Abstract
To examine metabolic differences between renal allograft acute cellular rejection (ACR) and ischemic-reperfusion injury (IRI), we transplanted MHC-mismatched kidneys and induced 28 minutes warm-IRI, and collected the ACR and IRI kidneys as well as their respective native and collateral control kidneys. We extracted metabolites from the kidney tissues and found the lysine catabolite saccharopine 12.5-fold enriched in IRI kidneys, as well as the immunometabolites itaconate and kynurenine in ACR kidneys. Saccharopine accumulation is known to be toxic to mitochondria and may contribute to IRI pathophysiology, while itaconate and kynurenine may be reflective of counterregulatory responses to immune activation in ACR.
Keywords: Metabolomic analysis, saccharopine, lysine catabolism, kynurenine, itaconate, tryptophan metabolism, NAD synthesis, NAD recycling, ischemia-reperfusion injury, renal transplant, allograft rejection
Introduction
Acute cellular rejection (ACR) is an important complication of kidney transplantation and must be distinguished from other causes of allograft dysfunction such as renal ischemia-reperfusion injury (IRI). Metabolomics of urine, blood and/or kidney tissue are of diagnostic interest (Christians et al, 2016). However, it is important to identify metabolites specific to a particular type of injury as opposed to general cell damage or impaired renal filtration. We hypothesized that unbiased tissue metabolomic profiling comparing IRI and ACR in murine models, each with intact control and native kidneys to maintain renal function, could identify new biomarkers and may provide new clues to pathophysiology.
Material and Methods
We induced renal IRI and ACR in murine models (Fig. 1A). For IRI, we clamped a renal pedicle in female C57Bl/6 mice under temperature-controlled conditions (36.0 ± 0.5 °C). Induction of ischemia was confirmed through visual inspection (expected color change after interruption of blood flow). The unclamped kidney served as control (CTR) and enabled maintenance of normal renal function. The IRI and CTR kidneys were obtained 24 hours after re-perfusion, at peak IRI injury based upon our previous studies (Levine et al, 2015). For allograft rejection, we transplanted C57Bl/6 kidneys into MHC-mismatched Balb/c recipients, and waited two weeks post-transplant until peak acute cellular rejection, as observed in our previous studies (Levine et al, 2016). The native Balb/c kidneys (NK) served as control condition to acute cellular rejection, and similar to the contralateral CTR kidneys in the IRI model, enabled the mice to maintain normal renal function. Additional comparisons between NK and CTR allowed to assess Balb/c versus C57Bl/6 differences (and systemic, not sufficiently cleared circulating metabolites, see Fig. S1). At the end of the experiment, kidney tissues were snap frozen and submitted to Metabolon for metabolite extraction and ultrahigh performance liquid chromatography-tandem mass spectrometry, with non-targeted metabolomic profiling of 879 biochemicals (Metabolon, Inc.). FDR-adjusted t-tests combined with a 2.5-fold difference were used to examine differences between ACR vs. NK and IRI vs. CTR metabolites (Fig. S1, Tables S1–4). For details, please refer to the detailed methods in the Supplementary Information.
Figure 1: Lysine catabolism is affected by renal ischemia-reperfusion injury.
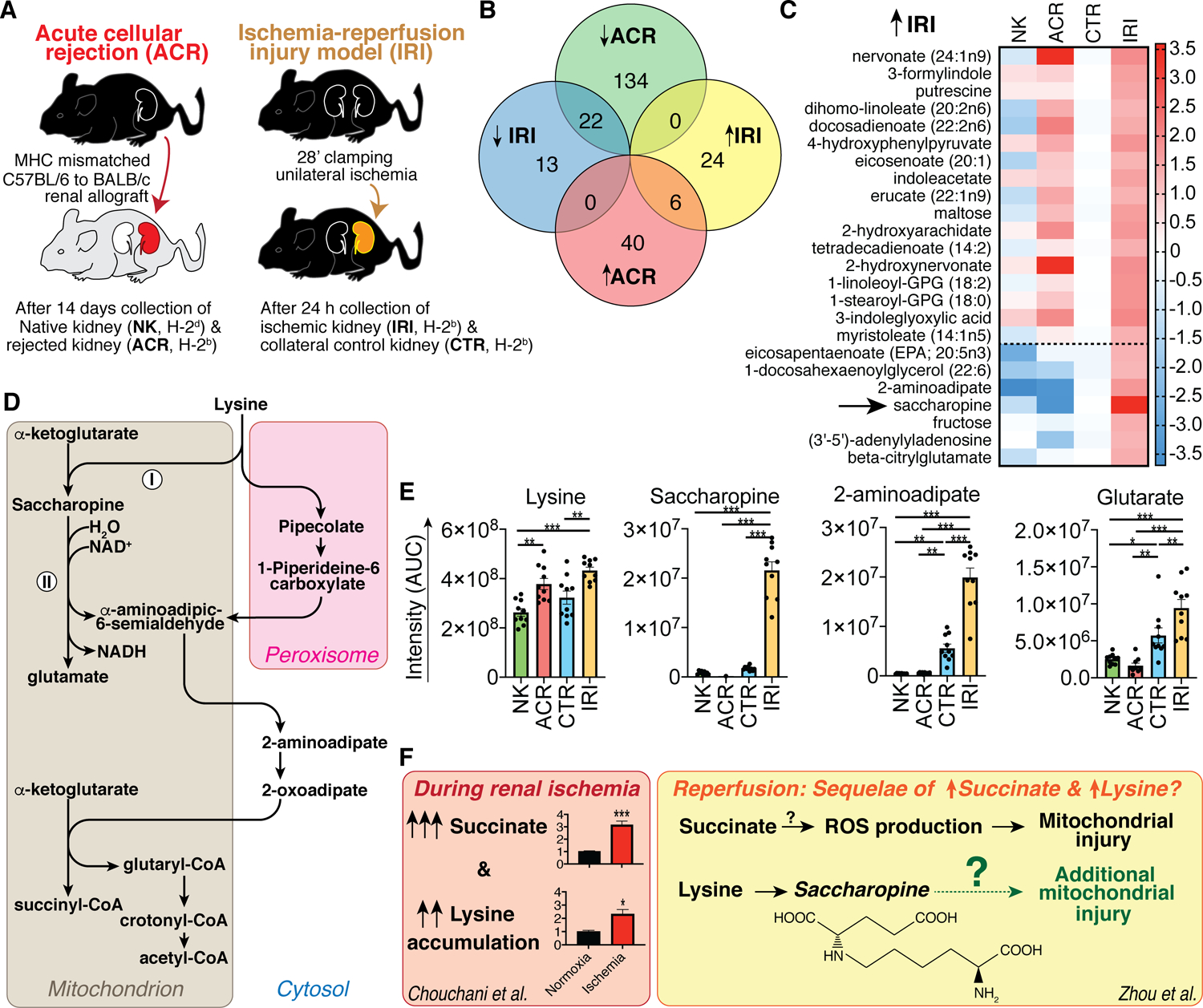
(A) Experimental design. (B) Venn diagram of statistically significant changes in detected 879 metabolites. (C) Heatmap of metabolite intensity area under the curve (AUC) measurements normalized to the average of the control (CTR) condition to the ischemia-reperfusion injury (IRI) model, and log2 transformed for display. Data shown include 24 metabolites with increased metabolite measurements in IRI vs. CTR, without overlap to the acute cellular rejection model (ACR). The dotted line separates metabolites that were only elevated in IRI and normal or decreased in the ACR condition. (D) Lysine catabolism, based upon (Danhauser et al, 2012). (I) and (II) indicate the LKR and SDH reactions catalyzed by the respective LKR and SDH domains of 2-aminoadipate-6-semialdehyde synthetase (AASS). (E) Intensity AUC measurements of metabolites involved in lysine catabolism (unpaired ANOVA). (F) Model of how saccharopine seen in our IRI model could link lysine accumulation in renal ischemia (data from (Chouchani et al, 2014)) with recently reported saccharopine-mediated mitochondrial injury (Zhou et al, 2019), further aggravating succinate-mediated mitopathy. *, **, and *** indicate p<0.05, p<0.01, and p<0.001, respectively. Data shown as mean ± SEM.
Results and Discussion
We found 30 metabolites elevated in IRI; of these, six (20%) were also elevated in ACR (Fig. 1B, 2A) and another 17 (57%) were similarly elevated in ACR but not at a statistically significant level (Fig. 1C, above dashed line). These overlapping metabolites between IRI and ACR consisted of cell membrane lipids and other components known to be increased with apoptosis, which may be nonspecific makers of tissue injury. We considered metabolites that were uniquely increased in IRI, and equal to, or lower than, control in ACR, to be of particular interest for insights into the pathogenesis of IRI (Fig. 1C, below dashed line). Among these, the highest fold difference was observed for the lysine catabolite saccharopine (arrow, Fig. 1C). Saccharopine is of particular interest due to the recent discovery of saccharopine-induced mitochondrial toxicity (Leandro & Houten, 2019; Zhou et al, 2019). Mitochondrial injury is a key mechanism in renal IRI pathophysiology (Malek & Nematbakhsh, 2015).
Figure 2: Itaconate and kynurenine accumulate during renal allograft rejection.
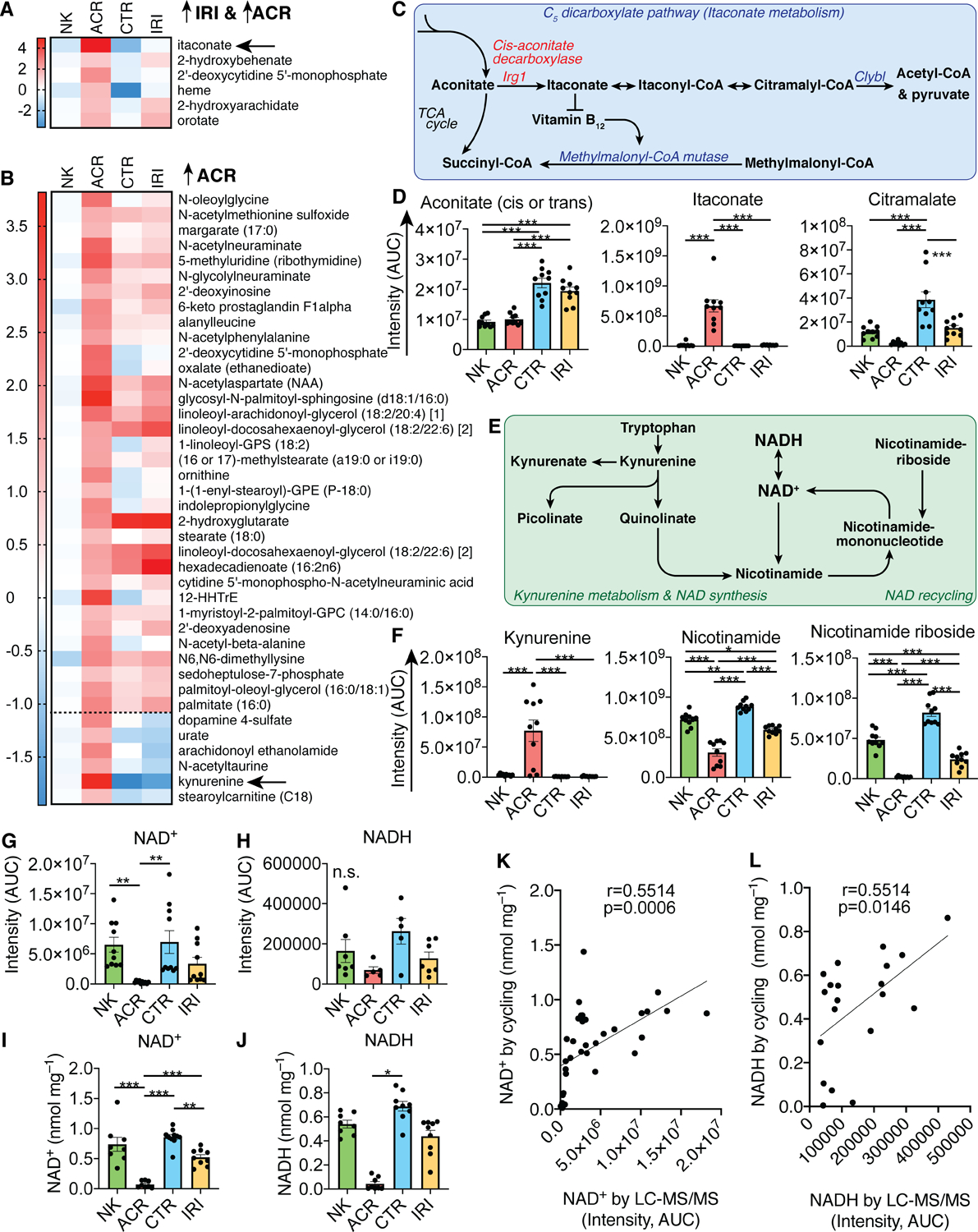
(A, B) Heatmaps of metabolite area under the curve (AUC) measurements normalized to the average of the native kidney (NK) condition, and log2 transformed for display. (A) Metabolites increased in both IRI and ACR, (B) just in ACR, dotted line separates metabolites only elevated in ACR and decreased in IRI. (C) Schematic of itaconate metabolism. (D) Metabolite intensity AUC measurements of C5-dicarboxylate pathway metabolites (one-way ANOVA). (E) Schematic of kynurenine and NAD metabolism. (F-H) Metabolite intensity AUC measurements of kynurenine and NAD metabolism metabolites (one-way ANOVA). (I, J) Confirmatory NAD+ and NADH measurements by enzymatic NAD(H) cycling (one-way ANOVA). (K, L) Correlation of NAD+ and NADH measurements by LC-MS/MS and NAD(H) cycling (Pearson’s correlation). Abbreviations: Irg1, Immune-responsive gene 1 (also known as aconitate decarboxylase 1, Acod1); Clybl, citrate lyase beta like; NAD, nicotinamide adenine dinucleotide. *, **, and *** indicate p<0.05, p<0.01, and p<0.001, respectively. Data shown as mean ± SEM.
To further investigate the IRI-associated increase in saccharopine, we examined other lysine catabolites in our samples (Fig. 1D, E) (Danhauser et al, 2012), and found that the downstream products 2-aminoadipate and glutarate, but not the parent substrates lysine and α-ketoglutarate, were increased in IRI (Figs. 1E, S2). Similarly, Fox et al. have observed cardiac 2-aminoadipate accumulation 24h after renal IRI and implicated protein oxidation and lysine degradation (Fox et al, 2019). The observation of lysine breakdown products in cardiac ischemia by Fox et al. and in renal ischemia in this study led us to question if saccharopine could be involved in IRI pathogenesis. Chouchani et al. have previously reported that succinate accumulates during ischemia of kidney, liver, heart and brain tissues and proposed that contributes to IRI by damaging mitochondria upon reperfusion (Chouchani et al, 2014). Interestingly, in the supplementary data of the same study, there was also persistent lysine accumulation during heart, kidney, and liver ischemia (1.24× p<0.05; 2.32× p<0.05; 6.4× p<0.001, respectively, Fig. 1F) (Chouchani et al, 2014). Our samples were obtained at 24 hours after reperfusion, and both succinate and lysine were close to CTR at this timepoint (Fig. 1E). This led us to speculate whether the elevated lysine observed by Chouchani et al. may lead saccharopine toxicity during reperfusion (Fig. 1F). Consistent with this hypothesis, the increase in cardiac 2-aminoadipate seen by Fox et al. 24 hours after renal IRI occurred also without a corresponding increase in lysine (Fox et al, 2019). We hypothesize that increased saccharopine/2-aminoadipate during reperfusion may be the result of prior lysine accumulation during renal ischemia, and may be relevant to subsequent mitochondrial injury given the reports of saccharopine-induced mitochondrial toxicity (Fig. 1F) (Leandro & Houten, 2019; Zhou et al, 2019).
Similar to the IRI-associated metabolites, we also noted that most metabolites increased in ACR consisted of cell membrane components (Fig. 2A, B). Two metabolites stood out based on their large fold increases in ACR kidneys, namely itaconate and kynurenine (arrows, Fig. 2A, B). Itaconate, an immune-modulatory metabolite that is produced from cis-aconitate in macrophages, has been reported to be immunosuppressive (Fig. 2C, D) (Lampropoulou et al, 2016). It is conceivable that its production may be associated with the high degree of inflammation seen in ACR. Itaconate also activates anti-oxidant responses via alkylation of cysteine residues on the protein Keap1 (KEAP1 - Kelch-like ECH-associated protein 1) which liberates the transcription factor Nrf2 (Nuclear factor erythroid 2-related factor 2) from Keap1-binding (Mills et al, 2018). Nrf2 is subject to post-translational control through Keap1, which keeps it from nuclear translocation and aids in its proteasomal degradation (McMahon et al, 2003). Thus, the effect of itaconate on Keap1 liberating Nrf2 increases Nrf2-mediated anti-inflammatory and anti-oxidant response gene expression (Mills et al, 2018; Perico et al, 2018). Beyond its immune modulatory and anti-oxidant effects, itaconate can be metabolized to citramalate, and accumulation of itaconate can inhibit vitamin B12-dependent methylmalonyl-CoA mutase, leading to a methyl-malonic acidemia phenotype (Shen et al, 2017). We observed a decrease in citramalate in ACR (Fig. 2D), and examined the possibility that high levels of itaconate could lead to a methyl-malonic acidemia phenotype; however, the data do not support this hypothesis as methylmalonyl-CoA mutase dependent metabolites were unaltered (Fig. S3).
Kynurenine is produced during tryptophan catabolism and can contribute to de-novo NAD-synthesis in the liver and kidneys (Fig. 2E) (Tran et al, 2016). Similar to itaconate, kynurenine has reported immunosuppressive properties, which are mediated through T cell apoptosis (Fallarino et al, 2002), lipid catabolism (Beier et al, 2019), and kynurenines functioning as agonists to the immunosuppressive Aryl hydrocarbon receptor (Mezrich et al, 2010; Quintana et al, 2008). Kynurenine and its derivatives are increasingly associated with chronic kidney disease (Addi et al, 2018). We found that NAD precursors from the kynurenine pathway were elevated, and nicotinamide, nicotinamide riboside, and NAD+ were decreased in ACR kidneys, supporting a defect in renal de-novo NAD synthesis and/or recycling (Figs. 2F–H, S4). Given that NAD+ and NADH measurements by mass spectrometry can be inaccurate, we validated our findings using enzymatic NAD(H) cycling (Fig. 2I–L).
In conclusion, ACR and IRI have distinct tissue metabolomic signatures. Saccharopine emerged as the most increased IRI metabolite and may have pathophysiological relevance due to its mitochondrial toxicity. Itaconate and kynurenine are highly increased in ACR and may point to immune-(counter)-regulatory mechanisms, as well defects in renal NAD synthesis and/or recycling. While confirmatory studies are necessary, these findings may aid in the future development of non-invasive biomarkers to discern rejection from IRI in kidney transplant recipients.
Supplementary Material
Funding:
This study was funded by the University of Pennsylvania (Penn) Institute for Translational Medicine and Therapeutics (to EAH, TPG, MHL, UHB); Laffey McHugh Foundation & American Society of Nephrology (to UHB); National Institutes of Health (DK109203 to EAH, AG043483 to JAB, OD021391 to TPG, DK106243 to MHL, and UL1TR001878 to Penn).
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Ethical approval (animal study): All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent: No humans were involved in the study.
References
- Addi T, Dou L & Burtey S (2018) Tryptophan-Derived Uremic Toxins and Thrombosis in Chronic Kidney Disease. Toxins (Basel), 10(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier UH, Cully MD, Siska PJ, Singer K, Jiao J, TeSlaa T, Quinn WJ, Hancock WW, Baur JA, Rabinowtiz JD & Kreutz M (2019) Fatty acid depletion is a reversible cause of kynurenine induced T cell apoptosis. J Immunol, 202(1 Supplement), 137.1. [Google Scholar]
- Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, Eyassu F, Shirley R, Hu CH, Dare AJ, James AM, Rogatti S, Hartley RC, Eaton S, Costa ASH, Brookes PS, Davidson SM, Duchen MR, Saeb-Parsy K, Shattock MJ, Robinson AJ, Work LM, Frezza C, Krieg T & Murphy MP (2014) Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature, 515(7527), 431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians U, Klawitter J & Klawitter J (2016) Biomarkers in Transplantation--Proteomics and Metabolomics. Ther Drug Monit, 38 Suppl 1, S70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhauser K, Sauer SW, Haack TB, Wieland T, Staufner C, Graf E, Zschocke J, Strom TM, Traub T, Okun JG, Meitinger T, Hoffmann GF, Prokisch H & Kolker S (2012) DHTKD1 mutations cause 2-aminoadipic and 2-oxoadipic aciduria. Am J Hum Genet, 91(6), 1082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, Fioretti MC & Puccetti P (2002) T cell apoptosis by tryptophan catabolism. Cell Death Differ, 9(10), 1069–77. [DOI] [PubMed] [Google Scholar]
- Fox BM, Gil HW, Kirkbride-Romeo L, Bagchi RA, Wennersten SA, Haefner KR, Skrypnyk NI, Brown CN, Soranno DE, Gist KM, Griffin BR, Jovanovich A, Reisz JA, Wither MJ, D’Alessandro A, Edelstein CL, Clendenen N, McKinsey TA, Altmann C & Faubel S (2019) Metabolomics assessment reveals oxidative stress and altered energy production in the heart after ischemic acute kidney injury in mice. Kidney Int, 95(3), 590–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC, Griss T, Weinheimer CJ, Khader S, Randolph GJ, Pearce EJ, Jones RG, Diwan A, Diamond MS & Artyomov MN (2016) Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab, 24(1), 158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leandro J & Houten SM (2019) Saccharopine, a lysine degradation intermediate, is a mitochondrial toxin. J Cell Biol, 218(2), 391–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MH, Wang Z, Bhatti TR, Wang Y, Aufhauser DD, McNeal S, Liu Y, Cheraghlou S, Han R, Wang L & Hancock WW (2015) Class-specific histone/protein deacetylase inhibition protects against renal ischemia reperfusion injury and fibrosis formation. Am J Transplant, 15(4), 965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MH, Wang Z, Xiao H, Jiao J, Wang L, Bhatti TR, Hancock WW & Beier UH (2016) Targeting Sirtuin-1 prolongs murine renal allograft survival and function. Kidney Int, 89(5), 1016–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek M & Nematbakhsh M (2015) Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev, 4(2), 20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon M, Itoh K, Yamamoto M & Hayes JD (2003) Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem, 278(24), 21592–600. [DOI] [PubMed] [Google Scholar]
- Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ & Bradfield CA (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol, 185(6), 3190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EL, Ryan DG, Prag HA, Dikovskaya D, Menon D, Zaslona Z, Jedrychowski MP, Costa ASH, Higgins M, Hams E, Szpyt J, Runtsch MC, King MS, McGouran JF, Fischer R, Kessler BM, McGettrick AF, Hughes MM, Carroll RG, Booty LM, Knatko EV, Meakin PJ, Ashford MLJ, Modis LK, Brunori G, Sevin DC, Fallon PG, Caldwell ST, Kunji ERS, Chouchani ET, Frezza C, Dinkova-Kostova AT, Hartley RC, Murphy MP & O’Neill LA (2018) Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature, 556(7699), 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perico L, Wyatt CM & Benigni A (2018) A new BEACON of hope for the treatment of inflammation? The endogenous metabolite itaconate as an alternative activator of the KEAP1-Nrf2 system. Kidney Int, 94(4), 646–649. [DOI] [PubMed] [Google Scholar]
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M & Weiner HL (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature, 453(7191), 65–71. [DOI] [PubMed] [Google Scholar]
- Shen H, Campanello GC, Flicker D, Grabarek Z, Hu J, Luo C, Banerjee R & Mootha VK (2017) The Human Knockout Gene CLYBL Connects Itaconate to Vitamin B12. Cell, 171(4), 771–782.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran MT, Zsengeller ZK, Berg AH, Khankin EV, Bhasin MK, Kim W, Clish CB, Stillman IE, Karumanchi SA, Rhee EP & Parikh SM (2016) PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature, 531(7595), 528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang X, Wang M, Chang Y, Zhang F, Ban Z, Tang R, Gan Q, Wu S, Guo Y, Zhang Q, Wang F, Zhao L, Jing Y, Qian W, Wang G, Guo W & Yang C (2019) The lysine catabolite saccharopine impairs development by disrupting mitochondrial homeostasis. J Cell Biol, 218(2), 580–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


