTATATE PET/CT). One additional patient had a LN metastasis solely detected by SRS and 68Ga-DOTATATE PET/CT. In three cases, 68Ga-DOTATATE PET/CT was negative and patients remained in remission. Two of them had false positive findings on SI. One patient had tumour relapse in the pancreatectomy bed (considered as a primary tumour) which was detected by all imaging modalities (Table S2).
3.4 |. Elevated serum neuroendocrine hormones or peptides
68Ga-DOTATATE PET/CT detected 4/6 pathologically proven NET patients (Table S3). Among the five patients with suspicion of ectopic Cushing, four had lung NETs (two typical and two atypical carcinoids) and one remained occult on all imaging studies. 68Ga-DOTATATE PET/CT could detect only 2/4 primary lesions, both were classified as nonpathological by CT (pulmonary arteriovenous malformation for one patient andsequelae following opportunistic infection in the other). Only 1/2 68Ga-DOTATATE-avid lesions was detected by SRS. In the remaining 2/5 patients, nodules were accurately classified as possibly NET on thoracic CT.
Five patients were evaluated for hypoglycaemia, 4/5 were retrospectively classified as organic hyperinsulinic hypoglycaemia and 1/5 was reclassified as functional hypoglycaemia related to amyloidosis, the latter being diagnosed by further investigations during follow-up. In three patients with organic hyperinsulinic hypoglycaemia, 68Ga-DOTATATE PET/CT as well as other imaging studies were negative. Since these patients had moderate hypoglycaemia, they were kept on regular follow-up and subsequently have no definitive diagnosis. In one case, the insulinoma was detected on CI and 68Ga-DOTATATE PET/CT but was negative on SRS.
Finally, three patients had elevation of various serum neuroendocrine markers. In one patient with elevated vasoactive intestinal peptide (VIP), imaging studies were negative or inconclusive and the patient became pregnant and hence, was not further evaluated. In one patient with only elevated CgA (423 ng/mL, URL: 94) in absence of proton pump inhibitor medication, the diagnosis of NET was ruled out based on negative imaging findings and medium-term follow-up. One patient had elevated serum NSE and found to have multiple foci on 68Ga-DOTATATE PET/CT corresponding to midgut NET and metastatic LNs. SRS detected only metastatic LN.
3.5 |. Overall results
Regarding detection of regions (n = 18), 68Ga-DOTATATE PET/CT detected more primary tumours (15 vs 10, P = .074) and more involved regions (29 vs 22, P = .023) than SRS. No statistical difference was observed between 68Ga-DOTATATE PET/CT and anatomical imaging (CT + MRI).
The missed tumours on 68Ga-DOTATATE PET/CT were located in the lung in two patients (two ectopic Cushing) (Figure 3) and duodenum (nonfunctioning) in one patient. For LN or distant metastasis only, no statistical difference was observed between imaging modalities (Table 1).
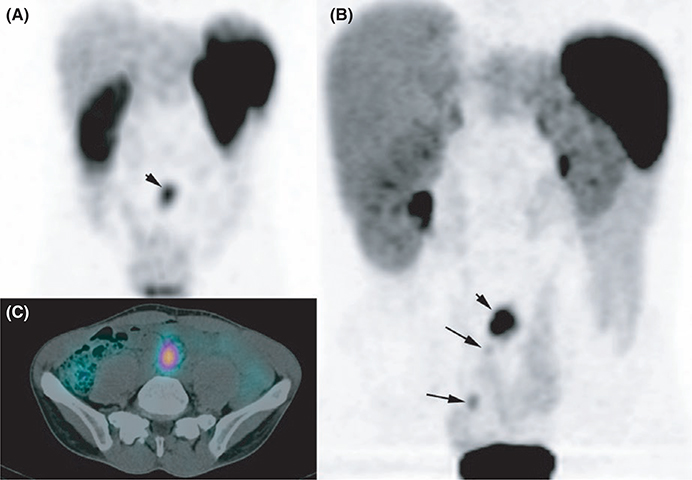
FIGURE 3.
Mutifocal midgut neuroendocrine tumours (NET). Somatostatin receptor scintigraphy A, attenuation corrected axial MIP image, C: axial fused single-photon emission computed tomography (SPECT)/CT image and 68Ga-DOTATATE PET/CT B, attenuation-corrected MIP image. Both imaging modalities identified a large mesenteric lymph node (LN) (small arrow). 68Ga-DOTATATE PET/CT identified multiple primaries in the ileum which were confirmed by pathological analysis (long arrows)
TABLE 1.
Comparison of the different imaging techniques for the localization and staging of neuroendocrine tumours (NETs)
| Per region analysis |
|||||
|---|---|---|---|---|---|
| Per patient analysis | Primary | LN | Metastases | Total | |
| n = 32 patients | n = 22 | n = 18* | n = 10 | n = 5 | n = 33 |
| 68Ga-DOTATATE (1) | 20 (91%) | 15 (83%) | 9 (90%) | 5 (100%) | 29 (88%) |
| SRS (2) | 16 (73%) | 10 (56%) | 8 (80%) | 4 (80%) | 22 (67%) |
| MRI + CT (3) | 20 (91%) | 13 (72%) | 7 (70%) | 5 (100%) | 25 (76%) |
| SRS + MRI + CT (4) | 20 (91%) | 15 (83%) | 8 (80%) | 5 (100%) | 28 (85%) |
| 68Ga-DOTATATE + MRI + CT (5) | 22 (100%) | 17 (94%) | 9 (90%) | 5 (100%) | 31 (94%) |
| P-values | .055 | .087 | .918 | 1.000 | .037 |
| (1) vs (2) P = .134 | (1) vs (2) P = .074 | (1) vs (2) P = 1.000 | (1) vs (2) P = 1.000 | (1) vs (2) P = .023 | |
| (1) vs (3) P = .617 | (1) vs (3) P = 1.000 | (1) vs (3) P = .480 | (1) vs (3) P = NA | (1) vs (3) P = .289 | |
| (1) vs (4) P = .617 | (1) vs (4) P = .617 | (1) vs (4) P = 1.000 | (1) vs (4) P = NA | (1) vs (4) P = 1.000 | |
| (5) vs (4) P = .480 | (5) vs (4) P = .480 | (5) vs (4) P = 1.000 | (5) vs (4) P = NA | (5) vs (4) P = .248 | |
LN, lymph nodes; SRS, somatostatin receptor scintigraphy; MRI, liver MRI; CT, multiphasic thoraco-abdomino-pelvic CT scan.
We have excluded one ectopic Cushing's syndrome and three organic hypoglycaemia that remained occult for all imaging studies at the time of the study and subsequent evaluations, with no definitive diagnosis from the analysis.
In comparison to 68Ga-DOTATATE PET/CT, SRS missed five primary tumours (2 nonfunctioning pancreatic tumours, one lung tumour, one insulinoma, one multiple ileal) (Figures 4 and 5), one distant metastatic region (liver) and one LN region (mesenteric LN from patient with primary midgut NET). In two cases, falses positive findings were described on SRS and not on 68Ga-DOTATATE PET/CT.
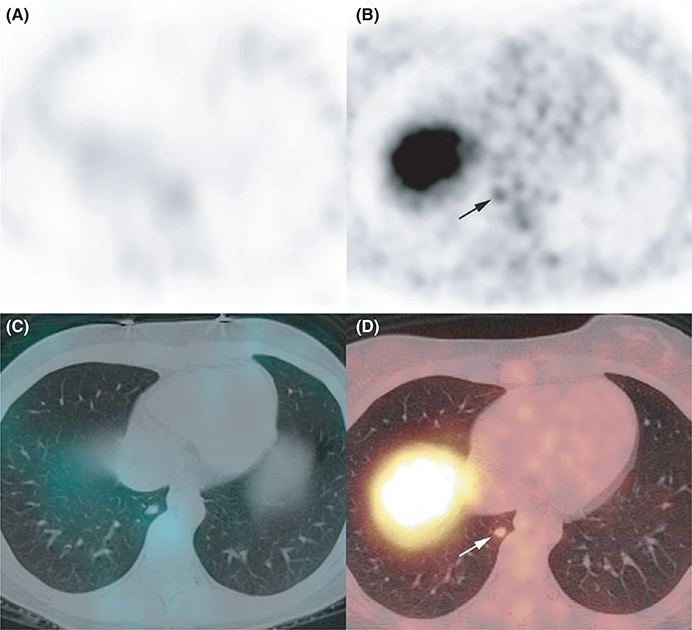
FIGURE 4.
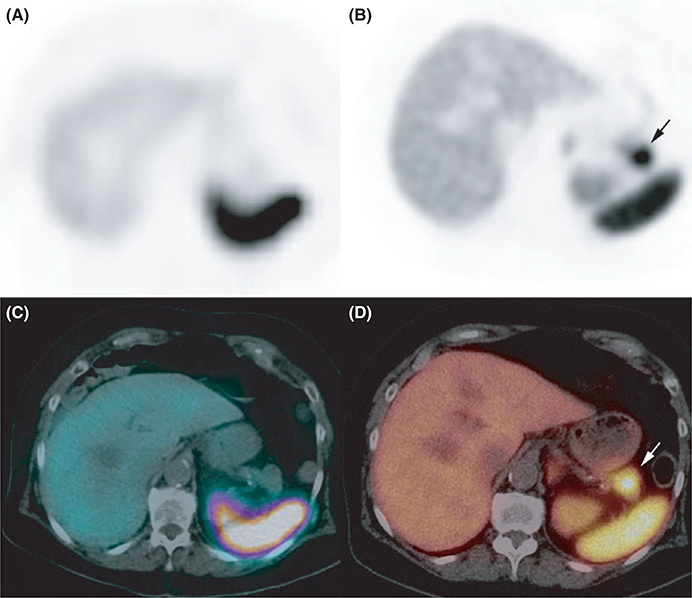
Ectopic Cushing’s syndrome. Somatostatin receptor scintigraphy A, attenuation corrected axial single-photon emission computed tomography (SPECT) image, C: axial fused SPECT/CT image failed to identify any abnormality, whereas PET/CT showed a lower right lobe 68Ga-DOTATATE-avid nodule B, attenuation-corrected axial PET image, D, axial fused PET/CT image
FIGURE 5.
Insulinoma. Somatostatin receptor scintigraphy A, attenuation corrected axial single-photon emission computed tomography (SPECT) image, C: axial fused SPECT/CT image) failed to identify any abnormality whereas PET/CT showed a 68Ga-DOTATATE-avid pancreatic mass B, attenuation-corrected axial PET image, C, axial fused PET/CT image, corresponding to an insulinoma
4 |. DISCUSSION
Our results confirm that 68Ga-DOTATATE PET/CT detects more primary NETs than SRS, with lack of significance that could be due to the limited number of patients (P = .074). 68Ga-DOTATATE PET/CT also detects statistically more involved regions than SRS (P = .023). This increased detection of the primary tumours can help to achieve the following: (1) resection of the primary tumour(s) that could enable resolution of paraneoplastic, prevent metastatic disease or improve disease-free and overall survival in well-differentiated midgut NETs even in the presence of metastases and (2) choice of chemotherapeutic agent, which is dependent upon the location of the primary tumour. Since these tumours can be very small and multicentric (especially for midgut NETs or in hereditary cases), localization of these tumours can be challenging and often relies on performing combination of endoscopic, anatomic and functional imaging.
In the present study, 68Ga-DOTATATE PET/CT missed 3/18 primary tumours. One of these three primaries was located in the genu superius and corresponded to a millimetric lesion. In this patient, nodal involvement was detected by 68Ga-DOTATATE PET/CT, illustrating that the tumour overexpressed somatostatin receptors and that the false negative finding was due to the spatial resolution limitation of PET imaging. The remaining two false negative cases corresponded to ACTH-producing lung carcinoids. Traditionally, the optimal functional imaging for ectopic Cushing syndrome relies on the use of SRS in addition to anatomic imaging.14–17 In contrast, 68Ga-DOTA-SSAs show excellent results, however, only few studies have been published so far and with a limited number of cases with head-to-head comparison between various imaging modalities.18–21 In a systematic review, functional imaging discovered 79.1% (53/67) of tumours unidentified by anatomic imaging. The pooled per patient sensitivity of various imaging modalities in tumour localization were found to be 66.2% (137/207) by CT, 51.5% (53/103) by MRI, 48.9% by Octreoscan® (84/172), 51.7% (46/89) by 18F-FDG-PET, 57.1% (12/21) by 18F-DOPA PET, 30.8% (4/13) by 131I/123I-metaiodobenzylguanidine, and 81.8% (18/22) by 68Ga-DOTA-SSA PET/CT.18 Although the number of patients associated with tumours causing ectopic Cushing syndrome is limited, our study showed that lung NETs represent an heterogenous family of tumours with various imaging phenotypes.
In three cases with organic hyperinsulinic hypoglycaemia, 68Ga-DOTATATE PET/CT as well as other imaging studies were negative. Since the patients have moderate hypoglycaemia, they are regularly followed up, however, without achieving any definitive diagnosis.
In other situations, we failed to identify any statistical difference between 68Ga-DOTATATE PET/CT and other imaging modalities in the detection of LN or distant metastases on per regional analysis most likely due to the small sample size and the rigid inclusion criteria which have selected patients with limited disease extension. However, 68Ga-DOTATATE PET/CT performed better than SRS on overall number of involved regions (primary + LN + distant metastases).
We acknowledge several limitations in the present study: first, there was a limited sample size, however, we would like to emphasize that these are rare tumours, hence it becomes important to report findings even from this small prospective study. Second, the histopatholgic confirmation were not available for all the suspicious lesions on 68Ga-DOTATATE due to ethical concerns, and third, the final diagnosis in few patients were lacking such as patients with mild hypoglycaemia. Hence, further multicentric trials are required to validate our results. Furthermore, comparison of diagnosis value of 68Ga-DOTATATE and 18F-FDOPA PET/CT in midgut NETs would merit further investigations despite the absence of theranostics value of 18F-FDOPA.
In conclusion, our prospective study shows that 68Ga-DOTATATE PET/CT is superior to SRS in the detection of primary NETs. In addition to the diagnostic superiority of 68Ga-DOTATATE PET/CT over SRS, 68Ga-DOTATATE can be easily produced and hence, has potential for widespread usage in the imaging of these tumours.
Supplementary Material
Acknowledgments
Funding information
This research was supported by the Assistance Publique des Hôpitaux de Marseille and the Conseil Général des Bouches du Rhône.
Footnotes
CONFLICT OF INTEREST
The authors have nothing to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. [DOI] [PubMed] [Google Scholar]
- 2.Deroose CM, Hindie E, Kebebew E, et al. Molecular imaging of gastroenteropancreatic neuroendocrine tumors: current status and future directions. J Nucl Med. 2016;57:1949–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofman MS, Kong G, Neels OC, Eu P, Hong E, Hicks RJ. High management impact of Ga-68 DOTATATE (GaTate) PET/CT for imaging neuroendocrine and other somatostatin expressing tumours. J Med Imaging Radiat Oncol. 2012;56:40–47. [DOI] [PubMed] [Google Scholar]
- 4.Sadowski SM, Neychev V, Millo C, et al. Prospective study of 68 Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol. 2016;34:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srirajaskanthan R, Kayani I, Quigley AM, Soh J, Caplin ME, Bomanji J. The role of 68 Ga-DOTATATE PET in patients with neuroendocrine tumors and negative or equivocal findings on 111In-DTPA-octreotide scintigraphy. J Nucl Med. 2010;51:875–882. [DOI] [PubMed] [Google Scholar]
- 6.Deppen SA, Blume J, Bobbey AJ, et al. 68 Ga-DOTATATE compared with 111In-DTPA-octreotide and conventional imaging for pulmonary and gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. J Nucl Med. 2016a; 57:872–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deppen SA, Liu E, Blume JD, et al. Safety and efficacy of 68 Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J Nucl Med. 2016b;57:708–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham MM, Gu X, Ginader T, Breheny P, Sunderland JJ. 68 Ga-DOTATOC imaging of neuroendocrine tumors: a systematic review and metaanalysis. J Nucl Med. 2017;58:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Binnebeek S, Vanbilloen B, Baete K, et al. Comparison of diagnostic accuracy of (111)In-pentetreotide SPECT and (68)Ga-DOTATOC PET/CT: a lesion-by-lesion analysis in patients with metastatic neuroendocrine tumours. Eur Radiol 2016;26:900–909. [DOI] [PubMed] [Google Scholar]
- 10.Buchmann I, Henze M, Engelbrecht S, et al. Comparison of 68 Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2007;34:1617–1626. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann M, Maecke H, Borner R, et al. Biokinetics and imaging with the somatostatin receptor PET radioligand (68)Ga-DOTATOC: preliminary data. Eur J Nucl Med. 2001;28:1751–1757. [DOI] [PubMed] [Google Scholar]
- 12.Krausz Y, Freedman N, Rubinstein R, et al. 68 Ga-DOTA-NOC PET/CT imaging of neuroendocrine tumors: comparison with (1)(1)(1)In-DTPA-octreotide (OctreoScan(R)). Mol Imaging Biol. 2011;13:583–593. [DOI] [PubMed] [Google Scholar]
- 13.Hawass NE. Comparing the sensitivities and specificities of two diagnostic procedures performed on the same group of patients. Br J Radiol. 1997;70:360–366. [DOI] [PubMed] [Google Scholar]
- 14.Tabarin A, Valli N, Chanson P, et al. Usefulness of somatostatin receptor scintigraphy in patients with occult ectopic adrenocorticotropin syndrome. J Clin Endocrinol Metab. 1999;84:1193–1202. [DOI] [PubMed] [Google Scholar]
- 15.Torpy DJ, Chen CC, Mullen N, et al. Lack of utility of (111)In-pentetreotide scintigraphy in localizing ectopic ACTH producing tumors: follow-up of 18 patients. J Clin Endocrinol Metab. 1999;84:1186–1192. [DOI] [PubMed] [Google Scholar]
- 16.de Herder WW, Krenning EP, Malchoff CD, et al. Somatostatin receptor scintigraphy: its value in tumor localization in patients with Cushing’s syndrome caused by ectopic corticotropin or corticotropin-releasing hormone secretion. Am J Med. 1994;96:305–312. [DOI] [PubMed] [Google Scholar]
- 17.Phlipponneau M, Nocaudie M, Epelbaum J, et al. Somatostatin analogs for the localization and preoperative treatment of an adrenocorticotropin-secreting bronchial carcinoid tumor. J Clin Endocrinol Metab. 1994;78:20–24. [DOI] [PubMed] [Google Scholar]
- 18.Isidori AM, Sbardella E, Zatelli MC, et al. Conventional and nuclear medicine imaging in ectopic cushing’s syndrome: a systematic review. J Clin Endocrinol Metab 2015;100:3231–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kakade HR, Kasaliwal R, Jagtap VS, et al. Ectopic ACTH-secreting syndrome: a single-center experience. Endocr Pract 2013;19:1007–1014. [DOI] [PubMed] [Google Scholar]
- 20.Ozkan ZG, Kuyumcu S, Balkose D, et al. The value of somatostatin receptor imaging with In-111 Octreotide and/or Ga-68 DOTATATE in localizing Ectopic ACTH producing tumors. Mol Imaging Radionucl Ther 2013;22:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkitaraman B, Karunanithi S, Kumar A, Bal C, Ammini AC, Kumar R. (6)(8)Ga-DOTATOC PET-CT in the localization of source of ectopic ACTH in patients with ectopic ACTH-dependent Cushing’s syndrome. Clin Imaging 2014;38:208–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.