Abstract
Households in poor countries are encouraged (and sometimes coerced) to increase investments in formal health care services during pregnancy and childbirth. Is this good policy? The answer to a large extent depends on its effects on child welfare. We study the effects of a cash transfer program in Nigeria in which households were offered a payment of $14 conditioned on uptake of health services. We show that the transfer led to a large increase in uptake and a substantial increase in child survival driven by a decrease in in-utero child deaths. We present evidence suggesting that the key driver is prenatal health investments.
Keywords: cash transfers, maternal health services, child mortality, developing countries, I10, I12, I15, O15
1. Introduction
Numerous policy statements emphasize the importance of receiving proper care during pregnancy and childbirth for child outcomes (Lawn et al., 2014; Bhutta et al., 2014). The World Health Organization notes, for example, in its recently released guidelines that: “increased access to, and use of, higher-quality health care during pregnancy and childbirth can prevent many of these [child] deaths” (World Health Organization et al., 2016).1 The policy stakes are consequential: about 6 million children under five die each year and nearly half of those deaths happen within the first month (Liu et al., 2015). Motivated by these statistics there has been renewed emphasis on strategies to promote the use of formal health care services early in life, and in particular during pregnancy and childbirth. There is, however, debate (and some controversy) about whether shifting only demand will lead to tangible improvements in child health (Powell-Jackson et al., 2015; Okeke and Chari, 2014; Godlonton and Okeke, 2016), especially in the light of evidence suggesting that quality differences between institutional and community settings may be marginal (Das and Hammer, 2014; Harvey et al., 2007; Das et al., 2018). There is limited credible evidence on this question (Glassman et al., 2013; Hunter et al., 2017).
This paper attempts to fill this gap in the literature by presenting new findings from a randomized conditional cash transfer program in Nigeria. In this program households with an expecting mother were offered payments of $14 conditioned on uptake of pregnancy and delivery care. This large-scale trial involved more than 2300 census areas (henceforth clusters), approximately half of which were randomly assigned to either get the program or to a control arm. Households in clusters assigned to receive the program were offered, at baseline, a cash payment of $14 to be made after the birth of the child if the mother attended at least three prenatal visits, delivered in a health facility, and attended a postnatal visit. Households in control communities received gifts of nominal value at follow-up to thank them for participating. Program staff revisited enrolled households a few months after the birth of the child to verify utilization of health services and to collect data on birth outcomes. They also recorded the survival status of the child that was in utero at enrollment (henceforth the treated child).
The effects of the intervention are quite striking. We also note upfront that they are extremely robust to a range of threats to internal validity. First, as expected, the program led to a large increase in uptake of the incentivized health care services. Uptake of the full package of health services more than doubled in the treatment group, increasing by 14 percentage points (off a base of 12% in the control arm). Second, we find that treated children were significantly more likely to survive to follow-up. The probability that a treated child was alive at follow-up increased by 6 percentage points (or 8% relative to children in the control group). Further analysis shows that the increase in child survival was entirely due to a reduction in in-utero child deaths. We document large and significant reductions in the probability of fetal losses and fetal deaths, but no effect on infant deaths (conditional on being born alive).
Having documented this result, next we examine the question of mechanisms. If one thinks of this as a (policy) prescription, what is the active ingredient? The answer has important policy implications. As noted earlier, there is a debate about the policy merits of trying to shift all births into health facilities and whether the health benefits from such policies outweigh the often significant costs (Okeke and Chari, 2014; Godlonton and Okeke, 2016).2 This is a legitimate question, and one that we can shed some light on. Are the documented child health gains attributable to care at birth? Our headline findings suggest that care received prior to birth was an important ingredient but the increase in uptake of childbirth care could also have played an important role by reducing child deaths during the process of birth (intrapartum deaths) (Lawn et al., 2014). To explore this question we dig a little deeper into the data making use of several complementary empirical strategies: first, we examine the effects of the intervention on intermediate (pathway) outcomes, second, we make use of data collected as part of a verbal autopsy to try to distinguish between deaths prior to, and deaths during, delivery, and finally, we exploit heterogeneity in effects of the intervention by pregnancy age at enrollment. The results all consistently point in the same direction. They indicate that the key ingredient was health investments during the prenatal period and not institutional care at birth.3
These results provide some of the first credible evidence that incentivizing utilization of formal health care services at the beginning of life can lower current rates of child mortality. We estimate that if the program were scaled up in Nigeria, it would result in about 85,000 fewer stillbirths annually and would reduce the global number of stillbirths by 3%. Our results on the mechanisms, however, call into question the current emphasis on institutional care at birth and suggest that policy priorities may be better served by focusing on increasing use of health services during the prenatal period. To be clear, this is not to say that institutional care at birth is not useful or valuable. However, it argues against programs that incentivize only facility births. If care in the prenatal period is a key pathway then it follows that programs should also incentivize this. There are clearly significant gains to be had given current levels of consumption (World Health Organization, 2015). Our back-of-the-envelope calculations indicate that conditional transfers are a cost-effective way of reducing child deaths. Making various assumptions, we estimate a cost per life saved of approximately $700, a cost that is well below accepted cost-effectiveness thresholds and in line with other widely used interventions (Horton et al., 2017).
This paper makes an important contribution to several strands of the literature. First we make a major contribution to a growing literature on the effect of maternal and child care incentive programs. For a systematic review and a critique of the literature see (Gopalan et al., 2014; Hunter et al., 2017). Up until now credible evidence of improvements in health outcomes has been lacking. Second, we make a contribution to a growing economic literature examining the returns to institutional care at birth (Daysal et al., 2015; Godlonton and Okeke, 2016). This is the first randomized trial, to our knowledge that addresses this question. We expand on existing work by examining the effects of care received prior to, as well as at, the time of birth. We show that while care at the time of birth may not significantly impact child outcomes, care received prior to birth appears to have large payoffs in terms of child health. Third, this paper makes a contribution to a nascent literature on the returns to health care in the formal sector in developing countries (see for example Adhvaryu and Nyshadham, 2015). We show that despite well-documented deficiencies in the health sector, there are large returns to care received during a key stage of human development, the in-utero period.
This paper also makes a contribution to the large literature on conditional cash transfers (CCT) and child health (de Brauw and Peterman, 2011; Gertler, 2004; Barber and Gertler, 2010; Barham, 2011; Attanasio et al., 2015). We demonstrate that increased health care consumption is a key channel through which conditional transfers might improve child health. This may seem intuitive but evidence is surprisingly limited. Many conditional cash transfer programs include health care conditionalities such as prenatal attendance, but because these programs move multiple levers at the same time, separating the effect of additional health consumption on child health has been difficult (Gaarder et al., 2010).4 A key issue is that CCT programs, by design, provide often substantial positive shocks to household income which obviously can feed into child health through multiple pathways.5 Several studies, in fact, point to the income-nutrition channel as a key pathway for improvements in child health (Maluccio and Flores, 2005; Amarante et al., 2016). Understanding the contribution of health care consumption is important and this study, by design, is able to shed some light on this pathway. In so doing we contribute to the longstanding debate in the literature about the importance of health conditionalities (Baird et al., 2011).
One must be careful in drawing lessons as this program differs in key ways from traditional CCT programs.6 In contrast with traditional CCT programs, the objective is not poverty alleviation, rather it is to incentivize health care utilization.7 Consequently, the cash transfers are typically much smaller and involve a one-off lump sum transfer, made often months after the utilization has occurred.8 There are an increasing number of these programs around the world. They include the Janani Suraksha Yojana program in India, the Bono Juana Azurduy Program in Bolivia, the SURE-P program in Nigeria, and the Safe Delivery Incentive Program in Nepal. They represent a class of conditional incentive or “pay-for-performance” programs in which individuals receive specified rewards for desired health behavior. The use of such conditional transfers is becoming increasingly popular. They have been used not only to encourage uptake of health care around pregnancy, but also uptake of HIV results (Thornton, 2008), HIV prevention and treatment adherence (Kohler and Thornton, 2012; Linnemayr et al., 2017), prevention of sexually transmitted infections (de Walque et al., 2012), and uptake of child immunization (Banerjee et al., 2010).
The remainder of the paper is structured as follows: Section 2 provides a description of the study context, details of the experimental design, sampling, and the randomization protocol, Section 3 describes data collection and provides descriptives of the study sample, Section 4 lays out the empirical analysis and results, Section 5 provides a discussion of the results and puts them into context, and Section 6 concludes.
2. Background
2.1. Study Setting
The intervention was implemented in Nigeria. Nigeria is the most populous country in Africa with an estimated population of over 180 million people and a gross national income per capita of $1,968 in 2017, making it a lower middle-income country (World Bank, 2018). It, however, scores poorly on most welfare indices with an average life expectancy of 53 years and an under-five child mortality rate of about 128 per 1,000 live births. In a 2015 United Nations Human Development Report, Nigeria was ranked 152nd out of 188 countries (Jahan and the Human Development Report 2015 Team, 2015).9 Of specific relevance to child health we note that Nigeria is one of five countries that account for more than half of all newborn deaths worldwide (Lawn et al., 2014).10
Health indicators in Nigeria lag behind those of other countries that spend less on health. At the time the intervention was implemented, population estimates indicated that 34% of women in Nigeria, for example, did not use any prenatal care and only 36% of births took place in a formal health care setting (National Population Commission and ICF International, 2014). Only one in four children aged 12–23 months were fully vaccinated, and 21% of eligible children received no vaccination at all (National Population Commission and ICF International, 2014). The data also showed significant heterogeneity across geographic regions, with the northern regions, particularly the north east and north west, the most worst-off. For example, while 38% of Nigerian women reported using a formal health facility for their last delivery in the 2013 Demographic and Health Survey, this plummeted to 22% and 11% respectively in these two regions (National Population Commission and ICF International, 2014). Similarly, while the average under-five child mortality rate was 128 per 1,000 live births, this increased to 160 and 185 in these two regions respectively.
Before describing the intervention, we provide some additional context about how the health care sector in Nigeria is organized. Nigeria operates a tiered health care system with primary health care facilities serving as the point of entry for most patients into the health care system. Each of these facilities is responsible for a defined catchment or service area (throughout the rest of the paper we refer to these as health service areas or HSAs).11 Nigeria has approximately 30,000 primary health care clinics, 78% of which are in the public sector. Primary health care facilities provide a set of services defined by federal guidelines. These include control of communicable diseases, child survival, maternal and newborn care, nutrition, non-communicable disease prevention, and health education (National Primary Health Care Development Agency, 2014). Primary health facilities are commonly staffed by mid-level health care providers - nurses, midwives, community health officers, and community health extension workers - the most senior of whom is called the ‘in-charge’ (or clinic manager).
2.2. Program Sites
The program was implemented in 180 primary health service areas across five states in Nigeria. Two states each were selected from the north-east and north-west, and one from the south to increase generalizability. The states are: Akwa Ibom (south-south region), Bauchi and Gombe (north-east region), Jigawa and Kano (north-west region). For reference, a map is provided in Figure A.1. These five states were in the bottom 20 in terms of institutional delivery rates, and three of the included states were in the bottom 10 (National Population Commission and ICF International, 2014). We chose these specific states in consultation with our local partners based on feasibility and support from policy makers. The program sites were distributed equally across the five states so there are 36 sites per state.
The specific HSAs in each state were chosen with the assistance of officials in the state primary health care agency and Ministry of Health. The included HSA facilities all offer delivery services and are predominantly located in rural and semi-rural areas. While they were not randomly sampled they were drawn from across the state and in that sense should be considered broadly representative. As one metric, 71% of Local Government Areas (a sub-administrative level similar to a US county) in Akwa Ibom, 100% of Local Government Areas in Bauchi and Gombe, 88% of Local Government Areas in Kano, and 61% of Local Government Areas in Jigawa are included in the sample. We note that the primary health care facilities serving these communities took part in another intervention in which a random subset of facilities were selected to receive an additional health worker.12 As we will describe later, the randomization of the cash transfer intervention was stratified by HSA. In all of the analysis that follows we include HSA fixed effects. Because of external validity implications we also examine whether there was an interaction effect (the results are in the Appendix).
2.3. Intervention and Enrollment
Census areas or tracts in each HSA were randomly assigned by the study investigators to either receive the conditional transfer program or to a control arm (moving forward whenever we use the term ‘cluster’ we are referring to the census area). Census areas are clusters of contiguously located households defined by the National Population Commission.13 Following randomization program staff then visited the study clusters to conduct enrollment (and roll out the program). The program was implemented by a local research group in collaboration with a well-known and highly respected local university.14 The program staff (we also refer to them interchangeably as field agents) were employed by the local implementation partner. They worked in teams of 3–4 led by a team leader or supervisor.
Enrollment visits took place between March and August 2017. Based on sample size and budgetary considerations the enrollment target was set at 60 women per HSA. The field agents visited randomly drawn clusters until the enrollment target was reached (or exceeded). We would first randomly draw a study cluster and the field agents would visit and enroll all eligible households in the cluster. If they did not meet the target, we repeated this process, drawing another random cluster, until they had enrolled enough women. We accomplished this by randomly sorting all census areas in the HSA and having the field teams follow this order until the enrollment target was reached or until they ran out of census areas. All eligible women in a cluster were enrolled so cluster sizes vary. Only 7 women in total did not agree to take part – four in the intervention arm and three in the control arm.
On arriving in a study cluster the field agents first met with community leaders and then carried out a household listing to identify potentially eligible households. Eligibility was limited to households with a 1st or 2nd trimester pregnant woman to ensure that women had enough time to attend the required number of prenatal visits. Pregnancy status was based on women’s self-reports (we will come back to this later). Following the household listing program staff then visited each identified household to collect baseline information from the eligible woman. During the visit all households were reminded of the importance of seeking proper care during pregnancy and delivery. We followed exactly the same protocol in all study clusters regardless of whether they were in the intervention or control arm. In clusters assigned to receive the program, households were additionally informed about the program and the necessary conditions. We discuss this in the next section.
2.3.1. The Conditional Transfer Program
Households in intervention clusters were informed that they would receive a cash payment of 5000 Naira (approximately $14 at the prevailing exchange rate) for each pregnant household member that regularly attended prenatal care (three or more times), delivered in a health facility, and attended postnatal care (at least once). To put this into context, the transfer amount is equivalent to about 30% of monthly household food expenditures (Nigerian National Bureau of Statistics, 2016). The primary constraint the program was meant to address was facility deliveries and so the transfer amount was calibrated to cover the average total cost of a facility delivery (including transportation costs for the woman and a companion).15 It represents about 225% of the average total unconditional cost of delivery, and is equivalent to the weighted average total cost of a facility delivery (weighting by the fraction of births in each facility type).16 Households were informed that we would return after the birth of the child for verification and payment. All three of the conditions had to be met to qualify for the payment. There were no partial or pro-rated payments.
2.4. Follow-up Visits
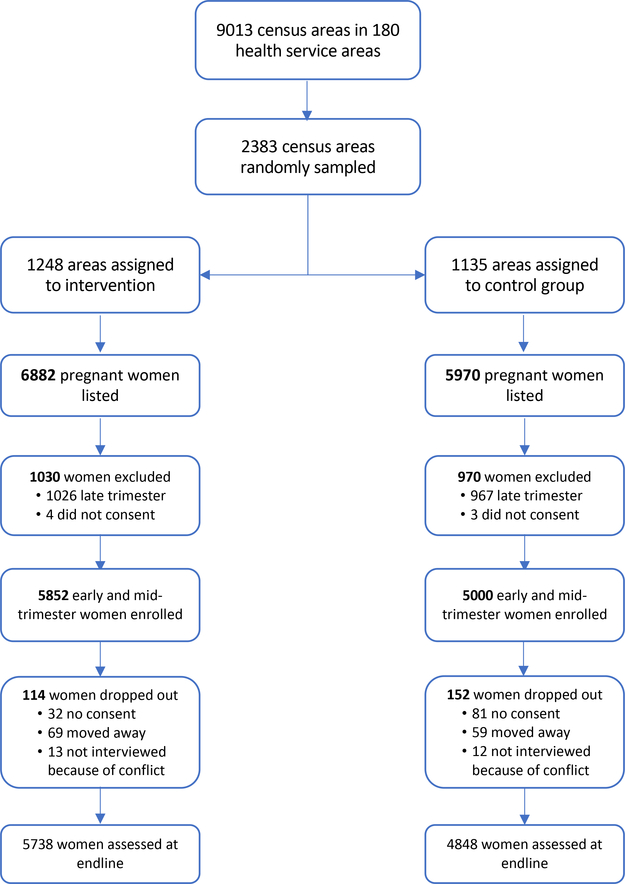
Follow-up visits took place between September 2017 and August 2018. These visits took place, on average, about eight months after enrollment. Effort was made to track down all participating households and 98.6% of enrolled participants were successfully re-contacted. A participant flowchart is provided in Figure A.2. In each household the mother was again interviewed to collect data on the outcome of the pregnancy and birth (more on this in the next section).17 Verification of health care utilization was done by program staff during these follow-up visits, with independent verification and payment (where indicated) by the team leader. Health cards and other documentation such as facility receipts were used for verification. In cases where satisfactory documentary proof could not be provided but a woman claimed to have used services, program staff visited the health facility to verify use from the health facility register. Households that qualified for the transfer were paid in cash. Regular audits were carried out to ensure that only women who qualified were paid. In clusters assigned to the control arm participating women received small gifts worth about $0.43 to thank them for their time (these gifts were not announced at baseline). In cases where the woman was deceased, the payment (or the gift) was given to the surviving spouse or to the household head.
3. Data
We have rich data on program participants including demographic characteristics, e.g., age, schooling, and ethnicity, birth history including number and outcomes of prior births, and household characteristics, e.g., household size, characteristics of the residential dwelling and ownership of various assets (such as a television set and refrigerator – we asked about 11 assets in total). We also have information about health behaviors during the pregnancy (such as use of malaria prophylaxis and iron supplementation), utilization of health services, and, finally, the outcome of the pregnancy – whether it ended in a fetal loss, in a live birth, or in a stillbirth (i.e., the infant was born dead). For infants that were born alive we also have data on survival status at the time of follow-up. Ethical approval for the study was given by RAND’s Human Subjects Protection Committee and by the Ethics Committee of Aminu Kano Teaching Hospital, Nigeria.
3.1. Outcomes
We define some of the key outcomes that will be used in the analysis. Overall child survival refers to the probability that a child who was in-utero at enrollment (the treated child) was alive at follow-up. A fetal loss is one where the pregnancy terminated early – before 28 weeks. A fetal death is a pregnancy that lasted longer than 28 weeks but where the infant was born dead. In order to correctly distinguish between a stillborn infant and one that died soon after birth, women were asked whether the child ever took a breath, whether the child ever moved, or whether the child ever cried (this is sometimes referred to as a verbal autopsy). An early infant death is one where the child was born alive but died before follow-up. In supplementary analysis we disaggregate this into neonatal deaths (a child death within the first 28 days) and post-neonatal deaths (a child death after the first 28 days but before follow-up).
3.2. Descriptives
We have baseline data for 10852 women. The average participant was 24.7 years old at baseline and had had two prior births (for 27% of participants this was their first birth). The vast majority of study participants (95%) were married, and most (70%) had no formal education (about half of these reported Islamic schooling). 23.7% of women used a health facility for their last delivery. The mean reported pregnancy age at baseline was 4.3 months. We also attempted to estimate pregnancy age at enrollment using information about the month of birth and assuming a standard pregnancy duration.18 One limitation is that this can only be done for pregnancies that resulted in a birth. To avoid dropping them from the analysis, for these women we rely on their reported pregnancy age. Average household sizes were large (5.7), and study participants were largely poor (the mean number of assets owned by the household was 2 out of a list of 11).
10699 (out of 10852) participants were successfully re-contacted. 113 declined to participate, leaving us with 10586 participants (an overall attrition rate of 2.5%). Attrition was slightly higher in control clusters compared to intervention clusters (3% vs. 2%). A flowchart is provided in Figure A.2. The 10586 pregnancies for which we have follow-up data resulted in 9126 liveborn and 395 stillborn infants. 1157 pregnancies ended in a fetal loss, and 19 participants died while still pregnant.19 78% of births were associated with some prenatal attendance (59% attended at least three times), and 42% of births took place in a health care facility. 35% of births were associated with some postnatal care. Next we examine whether randomization was successful.
3.3. Was randomization successful?
We approach this in two ways: first, we examine whether we have an equal number of participants in the intervention and control arms. Given the design, the sample should be evenly distributed. In Table 1 we report the number of participants in each arm: overall, and by state. We see that overall there are more participants in the intervention than in the control arm – 54% vs. 46%. When we disaggregate by state, we see that the imbalance is driven by one state, Gombe, where two-thirds of participants are in the intervention arm. In the other states the sample is evenly distributed as expected. The probability of obtaining this skewed distribution in Gombe by chance is very small suggesting that the field personnel may have deviated from the enrollment protocol. Our analysis of the household listing data shows that the field teams followed the randomized visit order – 52% of census areas visited were allocated to the intervention, which is similar to the other states20 – but were more likely to find eligible women in intervention clusters.21 This seems unlikely to be due to chance: 72% of census areas where no eligible women were reported are in the control arm compared to 28% in the intervention arm. Qualitative evidence from later debriefings suggests that there was a desire for as many women as possible to benefit from the cash transfer, which may have led to selective recruitment by the program staff in this state.22 An obvious concern is that this could lead to imbalance in characteristics between the treatment and control groups. We examine this next.
Table 1:
Allocation of sample to treatment and control arms (by state)
| State | Control | Treatment | Total | |||
|---|---|---|---|---|---|---|
| N | Percent (%) | N | Percent (%) | N | Percent (%) | |
| Akwa Ibom | 750 | 50.9 | 723 | 49.1 | 1473 | 100 |
| Bauchi | 1244 | 51.3 | 1183 | 48.7 | 2427 | 100 |
| Gombe | 763 | 33.6 | 1511 | 66.4 | 2274 | 100 |
| Jigawa | 1127 | 46.6 | 1294 | 53.4 | 2421 | 100 |
| Kano | 1116 | 49.4 | 1141 | 50.6 | 2257 | 100 |
| Total | 5000 | 46.1 | 5852 | 53.9 | 10852 | 100 |
Note: The table shows the number of participants in the treatment and control arms in each of the study states. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. We fail to reject the null of equality for every state except for Gombe. Standard errors are clustered at the level of the health service area (HSA). There are 180 HSAs included in the trial.
In Table 2 we examine whether participant characteristics are balanced overall between the intervention and control arms. The table shows means and standard deviations of each characteristic in the intervention and control arms, and p-values from tests of the null that the difference between arms is zero. Formally, we regress each characteristic on the treatment assignment indicator and strata (HSA) fixed effects. All models include HSA fixed effects to account for the blocked design (Bugni et al., 2018). Given the preceding discussion we examine balance for the full sample (Table 2); we also examine balance for a restricted sample that excludes observations from Gombe State (Table 3). We see that participant characteristics are well balanced in both cases, though in Gombe state women in the intervention arm are slightly older. In the analysis that follows, as a robustness check, we verify that all the key results hold with and without Gombe included in the sample.
Table 2:
Test of Balance (full sample)
| mean(C) | sd(C) | mean(T) | sd(T) | p-value | |
|---|---|---|---|---|---|
| Mother characteristics | |||||
| Age | 24.6 | 5.80 | 24.8 | 5.94 | 0.046 |
| Married | 0.95 | 0.22 | 0.95 | 0.21 | 0.48 |
| Moslem | 0.80 | 0.40 | 0.82 | 0.39 | 0.37 |
| Hausa or Fulani | 0.73 | 0.44 | 0.73 | 0.44 | 0.93 |
| Highest level of schooling | |||||
| None | 0.31 | 0.46 | 0.35 | 0.48 | 0.51 |
| Islamic school | 0.39 | 0.49 | 0.34 | 0.47 | 0.30 |
| Primary school | 0.080 | 0.27 | 0.090 | 0.28 | 0.88 |
| Secondary school | 0.20 | 0.40 | 0.20 | 0.40 | 0.70 |
| Tertiary school | 0.020 | 0.14 | 0.020 | 0.14 | 0.42 |
| Worked last 12 months | 0.44 | 0.50 | 0.42 | 0.49 | 0.92 |
| Owns mobile phone | 0.13 | 0.34 | 0.13 | 0.33 | 0.15 |
| Reported number of months pregnant | 4.20 | 1.56 | 4.27 | 1.57 | 0.42 |
| Number of prior births | 1.87 | 1.86 | 1.94 | 1.95 | 0.11 |
| Last birth was in a health facility | 0.21 | 0.41 | 0.26 | 0.44 | 0.18 |
| Pregnancy age at enrollment | |||||
| First trimester | 0.37 | 0.48 | 0.33 | 0.47 | 0.18 |
| Second trimester | 0.53 | 0.50 | 0.55 | 0.50 | 0.96 |
| Third trimester | 0.11 | 0.31 | 0.12 | 0.32 | 0.065 |
| Has had a previous miscarriage | 0.10 | 0.30 | 0.10 | 0.30 | 0.34 |
| Has had a previous stillbirth | 0.030 | 0.17 | 0.040 | 0.19 | 0.16 |
| Household characteristics | |||||
| Husband has other wives | 0.29 | 0.46 | 0.27 | 0.45 | 0.35 |
| Household size | 5.69 | 4.44 | 5.70 | 7.94 | 0.48 |
| Number of rooms in dwelling | 2.28 | 1.40 | 2.27 | 1.51 | 0.92 |
| Has no toilet | 0.050 | 0.22 | 0.050 | 0.23 | 0.86 |
| Number of assets (out of 11) | 2.06 | 1.74 | 2.03 | 1.69 | 0.47 |
| Household member has bank account | 0.15 | 0.35 | 0.14 | 0.35 | 0.56 |
| Distance to HSA health facility | |||||
| <1 kilometer | 0.37 | 0.48 | 0.41 | 0.49 | 0.78 |
| 1–3 kilometers | 0.22 | 0.41 | 0.20 | 0.40 | 0.99 |
| 3–5 kilometers | 0.14 | 0.35 | 0.14 | 0.35 | 0.33 |
| 5–10 kilometers | 0.16 | 0.37 | 0.16 | 0.37 | 0.77 |
| >10 kilometers | 0.11 | 0.31 | 0.090 | 0.29 | 0.14 |
| Number of months between baseline and follow-up | 7.84 | 2.24 | 7.80 | 2.26 | 0.41 |
| N | 5852 | . | 5000 | . | . |
Note: Table shows means, standard deviations (sd), and tests of balance for the full sample. C and T denote the control and treatment arms respectively. The last column reports p-values from a regression of each characteristic on the treatment indicator and strata (HSA) fixed effects. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. Standard errors are clustered at the level of the health service area (HSA). There are 180 HSAs. Trimester at enrollment was imputed based on the month of birth and assuming a standard pregnancy duration. It cannot be imputed if the pregnancy did not result in a birth so for these women we rely on their reported pregnancy age. Distance to the HSA health facility is measured from the center of each cluster.
Table 3:
Test of Balance (excluding Gombe state)
| mean(C) | sd(C) | mean(T) | sd(T) | p-value | |
|---|---|---|---|---|---|
| Mother characteristics | |||||
| Age | 24.8 | 5.81 | 24.9 | 6 | 0.41 |
| Married | 0.95 | 0.22 | 0.95 | 0.22 | 0.61 |
| Moslem | 0.81 | 0.39 | 0.82 | 0.39 | 0.86 |
| Hausa or Fulani | 0.74 | 0.44 | 0.76 | 0.43 | 0.48 |
| Highest level of schooling | |||||
| None | 0.28 | 0.45 | 0.30 | 0.46 | 0.69 |
| Islamic school | 0.44 | 0.50 | 0.42 | 0.49 | 0.30 |
| Primary school | 0.070 | 0.26 | 0.070 | 0.26 | 0.76 |
| Secondary school | 0.19 | 0.39 | 0.19 | 0.39 | 0.32 |
| Tertiary school | 0.020 | 0.12 | 0.020 | 0.13 | 0.32 |
| Worked last 12 months | 0.46 | 0.50 | 0.45 | 0.50 | 0.83 |
| Owns mobile phone | 0.14 | 0.34 | 0.14 | 0.35 | 0.19 |
| Reported number of months pregnant | 4.20 | 1.57 | 4.18 | 1.61 | 0.67 |
| Number of prior births | 1.96 | 1.88 | 2.01 | 1.98 | 0.90 |
| Last birth was in a health facility | 0.16 | 0.37 | 0.18 | 0.38 | 0.24 |
| Pregnancy age at enrollment | |||||
| First trimester | 0.38 | 0.49 | 0.37 | 0.48 | 0.56 |
| Second trimester | 0.50 | 0.50 | 0.50 | 0.50 | 1.00 |
| Third trimester | 0.12 | 0.32 | 0.13 | 0.34 | 0.38 |
| Has had a previous miscarriage | 0.10 | 0.30 | 0.10 | 0.30 | 0.51 |
| Has had a previous stillbirth | 0.030 | 0.18 | 0.040 | 0.19 | 0.29 |
| Household characteristics | |||||
| Husband has other wives | 0.31 | 0.46 | 0.30 | 0.46 | 0.21 |
| Household size | 5.84 | 4.47 | 5.88 | 8.92 | 0.95 |
| Number of rooms in dwelling | 2.27 | 1.38 | 2.24 | 1.35 | 0.57 |
| Has no toilet | 0.050 | 0.21 | 0.050 | 0.22 | 0.81 |
| Number of assets (out of 11) | 2.10 | 1.75 | 2.11 | 1.71 | 0.48 |
| Household member has bank account | 0.14 | 0.35 | 0.14 | 0.34 | 0.32 |
| Distance to HSA health facility | |||||
| <1 kilometer | 0.33 | 0.47 | 0.32 | 0.47 | 0.96 |
| 1–3 kilometers | 0.22 | 0.42 | 0.21 | 0.41 | 0.89 |
| 3–5 kilometers | 0.15 | 0.36 | 0.17 | 0.38 | 0.28 |
| 5–10 kilometers | 0.17 | 0.38 | 0.19 | 0.39 | 0.94 |
| >10 kilometers | 0.12 | 0.33 | 0.10 | 0.31 | 0.069 |
| Number of months between baseline and follow-up | 7.83 | 2.26 | 7.85 | 2.29 | 0.54 |
| N | 4341 | . | 4237 | . | . |
Note: Table shows means, standard deviations (sd), and tests of balance. The sample excludes observations in Gombe state. C and T denote the control and treatment arms respectively. The last column reports p-values from a regression of each characteristic on the treatment indicator and strata (HSA) fixed effects. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. Standard errors are clustered at the level of the health service area (HSA). There are 180 HSAs. Trimester at enrollment was imputed based on the month of birth and assuming a standard pregnancy duration. It cannot be imputed if the pregnancy did not result in a birth so for these women we rely on their reported pregnancy age. Distance to the HSA health facility is measured from the center of each cluster.
4. Analysis and Results
4.1. Effect of the Cash Transfer on Uptake of Health Services
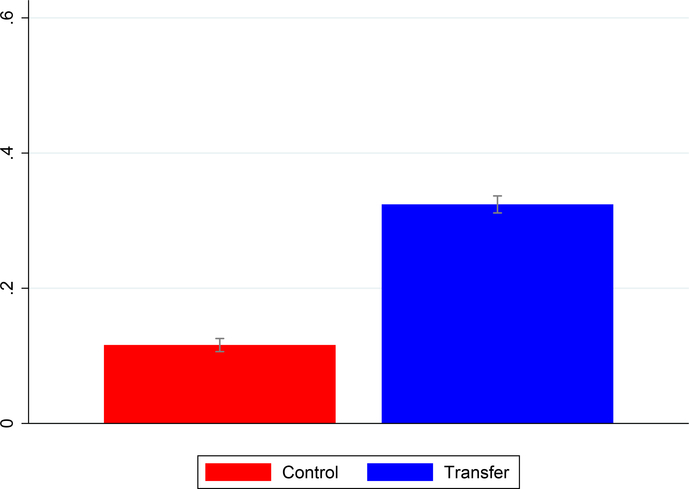
The first question is: did the cash transfer lead to greater use of health care? We begin by looking at uptake of the complete package of health services. We define a binary indicator equal to one if the mother attended prenatal care at least three times, delivered in a health facility, and had a postnatal visit. Figure 1 provides a graphical examination. We plot means and 95% confidence intervals for the intervention and control arms. The sample consists of pregnancies that ended in a birth. It is clear that the conditional transfer led to a significant increase in uptake.23 This mean comparison, however, does not account for the blocked design. For this we turn to the regression results. Regression analysis also allows us to include covariates. The basic regression specification is the following linear probability model:
Yijkm denotes the outcome for infant i born to mother j in cluster k in service area m. Transferk is an indicator denoting assignment to the intervention arm. X′j is a vector of included covariates. We control for mother’s age and schooling, ethnicity, prior birth history (number of prior births and prior history of fetal loss or a stillborn infant), and household wealth quintile dummies (derived by applying principal component analysis to the following variables: source of drinking water, cooking fuel, toilet ownership, dwelling characteristics, ownership of various durable assets, and ownership of a bank account). Controlling for a longer list of characteristics does not meaningfully affect the results.
Figure 1:
Effect of the Conditional Cash Payment on Uptake of Health Services
The figure shows the proportion of participants in each arm of the trial that attended at least three prenatal visits, gave birth in a health institution, and attended at least one postnatal visit. Means and 95% confidence intervals are shown.
Given that census areas were not randomly sampled from the population – we first sampled health service areas (HSA), and then within HSAs sampled census areas – we cluster the standard errors at the HSA level (there are 180 HSAs). In practice, this is more conservative than clustering at the census area level.24 MacKinnon and Webb (2017) have shown that inference based on cluster robust standard errors can become unreliable when cluster sizes vary substantially. To address this issue, we carry out permutation tests. Randomization inference does not depend on assumptions about cluster sizes (Heß, 2017). We report p-values from permutation tests based on 1000 draws from the distribution of the treatment effect estimate under the sharp null hypothesis of a zero treatment effect. Formally we randomly reassign clusters to the treatment or control group within strata and re-estimate the model. We replicate this 1000 times to generate an empirical distribution to which the coefficient from the model based on actual assignment can then be compared. The permutation test p-value is the probability that |T*| ≥ |T| where T denotes the statistic computed using the original dataset and T* is the statistic computed from a randomly permuted dataset.
The regression results are in Table 4. The dependent variable is the same as for Figure 1. Column 1 includes only strata (HSA) fixed effects, Column 2 adjusts for covariates, and Column 3 excludes observations from Gombe state. The intent-to-treat (ITT) coefficients indicate a 14-percentage-point increase in uptake in the intervention arm compared to a control group mean of 11.6% (a relative increase of 120%). The coefficients are not sensitive to inclusion of covariates. In the model that excludes observations in Gombe, the estimated ITT effect is slightly smaller, 10 percentage points, though the proportional increase is larger (173%). All of the results are highly statistically significant.
Table 4:
Effect of the conditional cash payment on uptake of the full package of health services
| (1) | (2) | (3) | |
|---|---|---|---|
| Intent-to-Treat | 0.139*** (0.0135) | 0.138*** (0.0134) | 0.0996*** (0.0131) |
| Controls | No | Yes | Yes |
| Observations | 9521 | 9521 | 7405 |
| Number of groups | 180 | 180 | 144 |
| p-value from permutation test | 0 | 0 | 0 |
| Control group mean | 0.116 | 0.116 | 0.0575 |
Note: The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The dependent variable is a dummy denoting uptake of this package of services. The sample consists of all births to study participants during the intervention period. Column 1 includes only strata (HSA) fixed effects. Column 2 adds in the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintile dummies. Column 3 excludes observations in Gombe state. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
We also report p-values from permutation tests based on 1,000 draws from the distribution of the treatment effect estimate under the sharp null hypothesis of a zero treatment effect.
Table 5 reports ITT effects on the individual components of uptake: the number of prenatal visits attended, the probability of a health facility birth, and postnatal attendance (at least one visit), separately. The results show that the number of prenatal visits increased by about 0.5 (the control group mean is 2.4 visits), the probability of a health facility birth increased by 14 percentage points (the control group mean is 29%), and the probability of receiving some postnatal care increased by 10 percentage points (the control group mean is 27%). Having established that the program significantly increased health care consumption, next we examine its effects on child health outcomes.
Table 5:
Effect of the conditional cash payment on on health care consumption
| Number of prenatal visits |
Childbirth in a health facility |
At least one postnatal visit |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | |
| Intent-to-Treat | 0.474*** (0.0663) | 0.471*** (0.0655) | 0.372*** (0.0765) | 0.142*** (0.0157) | 0.141*** (0.0153) | 0.151*** (0.0186) | 0.103*** (0.0132) | 0.103*** (0.0130) | 0.0784*** (0.0144) |
| Controls | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Observations | 10697 | 10697 | 8488 | 9521 | 9521 | 7405 | 9521 | 9521 | 7405 |
| Number of groups | 180 | 180 | 144 | 180 | 180 | 144 | 180 | 180 | 144 |
| p-value from permutation test | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Control group mean | 2.378 | 2.378 | 2.319 | 0.288 | 0.288 | 0.182 | 0.268 | 0.268 | 0.219 |
Note: The dependent variables are in the first row of the table. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The first column in each Panel includes only strata (HSA) fixed effects. The second column in each panel adds in the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintile dummies. The third column in each panel excludes observations in Gombe state. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
We also report p-values from permutation tests based on 1,000 draws from the distribution of the treatment effect estimate under the sharp null hypothesis of a zero treatment effect.
4.2. Effect of the Cash Transfer on Child Health
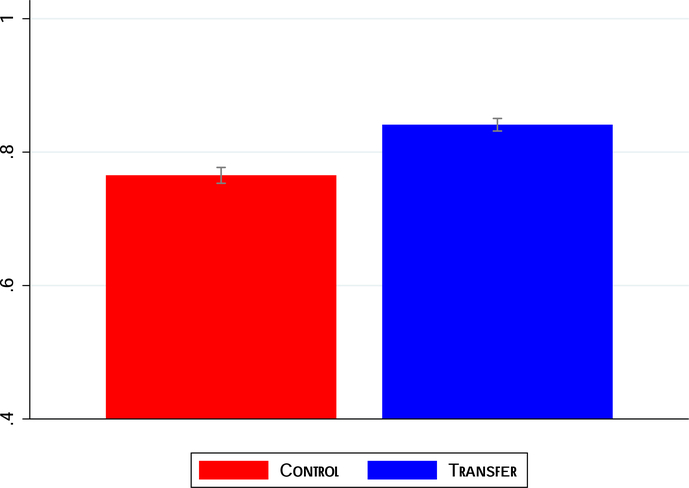
We begin by examining overall child survival. Figure 2 examines the probability that the treated child was alive at follow-up for each group (we plot means and 95% confidence intervals).25 We can clearly see that children in the intervention arm were significantly more likely to be alive at follow-up. The corresponding regression results are in Table 6. The results indicate a 6-percentage-point increase in child survival. Relative to the control group, this translates to an 8% increase. As before, controlling for covariates does not materially affect the point estimates. The treatment effect is slightly smaller (4.5 percentage points or a 6% increase) when we exclude observations from Gombe state, but all the results are highly significant at the 1% level.
Figure 2:
Effect of the Conditional Cash Payment on Child Survival
The figure shows the proportion of treated children in each arm of the trial that survived to follow-up. Means and 95% confidence intervals are shown.
Table 6:
Effect of the conditional cash payment on child survival
| (1) | (2) | (3) | |
|---|---|---|---|
| Intent-to-Treat | 0.0591*** (0.00992) | 0.0606*** (0.00980) | 0.0446*** (0.0110) |
| Controls | No | Yes | Yes |
| Observations | 10697 | 10697 | 8488 |
| Number of groups | 180 | 180 | 144 |
| p-value from permutation test | 0 | 0 | 0 |
| Control group mean | 0.765 | 0.765 | 0.752 |
Note: The dependent variable is an indicator equal to one if the treated child (who was in-utero at enrollment) was alive at follow-up. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. Column 1 includes only strata (HSA) fixed effects. Column 2 adds in the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintile dummies. Column 3 excludes observations in Gombe state. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
We also report p-values from permutation tests based on 1,000 draws from the distribution of the treatment effect estimate under the sharp null hypothesis of a zero treatment effect.
As a first step towards understanding why child survival increased in the treatment group, it is important first to establish where the mortality decrease is occurring. Is it occurring before or after birth? The answer helps us to start thinking about causal pathways (though we leave a more detailed discussion until later). A reduction in in-utero deaths might point, for example, to the potential importance of health investments in the prenatal period, while a reduction in deaths after delivery might suggest that the prevailing mechanism is care at birth (or potentially after birth). To explore this, we examine survival in three consecutive, non-overlapping time periods: the early in-utero period (fetal losses prior to 28 weeks); the late in-utero period, conditional on surviving the first period (a child that was born dead, i.e., a stillbirth), and early infancy, conditional on being born alive (a child death after birth).26
The results of this analysis are shown in Table 7. The specifications are the same as in previous tables. We see that the overall increase in child survival is driven by a large decrease in fetal losses (a 4–5 percentage point decrease in the treatment group or 29% relative to the control group mean) and fetal deaths (a decrease of 1.1–1.3 percentage points or 23% relative to the control group).27 There is no evidence of a decrease in early infant deaths (though in 2 of the 3 specifications the signs are in the right direction). The point estimate is close to zero and is fairly precisely estimated. Before getting into a detailed discussion of causal mechanisms, we first establish the robustness of these results by examining two important threats to validity in this context.
Table 7:
Where is the decrease in child mortality occurring?
| Early in-utero (fetal loss) |
Late in-utero (fetal death) |
Early infant death |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | |
| Intent-to-Treat | −0.052*** (0.0083) | −0.053*** (0.0081) | −0.039*** (0.0090) | −0.011*** (0.0044) | −0.012*** (0.0043) | −0.013** (0.0053) | −0.0023 (0.0052) | −0.0034 (0.0051) | 0.00036 (0.0060) |
| Controls | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Observations | 10697 | 10697 | 8488 | 9521 | 9521 | 7405 | 9126 | 9126 | 7019 |
| Number of groups | 180 | 180 | 144 | 180 | 180 | 144 | 180 | 180 | 144 |
| p-value from permutation test | 0 | 0 | 0 | 0.010 | 0.010 | 0.010 | 0.65 | 0.49 | 0.95 |
| Control group mean | 0.14 | 0.14 | 0.14 | 0.052 | 0.052 | 0.059 | 0.061 | 0.061 | 0.066 |
Note: The dependent variables are in the first row of the table: a fetal loss is a pregnancy that terminated before 28 weeks; a fetal death is a pregnancy that lasted past 28 weeks where the infant was born dead; and an early infant death is one where the child was born alive but died before the follow-up interview. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The first column in each Panel includes only strata (HSA) fixed effects. The second column in each panel adds in the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintile dummies. The third column in each panel excludes observations in Gombe state. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
We also report p-values from permutation tests based on 1,000 draws from the distribution of the treatment effect estimate under the sharp null hypothesis of a zero treatment effect.
4.2.1. Spillovers
Cluster randomization is intended to minimize spillovers between the treatment and control group, but given that we blocked by HSA, spillovers are a possibility that must carefully be ruled out. There are several kinds of spillovers one might be concerned with in this context. First, are interpersonal spillovers: women in intervention clusters might, for example, have transferred some of their winnings to their friends in control clusters, leading to a (smaller) increase in uptake in the control arm. There might also be peer effects. Both of these would imply that the treatment effect would be biased downwards (though this is arguably less of a problem than the alternative). We might also get a similar downward bias if women in control clusters erroneously thought that they were also eligible for the transfer. Second, are spillovers arising as a result of crowd-out at the health facility. For example, if health facilities were operating at full capacity and were more likely to accept women for delivery that registered for, and attended, prenatal care at the facility. Since, treated women were more likely to do so, this might inadvertently crowd-out care provision for women in the control arm. This could potentially lead to negative spillovers for women in the control arm if they were, as a consequence, more likely to use lower quality sources of childbirth care. An alternative is if health workers, who were aware of the cash transfer, attempted to tax participants’ winnings by raising informal fees, this would be more likely to deter women in the control arm since they were ineligible for transfers that would help to offset these informal payments. Both of these imply that the treatment effect would be overestimated.
The second type of spillovers – crowd-out – is more concerning and we examine this carefully. First, we carry out some back-of-the-envelope calculations to examine the plausibility of crowd-out. Recall that, on average, there are 60 women in a HSA, half of whom are in the intervention arm. Even if the facility delivery rate doubled as a result of the intervention, this would mean that nine additional women would show up at the health facility (0.3*30), or an average of 1 additional birth per month. The actual effect on facility deliveries is about half this, suggesting that crowd-out, on an a priori basis seems highly unlikely. But what about prenatal care? Women attend multiple times throughout the pregnancy suggesting that there could be more scope for crowd-out. We can again examine the likelihood of this using similar calculations. We estimate that the average number of prenatal visits increased by 0.5 (Table 5). This translates to 15 additional prenatal visits throughout the intervention period (30*0.5), or approximately 2 additional prenatal visits per month.
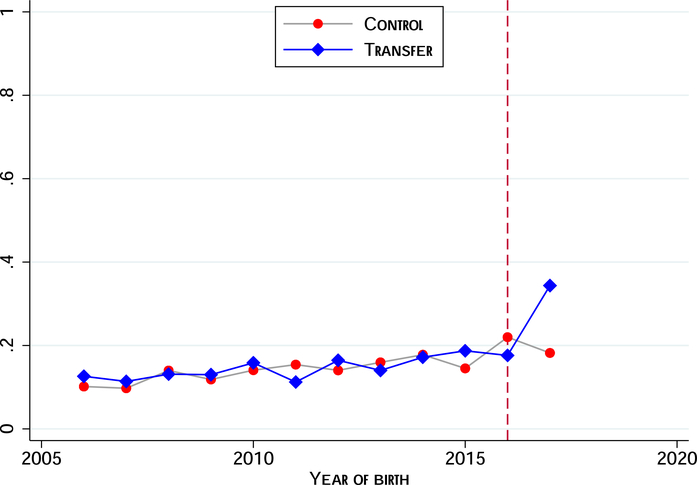
While these illustrative calculations suggest that crowd-out is a priori unlikely, we can examine whether this is backed up by the data. As a starting point we exploit the fact that we have data on place of birth for children born prior to program rollout.28 In Figure 3 we plot time trends in the intervention and control arms to visually examine whether there are any unusual changes in the control group post-intervention. The analysis excludes Gombe state. In the absence of spillovers we should not see any unusual jumps (up or down) for the control arm. We observe the expected increase in uptake for the intervention arm once the intervention is rolled out, but do not see any unusual changes for the control arm. We formally test this in Table A.3 where we regress facility deliveries on an exposure indicator (turned on if the birth occurred during the intervention period) and on a linear time trend. We report separate regressions for the intervention and control arms. In alternative specifications we relax the assumption of common time trends, allowing these to vary flexibly by state. These models all include mother fixed effects. In all cases, the coefficient for the control group is statistically indistinguishable from zero. These data do not provide any evidence of spillovers.
Figure 3:
Trends in Facility Births by Treatment Assignment
The figure shows the proportion of births to study participants, by year, that took place in a health care institution. The sample consists of births in the ten years preceding the intervention and excludes Gombe state. The vertical dashed line marks the last pre-intervention year, 2016. We have aggregated all post births.
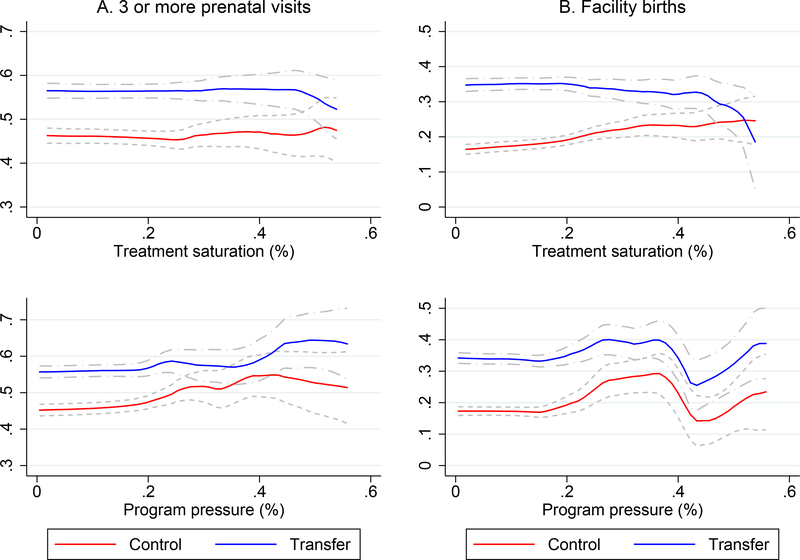
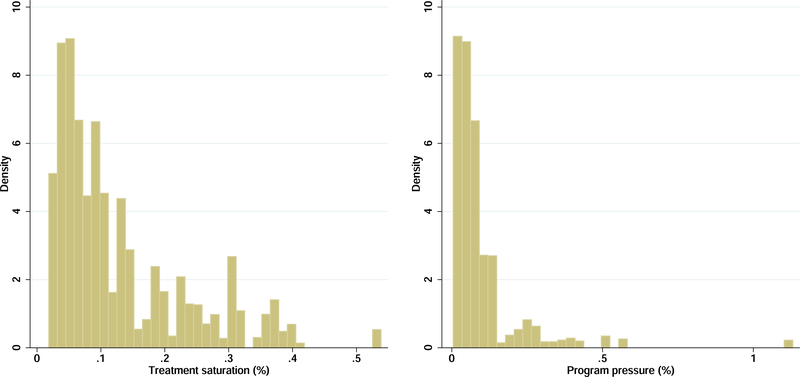
Another way to test for spillovers is to exploit (essentially random) variation across HSAs in the size of the treated population. We begin with a simple measure: the number of intervention clusters as a fraction of all census areas in the HSA (or the treatment saturation).29 We reason that the larger the treatment saturation, the greater the likelihood of spillovers. Recall that we randomly drew clusters out of the pool in a HSA for enrollment visits. Holding constant average cluster sizes, the number of intervention clusters in a HSA is essentially random. In Figure 4 we present non-parametric plots of utilization in the intervention and control groups across the saturation distribution (top panel). Evidence of crowd-out would be a divergence in outcomes at higher treatment saturation levels. One can see that there is no evidence of crowd-out for either prenatal care or facility births.
Figure 4:
Is there evidence of crowd-out?
The figure shows smoothed local polynomial plots (with 95% confidence bands) of utilization in the intervention and control groups over the distribution of treatment saturation (top) and program pressure (bottom). Treatment saturation is the fraction of EAs in the HSA that are treated, and program pressure is the number of treated women in the HSA divided by the baseline average monthly facility patient count. The latter is truncated at 1 for visual clarity. The utilization measure in Panel A (left) is an indicator for 3 or more prenatal visits, and in Panel B (right), an indicator for a facility birth.
We also define a measure of the additional pressure on the health facility created by the program. We relate the number of treated women in each HSA to HSA primary health facility capacity as measured by the average monthly number of patients seen in the facility at baseline. We reason that for a given number of treated women, crowd-out is more likely in smaller facilities (by volume). The distribution of program pressure is shown in Figure A.5. In the bottom panel of Figure 4 we again present non-parametric plots. As before there is little evidence of crowd-out. Finally, we re-estimate all the health outcome models, interacting the treatment dummy with each of these measures. These results are in Table A.4. One can clearly see that crowd-out is not a credible explanation for our findings.
4.2.2. Attrition
Loss to follow-up is another concern, though given the low attrition rate this would seem like an unlikely explanation for the results. 2.5% of the participants attrited between the baseline and follow-up (meaning that there is no data on their outcomes) and participants in the control arm were more likely to drop out – primarily because they were more likely to have refused consent for the follow-up interview. In Table A.5, we examine whether attriters are different from non-attriters. We find that older women, those with some secondary schooling, and those with a prior history of a fetal loss were less likely to attrit, while women with more birth experience were more likely to attrit. We examine whether the pattern of dropout is different between groups by interacting treatment assignment with each characteristic. The interaction terms are mostly insignificant (except for the interactions with islamic education and Hausa/Fulani ethnicity which are both significant at the 5% level).
Even though there is not a clear differential pattern of dropout, if attriters in the control arm were more likely to survive, we could be over-estimating the effect of the treatment on child survival (and vice-versa). We carry out two robustness checks. First, we construct a worst-case lower bound by assuming that all the unobserved attriters in the control arm experienced a ‘good’ outcome while all those in the treatment group experienced a ‘bad’ outcome. We impute these outcomes and re-estimate the models. As an additional check, we also estimate non-parametric Lee bounds with bootstrapped errors (Lee et al., 2009). Not surprisingly, given the very low rate of attrition the results are very similar to the main results. We report these in Table A.6. Even with the extremely conservative worst-case bounds, the key results hold. In the worst-case scenario, the coefficient on early infant deaths becomes positive and statistically significant but we believe that this is an artefact – a consequence of the fact that the influence of the attriters increases as the sample size reduces (going from left to right in the table). Recall that at each stage we are conditioning on surviving the previous stage but the number of attriters is the same. Given that we are assuming that attriters in the control group survived and those in the treatment group died, and attrition was more likely in the control arm, at each stage we are adding more deaths to the treatment arm than to the control arm. There aren’t very many deaths overall and so the influence of adding more deaths to the treatment arm grows as the number of observations reduces.
4.2.3. Additional Robustness
As we noted earlier, pregnancy was reported by participants.30 One concern might be that women in intervention clusters might be more likely to report being pregnant so as to be enrolled, in the hope that they would be able to get pregnant. If such women had systematically different birth outcomes, this might affect the treatment estimates. A priori this kind of strategic behavior seems unlikely because at the time field agents were identifying eligible households, information about the program was not yet public, thus limiting the potential for strategic behavior. However as a robustness check we drop all first trimester women (based on reports) and check whether the main results hold. We reason that women claiming to be pregnant (but who were not) would more likely report that they were at an early stage of pregnancy. As an additional robustness check we also separately drop 3rd trimester pregnancies (based on our imputed estimates of pregnancy age). This is another margin along which differential misreporting might occur. The imputation is based on the month of birth which means that we cannot do this imputation for women whose pregnancies terminated early. However, a pregnancy loss must have occurred prior to the 3rd trimester and so for these women we use their reported pregnancy age at enrollment so as to retain them in the analysis. Tables A.7 and A.8 presents these results. In all cases we see that the results are robust. A second concern is inadvertent reporting error. It is likely that there were some women who were in fact pregnant but were not aware of their status. Such women would not be included in the sample. Inadvertent reporting error should not be different between the intervention and control arms and, as such, should not pose a threat to internal validity.
4.3. Heterogeneous Effects
We examine whether the treatment effects vary by participant characteristics. Of particular interest is whether there is treatment heterogeneity by socioeconomic status. There are well-documented socioeconomic inequalities in health outcomes (World Health Organization, 2015). This is one of the reasons why cash transfers are often explicitly targeted towards less well-off households (Alatas et al., 2012). Policy makers might therefore be interested in whether the cash transfer may have helped to narrow or close existing socioeconomic gaps. We explore this in Table A.9 where we estimate separate outcome models for households in the top 2 and bottom 3 wealth quintiles. We also examine heterogeneity by mother’s education (no formal schooling vs. some education), and by pregnancy risk (we define an indicator for higher risk if the woman one of the following risk factors: first-time mother, five or more previous births, age less than 15 years or older than 35, prior history of a stillbirth). These results are in Table A.10 and A.11. P-values from a test of difference in the ITT coefficients are reported at the bottom of the table.
In Table A.12 we test for heterogeneity by gender. There is a well-known mortality disadvantage for male infants (Naeye et al., 1971), which we also find in our sample. It is possible that health care could help to offset some of this disadvantage. Overall, we do not find any evidence of heterogeneity along any of these dimensions except for fetal losses where we find some evidence of larger reductions for higher-risk women (this result is significant at the 10% level). Finally to round out the analysis we examine whether there is any heterogeneity by whether the health facility received an additional health worker (see Table A.13). This would have implications for external validity. Once again, we find no evidence of a differential effect.
4.4. Mechanisms
We have shown in the preceding sections that the conditional transfer led to a reduction in child mortality. While this is an important finding, it is important to understand why child mortality decreased. The most obvious explanation is that mortality decreased because the conditional incentive induced women to use health care that they would not have used otherwise, and this proved beneficial for child health.31 The preceding results suggest that we can rule out health care received after birth as a causal mechanism, but is the mortality decrease due to greater uptake of formal care at birth? The conditional transfer also increased uptake of pregnancy care, which could also help explain the improvement in child health (Joyce, 1994; Gajate-Garrido, 2013). The effect on fetal losses provides strong a priori evidence for the latter channel, but the effect on fetal deaths muddies the water as both could in theory be responsible. To try to tease this out, we implement three complementary strategies:
First, we examine whether the treatment had an effect on intermediate prenatal and delivery outcomes known to be associated with a fetal death. Specifically, we examine whether women in the intervention arm were more likely to have received iron supplementation, malaria prophylaxis, and HIV testing during the pregnancy. Anemia in pregnancy is known to be associated with higher rates of stillbirths (Nair et al., 2017; Zhang et al., 2009), malaria is a major cause of stillbirths and other adverse outcomes (Moore et al., 2017; McClure et al., 2013), and maternal HIV infection is associated with both an increase in spontaneous abortions and stillbirths (Brocklehurst and French, 1998; Wedi et al., 2016). We also examine whether participants in the intervention arm were more likely to receive treatment for problems experienced during the pregnancy (conditional on experiencing any problems).32 For labor/delivery, we examine whether the treatment had any effect on two intermediate outcomes: whether the labor was obstructed and whether the woman had hypertensive complications (Lawn et al., 2011).33 Care at birth, by reducing the incidence, or by more effective treatment, of these outcomes, could have contributed to the reduction in mortality.
The results of this analysis are in Table 8. We find significant effects of the conditional transfer on intermediate prenatal, but not labor/delivery, outcomes. Women in the intervention arm were 7.1 percentage points more likely to have received HIV testing and counseling, 6.6 percentage points more likely to have taken iron supplements during the pregnancy, and 4.5 percentage points more likely to have received malaria prophylaxis. Additionally, we show that while women in the intervention arm were no more likely to report problems during the pregnancy, they were significantly more likely to have sought treatment for these problems. In contrast, there is no effect of the intervention on the intermediate labor and delivery outcomes.34 These results point towards prenatal health investments as the key mechanism.
Table 8:
Effect of the treatment on intermediate outcomes
| (1) HIV testing & counseling | (2) Iron supplements | (3) Antimalarial prophylaxis | (4) Pregnancy problems | (5) Treated for problem | (6) Labor was obstructed | (7) Hypertensive complications | |
|---|---|---|---|---|---|---|---|
| Intent-to-Treat | 0.077*** (0.014) | 0.072*** (0.013) | 0.050*** (0.011) | 0.0025 (0.0036) | 0.048*** (0.013) | −0.0019 (0.0046) | 0.0055 (0.0037) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 10697 | 10697 | 10697 | 10697 | 7540 | 9521 | 9521 |
| Number of groups | 180 | 180 | 180 | 180 | 180 | 180 | 180 |
| p-value from permutation test | 0 | 0 | 0 | 0.49 | 0 | 0.65 | 0.14 |
| Control group mean | 0.61 | 0.71 | 0.74 | 0.15 | 0.30 | 0.041 | 0.021 |
Note: This table examines the effect on intermediate outcomes. The dependent variables are in the first row of the table. Intermediate prenatal indicators are in Columns 1–3, and labor/delivery indicators are in Columns 6 and 7. These are all binary indicators. Pregnancy problems (column 4) is an index created by taking an average across women’s responses to a series of questions about problems experienced during the pregnancy. Column 5 asks if the woman received treatment for the problem. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. All models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintile dummies. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
We also report p-values from permutation tests based on 1,000 draws from the distribution of the treatment effect estimate under the sharp null hypothesis of a zero treatment effect.
Our second strategy to try to distinguish between these two channels is to identify fetal deaths that likely happened long before labor, to separate them from those that could have happened during labor/delivery. Only the latter could have been influenced by labor/delivery care. If we drop fetal deaths that likely happened long before labor and the mortality results diminish significantly or disappear, it would suggest that delivery care was not a primary mechanism for the effects. To do this, we utilize the verbal autopsy data. Deaths where the mother reported that the baby was not moving when labor started, suggesting that the infant had already died, are coded as a fetal death prior to labor and dropped from the analysis. We also drop cases where the mother reported that the child had skin and body changes as these are also likely to have occurred long before labor.35 This is of course an imprecise exercise, but the results are nevertheless instructive. When we exclude deaths that are likely to have occurred prior to labor, the mortality coefficient reduces by more than half to 0.0057. When we exclude observations in Gombe state, the coefficient becomes only borderline significant.
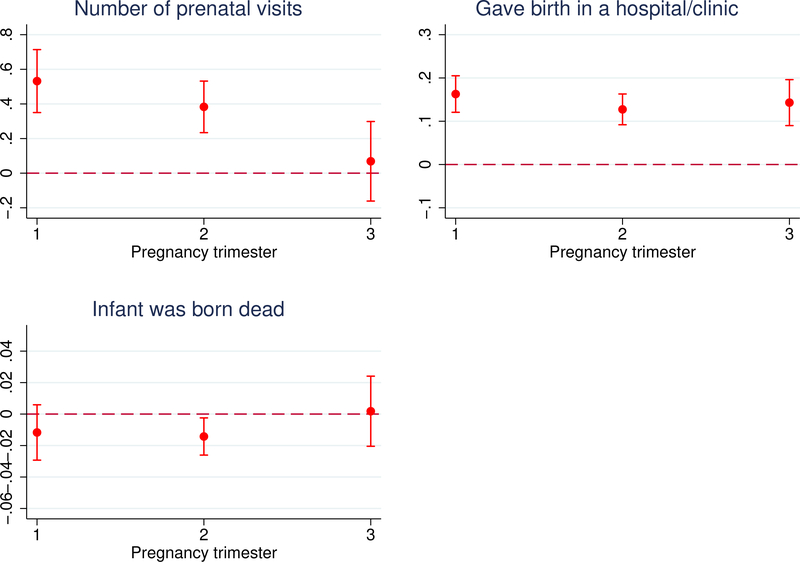
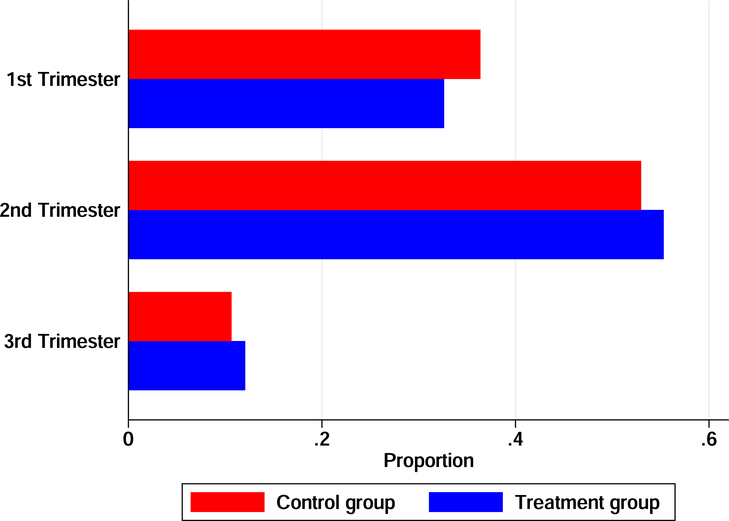
Finally, we exploit differences in pregnancy age at enrollment. Women in late trimester pregnancy at enrollment faced similar incentives as early and mid-trimester women to increase uptake of delivery care but would have received less prenatal care because they simply had less time.36 We can therefore exploit this variation to examine whether the effect of the treatment varies by pregnancy age at enrollment. A strong effect for late-trimester women would potentially point to the importance of care at birth. However, if the primary pathway is care in the prenatal period, the mortality effect should be stronger for early, and mid-trimester women. In Figure A.4 we present the distribution of pregnancy trimester at enrollment for the treatment and control arms. As Table 2 has already shown, the distribution is balanced.
Figure 5 confirms that late trimester women consumed less prenatal care. The top left figure shows coefficients and 95% confidence intervals from a regression of prenatal attendance on treatment interacted with pregnancy trimester dummies (the corresponding regression results are in Table A.15). In the top right figure, we repeat the same exercise for facility childbirth care and show that it does not vary with pregnancy age. In the bottom left figure we examine the effect on fetal deaths. We see a decrease for early and mid-trimester women, of roughly similar magnitudes, but the point estimate is quite close to zero for late-trimester women though the result is not precisely estimated. This is not totally surprising given that only 12% of the sample are estimated to have been late trimester at enrollment. We caution, however, that these differences are not statistically significant. That said, this result lines up with the previous results and continues to point towards the importance of health care prior to birth.
Figure 5:
Effect of the Conditional Cash Payment by Pregnancy Trimester at Enrollment
The figure shows the effect of the conditional cash payment on (i) the number of prenatal visits, (ii) the probability of a health facility birth, and (iii) the probability of a fetal death, by pregnancy trimester at enrollment. Trimester at enrollment was imputed using month of birth and assuming a standard pregnancy duration. It cannot be imputed if the pregnancy did not result in a birth so for these women we rely on their reported pregnancy age. We plot coefficients and 95% confidence intervals from a linear regression of each outcome on the treatment indicator interacted with dummies for each trimester. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintile dummies.
4.4.1. Other Mechanisms
There are other (complementary) pathways through which the conditional transfer could have affected child health. We can think of at least two. First, the conditional transfer likely raised the expected value of a birth in treated households (if the woman lost the pregnancy then the household had no chance of qualifying for the transfer). This gave households an additional reason to be invested in the health of the pregnancy. One way to try to ensure this would have been to reduce maternal work, e.g., by assigning women fewer chores around the house.37 Reduced maternal work and stress could then in turn lead to better outcomes (Chari et al., 2019; Goldenberg et al., 2008). To test this channel, we restrict the sample to women in households with no co-wives and no female children older than 7, both of whom could potentially substitute for maternal labor. We reason that the scope for substitution is significantly smaller for such women. The mortality results still hold in this sample and the coefficient sizes are similar to the unrestricted sample (see Table A.16 Column 1). We also test whether there are differential effects by employment (we define a woman as employed if she reported working outside the home within the 12 months preceding the baseline interview). The results (not shown) are similar. While these are by no means definitive tests, they suggest that this is unlikely to be an important pathway.
A second pathway is through improved maternal nutrition (Imdad and Bhutta, 2012). Even though income effects are minimized here because the cash transfer was made after the birth, households in intervention clusters could still have allocated more/better food to enrolled women. We know that maternal nutrition is related to child birth weight (Imdad and Bhutta, 2012), suggesting one way to test this channel. We do not have birthweight for children born outside of health facilities and so we rely on a proxy, the child’s relative size at birth as reported by the mother (very small, smaller than average, average, larger than average, and very large). We examine whether the transfer decreased the probability of giving birth to a smaller than average or very small infant. As Table A.16 Column 2 shows, there is no evidence of this. Again, we admit that this is not a definitive test but the results do not support the nutrition channel.
Lastly we examine the possibility that women in the intervention arm could have received substantially better care in service area facilities than women in the control group. In the extreme, even if the transfer had no effect on uptake we could still see a decrease in mortality via this pathway. But why might health clinics provide better care to women in the intervention arm? At first glance this is not obvious; plus in order to discriminate, clinics would have needed to know assignment status (which they did not know and which participants had little incentive to reveal). One possibility alluded to earlier, is that women in the intervention arm would have had greater ability to pay informal fees. If that were the case, then providers could simply have been responding to this, i.e., they were providing better treatment to those that paid for it (in which case knowledge of treatment assignment would not be necessary). It would have to be the case though that they received better medical treatment, for this to be a credible explanation for the study findings. While this would not explain the effect on fetal losses, it could explain the difference in stillbirth rates.
We examine this in two ways: first we test whether, conditional on a birth in the service area clinic, participants in the treatment group reported paying more than participants in the control group; second, we examine various measures of delivery care quality to test whether participants in the treatment group who delivered in the local clinic received better care. The answer to both questions appears to be no. The difference in reported payment for delivery is trivial (54 Naira; p=0.5), and on the indicators that we consider such as whether labor was unduly prolonged, whether oxytocin (a drug that helps with uterine contractions) was administered after birth, and whether the woman was physically or verbally abused, we find no difference between groups; though we find weak evidence that treated women were more likely to have received medication to help ease labor pains (see Table A.16 Columns 3–7). In sum, the data do not support this as an important channel.
5. Discussion and Conclusion
This paper has examined the effect of cash transfers conditioned on utilization of health services during pregnancy on child health, and specifically child mortality. We have shown that it led to a large increase in utilization of health services that, in turn, led to improvements in child health. This study provides some of the first credible evidence linking utilization incentives to improvements in child health. A noteworthy finding is that this occurred in the absence of any major investments or improvements in service delivery, suggesting that there is value to policies that promote utilization even under status quo conditions.38 An important qualification to this is that our analysis of mechanisms suggests that the key driver appears to be prenatal care. So while these findings provide a strong endorsement for promoting utilization of health care early in life, the weight of evidence is slanted more strongly towards care prior to birth. In that sense, our findings are congruent with the findings from studies such as Godlonton and Okeke (2016) and Powell-Jackson et al. (2015) which have generally found little evidence that steering births into health facilities leads to improvements in child health.
How large are the child health effects we find? The smallest intent-to-treat estimate implies a 4.5-percentage-point increase in survival. This translates to about a 6% increase (or 20% decrease in mortality) relative to the control group. Converting this to lives saved, we estimate that about 260 children (0.045*5852) survived to follow-up only because of the intervention. To better put this into context, we focus only on the effect of the conditional transfer on stillbirths, which is a key outcome of policy interest and (perhaps not coincidentally) an outcome for which we have reliable data. We estimate that scaling up the intervention in Nigeria, assuming a constant effect size, would result in about 85,000 fewer stillbirths annually.39 It would also reduce Nigeria’s stillbirth rate from its current level which is 1.5 times the average for sub-Saharan Africa, down to the average (Lawn et al., 2011).40 Given that Nigeria accounts for 12% of global stillbirths (Lawn et al., 2016), this would reduce the global number of stillbirths by 3%. These calculations assume that health facilities are able to handle the resulting increase in demand without compromising quality of care. While we have shown that the average health facility in this sample is operating significantly under capacity, a situation that is true of many primary health facilities, if this program were to be scaled up, capacity constraints may become important and this is something policy makers would have to take into consideration.
The size of these child health effects are plausible. Lawn et al. (2016) estimated that up to half of all stillbirths in sub-Saharan Africa occur prior to labor. In this study we have shown that the treatment led to a 22% reduction in the stillbirth rate. We know from the medical and epidemiological literature that malaria, anemia and HIV are all important causes of fetal deaths. In sub-Saharan Africa, malaria is thought to be responsible for between 12–20% of all stillbirths (Moore et al., 2017); Watson-Jones et al. (2007) have estimated that up to 60% of stillbirths can be attributed to maternal anemia; and Nair et al. (2017) have shown that the odds of a stillbirth are five-fold higher among women with moderate to severe anemia compared to women with no anemia. Our results indicate that the treatment led to significant increases in malaria and anemia prevention, and HIV testing and counseling. We also showed that it increased the probability of women seeking treatment for pregnancy complications.
Next we examine how these results compare to others in the literature. A caveat is that it is difficult to make direct comparisons because of differences in how mortality is defined (and over what periods). We start by looking at estimates from the broader CCT literature. Barham (2011) is one of the few CCT papers that has child mortality as an outcome. She finds that Progresa in Mexico led to an 8% decrease in infant mortality (deaths among children younger than one year) though she finds no effect on newborn deaths (deaths within the first 30 days). Another paper by Rasella et al. (2013) that evaluated the effect of the Bolsa Familia program in Brazil found that it reduced under-five child deaths by 12% in high-coverage areas. Moving to cash transfer programs more similar to this one, Powell-Jackson et al. (2015), found no effect of the JSY program in India on either neonatal mortality or one-day mortality. The JSY program, however, conditioned only on a health facility birth, which may help to explain this result.
There are several policy implications that emerge from our findings. First, policies that encourage greater household investments in health care, particularly during the in-utero period, are likely to have substantial payoffs. We have shown effects on mortality in the short-run but there may also be long-run effects (see for example Almond and Mazumder, 2011). Monetary incentives and subsidies are one policy tool for modifying behavior, but there may be scope for other kinds of interventions. Second, they indicate that conditionalities have an important role in cash transfer programs. Cash transfers without strong conditionalities have often found small (or no) effects on health utilization (Urquieta et al., 2009; Amarante et al., 2016; Handa et al., 2016; Cohen et al., 2017). If households are under-investing in care with high private returns even when financial barriers are removed, it may suggest that they may not be fully aware of these returns (Jensen, 2010; Boneva and Rauh, 2018). This may argue for informational types of interventions.41 Third, our findings suggest that focusing only on shifting births into health facilities may not be the optimal strategy. This does not mean that care at birth offers no health benefits. However, there is growing evidence that unlocking these benefits will likely require improvements in quality (Godlonton and Okeke, 2016; Leslie et al., 2016; Okeke, 2019).
While a full cost-benefit analysis is beyond the scope of this paper, we carry out some back-of-the-envelope calculations to examine the cost-effectiveness of the intervention. We estimate a program cost per life saved, including only the cost of the cash transfers, of $143. Administrative costs are not straightforward to calculate in this context, and will be different in the context of scale-up, so we turn to the literature for estimates of administrative costs. Borghi et al. (2015), estimated that the cost of managing a health worker incentive program in Tanzania exceeded the costs of financial incentives by between 1.7–1.9 times. Another study in the UK found that management costs exceeded incentive payments by about 1.4 times (Meacock et al., 2014). Assuming that administrative costs are conservatively two times the cost of incentives, the cost per life saved would increase to $429. Given average life expectancy, this works out to about $8 per life year saved. Even adding in the incremental cost of the additional care used, the cost per life saved would only increase to $693.42 These estimates are well under typically used thresholds for cost-effectiveness (Marseille et al., 2014; Horton et al., 2017).
This study, of course, has some limitations. Many of the outcomes are self-reported, which is often unavoidable in contexts like these, but we note that the key outcome – child mortality – is objectively measured and less subject to potential accusations of measurement error or recall bias. Also given the relatively short follow-up period (a few months), we cannot speak to longer run effects on child health. These could be larger than the short run effects we have demonstrated if uptake early in life has dynamic downstream effects on uptake later in the child’s life (Takasaki and Sato, 2017), or could be smaller if the marginal child saved by the intervention is frailer, i.e., mortality is deferred, or alternatively, if there is ‘culling’ of fetuses such that those in the control group that survive birth are the hardiest ones so that they are more likely to survive after birth. This might also interact in interesting ways with compensatory behavior by parents (Liu et al., 2009). This is an interesting area for future work.
Lastly we consider external validity. A strength of this study is that the conditional transfer program was implemented in multiple states in different regions. This may have added to complexity but it strengthens the external validity. In applying these results to other contexts it is obviously important to pay careful attention to the peculiarities of each context. Incentives do not work in isolation. It is important to ensure, for example, that services will be available when needed (Okeke and Chari, 2018; Banerjee et al., 2010). Though this was a large-scale trial across multiple states, it was nevertheless a controlled trial. Scaling-up brings up administrative considerations. If women do not get paid on time (or at all), or there are significant hassle costs to obtain payment, this is likely to have dynamic effects that will dilute program effects (Hunter and Murray, 2017). Finally, the cash transfer in this study was conditioned on uptake of the full package of health services. This is in contrast to an à la carte design in which ‘prices’ are attached to each component, allowing households to pick and choose which component to consume (see for example Okoli et al., 2014). It is possible that this alternative design might result in larger effects on prenatal care attendance (though potentially smaller effects on facility births), as it gives beneficiaries more flexibility to choose a bundle that better matches with their preferences. Making the package a lumpy unit, could have induced some women, e.g., those with a strong preference for a home birth, to not use any care at all since they would not receive the transfer unless they used all of it. The optimal design of incentives will be an interesting area for future work. We are cautiously optimistic that the results will carry over to other settings and we look forward to future studies that will replicate these findings.
We examine the effect of cash transfers conditioned on health care utilization during pregnancy on child health
The cash transfer program was implemented in Nigeria and selection of communities for the program was random
In intervention areas use of recommended health services more than doubled
We find significant reductions in child mortality driven by reductions in in-utero child deaths
The ley mechanism appears to be prenatal health investments
Acknowledgments
This study could not have been carried out without the help and support of many individuals. We are grateful to Peter Glick and A.V. Chari for their involvement as collaborators in the early stages of this project and for many helpful discussions. We thank Drs. Christie Akwaowo, Usman Bashir, and Nnenna Ihebuzor (RIP) who helped to facilitate implementation. We also thank Professor Obi Onwujekwe and the staff of the Health Policy Research Group, University of Nigeria for hosting the project and providing logistical support and infrastructure. We thank Susan Lovejoy, Onyinye Stephen-Gow, and Laura Pavlock- Albright for project management support, and Juliana Chen, Crystal Huang, Stephen Okpalaononuju, Adeyemi Okunogbe, and Victor Olajide for excellent research assistance. We are grateful for the dedicated work of Bamidele Aderibigbe, Saidu Abubakar, Sadia Aliyu, Sadiya Awala, Yakubu Suleiman, and Edidiong Umoh who oversaw the data collection and field work. Lastly, we are grateful to all the study participants who gave generously of their time. We are appreciative of comments provided by participants at various seminars and conferences. This study was supported by a grant from the Eunice Kennedy Shriver National Institute for Child Health and Human Development (R01HD083444). The funder played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Appendix Material
Figure A.1:
Map of Nigeria showing the Program States
The program sites were drawn from five states (shaded areas) representing three of Nigeria’s six geopolitical regions: Akwa Ibom (south-south), Bauchi and Gombe (north-east), Jigawa and Kano (north-west).
Figure A.2:
Participant Flowchart
Figure A.3:
Effect of the Conditional Cash Payment by State
The figure shows the effect of the conditional cash payment on each component of the care package and on child survival by state (AK = Akwa Ibom; BA = Bauchi; GO = Gombe; JG = Jigawa; KN = Kano). The full care package consists of all three components. We plot coefficients and 95% confidence intervals from a linear regression of each outcome on the treatment indicator interacted with dummies for each state. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintiles. Standard errors in parentheses are clustered at the level of the health service area (HSA). *p < 0.1,** p < 0.05,*** p < 0.01.
Figure A.4:
Distribution of estimated pregnancy trimester at enrollment by treatment and control arms
The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. We impute pregnancy at enrollment using the month of birth and assuming a standard pregnancy duration. Pregnancy age cannot be imputed for women with a fetal loss so for these women we rely on their reported pregnancy age.
Figure A.5:
Distribution of treatment saturation and program pressure variables
Treatment saturation is the fraction of EAs in the HSA that are treated, and program pressure is the number of treated women in the HSA divided by the baseline average monthly facility patient count.
Table A.1:
Effect of the conditional cash payment on neonatal and post-neonatal deaths
| Neonatal death |
Post-neonatal death |
|||||
|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | |
| Intent-to-Treat | −0.0016 (0.0049) | −0.0025 (0.0049) | 0.0027 (0.0058) | −0.00083 (0.0022) | −0.0010 (0.0022) | −0.0025 (0.0026) |
| Controls | No | Yes | Yes | No | Yes | Yes |
| Observations | 9126 | 9126 | 7019 | 8706 | 8706 | 6634 |
| Number of groups | 180 | 180 | 144 | 180 | 180 | 144 |
| p-value from permutation test | 0.75 | 0.62 | 0.67 | 0.71 | 0.64 | 0.35 |
| Control group mean | 0.050 | 0.050 | 0.053 | 0.012 | 0.012 | 0.013 |
Note: The dependent variables are in the first row of the table: a neonatal death is a death within the first 28 days after birth; and a post-neonatal infant death is one where the child survived the neonatal period but died before the follow-up interview. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The first column in each Panel includes only strata (HSA) fixed effects. The second column in each panel adds in the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintile dummies. The third column in each panel excludes observations in Gombe state. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
We also report p-values from permutation tests based on 1,000 draws from the distribution of the treatment effect estimate under the sharp null hypothesis of a zero treatment effect.
Table A.2:
Where is the decrease in child mortality occurring? (Unconditional results)
| Early in-utero (fetal loss) |
Late in-utero (fetal death) |
Early infant death |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | |
| Intent-to-Treat | −0.052*** (0.0083) | −0.053*** (0.0081) | −0.039*** (0.0090) | −0.0080** (0.0037) | −0.0081** (0.0036) | −0.0086* (0.0044) | 0.00088 (0.0044) | 0.000096 (0.0044) | 0.0033 (0.0050) |
| Controls | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Observations | 10697 | 10697 | 8488 | 10697 | 10697 | 8488 | 10697 | 10697 | 8488 |
| Number of groups | 180 | 180 | 144 | 180 | 180 | 144 | 180 | 180 | 144 |
| p-value from permutation test | 0 | 0 | 0 | 0.030 | 0.030 | 0.070 | 0.86 | 0.99 | 0.55 |
| Control group mean | 0.14 | 0.14 | 0.14 | 0.045 | 0.045 | 0.051 | 0.050 | 0.050 | 0.053 |
Note: The dependent variables are in the first row of the table: a fetal loss is a pregnancy that terminated before 28 weeks; a fetal death here is not conditioned on the pregnancy extending beyond 28 weeks, it is a simple indicator for whether the enrolled pregnancy resulted in a fetal death, i.e., the infant was born dead; similarly an early infant death here is not conditioned on a live birth, it is simply equal to one if the child died after birth (before the follow-up interview). The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The first column in each Panel includes only strata (HSA) fixed effects. The second column in each panel adds in the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintile dummies. The third column in each panel excludes observations in Gombe state. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
We also report p-values from permutation tests based on 1,000 draws from the distribution of the treatment effect estimate under the sharp null hypothesis of a zero treatment effect.
Table A.3:
Test of spillovers
| Control arm |
Treatment arm |
|||
|---|---|---|---|---|
| (1) Linear trend | (2) State-specific trend | (3) Linear trend | (4) State-specific trend | |
| Exposed birth | 0.0023 (0.014) | 0.018 (0.014) | 0.14*** (0.028) | 0.15*** (0.030) |
| Time trend | 0.0044*** (0.0015) | 0.0079*** (0.0018) | ||
| Observations | 9779 | 9779 | 10252 | 10252 |
| Number of groups | 144 | 144 | 144 | 144 |
Note: The dependent variable in all columns is an indicator equal to one if the child was born in a health facility. Exposed is an indicator equal to 1 if the birth occured during the intervention period and 0 if it occurred before. The sample consists of births in the ten years preceding the intervention (since 2006) and excludes Gombe state. In each column the dependent variable is regressed on the exposure indicator and time trends. All models include mother fixed effects. Separate models are estimated for participants in the treatment and control groups. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.4:
Are the child health effects explained by crowdout?
| Child survival |
Fetal loss |
Fetal death |
Early infant death |
|||||
|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
| Treatment | −0.037** (0.017) | −0.048*** (0.014) | −0.028** (0.014) | −0.036*** (0.012) | −0.022** (0.0085) | −0.018*** (0.0063) | 0.0044 (0.0097) | −0.0020 (0.0071) |
| Treatment × Saturation | −0.055 (0.10) | −0.087 (0.091) | 0.068 (0.053) | −0.032 (0.056) | ||||
| Treatment × Pressure | 0.036 (0.10) | −0.031 (0.096) | 0.049 (0.033) | 0.025 (0.037) | ||||
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 8488 | 8488 | 8488 | 8488 | 7405 | 7405 | 7019 | 7019 |
| Number of groups | 144 | 144 | 144 | 144 | 144 | 144 | 144 | 144 |
| Control group mean | 0.25 | 0.25 | 0.14 | 0.14 | 0.059 | 0.059 | 0.066 | 0.066 |
Note: The dependent variables are in the first row of the table: child survival is the probability that a treated child (who was in-utero at enrollment) was alive at follow-up; a fetal loss is a pregnancy that terminated before 28 weeks; a fetal death is a child that was delivered after 28 weeks but was born dead; and an early infant death is one where the child was born alive but died before the follow-up interview. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. Saturation is the fraction of EAs in the HSA that are treated and Pressure is the number of treated women in the HSA/baseline average monthly facility patient count. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintiles. The sample excludes observations in Gombe state. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.5:
Are attriters different from non-attriters?
| (1) Attrition | (2) Attrition | |
|---|---|---|
| Treatment | −0.012*** (0.0037) | |
| 18–24 years | −0.0060 (0.0057) | −0.018* (0.010) |
| 25–29 years | 0.0030 (0.0067) | −0.010 (0.012) |
| 30–34 years | −0.018** (0.0078) | −0.026** (0.013) |
| 35 and older | −0.021** (0.0092) | −0.032** (0.015) |
| Islamic school | −0.0040 (0.0035) | −0.011** (0.0047) |
| Primary school | −0.0070 (0.0066) | 0.0024 (0.012) |
| Secondary school | −0.015** (0.0066) | −0.015 (0.011) |
| Tertiary school | 0.0047 (0.017) | −0.013 (0.026) |
| No. of previous births | 0.0049*** (0.0014) | 0.0052** (0.0021) |
| History of previous stillbirth=1 | −0.0034 (0.0082) | −0.0017 (0.014) |
| Previous miscarriage | −0.012** (0.0053) | −0.011 (0.0098) |
| Wealth quintiles=2 | −0.00033 (0.0051) | −0.0012 (0.0067) |
| Wealth quintiles=3 | −0.0063 (0.0053) | −0.011 (0.0069) |
| Wealth quintiles=4 | 0.0093* (0.0056) | 0.015** (0.0077) |
| Wealth quintiles=5 | −0.0038 (0.0078) | 0.00098 (0.011) |
| Treatment | −0.049*** (0.017) | |
| Treatment × 18–24 years | 0.022* (0.012) | |
| Treatment × 25–29 years | 0.023* (0.014) | |
| Treatment × 30–34 years | 0.013 (0.014) | |
| Treatment × 35 and older | 0.019 (0.017) | |
| Hausa or Fulani extraction=1 | −0.012 (0.0086) | |
| Treatment × Hausa or Fulani extraction=1 | 0.024** (0.010) | |
| Treatment × Islamic school | 0.014** (0.0065) | |
| Treatment × Primary school | −0.017 (0.015) | |
| Treatment × Secondary school | 0.00053 (0.011) | |
| Treatment × Tertiary school | 0.033 (0.036) | |
| Treatment × No. of previous births | −0.00037 (0.0023) | |
| Treatment × History of previous stillbirth=1 | −0.0027 (0.017) | |
| Treatment × Previous miscarriage | −0.0032 (0.011) | |
| Treatment × Wealth quintiles=2 | 0.0015 (0.0076) | |
| Treatment × Wealth quintiles=3 | 0.0070 (0.0082) | |
| Treatment × Wealth quintiles=4 | −0.012 (0.0095) | |
| Treatment × Wealth quintiles=5 | −0.0085 (0.011) | |
| Controls | Yes | No |
| Observations | 10852 | 10852 |
Note: The dependent variable is a dummy indicating women who dropped out of the study. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.6:
Bounding child health effects to account for attrition
| Child survival |
Fetal loss |
Fetal death |
Early infant death |
|||||
|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | |
| Worst-case bounds | −0.038*** (0.010) | −0.023* (0.012) | −0.031*** (0.0088) | −0.018* (0.0098) | 0.011* (0.0059) | 0.010 (0.0073) | 0.020*** (0.0063) | 0.024*** (0.0075) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 10963 | 8677 | 10963 | 8677 | 9787 | 7594 | 9392 | 7208 |
| Number of groups | 180 | 144 | 180 | 144 | 180 | 144 | 180 | 144 |
| p-value from permutation test | 0 | 0.030 | 0 | 0.020 | 0.020 | 0.090 | 0 | 0 |
| Control group mean | 0.23 | 0.25 | 0.14 | 0.14 | 0.052 | 0.059 | 0.061 | 0.066 |
| Lee upper bound | −0.054 | −0.041 | −0.047 | −0.036 | −0.012 | −0.013 | −0.00084 | 0.0020 |
| Lee lower bound | −0.063 | −0.046 | −0.052 | −0.041 | −0.018 | −0.019 | −0.010 | −0.0053 |
Note: To estimate the worst-case bound, we assume that all the unobserved attriters in the control group experienced a ‘good’ outcome while all those in the treatment group experienced a ‘bad’ outcome. The dependent variables are in the first row of the table: child survival is the probability that an exposed child (who was in-utero at enrollment) was alive at follow-up; a fetal loss is a pregnancy termination before 28 weeks; a fetal death is a child that was delivered after 28 weeks but was born dead; and an early infant death is one where the child was born alive but died before the follow-up interview. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintiles. The second column in each panel excludes observations in Gombe state. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.7:
Robustness to excluding 1st trimester pregnancies at baseline (self-reported)
| Uptake |
Child survival |
Fetal loss |
Fetal death |
Early infant death |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | |
| Intent-to-Treat | 0.12*** (0.014) | 0.076*** (0.013) | 0.062*** (0.011) | 0.050*** (0.012) | −0.044*** (0.0088) | −0.032*** (0.0094) | −0.014*** (0.0051) | −0.016** (0.0064) | −0.011* (0.0061) | −0.0086 (0.0075) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 6463 | 4915 | 6960 | 5359 | 6960 | 5359 | 6463 | 4915 | 6223 | 4681 |
| Number of groups | 180 | 144 | 180 | 144 | 180 | 144 | 180 | 144 | 180 | 144 |
| p-value from permutation test | 0 | 0 | 0 | 0 | 0 | 0 | 0.010 | 0.010 | 0.080 | 0.24 |
| Control group mean | 0.12 | 0.066 | 0.81 | 0.80 | 0.096 | 0.096 | 0.049 | 0.056 | 0.061 | 0.066 |
Note: The dependent variables are in the first row of the table: uptake denotes at least three antenatal visits, a health facility delivery, and one postnatal visit; child survival is the probability that an exposed child (who was in-utero at enrollment) was alive at follow-up; a fetal loss is a pregnancy termination before 28 weeks; a fetal death is a child that was delivered after 28 weeks but was born dead; and an early infant death is one where the child was born alive but died before the follow-up interview. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), a dummy denoting Hausa or Fulani extraction, dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintiles. The second column in each panel excludes observations in Gombe state. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.8:
Robustness to excluding 3rd trimester pregnancies at baseline (estimated)
| Uptake |
Child survival |
Fetal loss |
Fetal death |
Early infant death |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | (7) | (8) | (9) | (10) | |
| Intent-to-Treat | 0.14*** (0.014) | 0.10*** (0.014) | 0.066*** (0.011) | 0.049*** (0.012) | −0.058*** (0.0089) | −0.044*** (0.010) | −0.013*** (0.0048) | −0.015** (0.0060) | −0.0021 (0.0055) | 0.0019 (0.0064) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 8285 | 6336 | 9418 | 7377 | 9418 | 7377 | 8285 | 6336 | 7933 | 5993 |
| Number of groups | 180 | 144 | 180 | 144 | 180 | 144 | 180 | 144 | 180 | 144 |
| p-value from permutation test | 0 | 0 | 0 | 0 | 0 | 0 | 0.010 | 0.020 | 0.70 | 0.78 |
| Control group mean | 0.12 | 0.056 | 0.75 | 0.73 | 0.16 | 0.16 | 0.054 | 0.062 | 0.062 | 0.067 |
Note: The dependent variables are in the first row of the table: uptake denotes at least three antenatal visits, a health facility delivery, and one postnatal visit; child survival is the probability that an exposed child (who was in-utero at enrollment) was alive at follow-up; a fetal loss is a pregnancy termination before 28 weeks; a fetal death is a child that was delivered after 28 weeks but was born dead; and an early infant death is one where the child was born alive but died before the follow-up interview. Pregnancy age cannot be imputed for women with a fetal loss because the imputation is based on the month of birth so for these women we rely on their reported pregnancy age. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), a dummy denoting Hausa or Fulani extraction, dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintiles. The second column in each panel excludes observations in Gombe state. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.9:
Heterogeneity by household wealth: Top 2 vs. Bottom 3 quintiles
| Uptake |
Child survival |
Fetal loss |
Fetal death |
Early infant death |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) Top 2 | (2) Bottom 3 | (3) Top 2 | (4) Bottom 3 | (5) Top 2 | (6) Bottom 3 | (7) Top 2 | (8) Bottom 3 | (9) Top 2 | (10) Bottom 3 | |
| Intent-to-Treat | 0.12*** (0.011) | 0.14*** (0.010) | −0.044*** (0.014) | −0.069*** (0.0100) | −0.040*** (0.011) | −0.057*** (0.0079) | −0.012 (0.0075) | −0.0093* (0.0054) | 0.0018 (0.0091) | −0.0093 (0.0064) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 3606 | 5915 | 4100 | 6597 | 4100 | 6597 | 3606 | 5915 | 3442 | 5684 |
| Control group mean | 0.076 | 0.14 | 0.24 | 0.23 | 0.14 | 0.14 | 0.055 | 0.050 | 0.067 | 0.058 |
| p-value (difference) | 0.55 | 0.55 | 0.19 | 0.19 | 0.34 | 0.34 | 0.80 | 0.80 | 0.30 | 0.30 |
Note: The dependent variables are in the first row of the table: uptake denotes at least three antenatal visits, a health facility delivery, and one postnatal visit; child survival is the probability that an exposed child (who was in-utero at enrollment) was alive at follow-up; a fetal death is a child that was delivered after 28 weeks but was born dead; and an early infant death is one where the child was born alive but died before the follow-up interview. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), a dummy denoting Hausa or Fulani extraction, dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), dummies for mother’s number of prior births, and dummies indicating a prior fetal loss or a stillbirth. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.10:
Heterogeneity by maternal education
| Uptake |
Child survival |
Fetal loss |
Fetal death |
Early infant death |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) None | (2) Some | (3) None | (4) Some | (5) None | (6) Some | (7) None | (8) Some | (9) None | (10) Some | |
| Intent-to-Treat | 0.14*** (0.0084) | 0.14*** (0.016) | −0.052*** (0.0095) | −0.069*** (0.015) | −0.044*** (0.0074) | −0.066*** (0.012) | −0.015*** (0.0054) | −0.0015 (0.0074) | 0.00044 (0.0064) | −0.0067 (0.0088) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 6718 | 2803 | 7514 | 3183 | 7514 | 3183 | 6718 | 2803 | 6417 | 2709 |
| Control group mean | 0.091 | 0.18 | 0.24 | 0.23 | 0.13 | 0.16 | 0.057 | 0.038 | 0.065 | 0.052 |
| p-value (difference) | 0.96 | 0.96 | 0.42 | 0.42 | 0.23 | 0.23 | 0.20 | 0.20 | 0.50 | 0.50 |
Note: None denotes no formal education and Some denotes at least primary school. The dependent variables are in the first row of the table: uptake denotes at least three antenatal visits, a health facility delivery, and one postnatal visit; child survival is the probability that an exposed child (who was in-utero at enrollment) was alive at follow-up; a fetal death is a child that was delivered after 28 weeks but was born dead; and an early infant death is one where the child was born alive but died before the follow-up interview. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintiles. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.11:
Heterogeneity by pregnancy risk
| Uptake |
Child survival |
Fetal loss |
Fetal death |
Early infant death |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) Low | (2) High | (3) Low | (4) High | (5) Low | (6) High | (7) Low | (8) High | (9) Low | (10) High | |
| Intent-to-Treat | 0.13*** (0.0098) | 0.15*** (0.011) | −0.052*** (0.011) | −0.069*** (0.012) | −0.040*** (0.0084) | −0.065*** (0.0094) | −0.012** (0.0059) | −0.012* (0.0064) | −0.0055 (0.0069) | −0.00021 (0.0078) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 4901 | 4620 | 5439 | 5258 | 5439 | 5258 | 4901 | 4620 | 4709 | 4417 |
| Control group mean | 0.095 | 0.14 | 0.21 | 0.26 | 0.12 | 0.16 | 0.049 | 0.056 | 0.056 | 0.067 |
| p-value (difference) | 0.48 | 0.48 | 0.28 | 0.28 | 0.073 | 0.073 | 0.95 | 0.95 | 0.61 | 0.61 |
Note: High(er) risk indicates women with at least one of the following risk factors: first-time mother, five or more previous births, age less than 15 years or older than 35, prior history of a stillbirth. Low(er) risk indicates women with none of these factors. The dependent variables are in the first row of the table: uptake denotes at least three antenatal visits, a health facility delivery, and one postnatal visit; child survival is the probability that an exposed child (who was in-utero at enrollment) was alive at follow-up; a fetal death is a child that was delivered after 28 weeks but was born dead; and an early infant death is one where the child was born alive but died before the follow-up interview. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The models include strata (HSA) fixed effects and the following controls: a dummy denoting Hausa or Fulani extraction, dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy indicating a prior fetal loss, and household wealth quintiles. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.12:
Heterogeneity by infant gender
| Uptake |
Fetal death |
Early infant death |
Neonatal death |
|||||
|---|---|---|---|---|---|---|---|---|
| (1) Female | (2) Male | (3) Female | (4) Male | (5) Female | (6) Male | (7) Female | (8) Male | |
| Intent-to-Treat | 0.13*** (0.011) | 0.15*** (0.010) | −0.0078 (0.0062) | −0.016*** (0.0061) | −0.0034 (0.0068) | −0.0041 (0.0076) | −0.0039 (0.0062) | −0.0013 (0.0069) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 4474 | 5047 | 4474 | 5047 | 4301 | 4825 | 4301 | 4825 |
| Control group mean | 0.11 | 0.12 | 0.046 | 0.057 | 0.049 | 0.073 | 0.041 | 0.058 |
| p-value (difference) | 0.46 | 0.46 | 0.35 | 0.35 | 0.95 | 0.95 | 0.80 | 0.80 |
Note: The sex of the infant is only observed if the pregnancy resulted in a birth. The dependent variables are in the first row of the table: uptake denotes at least three antenatal visits, a health facility delivery, and one postnatal visit; child survival is the probability that an exposed child (who was in-utero at enrollment) was alive at follow-up; a fetal death is a child that was delivered after 28 weeks but was born dead; and an early infant death is one where the child was born alive but died before the follow-up interview. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), a dummy denoting Hausa or Fulani extraction, dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintiles. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.13:
Heterogeneity by whether the HSA facility received an additional health worker
| Uptake |
Child survival |
Fetal loss |
Fetal death |
Early infant death |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) No | (2) Yes | (3) No | (4) Yes | (5) No | (6) Yes | (7) No | (8) Yes | (9) No | (10) Yes | |
| Intent-to-Treat | 0.11*** (0.013) | 0.15*** (0.0092) | −0.054*** (0.014) | −0.065*** (0.0096) | −0.044*** (0.011) | −0.058*** (0.0076) | −0.014** (0.0070) | −0.011** (0.0054) | −0.0035 (0.0090) | −0.0036 (0.0063) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 3114 | 6407 | 3502 | 7195 | 3502 | 7195 | 3114 | 6407 | 3005 | 6121 |
| Control group mean | 0.11 | 0.12 | 0.22 | 0.24 | 0.13 | 0.14 | 0.044 | 0.055 | 0.061 | 0.061 |
| p-value (difference) | 0.41 | 0.41 | 0.65 | 0.65 | 0.50 | 0.50 | 0.76 | 0.76 | 0.99 | 0.99 |
Note: The dependent variables are in the first row of the table: uptake denotes at least three antenatal visits, a health facility delivery, and one postnatal visit; child survival is the probability that an exposed child (who was in-utero at enrollment) was alive at follow-up; a fetal death is a child that was delivered after 28 weeks but was born dead; and an early infant death is one where the child was born alive but died before the follow-up interview. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), a dummy denoting Hausa or Fulani extraction, dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintiles. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.14:
Effect of the treatment on intermediate outcomes (excluding Gombe state)
| (1) HIV testing & counseling | (2) Iron supplements | (3) Antimalarial prophylaxis | (4) Pregnancy problems | (5) Treated for problem | (6) Labor was obstructed | (7) Hypertensive complications | |
|---|---|---|---|---|---|---|---|
| Intent-to-Treat | 0.072*** (0.016) | 0.058*** (0.015) | 0.036*** (0.013) | 0.00032 (0.0041) | 0.037** (0.015) | −0.0075 (0.0053) | 0.0019 (0.0041) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 8488 | 8488 | 8488 | 8488 | 6356 | 7405 | 7405 |
| Number of groups | 144 | 144 | 144 | 144 | 144 | 144 | 144 |
| p-value from permutation test | 0 | 0 | 0 | 0.95 | 0.010 | 0.13 | 0.68 |
| Control group mean | 0.57 | 0.69 | 0.72 | 0.16 | 0.31 | 0.045 | 0.022 |
Note: This table examines the effect on intermediate outcomes. The sample excludes Gombe state. The dependent variables are in the first row of the table. Intermediate prenatal indicators are in Columns 1–3, and labor/delivery indicators are in Columns 6 and 7. They are binary indicators. Pregnancy problems (column 4) is an index created by taking an average across women’s responses to a series of questions about problems experienced during the pregnancy. Column 5 asks if the woman received treatment for the problem. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. All models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintile dummies. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
We also report p-values from permutation tests based on 1,000 draws from the distribution of the treatment effect estimate under the sharp null hypothesis of a zero treatment effect.
Table A.15:
Effect of the conditional cash payment by pregnancy trimester at enrollment
| (1) Number of prenatal visits | (2) Childbirth in a health facility | (3) Infant was born dead | |
|---|---|---|---|
| Transfer | 0.61*** (0.092) | 0.16*** (0.021) | −0.012 (0.0089) |
| 2nd trimester | 0.30*** (0.069) | 0.027 (0.016) | −0.0015 (0.0087) |
| 3rd trimester | 0.74*** (0.12) | 0.022 (0.025) | −0.015 (0.012) |
| Transfer × 2nd trimester | −0.15 (0.097) | −0.035 (0.023) | −0.0026 (0.011) |
| Transfer × 3rd trimester | −0.57*** (0.14) | −0.020 (0.031) | 0.013 (0.015) |
| Controls | Yes | Yes | Yes |
| Observations | 10632 | 9499 | 9499 |
| Number of groups | 180 | 180 | 180 |
| p-value (B1+B4=0) | 0.000000013 | 3.2e-11 | 0.018 |
| p-value (B1+B5=0) | 0.70 | 0.00000031 | 0.87 |
| Control group mean | 2.38 | 0.29 | 0.052 |
Note: The dependent variables are in the first row of the table. Trimester at enrollment was imputed using month of birth and assuming a standard pregnancy duration. It cannot be imputed if the pregnancy did not result in a birth so for these women we rely on their reported pregnancy age. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. The models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), a dummy denoting Hausa or Fulani extraction, dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintiles. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
Table A.16:
Other causal pathways
| (1) Child survival | (2) Small-size infant | (3) Cost of delivery | (4) Labor was prolonged | (5) Received oxytocin | (6) Physical/verbal mistreatment | (7) Pain medication | |
|---|---|---|---|---|---|---|---|
| Intent-to-Treat | 0.0637*** (0.0117) | 0.00356 (0.00482) | 54.33 (76.19) | −0.00960 (0.0121) | 0.00374 (0.0198) | −0.00582 (0.00830) | 0.0357* (0.0193) |
| Controls | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Observations | 6538 | 8910 | 3460 | 3468 | 3459 | 3459 | 3459 |
| Number of groups | 180 | 180 | 161 | 161 | 161 | 161 | 161 |
| p-value from permutation test | 0 | 0.500 | 0.550 | 0.310 | 0.820 | 0.520 | 0.0400 |
| Control group mean | 0.775 | 0.0537 | 1375.8 | 0.0870 | 0.780 | 0.0521 | 0.193 |
Note: This tables examines other potential causal pathways. In Column 1 we test whether reduced household work and stress can potentially account for the findings. We re-estimate the mortality results restricting the sample to women in households with no co-wives and no female children older than 7, both of whom could potentially substitute for maternal labor. The dependent variable is an indicator equal to one if the exposed child (who was in-utero at enrollment) was alive at follow-up. In Column 2 we examine the maternal nutrition channel by examining whether the intervention had an effect on the probability of giving birth to a very small or smaller than average infant. In Column 3 we examine whether participants in the treatment group paid more than participants in the control group for delivery at the local health facility. In Columns 4–7 we examine whether women in the treatment group received better delivery care at the local health facility than women in the control group. The treatment is a cash payment of $14 paid to households if eligible pregnant women used a package of health services consisting of at least three antenatal visits, a health facility delivery, and one postnatal visit. All models include strata (HSA) fixed effects and the following controls: dummies for mother’s age (<18, 18–24, 25–29, 30–34, and >35 years), dummies for mother’s educational attainment (no schooling, Islamic schooling, some primary school, some secondary school, and some tertiary schooling), a dummy denoting Hausa or Fulani extraction, dummies for mother’s number of prior births, dummies indicating a prior fetal loss or a stillbirth, and household wealth quintiles. Standard errors in parentheses are clustered at the level of the health service area (HSA).
p < 0.1
p < 0.05
p < 0.01.
We also report p-values from permutation tests based on 1,000 draws from the distribution of the treatment effect estimate under the sharp null hypothesis of a zero treatment effect.
Footnotes
In high mortality regions such as sub-Saharan Africa and South Asia, more than half of all births take place outside of health facilities (Darmstadt et al., 2009).
While the epicenter of this debate is in developing countries, there is a related discussion in high-income countries, where home births have been on the rise, about whether low-risk births can safely take place at home. See exchange between de Jonge et al. (2016) and Daysal et al. (2016).
That care at birth does not seem to result in better outcomes may be indicative of constraints in the medical technology available in typical health facilities.
Attanasio et al. (2015) is an exception. They are able to link health improvements to child preventive care visits.
In the context of the Oportunidades program which has been widely studied, Bobonis (2011) has shown that it affected rates of marriage and divorce, and Angelucci and De Giorgi (2009) has found that it generated general equilibrium effects. Many CCT programs are also bundled with additional interventions. Oportunidades, for example, required attendance at health promotion talks and distributed nutritional supplements to women and children (Urquieta et al., 2009).
The reverse is also true in that it also limits what inferences we can draw from the existing CCT literature.
By ‘traditional’ CCT programs we mean social welfare programs such as Oportunidades. Historically these programs have been popular in Latin America.
A practical implication of this is that income effects are much less important both because of the size and the timing of the transfer.
For comparison, the United States and South Africa were ranked 8th and 116th respectively.
The other countries are India, Pakistan, Democratic Republic of Congo, and Ethiopia (UNICEF, 2017). Newborn deaths are child deaths within the first month.
HSAs in our sample serve, on average, about 7,000 people.
Two-thirds of participating facilities received an additional health worker. The results of this study are reported elsewhere (Okeke and Abubakar, 2019). The health workers were present in these facilities during this cash transfer intervention.
A census area may contain anywhere from a few dozen to more than one hundred households (the sample mean is 30).
Program credibility was critical.
There is a high level of acceptability of prenatal care, with a 3 in 4 women attending at least once, suggesting that the marginal cost of an additional visit is low.
Facility types are public hospital, public primary health facility, private hospital/clinic, or other.
In cases where the woman was deceased, this information was collected from a knowledgeable household member.
We used two alternative methods: (i) assigning each birth the same standard pregnancy duration, and (ii) assigning each woman a random draw from a normal distribution of pregnancy duration (Jukic et al., 2013). They yield slightly different percentages by trimester but produce similar results in the analysis. The reported results use the first imputation method.
The sum exceeds the number of pregnancies because 1% of pregnancies resulted in a multiple birth.
This is not surprising as this was closely monitored.
We verified that the number of households in intervention clusters was the same as in control clusters.
Given that it is unlikely that the program staff in this state were more inherently altruistic than in other states, it is likely that deficiencies in supervision by the field manager also played a role.
There is a significant increase in all states except for Akwa Ibom. In Akwa Ibom, there was a small significant increase in prenatal attendance but no effect on facility births. See Figure A.3.
Clustering at the census area level fails to take into account the nesting within HSAs. We also estimated multilevel models using maximum likelihood that more efficiently account for the nesting structure of the data. The results were nearly identical to the OLS model so we report results from the simpler model.
Participants were re-interviewed a median of three months after the conclusion of the pregnancy.
For comparison with prior work we also disaggregate this into neonatal and post-neonatal deaths (see Table A.1). For interested readers we also report unconditional results in Table A.2.
Since pregnancy was self-reported, one concern might be that in intervention clusters, women who were not pregnant at the time were more likely to report being pregnant (in the hope of getting pregnant after enrollment) to be eligible. This could, in part, explain the higher rate of fetal losses. As a robustness check, we exclude women who reported an early stage pregnancy (≤ 3 months). The results are similar. The treatment effect on fetal losses in this sample is 4.2 percentage points and still highly significant.
We do not have similar data for prenatal care.
Treatment saturation varies from 2% to 54%. We graph the distribution in Figure A.5 Panel A.
We considered using pregnancy tests but decided against it because of the expense and because of potential ethical considerations involved in asking women to take pregnancy tests.
In Figure A.3 we show that the pattern of the mortality decrease closely follows that of the first stage effect. Mortality decreases only in states where there was an intervention effect on uptake. In addition, the size of the mortality effect is correlated with that of the uptake effect. This serves as additional validation for the results.
Women were asked whether they experienced any of the following problems during the pregnancy: swelling of hands, feet and face; paleness, giddiness, weakness; blurred vision or other visual disturbance; weak or no movement of the fetus; excessive fatigue/tiredness; convulsions (not from fever); high blood pressure; vaginal bleeding; excessive vomiting; abnormal position of the fetus; high fever; jaundice; water break without labor; and any other problems not specifically asked about. The dependent variable is an index created by taking an average across women’s responses.
The latter was measured by asking study participants if they experienced convulsions or seizures during labor but without fever (a medical condition known as eclampsia).
In Table A.14 we show that the results are similar if Gombe is excluded.
These are referred to as macerated stillbirths in the medical literature. We asked study participants the following question: Did the baby look like a normal baby, or had the skin and body changed and become pulpy/puffy/mushy/swollen?
One might wonder why this might be the case. If a woman knew that she did not have a sufficient number of prenatal visits, why would she bother with a facility birth? We believe that there are several possible reasons. First, she could have learned about the value of a facility birth during prenatal health sessions and decided it was worth paying the full price (without the implicit subsidy). She could also have faced pressure from health providers. More likely, it is possible that she hoped that she might still be paid if she met most of the conditions. Finally, she may have mistakenly believed that she had a sufficient number of prenatal visits when she did not.
There seems to be an understanding in this setting that hard work, and “stress”, negatively impact birth outcomes.
As noted earlier, some of the health facilities received an additional health worker, but no attempt was made to influence patterns of care delivery or health worker behavior. Besides as Table A.13 has shown there is no evidence of an interaction with the health worker intervention.
We estimated the annual number of births in Nigeria by multiplying the crude birth rate (≈ 38 per 1000) by the estimated population (≈ 186 million). The estimated decrease in stillbirths is then calculated by multiplying the annual number of births by the coefficient in Table 7 Column 5.
Nigeria has a stillbirth rate of 42 per 1000 births. Even though it is a lower middle-income country, its stillbirth rate resembles that of a low-income country. Countries like Moldova (7.8 per 1000), Nicaragua (14.5 per 1000), Sudan (23.9 per 1000), Ukraine (8.2 per 1000), Uzbekistan (6.5 per 1000), and Vietnam (13.9 per 1000), all of which have similar income levels, have substantially lower stillbirth rates (Lawn et al., 2011).
This is the subject of ongoing work.
Primary health facilities are heavily subsidized by the government and prices are not reflective of opportunity costs. Costs in the private sector may be a better proxy but we have only a few dozen observations. As a compromise we use reported costs paid in government hospitals. The cost of a delivery in a government hospital is $51 compared to $4 in a primary health care clinic. We value time costs at the statutory monthly minimum wage of 18,000 Naira ($50).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adhvaryu A and Nyshadham A (2015). Returns to treatment in the formal health care sector: Evidence from Tanzania. American Economic Journal: Economic Policy, 7(3):29–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatas V, Banerjee A, Hanna R, Olken BA, and Tobias J (2012). Targeting the poor: Evidence from a field experiment in Indonesia. American Economic Review, 102(4):1206–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond D and Mazumder BA (2011). Health capital and the prenatal environment: the effect of ramadan observance during pregnancy. American Economic Journal: Applied Economics, 3(4):56–85. [Google Scholar]
- Amarante V, Manacorda M, Miguel E, and Vigorito A (2016). Do cash transfers improve birth outcomes? evidence from matched vital statistics, program, and social security data. American Economic Journal: Economic Policy, 8(2):1–43. [Google Scholar]
- Angelucci M and De Giorgi G (2009). Indirect effects of an aid program: how do cash transfers affect ineligibles’ consumption? American Economic Review, 99(1):486–508. [Google Scholar]
- Attanasio OP, Oppedisano V, and Vera-Hernandez M (2015). Should cash transfers be conditional? conditionality, preventive care, and health outcomes. American Economic Journal: Applied Economics, 7(2):35–52. [Google Scholar]
- Baird S, McIntosh C, and Özler B (2011). Cash or condition: Evidence from a randomized cash transfer program. Quarterly Journal of Economics, 126(4):1709–1753. [Google Scholar]
- Banerjee AV, Duflo E, Glennerster R, and Kothari D (2010). Improving immunisation coverage in rural India: clustered randomised controlled evaluation of immunisation campaigns with and without incentives. BMJ, 340:c2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SL and Gertler PJ (2010). Empowering women: how Mexico’s conditional cash transfer programme raised prenatal care quality and birth weight. Journal of Development Effectiveness, 2(1):51–73. [Google Scholar]
- Barham T (2011). A healthier start: The effect of conditional cash transfers on neonatal and infant mortality in rural Mexico. Journal of Development Economics, 94(1):74–85. [Google Scholar]
- Bhutta ZA, Das JK, Bahl R, Lawn JE, Salam RA, Paul VK, Sankar MJ, Blencowe H, Rizvi A, Chou VB, and Walker N (2014). Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? The Lancet, 384(9940):347–370. [DOI] [PubMed] [Google Scholar]
- Bobonis GJ (2011). The impact of conditional cash transfers on marriage and divorce. Economic Development and cultural change, 59(2):281–312. [DOI] [PubMed] [Google Scholar]
- Boneva T and Rauh C (2018). Parental Beliefs about Returns to Educational Investments—The Later the Better? Journal of the European Economic Association, 16(6):1669–1711. [Google Scholar]
- Borghi J, Little R, Binyaruka P, Patouillard E, and Kuwawenaruwa A (2015). In Tanzania, the many costs of pay-for-performance leave open to debate whether the strategy is cost-effective. Health Affairs, 34(3):406–414. [DOI] [PubMed] [Google Scholar]
- Brocklehurst P and French R (1998). The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. BJOG: An International Journal of Obstetrics & Gynaecology, 105(8):836–848. [DOI] [PubMed] [Google Scholar]
- Bugni FA, Canay IA, and Shaikh AM (2018). Inference under covariate-adaptive randomization. Journal of the American Statistical Association, pages 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari A, Glick P, Okeke EN, and Srinivasan S (2019). Does workfare worsen infant health? evidence from India’s public works program. Journal of Development Economics, 138:116–134. [Google Scholar]
- Cohen J, Rothschild C, Golub G, Omondi GN, Kruk ME, and McConnell M (2017). Measuring the impact of cash transfers and behavioral ‘nudges’ on maternity care in Nairobi, Kenya. Health Affairs, 36(11):1956–1964. [DOI] [PubMed] [Google Scholar]
- Darmstadt GL, Lee AC, Cousens S, Sibley L, Bhutta ZA, Donnay F, Osrin D, Bang A, Kumar V, Wall SN, Baqui A, and Lawn JE (2009). 60 million non-facility births: Who can deliver in community settings to reduce intrapartum-related deaths? International Journal of Gynecology & Obstetrics, 107, Supplement(0):S89–S112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J and Hammer J (2014). Quality of primary care in low-income countries: Facts and economics. Annual Review of Economics, 6(1):525–553. [Google Scholar]
- Das J, Woskie L, Rajbhandari R, Abbasi K, and Jha A (2018). Rethinking assumptions about delivery of healthcare: implications for universal health coverage. BMJ, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daysal N, Trandafir M, and van Ewijk R (2016). Re: A recent study by economists on the impact of home births on infant outcomes confuses the debate on home birth. BJOG: An International Journal of Obstetrics & Gynaecology, 123(10):1713–1714. [DOI] [PubMed] [Google Scholar]
- Daysal NM, Trandafir M, and van Ewijk R (2015). Saving lives at birth: The impact of home births on infant outcomes. American Economic Journal: Applied Economics, 7(3):28–50. [Google Scholar]
- de Brauw A and Peterman A (2011). Can conditional cash transfers improve maternal health and birth outcomes?: Evidence from El Salvador’s Comunidades Solidarias Rurales IFPRI discussion paper 1080, International Food Policy Research Institute (IFPRI). [Google Scholar]
- de Jonge A, Verhoeven C, and Thornton J (2016). Re: Perinatal mortality and morbidity up to 28 days after birth among 743 070 low-risk planned home and hospital births: a cohort study based on three merged national perinatal databases. BJOG: An International Journal of Obstetrics & Gynaecology, 123(7):1235–1236. [DOI] [PubMed] [Google Scholar]
- de Walque D, Dow WH, Nathan R, Abdul R, Abilahi F, Gong E, Isdahl Z, Jamison J, Jullu B, Krishnan S, Majura A, Miguel E, Moncada J, Mtenga S, Mwanyangala MA, Packel L, Schachter J, Shirima K, and Medlin CA (2012). Incentivising safe sex: a randomised trial of conditional cash transfers for HIV and sexually transmitted infection prevention in rural tanzania. BMJ Open, 2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaarder MM, Glassman A, and Todd JE (2010). Conditional cash transfers and health: unpacking the causal chain. Journal of Development Effectiveness, 2(1):6–50. [Google Scholar]
- Gajate-Garrido G (2013). The impact of adequate prenatal care on urban birth outcomes: An analysis in a developing country context. Economic Development and Cultural Change, 62(1):95–130. [Google Scholar]
- Gertler P (2004). Do conditional cash transfers improve child health? evidence from PROGRESA’s control randomized experiment. American economic review, 94(2):336–341. [DOI] [PubMed] [Google Scholar]
- Glassman A, Duran D, Fleisher L, Singer D, Sturke R, Angeles G, Charles J, Emrey B, Gleason J, Mwebsa W, Saldana K, Yarrow K, and Koblinsky M (2013). Impact of conditional cash transfers on maternal and newborn health. Journal of Health, Population, and Nutrition, 31(4 Suppl 2):S48–S66. [PubMed] [Google Scholar]
- Godlonton S and Okeke EN (2016). Does a ban on informal health providers save lives? evidence from malawi. Journal of Development Economics, 118:112–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, and Romero R (2008). Epidemiology and causes of preterm birth. The lancet, 371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalan SS, Mutasa R, Friedman J, and Das A (2014). Health sector demand-side financial incentives in low- and middle-income countries: A systematic review on demand- and supply-side effects. Social Science & Medicine, 100:72–83. [DOI] [PubMed] [Google Scholar]
- Handa S, Peterman A, Seidenfeld D, and Tembo G (2016). Income transfers and maternal health: Evidence from a national randomized social cash transfer program in Zambia. Health Economics, 25(2):225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SA, Blandón YC, McCaw-Binns A, Sandino I, Urbina L, Rodríguez C, Gómez I, Ayabaca P, Djibrina S, and the Nicaraguan maternal and neonatal health quality improvement group (2007). Are skilled birth attendants really skilled? A measurement method, some disturbing results and a potential way forward. Bulletin of the World Health Organization, 85(10):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heß S (2017). Randomization inference with stata: A guide and software. Stata Journal, 17(3):630–651. [Google Scholar]
- Horton S, Gelband H, Jamison D, Levin C, Nugent R, and Watkins D (2017). Ranking 93 health interventions for low-and middle-income countries by cost-effectiveness. PloS one, 12(8):e0182951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter BM, Harrison S, Portela A, and Bick D (2017). The effects of cash transfers and vouchers on the use and quality of maternity care services: A systematic review. PloS one, 12(3):e0173068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter BM and Murray SF (2017). Demand-side financing for maternal and newborn health: what do we know about factors that affect implementation of cash transfers and voucher programmes? BMC Pregnancy and Childbirth, 17(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad A and Bhutta ZA (2012). Maternal nutrition and birth outcomes: Effect of balanced protein-energy supplementation. Paediatric and Perinatal Epidemiology, 26:178–190. [DOI] [PubMed] [Google Scholar]
- Jahan S and the Human Development Report 2015 Team (2015). Human development report 2015: Work for human development. Technical report, United Nations Development Programme, 1 UN Plaza, New York, NY 10017, USA. [Google Scholar]
- Jensen R (2010). The Perceived Returns to Education and the Demand for Schooling. Quarterly Journal of Economics, 125(2):515–548. [Google Scholar]
- Joyce T (1994). Self-selection, prenatal care, and birthweight among blacks, whites, and hispanics in new york city. The Journal of Human Resources, 29(3):762–794. [Google Scholar]
- Jukic AM, Baird DD, Weinberg CR, McConnaughey DR, and Wilcox AJ (2013). Length of human pregnancy and contributors to its natural variation. Human Reproduction, 28(10):2848–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler H and Thornton RL (2012). Conditional cash transfers and HIV/AIDS prevention: unconditionally promising? The World Bank Economic Review, 26(2):165–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, Lalli M, Bhutta Z, Barros AJ, Christian P, et al. (2014). Every newborn: progress, priorities, and potential beyond survival. The Lancet, 384(9938):189–205. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, Ibiebele I, Gardosi J, Day LT, Stanton C, et al. (2011). Stillbirths: Where? when? why? how to make the data count? The Lancet, 377(9775):1448–1463. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, Flenady V, Frøen JF, Qureshi ZU, Calderwood C, et al. (2016). Stillbirths: rates, risk factors, and acceleration towards 2030. The Lancet, 387(10018):587–603. [DOI] [PubMed] [Google Scholar]
- Lee AC, Lawn JE, Cousens S, Kumar V, Osrin D, Bhutta ZA, Wall SN, Nandakumar AK, Syed U, and Darmstadt GL (2009). Linking families and facilities for care at birth: What works to avert intrapartum-related deaths? International Journal of Gynecology & Obstetrics, 107, Supplement(0):S65–S88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie HH, Fink G, Nsona H, and Kruk ME (2016). Obstetric facility quality and newborn mortality in Malawi: a cross-sectional study. PLoS medicine, 13(10):e1002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemayr S, Stecher C, and Mukasa B (2017). Behavioral economic incentives to improve adherence to antiretroviral medication. AIDS (London, England), 31(5):719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Mroz T, and Adair L (2009). Parental compensatory behaviors and early child health outcomes in cebu, philippines. Journal of Development Economics, 90(2):209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, and Black RE (2015). Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. The Lancet, 385(9966):430–440. [DOI] [PubMed] [Google Scholar]
- MacKinnon JG and Webb MD (2017). Wild bootstrap inference for wildly different cluster sizes. Journal of Applied Econometrics, 32(2):233–254. [Google Scholar]
- Maluccio J and Flores R (2005). Impact evaluation of a conditional cash transfer program: the nicaraguan red de protección social Technical report, International Food Policy Research Institute (IFPRI). [Google Scholar]
- Marseille E, Larson B, Kazi DS, Kahn JG, and Rosen S (2014). Thresholds for the cost–effectiveness of interventions: alternative approaches. Bulletin of the World Health Organization, 93:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EM, Goldenberg RL, Dent AE, and Meshnick SR (2013). A systematic review of the impact of malaria prevention in pregnancy on low birth weight and maternal anemia. International Journal of Gynecology & Obstetrics, 121(2):103–109. [DOI] [PubMed] [Google Scholar]
- Meacock R, Kristensen SR, and Sutton M (2014). The cost-effectiveness of using financial incentives to improve provider quality: A framework and application. Health economics, 23(1):1–13. [DOI] [PubMed] [Google Scholar]
- Moore KA, Simpson JA, Scoullar MJL, McGready R, and Fowkes FJI (2017). Quantification of the association between malaria in pregnancy and stillbirth: a systematic review and meta-analysis. The Lancet Global Health, 5(11):e1101–e1112. [DOI] [PubMed] [Google Scholar]
- Naeye RL, Burt LS, Wright DL, Blanc WA, and Tatter D (1971). Neonatal mortality, the male disadvantage. Pediatrics, 48(6):902–906. [PubMed] [Google Scholar]
- Nair M, Churchill D, Robinson S, Nelson-Piercy C, Stanworth SJ, and Knight M (2017). Association between maternal haemoglobin and stillbirth: a cohort study among a multi-ethnic population in England. British Journal of Haematology, 179(5):829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Population Commission and ICF International (2014). Nigeria demographic and health survey 2013 Technical report, NPC and ICF International, Abuja, Nigeria, and Rockville, Maryland, USA. [Google Scholar]
- National Primary Health Care Development Agency (2014). Minimum standards for primary health care in Nigeria Technical report, Department of Planning, Research and Statistics, NPHCDA; Plot 681/682 Port-Harcourt Crescent, Off Gimbiya Street, Garki II, Abuja. [Google Scholar]
- Nigerian National Bureau of Statistics (2016). LSMS-Integrated Surveys on Agriculture General Household Survey Panel 2015/2016 Technical report, Nigerian National Bureau of Statistics in Collaboration with the Federal Ministry of Agriculture and Rural Development and the World Bank. [Google Scholar]
- Okeke E (2019). Working hard or hardly working: Health worker effort and health outcomes. Economic Development and Cultural Change. [Google Scholar]
- Okeke EN and Abubakar IS (2019). When a doctor falls from the sky: Physician supply and health outcomes. Working Paper. [Google Scholar]
- Okeke EN and Chari A (2014). Can Institutional Deliveries Reduce Newborn Mortality? Evidence from Rwanda: RAND Working Paper 1072. [Google Scholar]
- Okeke EN and Chari A (2018). Health care at birth and infant mortality: Evidence from nighttime deliveries in Nigeria. Social Science and Medicine, 196(Supplement C):86–95. [DOI] [PubMed] [Google Scholar]
- Okoli U, Morris L, Oshin A, Pate MA, Aigbe C, and Muhammad A (2014). Conditional cash transfer schemes in Nigeria: potential gains for maternal and child health service uptake in a national pilot programme. BMC pregnancy and childbirth, 14(1):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Jackson T, Mazumdar S, and Mills A (2015). Financial incentives in health: New evidence from India’s Janani Suraksha Yojana. Journal of Health Economics, 43:154–169. [DOI] [PubMed] [Google Scholar]
- Rasella D, Aquino R, Santos CA, Paes-Sousa R, and Barreto ML (2013). Effect of a conditional cash transfer programme on childhood mortality: a nationwide analysis of Brazilian municipalities. The lancet, 382(9886):57–64. [DOI] [PubMed] [Google Scholar]
- Takasaki Y and Sato R (2017). Short-run incentive and information in sequential adoptions: An antenatal care experiment in rural nigeria. CIRJE Discussion Paper F-1070. [Google Scholar]
- Thornton R (2008). The demand for, and impact of, learning HIV status. American Economic Review, 98(5):1829–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF (2017). Levels and trends in child mortality. report 2014. Estimates developed by the UN inter-agency group for child mortality estimation. United Nations Children’s Fund. [Google Scholar]
- Urquieta J, Angeles G, Mroz T, Lamadrid-Figueroa H, and Hernández B (2009). Impact of Oportunidades on skilled attendance at delivery in rural areas. Economic Development and Cultural Change, 57(3):539–558. [Google Scholar]
- Watson-Jones D, Weiss HA, Changalucha JM, Todd J, Gumodoka B, Bulmer J, Balira R, Ross D, Mugeye K, Hayes R, et al. (2007). Adverse birth outcomes in United Republic of Tanzania: impact and prevention of maternal risk factors. Bulletin of the World Health Organization, 85(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedi CO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, and Hemelaar J (2016). Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. The Lancet HIV, 3(1):e33–e48. [DOI] [PubMed] [Google Scholar]
- World Bank (2018). World Development Indicators, World Bank, Washington. [Google Scholar]
- World Health Organization (2015). World health statistics. Health Organization, Switzerland, WHO Press, 20 Avenue Appia, 1211 Geneva 27, Switzerland. [Google Scholar]
- World Health Organization et al. (2016). WHO recommendations on antenatal care for a positive pregnancy experience. World Health Organization. [PubMed] [Google Scholar]
- Zhang Q, Ananth CV, Rhoads GG, and Li Z (2009). The impact of maternal anemia on perinatal mortality: a population-based, prospective cohort study in China. Annals of Epidemiology, 19(11):793–799. [DOI] [PubMed] [Google Scholar]