Abstract
In the current study, we tested for Gene × Environment interactions in the association between pubertal timing and adolescent depression by examining how socioeconomic factors might moderate age at menarche’s relation with depressive symptoms. Participants comprised 630 female twin and sibling pairs from the National Longitudinal Study of Adolescent Health. Consistent with previous studies, results showed that genetic predispositions toward later menarche were associated with fewer depressive symptoms and that genetic predispositions toward earlier menarche were associated with more depressive symptoms. However, this pattern was subtle and evident only in girls from higher socioeconomic backgrounds. Although girls from lower socioeconomic families showed the highest overall levels of depression, their symptoms appeared unrelated to timing of physical development through either a genetic or an environmental path.
Keywords: puberty, menarche, poverty, depression, Gene × Environment interactions
Puberty represents a pivotal time for affective difficulties for girls. The striking discrepancy in depression rates between males and females first emerges during this transition, with girls’ internalizing and poor self-esteem typically increasing during the course of puberty (Cyranowski, Frank, Young, & Shear, 2000). Although puberty presents social and emotional challenges for all girls, individual differences in maturation play a key role; girls who mature ahead of peers are at particularly high risk for depression (e.g., Mendle, Turkheimer, & Emery, 2007). This correlation is complicated by two factors. First, both depression and pubertal timing are heritable. Second, both depression and earlier pubertal timing have been associated with socioeconomic disadvantage. In the present study, we investigate Gene × Environment (G × E) interactions in these associations. Specifically, we examine how girls’ socioeconomic background may moderate the genetic and environmental pathways between age at menarche and adolescent depressive symptoms.
Puberty and Depression in Adolescent Girls
Early maturing girls are psychologically vulnerable. Compared with later developing peers, early maturers are more likely to exhibit internalizing symptoms (Graber, Lewinsohn, Seeley, & Brooks-Gunn, 1997), to meet criteria for a Diagnostic and Statistical Manual of Mental Disorders (4th ed., DSM–IV; American Psychiatric Association, 1994) diagnosis of major depression (Stice, Presnall, & Bearman, 2001), and to attempt suicide (Graber et al., 1997; Wichstrøm, 2000). These findings remain robust across a broad range of measurement methods for both puberty and depression, which suggests that early puberty in girls is both “uniquely associated with substantial risk” (Graber, Nichols, & Brooks-Gunn, 2010) and carries “profound psychological effects” (Grumbach & Styne, 2003). Because adolescent depression creates susceptibility for future depressive episodes (e.g., Rutter, Kim-Cohen, & Maughan, 2006), it is perhaps not surprising that early pubertal timing continues to predict higher risk for depression during the course of adolescence and into early adulthood (Graber, Seeley, Brooks-Gunn, & Lewinsohn, 2004).
The most common explanation for the association between pubertal timing and depression hinges on the mismatch between physical, cognitive, and emotional maturation (also known as the maturation-disparity hypothesis; reviewed in Ge & Natsuaki, 2009). Puberty is characterized by a high degree of change, transformation, and challenge. Shifting friendships, new family roles and expectations, increases in parent-child conflict, novel romantic encounters, and either unwanted or unexpected sexual attention are all common during this time. Despite an outwardly mature appearance, girls who experience early puberty often maintain an age-appropriate level of cognitive and emotional development. This requires them to contend with the changes of puberty with fewer resources than peers who reach the same developmental milestones at a later chronological age—a predicament hypothesized to instill or to amplify feelings of isolation, loneliness, and helplessness.
Socioeconomic Status, Puberty, and Depression
Early puberty is hardly random. Among industrialized nations, the United States is unique in its inequitable distribution of wealth across citizens (Davies, Sandström, Shorrocks, & Wolff, 2009). In general, girls from socioeconomically disadvantaged backgrounds tend to reach menarche before girls from more affluent circumstances (James-Todd, Tehranifar, Rich-Edwards, Titievsky, & Terry, 2010). Earlier puberty is also related to factors often confounded with lower socioeconomic status (SES), such as family structure, race/ethnicity, or obesity (e.g., Bogaert, 2008; Freedman et al., 2003; Obeidallah, Brennan, Brooks-Gunn, & Earls, 2004). These associations typically are considered sequelae of environmental stress: Early childhood adversities are believed to accelerate timing of reproductive maturation either through an evolved life-history strategy (Belsky, Steinberg, & Draper, 1991) or as part of “weathering,” the process of premature aging in individuals from disadvantaged backgrounds (Allsworth, Weitzen, & Boardman, 2005). Although most research on weathering considers health disparities later in life, timing of menarche may reflect one of the earliest signs that weathering begins in childhood.
SES is relevant not just for pubertal timing but also for psychological well-being. In general, there tends to be an inverse relation between SES and mental health; depression is disproportionately prevalent among both adults (e.g., Galea et al., 2007; Lorant et al., 2003) and adolescents from lower SES backgrounds (e.g., Dupéré, Leventhal, & Lacourse, 2009; Henderson et al., 2005). Almost one half of women receiving public assistance report a clinically significant level of depressive symptoms and approximately 20% meet criteria for major depressive disorder (Lennon, Blome, & English, 2002). Consequently, low-SES teens come of age in families in which parents are often struggling and depressed themselves. They tend to live in neighborhoods with high degrees of violence, substance abuse, and social stigma. It has been suggested that these circumstances contribute to beliefs that negative experiences are far more common than are positive ones and that people have neither the ability nor the resources to change their own futures (Bolland, 2003; Bolland, Lian, & Formicella, 2005).
G × E Interactions
As the field of developmental psychopathology has matured, interest in the dichotomous “nature-versus-nurture debate” has waned in the face of more empirically supported and conceptually logical biopsychosocial models in which genes and environment are jointly assumed to play a role in development. In the case of the phenotypes of interest in the present study, twin and family studies yield heritability estimates for menarche in the .4 to .8 range (e.g., Anderson, Duffy, Martin, & Visscher, 2007; Doughty & Rodgers, 2000; Rowe, 2000; Treloar & Martin, 1990). More than 100 genomic loci contribute to this variation—most prominently for genes related to ovarian hormone receptors, biosynthesis, and metabolism, as well as for body mass index, regulation of weight, appetite, and satiety (Elks et al., 2010; Kim et al., 2012; Perry et al., 2014). Likewise, concordance for depressive symptoms is consistently higher among monozygotic (MZ) than dizygotic (DZ) twins (McGuffin, Katz, Watkins, & Rutherford, 1996); molecular studies have implicated polymorphisms in a variety of genes, most commonly serotonergic (Caspi et al., 2003; Eley et al., 2004; Karg, Burmeister, Shedden, & Sen, 2011).
The portion of variance in a trait not accounted for by genes is, of course, accounted for by environmental influences, including SES. Yet the extent to which genes versus environments influence a given phenotype may vary across environmental conditions. Specifically, a G × E interaction occurs when environmental conditions moderate the magnitude of genetic effects on a phenotype or when genetic influences moderate the response to environmental conditions. More simply, in the case of the present study, a G × E interaction would suggest that individual genetic differences may help explain how girls respond to the environments they encounter at puberty. Genes influence the timing of puberty itself, as well as tendencies toward mood and emotion regulation, which are critical components of the psychological response to puberty. This genetically influenced psychological response may, in turn, be moderated by socioeconomic factors, given that becoming sexually and reproductively mature likely presents different repercussions across socioeconomic strata. How girls view, interpret, and plan for their futures may vary according to economic and career options. The physiological changes of puberty, including curviness and breast development, may be considered more or less desirable on the basis of social norms within a girl’s world. Adulthood may be seen as either imminent or distant because low-income girls tend to enter the labor market and parent children at younger ages than do more affluent girls, for whom adolescence will often lengthen into a period of “emerging adulthood.” Consequently, if the genetic influences underlying the menarche-depression association were moderated by socioeconomic conditions, it would be evidence of a G × E interaction.
The Present Study
In the present study, we tested for G × E interactions in the association of menarche and depressive symptoms during adolescence. Our analyses were shaped by the core hypothesis that girls are genetically predisposed toward earlier or later menarche but that these predispositions may convey different psychological experiences based on socioeconomic context. We therefore considered (a) the genetic and environmental pathways between age at menarche and depressive symptoms and (b) the moderating role of SES in these associations.
Method
Participants
Data are drawn from the National Longitudinal Study of Adolescent Health (Add Health; Harris, 2009), a nationally representative study designed to evaluate adolescent health and risk behaviors. The Add Health study used stratified random sampling of U.S. high schools, and 79% of targeted schools agreed to participate. From the participating schools, 90,118 students completed a confidential in-school survey during the 1994–1995 academic year. School rosters were then used to select a random sample of more than 20,000 adolescents to complete a comprehensive, 90-min, in-home assessment between April and December 1995. Participants ranged in age from 11 to 21 years (mean age = 16 years, 25th percentile = 14 years, 75th percentile = 17 years). There have been three follow-up interviews with the Add Health participants: Wave II in 1996, Wave III in August 2001–2002, and Wave IV in 2007–2008.
For the current analyses, we used all 630 female-female sibling pairs available in the Add Health data: 145 MZ twin pairs, 116 DZ twin pairs, and 369 full-sibling (FS) pairs.1 During the in-school interview, adolescents were asked whether they currently lived with another adolescent in the same household. The information was used to oversample adolescent sibling pairs who resided in the same home deliberately, even if one member of the pair did not attend a high school in the original probability sample. Jacobson and Rowe (1999) compared the sociodemographic composition of sibling pairs in Add Health with the full Add Health sample and found negligible differences. Twin zygosity was determined by 11 molecular genetic markers and responses to four questionnaire items that concerned similarity of appearance and frequency of being mistaken for one’s twin (Harris, Halpern, Smolen, & Haberstick, 2006). Similar questionnaires have been used widely in twin research and have been repeatedly cross-validated with zygosity determinations based on DNA (e.g., Spitz et al., 1996). Within this subsample, 64% identified as Caucasian, 22% as African American, 4% as Native American, 6% as Asian American, and the remainder as other.
Measures
Menarche.
In the in-home interview for both Waves I and II, participants were asked whether they had ever had a menstrual period and, if so, which month and year it first occurred. At Wave III, participants were asked, “How old were you when you got your period for the first time?” In general, age at menarche is reliably reported by women, with 85%-to-90% accuracy compared with historical medical records (Casey et al., 1991). In the full Add Health sample, test-retest reliability for age at menarche measured at Waves 1, 2, and 3, assessed using Cronbach’s coefficient alpha, was .80. This result is consistent with findings from previous studies (reviewed in Dorn, Dahl, Woodward, & Biro, 2006). The Pearson correlation between age at menarche reported at Waves 1 and 2 (r = .76, p < .001) was higher than that between Waves 2 and 3 (r = .53, p < .001) and between Waves 1 and 3 (r = .53, p < .001). (The lower correlation between the Wave 3 report and Wave 1 and 2 reports is likely because menarche at Wave 3 was reported in years, whereas menarche at Waves 1 and 2 was reported in months and years.) We used the first reported age at menarche to reduce the likelihood of a memory bias, referred to as telescoping, in which events are remembered as closer to the date of an interview than they actually occurred (Janssen, Chessa, & Murre, 2006). This was most often the Wave 1 report (87.6% of participants). The mean age of menarche in the sample was 12.29 years (SD = 1.39, range = 8.0–19.0).
Depressive symptoms.
A 19-item version of the Center for Epidemiological Studies–Depression Scale (CES-D; Radloff, 1977) was used to assess level of depressive symptoms at Wave 1. The CES-D is a self-report measure of cognitive, affective, and physiological symptoms of depression. A score of 18 on the CES-D is typically used to demarcate a clinically significant level of depression (Radloff & Locke, 1986); in this sample, scores ranged from 0 to 48 (M = 12.41, SD = 8.08), and 24.42% of the sample reported a CES-D score in the clinical range. Internal reliability was adequate (α = .88). Because CES-D scores tend to be higher among older adolescents within the Add Health sample (Rushton, Forcier, & Schectman, 2002), CES-D scores were standardized by age (in years) so that higher scores represent more symptoms of depression relative to girls of the same age.
SES.
We used level of educational attainment of the parent present during the in-home assessment (most often mothers) as a proxy measure for SES. SES, of course, is not defined solely by education level but by income and type of occupation, as well as numerous subtle indicators of prestige and social standing. Not all of these sources of information are available in Add Health. We chose to use educational attainment because it is a commonly used proxy for SES (Bradley & Corwyn, 2002) and believed to be a more stable and accurate indicator than family income, which is often confounded by family structure, the number of working parents in two-parent families, temporary episodes of unemployment, and so forth (U.S. Treasury Department, 2007). In the Add Health data, educational attainment was coded on a 9-point ordinal scale ranging from 8th grade or less to professional training beyond a 4-year degree. The median level of SES was equivalent to a general equivalency diploma or high school graduate (Mdn = 5, SD = 2.42). In a follow-up set of sensitivity analyses (described in more detail later and in Table S1, Fig. S1, and the Methods and Results for Sensitivity Analysis Using SES Composite section of the Supplemental Material available online), we tested a composite measure of parental education and family income.
Race/ethnicity.
For analyses, race was dummy coded such that 0 corresponded to European American and 1 corresponded to non-European American. (The Add Health twin sample, although notable for its diversity relative to other behavioral-genetic studies, does not have sufficient numbers of racial/ethnic minority twins to test for G × E interactions within each minority group separately.) All models statistically controlled for the main effects of race on depressive symptoms and age at menarche.
Analyses
Data were analyzed using structural equation modeling in Mplus (Muthén & Muthén, 1998–2010) and full information maximum likelihood to account for missing data. Nested models were compared using differences in log likelihood, which are distributed as chi-square results. Absolute model fit was assessed using the comparative fit index (CFI; Bentler, 1990) and the root-mean-square error of approximation (RMSEA; Steiger, 1990). On the basis of recommendations by Hu and Bentler (1999), values of RMSEA less than or equal to .05 and CFI greater than or equal to .95 indicate good model fit and RMSEA values of less than .08 indicate acceptable fit.
Analyses estimated genetic and environmental influences on age at menarche and CES-D scores using a bivariate biometric model (Neale & Maes, 2007). This model partitions the variance for each phenotype (i.e., menarche and depression) into three latent factors: variance due to additive genes (A); variance due to environmental influences that make twins and siblings similar to each other, also known as the shared environment (C); and variance due to environmental influences that make twins and siblings different from each other (the nonshared environment) plus measurement error (E). The correlation between the additive genetic factors for the first and second sibling in each pair is fixed according to genetic theory: 1.0 for MZ twins, 0.5 for DZ twins and FS pairs. This is because MZ twins share 100% of their genetic code and DZ twins and FS pairs, on average, share 50% of their segregating genetic code.2
Previous analyses of the Add Health data have indicated that shared environmental influences for both menarche (Harden & Mendle, 2012; Moore, Harden, & Mendle, 2013) and CES-D scores (McCaffery, Papandonatos, Stanton, Lloyd-Richardson, & Niaura, 2008) contribute negligibly to the overall variance for each phenotype and can be dropped from the model without significant decrements in model fit. This was also the case in our preliminary models for the current study (available from the first author on request); consequently, the final models presented here include only genetic and nonshared environmental influences for menarche and CES-D scores. The bivariate Cholesky model, with SES and race included as covariates, is depicted in Figure 1a and can be considered a “main effects” model. In this model, the parameters of interest are the regressions of CES-D scores on the A and E factors of age at menarche (respectively labeled aMD and eMD). These regressions test whether symptoms of depression are predicted by the same genes that influence timing of menarche (aMD) or by environmental experiences that influence timing of menarche (eMD).
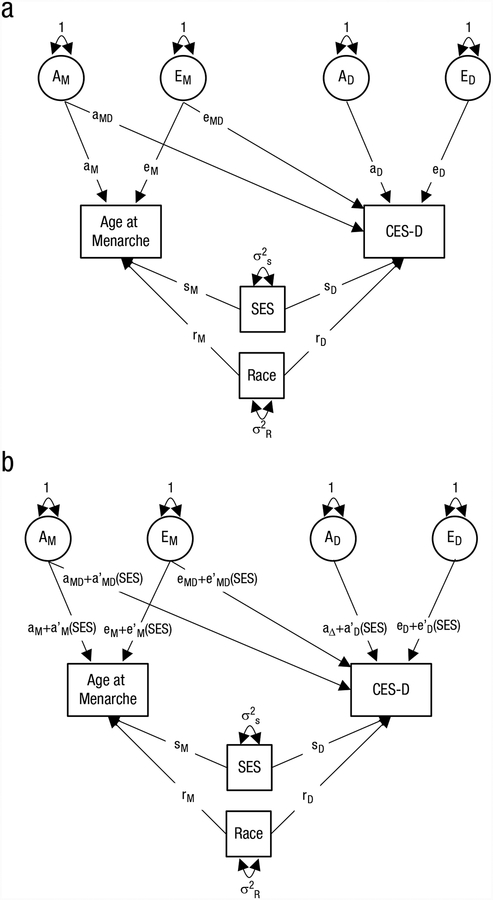
Fig. 1.
Path diagrams: The schematics depict (a) the bivariate Cholesky model and (b) the parametric Purcell model. Only one twin per pair is shown. AM and EM represent the additive genetic and nonshared environmental variance components for age at menarche; AD and ED represent the additive genetic and nonshared environmental variance components for CES-D scores independent of menarche. sM, sD, rM, and rD represent the main effects of SES and race on menarche and on CES-D scores. aM and eM represent the effect of genes and nonshared environment on age at menarche. aMD and eMD represent the effect of the genes and nonshared environmental influences that underlie age at menarche on CES-D scores. aD and eD represent the effect of genetic and nonshared environmental unique to CES-D. a′M, a′MD, a′D, e′M, e′MD, and e′D represent the moderated components of each a and e path. CES-D = Center for Epidemiological Studies-Depression Scale self-reported depressive symptoms score; Race = European American (reference) versus non-European American.
We then expanded the bivariate Cholesky model to test SES as a moderator of the genetic and environmental associations between menarche and depressive symptoms. This was tested in three ways. First, we used locally weighted structural equation modeling (LOSEM; Hildebrandt, Wilhelm, & Robitzsch, 2009) for the analysis of G × E (Briley, Harden, & Tucker-Drob, in press). LOSEM can be considered analogous to LOESS (locally weighted regression), which researchers use to draw a smoothed line through a scatter plot of observed data points by fitting regression lines to local subsets of the data using kernel regression techniques (Li & Racine, 2007). These local regressions are then connected to create a smooth, continuous, nonparametric curve. Like LOESS, LOSEM is primarily an exploratory statistical technique that is particularly useful for detecting potential nonlinearity or discontinuity in the association between variables. When applied to behavior-genetic analyses, the main parameters of interest are the pathways between the latent ACE factors and the observed variables. LOSEM focuses on how these parameter estimates (i.e., genetic and environmental influences) may differ across levels of a moderator (i.e., SES). Because this approach does not impose an a priori form to the relation between SES and genetic and environmental parameter estimates, it is helpful for evaluating potential nonlinearity.
In the Add Health data, as noted earlier, parental education is coded on a 9-point ordinal scale. We estimated the bivariate Cholesky model shown in Figure 1a nine times, once for each possible or “focal” value of SES (1 to 9). Each time, data were weighted by their distance from the focal value of SES. That is, when the focal value of SES was 1, data from pairs with very low levels of SES were weighted most heavily; when the focal value of SES was 9, data from pairs with very high levels of SES were weighted most heavily. Every LOSEM model uses data from the entire sample, but data receive less weight in the analysis with greater distance from the focal value of SES. Estimates were then aggregated across models to obtain a continuous, smoothed, nonparametric estimate of how the genetic and environmental associations between menarche and depression differ across socioeconomic levels.
Second, we tested G × E using the parametric models described by Purcell (2002; see Fig. 1b for a schematic). This model estimated the paths from the A and E factors as a linear function of SES. The presence of moderation can be inferred when an interaction term is significantly different from 0. Specifically, a gene-environment interaction in the association between menarche and depressive symptoms would be indicated by a significant interaction on the cross-path from genes influencing menarche to depression (which is labeled a′MD in Fig. 1b). This model also controls for potential gene-environment correlations (which may bias estimates of G × E) because it partitions out the variance in menarche and in CES-D scores that overlaps with SES (the main effect of SES).
Third, on the basis of the nonparametric LOSEM results, we split pairs into two groups based on parental education: (a) high SES, whose parents had a college degree or higher; and (b) low/moderate SES, whose parents had less education than a college degree. We then refit the Purcell model shown in Figure 1b except that rather than use the continuous measure of SES as the moderator, a dummy-coded indicator comparing highSES with low/moderate-SES groups was used.
Results
Descriptive analyses
Correlations among all study variables are presented in Table 1. Consistent with prior epidemiological literature, results showed that race and SES were significantly correlated, with non-European American girls experiencing greater socioeconomic disadvantage. Girls who reported earlier menarche reported higher levels of depressive symptoms on the CES-D. Socioeconomic disadvantage was also related to depressive symptoms. Last, non-European Americans reported earlier ages of menarche (M = 12.09, SD = 1.38) than did European Americans (M = 12.31, SD = 1.44).
Table 1.
Correlations Among Study Variables and Within-Pair Correlations
| Variable | Menarche | CES-D | SES | Race | MZ | DZ | FS |
|---|---|---|---|---|---|---|---|
| Menarche | — | ||||||
| CES-D | −.09 | — | |||||
| SES | .04 | −.08 | — | ||||
| Race | −.07 | .05 | −.21 | — | |||
| Within-pair correlations | |||||||
| Menarche | .61 | .31 | .30 | ||||
| CES-D | .54 | .24 | .25 |
Note: Correlations among study variables are based on one twin per pair selected at random. Pearson correlations are presented for continuous variables; point biserial coefficients are presented for associations among race (coded dichotomously: 0 = European American, 1 = non-European American) and other variables. Boldface indicates values significantly different from 0 (at p < .05). CES-D = Center for Epidemiological Studies–Depression Scale; SES = socioeconomic status; MZ = monozygotic twins; DZ = dizygotic twins; FS = full-sibling pairs.
Model 1: Bivariate Cholesky model (no interaction)
Table 2 presents the parameter estimates from the bivariate Cholesky model. The model fit the data well (CFI = .95, RMSEA = .03). The proportion of variance due to genetic and environmental factors can by calculated by dividing the squared A and E paths to the phenotype of interest (either age at menarche or CES-D scores) by the total variance for that phenotype. Following this formula, we found that genes accounted for 58% of the variance in age at menarche and nonshared environmental influences accounted for 42%. Of the variation in CES-D scores that was independent of menarche, genes accounted for 53% and nonshared environmental influences accounted for 47%. SES was inversely related to CES-D scores (b = −0.14, p < .01) but not significantly related to menarche. Conversely, race was related to age at menarche (b = −0.22) but not to CES-D scores. The regressions of CES-D scores on both A and E factors for menarche were not significantly different from 0.
Table 2.
Parameter Estimates From Behavioral-Genetic Models
| Parameter | Model 1 (interaction) | Model 2 (interaction: continuous SES) | Model 3 (interaction: dichotomous SES) |
|---|---|---|---|
| Intercept | |||
| Menarche | 12.339 (0.061) | 12.34 (0.062) | 12.343 (0.074) |
| CES-D | 0.118 (0.047) | 0.117 (0.048) | 0.160 (0.058) |
| Covariate | |||
| SES → Menarche (sM) | 0.009 (0.020) | −0.009 (0.02) | −0.020 (0.098) |
| Race → Menarche (rM) | −0.171 (0.102) | −0.169 (0.103) | −0.167 (0.101) |
| SES → CES-D (sD) | −0.051 (0.015) | −0.050 (0.016) | −0.162 (0.076) |
| Race → CES-D (rD) | 0.145 (0.079) | 0.149 (0.079) | 0.169 (0.079) |
| Genetic and environmental influences on menarche | |||
| aM | 1.044 (0.054) | 1.067 (0.054) | 1.067 (0.076) |
| a′M | — | −0.015 (0.022) | −0.034 (0.106) |
| eM | 0.895 (0.046) | 0.870 (0.048) | 0.902 (0.066) |
| e′M | — | 0.020 (0.019) | −0.032 (0.091) |
| Menarche → CES-D | |||
| aMD | −0.108 (0.063) | −0.017 (0.064) | 0.033 (0.085) |
| a′MD | — | −0.038 (0.024) | −0.289 (0.125) |
| eMD | 0.041 (0.056) | −0.008 (0.059) | −0.113 (0.076) |
| e′MD | — | 0.050 (0.022) | 0.326 (0.110) |
| Residual genetic and environmental influences on CES-D | |||
| aD | 0.774 (0.049) | 0.787 (0.049) | 0.812 (0.060) |
| a′D | — | −0.029 (0.020) | −0.080 (0.104) |
| eD | 0.742 (0.040) | 0.725 (0.041) | 0.711 (0.052) |
| e′D | — | 0.003 (0.016) | 0.011 (0.081) |
Note: See Figure 1a and 1b for path diagrams. Standard errors are shown in parentheses; boldface indicates parameters significantly different from 0 (at p < .05). For Models 1 and 2, SES centered at parental education equals 5. For Model 3, SES dichotomized into less than a college degree—parental education equals less than 7 (reference group) versus a college degree or higher. SES = socioeconomic status; CES-D = Center for Epidemiological Studies–Depression Scale.
G × E interaction models
LOSEM results.
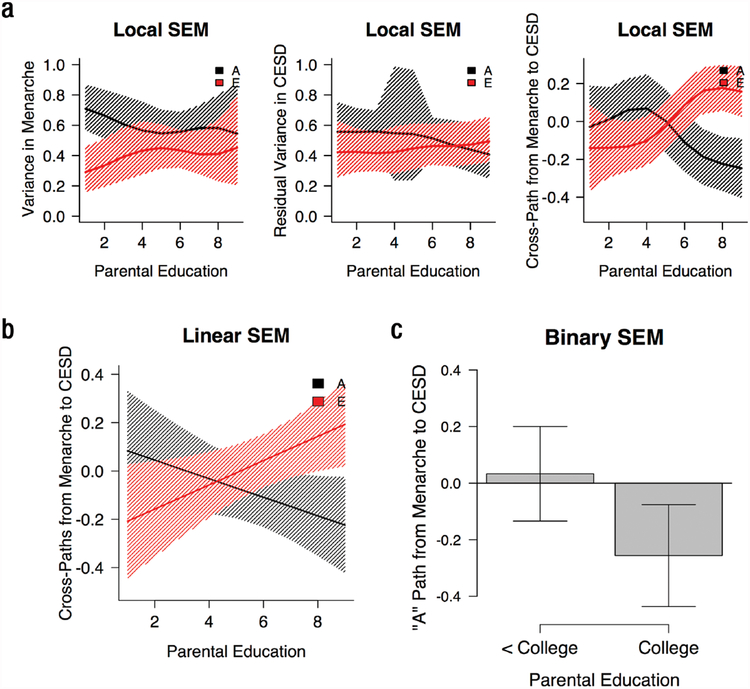
Figure 2a displays the locally weighted trends in genetic and environmental parameter estimates across levels of parental education, along with 95% confidence intervals. The left panel shows the standardized genetic and environmental variances in age at menarche. There is a slight trend toward increasing nonshared environmental influence and decreasing genetic influence as SES increases. The middle panel shows the standardized genetic and environmental variances in CES-D scores that are unique of age at menarche. Again, there is a slight trend toward increasing nonshared environmental influences and decreasing genetic influence as SES increases. In addition, when pairs with average levels of SES were weighted most heavily (focal SES approximately 5), there was considerably more uncertainty regarding the residual genetic influence. Finally, the right panel shows the standardized cross-paths from the A and E factors of menarche to CES-D scores. At lower levels of SES, the A and E cross-paths are both close to 0 and the 95% confidence intervals are wide and include 0. At higher levels of SES, the A and E cross-paths diverge: The A path becomes more negative, whereas the E path becomes more positive. The A path suggests that, at higher levels of SES, girls who are genetically predisposed toward later menarche show lower levels of depressive symptoms and, conversely, girls who are genetically predisposed toward earlier menarche show higher levels of depressive symptoms. At the same time, as indicated by the positive E path, within a pair of identical twins, the twin who experiences later menarche shows higher levels of depression than does her co-twin. We tested the statistical significance of these trends in our subsequent parametric analyses.
Fig. 2.
Model results: The graphs in (a) display the local structural equation modeling (SEM) trends in genetic and environmental parameter estimates across levels of parental education. The graph in (b) shows Purcell model linear SES results. The graph in (c) shows Purcell model binary high-SES versus low/moderate-SES results (group differences are statistically significant, p = .02). Bands and error bars represent 95% confidence intervals. Cross-paths from menarche to CES-D are standardized paths. CES-D = Center for Epidemiological Studies–Depression Scale; A = additive genetic; E = nonshared environmental.
Purcell model results: Linear SES.
Parameter estimates for the bivariate interaction model are shown in the second column of Table 2. SES did not significantly moderate the genetic and environmental influences on menarche or the residual genetic and environmental influences on depressive symptoms unique of menarche. There was a significant E × SES interaction in the association between menarche and CES-D scores (p = .02; see Fig. 2b for Purcell model results). The E factor, as noted earlier, represents the variation in a phenotype that is unique to each twin in a pair. The path from the E factor of menarche to CES-D, therefore, represents the extent to which twins who differ in menarche show differences in depressive symptoms. Accordingly, the E × SES effect indicates that this within-twin-pair effect of menarche on depressive symptoms varies across socioeconomic strata: Among high-SES girls, twins who have later menarche than their co-twins show a higher number of depressive symptoms. This within-twin effect of menarche is attenuated among girls at lower levels of SES.
The estimate for the A × SES interaction in the Purcell model was consistent with what was observed in the LOSEM results: Later menarche was associated with fewer depressive symptoms among girls at higher levels of SES. This effect, however, was not statistically significant. However, a reduced model that included interaction effects only on the cross-paths from the AE components of age at menarche to depressive symptoms fit significantly better than did a model with no interaction effects, Δχ2(2, N = 550 pairs) = 6.578, p = .037 (full parameter estimates are available on request).
Purcell model results: High SES versus low/moder-ate SES.
The LOSEM results suggested the relation between SES and the A path was flat in the lower range of parental education. Our third analysis therefore directly targeted a potential nonlinear pattern in SES. To test this, we dichotomized parental education to separate participants with high SES (whose parent had a college degree) versus participants of low-to-moderate SES (less than a college degree). Results from this model are summarized as Model 3 in Table 2. There was evidence for significant A × SES and E × SES effects (see Fig. 2c for Purcell model results). Among girls whose parents were college educated, a genetic predisposition toward later menarche was significantly associated with lower CES-D scores (b = −0.26, SE = 0.09, p = .005), but this effect was not present among girls whose parents had less than a college education (b = 0.03, SE = 0.09, p = .70). This difference between the groups was statistically significant (b = −0.29, SE = 0.13, p = .02). Moreover, among girls whose parents were college educated, within-twin-pair differences in menarche were associated with depression such that the twin who matured later reported more depressive symptoms (b = 0.21, SE = 0.08, p = .007). There was no within-twinpair association (i.e., E cross-path) among girls whose parents had less than a college education (b = −0.11, SE= 0.08, p = .14). Again, this difference between the groups was statistically significant (b = 0.33, SE = 0.11, p = .003).
Sensitivity analyses using alternative SES variable
Parental education is a single indicator of SES. SES is also conventionally measured using income, wealth, and occupational status and, as noted earlier, is more complex than can be captured by education level. We elected to use parental education for our primary analyses because (a) parental education is a more stable indicator of social class than income and (b) a nontrivial portion of the sample is missing parental report of income (24.1%). Nevertheless, to examine whether the current results were consistent with a broader operationalization of SES, we constructed an SES composite on the basis of parental education and income. We then refit the LOSEM and dichotomous SEM models using the SES composite variable in lieu of parental education. Details and complete results from analyses using the SES composite are described in the Supplemental Material. Overall, the LOSEM analyses suggested a similar pattern of results between age at menarche and depressive symptoms regardless of whether the SES composite or education alone was used. The dichotomous interaction models yielded highly similar parameter estimates across the two measures of SES. There were significant interaction effects on the cross-paths from the A and E components of menarche to depression regardless of which measure of SES was used.
Discussion
Puberty is a universal transition navigated by all children on their path to adulthood, but it is also highly individualized. Variations in puberty across children include differences in the timing and rate of development and the subjective meaning of maturation within a child’s time, place, and culture (Mendle, 2014). In accordance with previous research, our findings indicate that a genetic predisposition for earlier menarche is psychologically risky and a genetic predisposition for later menarche is psychologically advantageous. However, these effects emerged only for girls in higher socioeconomic contexts; for girls from lower socioeconomic backgrounds, depressive symptoms were not related to age at menarche by either a genetic or a nonshared environmental pathway.
These findings are broadly consistent with the social-push hypothesis (e.g., Raine, 2002). The social-push model of G × E interaction is the major alternative model to a diathesis-stress model. Although developed originally with regard to antisocial behavior, the principles of social push can apply to a number of phenotypes, including the depressive symptoms studied here. Social push predicts that environmental adversities “push” children into negative psychological outcomes. In contrast, genetic predispositions become more influential in contexts with low environmental risk. Therefore, the association between genetic risk (in this case, genetic predispositions toward earlier menarche) and depressive symptoms will emerge only if environmental conditions are relatively secure. The social-push pattern of G × E observed here can be contrasted with the more familiar diathesis-stress model, in which genetic predispositions are expected to be more strongly predictive of behavior in conditions of environmental adversity.
Consideration of social push and the divergence between high and low-SES contexts yields two related questions. First, why do low-SES contexts lack an association between menarche and depressive symptoms? Second, what are the qualities of high-SES contexts that foster an association between genetic predispositions for menarche and depressive symptoms? With regard to the first question, it is essential to recognize that these results do not indicate an absence of depressive symptoms for girls from low-SES backgrounds. In fact, we observed a main effect consistent with previous research; girls at lower levels of SES displayed the highest levels of depressive symptoms in the sample. However, their symptoms were unrelated to the timing of puberty, which we suspect reflects their life experiences. Girls growing up in communities with lower levels of SES have a lengthy and chronic history of stress exposure across their entire lives. They must navigate numerous pathways of potential risk and may show a dysregulated stress response even before puberty begins. Multiple genes correlated with depression have been shown to be sensitive to stressful environments, including several serotonergic genes and the BDNF gene, which plays a role in synaptic growth. One explanation for the present results is that the genes related to menarche are not the genes relevant for depression in low-SES contexts. That is, the biological and social challenges that accompany early puberty—and the advantages conferred by later maturity—are less consequential in communities in which there are numerous stressors that may activate propensity for depression. In contrast, in more advantaged contexts, risks are reduced and the overall frequency of depression is less common. For girls growing up in this environment, puberty may represent one of the first serious sources of life stress, and its influence is less likely to be obscured by conflicting stressors.
Developing girls, of course, lack knowledge of the genes they carry and whether these genes have been expressed. For them, depression is a subjective experience often viewed through the lens of their daily lives and experiences. This is relevant for the second question outlined earlier: identifying the aspects of environments with higher levels of SES that underlie the association between genetic predispositions for menarche and depressive symptoms. The genes that control timing of menarche are most commonly related to the synthesis and secretion of ovarian hormones, which are known to play a role in regulation of weight and eating (reviewed in Asarian & Geary, 2006), as well as genes that directly regulate body mass index, food intake, and satiety (Kim et al., 2012; Perry et al., 2014). Adolescent girls are highly attuned to their bodies, particularly to the extent to which they may be perceived as attractive and desirable to others. What constitutes attractive—and the extent to which the physiological changes of puberty are congruent with this ideal—differs across SES. Although girls from lowSES backgrounds tend to weigh more than do girls from high-SES backgrounds, they also tend to have better body image and are less likely to diet or engage in other weight-control behaviors (O’Dea & Caputi, 2001). Because poor body image is one of the strongest prospective predictors of depression among adolescent girls (Stice & Bearman, 2001), socioeconomic variation in how girls interpret and respond to normative changes in weight, curviness, and breast development may explain variation in mood. Given that girls with later timing of menarche are genetically predisposed to maintain lower body weight and a slimmer, less curvy body for a longer period of time, their depressive risk may be attenuated in the high-SES contexts that overvalue a thin body ideal.
Last, in addition to our G × E interaction, we also observed an “Environment × Environment interaction.” That is, in addition to the significant interaction of genes with SES, results suggested an interaction between SES and the environmental influences unique to each twin (the significant E parameter in Models 2 and 3). It is important that this interaction is in the opposite direction from the genetic interaction. For girls from high-SES backgrounds, later menarche is overall associated with less depression, yet within a pair of sisters, the pattern is reversed: The sister who goes through menarche first reports fewer symptoms of depression than does the sister who goes through menarche later. We suspect this may reflect how aspects of puberty unfold within sisterly relationships and, particularly, within the intimate bonds shared by twins. Foundational studies of puberty explored the possibility that any aberrance in pubertal timing-either early or late—might carry psychological risk (sometimes termed the “off-time” hypothesis; e.g., Simmons & Blyth, 1987). Because twins naturally serve as a reference point for each other, it may be the case that maturing later than one’s twin conveys a sense of being off time or even left behind.
Limitations and Future Directions
There are a number of methodological aspects of our study worth consideration. First and foremost, the temporal relationship of menarche and depressive symptoms is ambiguous. We have limited knowledge of the premenarcheal affective state of the majority of our participants. Although many theories of pubertal timing assume puberty provokes psychological distress, those girls most likely to respond adversely to menarche may be those with preexisting psychological difficulties. In this case, puberty represents an exacerbation or reactivation of difficulties among vulnerable girls. In support of this, early puberty has been more closely linked with depression among girls who are already at risk in some way (e.g., girls who have histories of maltreatment, Mendle, Leve, Van Ryzin, & Natsuaki, 2014, or who are prone to cognitive distortions and ruminative coping, Hamilton et al., 2013). Second, pubertal timing in this study was solely determined using age at menarche. Although menarche is among the most commonly used indicators of pubertal timing, it is one of the last biological events during puberty and typically occurs at Tanner Stage 4 or 5. By the time early maturing girls reach menarche, their peers have typically started puberty. Yet when other pubertal changes- such as changes in skin, body shape, or hair—occur, early maturing girls are the only members of their peer network navigating puberty, which may heighten a sense of isolation or difference from others around them. Because of its timing in the pubertal process, menarche may therefore not capture important cognitive and emotional reactions to development that occur in the beginning stages of puberty. In addition, because of its relatively concealed nature, it may lack some of the social repercussions of changes that are more visibly obvious to others.
In general, behavior-genetics research on puberty and its psychological correlates is uncommon. Our study, like the majority of research, concentrated on female sister dyads. Because boys’ puberty is more difficult to measure than girls’, boys tend to be understudied relative to girls; in fact, there currently are no genetically informed studies of puberty and boys’ depression. Whether the results obtained in the current study would hold true for boys is unclear, but we believe there is good reason that boys might show a different pattern of results. All extant research suggests that puberty is more challenging for girls than for boys and that girls experience more emotional change and symptoms of a variety of different clinical disorders (in addition to depression). However, this interpretation is purely speculative and the psychology of boys’ development remains an open field for behavior geneticists.
Last, it is worth commenting on our measure of SES. Although SES is frequently addressed as either a focus or necessary covariate in research, it has proved to be multidimensional and difficult to capture through simple metrics. In the present study, we used educational attainment of parents as a proxy for SES. This is a common technique, particularly within the fields of economics, demography, and sociology. But it is also incomplete. As noted earlier, SES is a blend of not just education but also income, occupational prestige, and more subtle indicators of social standing. It is these more subtle elements that contribute to individuals’ sense of their place in society, the availability of resources, and prospects for the future. In other words, the elements of SES that are most psychologically relevant may also be the most difficult to measure.
Conclusion
Despite conventional scientific wisdom that genes and environment jointly influence health and well-being, there is limited understanding of gene-environment interplay with regard to puberty and its psychosocial correlates. In the present study, we considered some of the multiple influences—personal, familial, and societal- that help link timing of puberty with adolescent mood. It is logical that girlhood, and by extension the process of growing out of girlhood, would hold varying salience for girls from different backgrounds. Although preliminary, the current results suggest that the association of pubertal timing with depression may be both more complicated and more difficult to capture than previously assumed.
Supplementary Material
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Supplemental Material
Additional supporting information may be found at http://cpx.sagepub.com/content/by/supplemental-data
Notes
Add Health also includes half siblings, cousins raised together, and biologically unrelated siblings. We elected to include only twins and FS pairs in our analyses. This allowed us to ascertain that the report of maternal education used in analyses came from the same mother and applied to both members of a sibling pair.
Although conventionally labeled the nonshared environmental factor, the E factor represents variation due to factors that differ within MZ twin pairs. To the extent that MZ twins are not, in fact, perfectly genetically identical (Charney, 2012), the effects of that within-MZ variation will be reflected in E. Also, in nontwin biometrical analyses, measurement error may be estimated separately from nonshared environment variance.
References
- Allsworth JE, Weitzen S, & Boardman LA (2005). Early age at menarche and allostatic load: Data from the Third National Health and Nutrition Examination Survey. Annals of Epidemiology, 15, 438–444. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1994). Diagnostic and sta-tistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- Anderson CA, Duffy DL, Martin NG, & Visscher PM (2007). Estimation of variance components for age at menarche in twin families. Behavior Genetics, 37, 668–677. [DOI] [PubMed] [Google Scholar]
- Asarian L, & Geary N (2006). Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society B: Biological Sciences, 361, 1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Steinberg L, & Draper P (1991). Childhood experience, interpersonal development, and reproductive strategy. Child Development, 62, 647–670. [DOI] [PubMed] [Google Scholar]
- Bentler PM (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107, 238–246. [DOI] [PubMed] [Google Scholar]
- Bogaert AF (2008). Menarche and father absence in a national probability sample. Journal of Biosocial Science, 40, 623–636. [DOI] [PubMed] [Google Scholar]
- Bolland JM (2003). Hopelessness and risk behaviour among adolescents living in high-poverty inner-city neighbourhoods. Journal of Adolescence, 26, 145–158. [DOI] [PubMed] [Google Scholar]
- Bolland JM, Lian BE, & Formichella CM (2005). The origins of hopelessness among inner-city African-American adolescents. American Journal of Community Psychology, 36, 293–305. [DOI] [PubMed] [Google Scholar]
- Bradley RH, & Corwyn RF (2002). Socioeconomic status and child development. Annual Review of Psychology, 53, 371–399. [DOI] [PubMed] [Google Scholar]
- Briley DA, Harden KP, & Tucker-Drob EM (in press). Genotype × cohort interaction on completed fertility and age at first birth. Behavior Genetics. doi: 10.1007/s10519014-9693-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey VA, Dwyer JT, Coleman KA, Krall EA, Gardner J, & Valadian I (1991). Accuracy of recall by middle-aged participants in a longitudinal study of their body size and indices of maturation earlier in life. Annals of Human Biology, 18, 155–166. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, … Poulton R (2003). Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science, 301, 386–389. [DOI] [PubMed] [Google Scholar]
- Charney E (2012). Behavior genetics and postgenomics. Behavioral & Brain Sciences, 35, 331–358. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, & Shear MK (2000). Adolescent onset of the gender difference in lifetime rates of major depression: A theoretical model. Archives of General Psychiatry, 57, 21–27. doi: 10.1001/archpsyc.57.1.21 [DOI] [PubMed] [Google Scholar]
- Davies JB, Sandström S, Shorrocks A, & Wolff E (2009). The global pattern of household wealth. Journal of International Development, 2, 1111–1124. [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, & Biro F (2006). Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science, 10, 30–56. [Google Scholar]
- Doughty D, & Rodgers JL (2000). Behavior genetic modeling of menarche in U.S. females In Rodgers JL, Rowe DC, & Miller WB (Eds.), Genetic influences on human fertility and sexuality: Theoretical and empirical contributions from the biological and behavioral sciences (pp. 169–182). Boston, MA: Kluwer. [Google Scholar]
- Dupéré V, Leventhal T, & Lacourse E (2009). Neighborhood poverty and suicidal thoughts and attempts in late adolescence. Psychological Medicine, 39, 1295–1306. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, … Craig IW (2004). Gene–environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry, 9, 908–915. [DOI] [PubMed] [Google Scholar]
- Elks CE, Perry JRB, Sulem P, Chasman DI, Franceschini N, He C, … Murray A (2010). Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nature Genetics, 42, 1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, & Berenson GS (2003). The relation of menarcheal age to obesity in childhood and adulthood: The Bogalusa Heart Study. BMC Pediatrics, 3, 3 Retrieved from http://www.biomedcentral.com/1471-2431/3/3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Ahern J, Nandi A, Tracy M, Beard J, & Vlahov D (2007). Urban neighborhood poverty and the incidence of depression in a population-based cohort study. Annals of Epidemiology, 17, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, & Natsuaki MN (2009). In search of explanations for early pubertal timing effects on developmental psychopathology. Current Directions in Psychological Science, 18, 327–331. [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, & Brooks-Gunn J (1997). Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child and Adolescent Psychiatry, 36, 1768–1776. [DOI] [PubMed] [Google Scholar]
- Graber JA, Nichols TR, & Brooks-Gunn J (2010). Putting pubertal timing in developmental context: Implications for prevention. Developmental Psychobiology, 52, 254–262. [DOI] [PubMed] [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, & Lewinsohn PM (2004). Is pubertal timing associated with psychopathology in young adulthood? Journal of the American Academy of Child and Adolescent Psychiatry, 43, 718–726. [DOI] [PubMed] [Google Scholar]
- Grumbach MM, & Styne DM (2003). Puberty: Ontogeny, neuroendocrinology, physiology, and disorders In Wilson JD, Foster DW, & Kronenberg HM (Eds.), Williams textbook of endocrinology (pp. 1509–1625). Philadelphia, PA: Saunders. [Google Scholar]
- Hamilton JL, Stange JP, Kleiman EM, Hamlat EJ, Abramson LY, & Alloy LB (2013). Cognitive vulnerabilities amplify the effect of early pubertal timing on interpersonal stress generation during adolescence. Journal of Youth and Adolescence, 41, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, & Mendle J (2012). Gene-environment interplay in the association between pubertal timing and delinquency in adolescent girls. Journal of Abnormal Psychology, 12, 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM (2009). The National Longitudinal Study of Adolescent Health (Add Health), Waves I & II, 1994–1996; Wave III, 2001–2002; Wave IV, 2007–2009 [Machinereadable data file and documentation]. Chapel Hill: University of North Carolina at Chapel Hill, Carolina Population Center. doi: 10.3886/ICPSR27021.v9 [DOI] [Google Scholar]
- Harris KM, Halpern CT, Smolen A, & Haberstick BC (2006). The National Longitudinal Study of Adolescent Health (Add Health) twin data. Twin Research and Human Genetics, 9, 988–997. [DOI] [PubMed] [Google Scholar]
- Henderson C, Roux AVD, Jacobs DR, Kiefe CI, West D, & Williams DR (2005). Neighbourhood characteristics, individual level socioeconomic factors, and depressive symptoms in young adults: The CARDIA study. Journal of Epidemiology and Community Health, 59, 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt A, Wilhelm O, & Robitzsch A (2009). Complementary and competing factor analytic approaches for the investigation of measurement invariance. Review of Psychology, 16, 87–102. [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. [Google Scholar]
- Jacobson KC, & Rowe DC (1999). Genetic and environmental influences on the relationships between family connectedness, school connectedness, and adolescent depressed mood. Developmental Psychology, 35, 926–939. [DOI] [PubMed] [Google Scholar]
- James-Todd T, Tehranifar P, Rich-Edwards J, Titievsky L, & Terry MB (2010). The impact of socioeconomic status across early life on age at menarche among a racially diverse population of girls. Annals of Epidemiology, 20, 836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen SMJ, Chessa AG, & Murre JMJ (2006). Memory for time: How people date events. Memory & Cognition, 34, 138–147. doi: 10.3758/BF03193393 [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, & Sen S (2011). The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry, 68, 444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-Z, Shin A, Lee Y-S, Kim S-Y, Kim Y, & Lee E-S (2012). Polymorphisms in adiposity-related genes are associated with age at menarche and menopause in breast cancer patients and healthy women. Human Reproduction, 27, 2193–2200. [DOI] [PubMed] [Google Scholar]
- Lennon MC, Blome J, & English K (2002). Depression among women on welfare: A review of the literature. Journal of the American Medical Women’s Association, 57, 27–32. [PubMed] [Google Scholar]
- Li Q, & Racine J (2007). Nonparametric econometrics: Theory and practice. Princeton, NJ: Princeton University Press. [Google Scholar]
- Lorant V, Deliège D, Eaton W, Robert A, Philippot P, & Ansseau M (2003). Socioeconomic inequalities in depression: A meta-analysis. American Journal of Epidemiology, 157, 98–112. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Papandonatos GD, Stanton C, LloydRichardson EE, & Niaura R (2008). Depressive symptoms and cigarette smoking in twins from the National Longitudinal Study of Adolescent Health. Health Psychology, 27, S207–S215. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Katz R, Watkins S, & Rutherford J (1996). A hospital-based twin register of the heritability of DSM-IV unipolar depression. Archives of General Psychiatry, 53, 129–136. [DOI] [PubMed] [Google Scholar]
- Mendle J (2014). Beyond pubertal timing: New directions for studying individual differences in development. Current Directions in Psychological Science, 23, 215–219. [Google Scholar]
- Mendle J, Leve LD, Van Ryzin M, & Natsuaki MN (2014). Linking childhood maltreatment with girls’ internalizing symptoms: Early puberty as a tipping point. Journal of Research on Adolescence, 24, 689–702. doi: 10.1111/jora.12075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Turkheimer E, & Emery RE (2007). Detrimental psychological outcomes associated with early pubertal timing in adolescent girls. Developmental Review, 27, 151–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SR, Harden KP, & Mendle J (2013). Pubertal timing and adolescent sexual behavior in girls. Developmental Psychology, 50, 1734–1745. 10.1037/a0036027 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2010). Mplus user’s guide (4th ed.). Los Angeles, CA: Authors. [Google Scholar]
- Neale MC, & Maes HH (2007). Methodology for genetic studies of twins and families. Dordrecht, The Netherlands: Kluwer Academic. [Google Scholar]
- Obeidallah D, Brennan RT, Brooks-Gunn J, & Earls F (2004). Links between pubertal timing and neighborhood contexts: Implications for girls’ violent behavior. Journal of the American Academy of Child and Adolescent Psychiatry, 43, 1460–1468. [DOI] [PubMed] [Google Scholar]
- O’Dea JA, & Caputi P (2001). Association between socioeconomic status, weight, age and gender, and the body image and weight control practices of 6- to 19-year-old children and adolescents. Health Education Research, 16, 521–532. [DOI] [PubMed] [Google Scholar]
- Perry JRB, Day F, Elks CE, Sulem P, Thompson DJ, Ferreira T, … Ong KK (2014). Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature, 514, 92–97. doi: 10.1038/nature13545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S (2002). Variance components models for gene-environment interaction in twin analysis. Twin Research, 5, 554–571. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. [Google Scholar]
- Radloff LS, & Locke BZ (1986). The community mental health assessment survey and the CES-D Scale In Weissman MM, Myers JK, & Ross CE (Eds.), Community surveys of psychiatric disorders (pp. 177–189). Rutgers, NJ: Rutgers University Press. [Google Scholar]
- Raine A (2002). Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology, 30, 311–326. [DOI] [PubMed] [Google Scholar]
- Rowe DC (2000). Environmental and genetic influences on pubertal development: Evolutionary life history traits? In Rodgers JL, Rowe DC, & Miller WB (Eds.), Genetic influences on human fertility and sexuality: Theoretical and empirical contributions from the biological and behav-ioral sciences (pp. 147–168). Boston, MA: Kluwer. [Google Scholar]
- Rushton JL, Forcier M, & Schectman RM (2002). Epidemiology of depressive symptoms in the National Longitudinal Study of Adolescent Health. Journal of the American Academy of Child and Adolescent Psychiatry, 41, 199–205. [DOI] [PubMed] [Google Scholar]
- Rutter M, Kim-Cohen J, & Maughan B (2006). Continuities and discontinuities in psychopathology between childhood and adult life. Journal of Child Psychology and Psychiatry, 47, 276–295. [DOI] [PubMed] [Google Scholar]
- Simmons RG, & Blyth DA (1987). Moving into adoles-cence: The impact of pubertal change and school context. Hawthorne, NY: Aldine. [Google Scholar]
- Spitz E, Moutier R, Reed T, Busnel MC, Marchaland C, Roubertoux PL, & Carlier M (1996). Comparative diagnoses of twin zygosity by SSLP variant analysis, questionnaire, and dermatoglyphic analysis. Behavior Genetics, 26, 55–63. [DOI] [PubMed] [Google Scholar]
- Steiger H (1990). Structural model evaluation and modification. Multivariate Behavioral Research, 25, 173–180. [DOI] [PubMed] [Google Scholar]
- Stice E, & Bearman SK (2001). Body-image and eating disturbances prospectively predict increases in depressive symptoms in adolescent girls: A growth curve analysis. Developmental Psychology, 37, 597–607. [DOI] [PubMed] [Google Scholar]
- Stice E, Presnell K, & Bearman SK (2001). Relation of early menarche to depression, eating disorders, substance abuse, and comorbid psychopathology among adolescent girls. Developmental Psychology, 37, 608–619. [DOI] [PubMed] [Google Scholar]
- Treloar SA, & Martin NG (1990). Age at menarche as a fitness trait: Nonadditive genetic variance detected in a large twin sample. American Journal of Human Genetics, 47, 137–148. [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of the Treasury. (2007). Income mobility in the U.S. from 1996–2005: Report from the Department of Treasury. Washington, DC: Author. [Google Scholar]
- Wichstrøm L (2000). Predictors of adolescent suicide attempts: A nationally representative longitudinal study of Norwegian adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 603–610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.