Abstract
We present a series of patients with recurrent acute pancreatitis caused by a duplicated pancreatic head connected to a gastric duplication and successfully treated by conservative surgery. This retrospective study included consecutive adult patients referred to our institution for recurrent acute pancreatitis. All patients underwent a preoperative non-invasive imaging examination including contrast-enhanced computed tomography and magnetic resonance cholangiopancreatography (MRCP). The final diagnosis of this developmental anomaly was based on surgical and pathological examinations. The four patients in this study had the same typical imaging pattern including a duplicated duct. There was no recurrent acute pancreatitis after surgical treatment, which involved atypical resection of the duplicated pancreatic head and segmental gastric resection, without a Whipple procedure. The discovery of an accessory pancreatic head with a duct terminating in a cyst identified on MRCP in a patient with recurrent acute pancreatitis could suggest this rare and surgically treatable cause of acute pancreatitis.
INTRODUCTION
Developmental anomalies are an uncommon cause of acute pancreatitis in adults. This study reports a rare anatomical variant in which a gastric duplication cyst communicates with the main pancreatic duct through a duplicated pancreatic head (Fig. 1).
Figure 1.
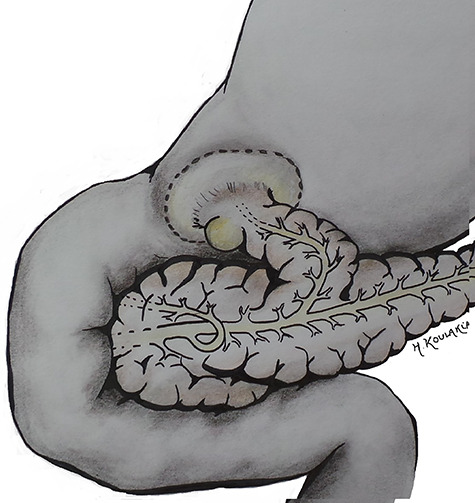
Schematic representation of the anatomic variant: duplicated head (the accessory parenchyma is a parenchymal band heading toward the pyloric region, containing an accessory duct connected with the pancreatic head on one side), and with a gastric duplication in the pyloric part of stomach on the other side.
A previous review of the literature including 23 individual case reports of this rare anatomic variant showed that most of the population was pediatric (n = 15), with a few young adults (n = 8, mean age = 33.8 years old). Treatment of these patients varied [1]. We report the first series of adult patients with this anatomical variant, treated by surgical resection of the duplications alone and show that this variant may be associated with recurrent acute pancreatitis.
The aim of this study was to describe this variant, its typical pattern on non-invasive imaging and show that this rare but curable cause of acute pancreatitis may be surgically managed.
CASE SERIES
In this retrospective study in a single tertiary referral center for pancreatic disease, we included all consecutive adult patients who were referred to our institution between 2010 and 2018 for recurrent acute pancreatitis in whom this rare variant was suspected. All patients underwent a complete, preoperative, non-invasive imaging examination including contrast-enhanced computed tomography (CECT) and magnetic resonance cholangiopancreatography (MRCP). Exclusion criteria were patients who were <18 years old or who did not undergo surgery in our institution.
This study was performed according to the principles of the Declaration of Helsinki.
Imaging protocol
CT examinations were performed with a LightSpeed VCT 64 (GE Healthcare, Milwaukee, WI, USA). After performing a non-enhanced CT, patients were administered 2 ml/kg of bodyweight of non-ionic contrast agent (350–400 mg/ml of iodine) through an 18-gauge angiographic catheter. Contrast was administered intravenously at 3–4 ml/s and multidetector CT was performed with two phases according to a recommended protocol.
Magnetic resonance imaging (MRI) was performed with 1.5-Tesla (Intera n = 1) or 3-Tesla (Ingenia n = 3) (Philips Medical System, Best, the Netherlands) systems with a phased array body coil. The MRI protocol included transverse and coronal T2-weighted images and a transverse breath-hold T1-weighted (dynamic gadolinium enhanced) sequence before and after the dynamic phases. MRCP was performed in all patients with radial coronal or coronal oblique thick slab MRCP acquisitions that provide the best visualization of the pancreatic duct system and the biliary tree. Coronal respiratory-triggered 3D half-Fourier MRCP was also performed. All images were archived with permanent storage.
Imaging analysis
CT and MRCP examinations were available for all included patients.
Results of imaging studies were evaluated by two radiologists in consensus.
We report the radiological features of acute pancreatitis such as the presence of ascites as well as focal or diffuse pancreatitis. The precise location of focal pancreatitis was identified (groove, head, duplicated head, body and tail).
The appearance of the gastric duplication (size, signal, multi-layer aspect and location) and the duplicated pancreatic head (pancreatic accessory parenchyma and visibility of the duplicated duct inside) were reported on both CT and MRCP. Multiplanar reconstruction was used for analysis of the accessory head. Any other anatomical variations of the pancreas or digestive structures were also searched for.
Surgical procedure
Surgical management was similar in all patients and was performed by a single surgeon. The Clavien–Dindo classification [2] was used as well as the length of hospital stay to evaluate postoperative morbidity.
Any postoperative pancreatic fistula was scored according to the International Study Group of Pancreatic Fistula classification [3].
The presence of any new episodes of pancreatitis or digestive symptoms was reported during the postoperative clinical follow-up, and all patients systematically underwent non-invasive imaging following surgery.
Pathology analysis
Samples were fixed in formalin for 24 hours, sampled extensively and embedded in paraffin according to the routine practices of our hospital. The diagnosis was confirmed on hematein–eosin–safran stains by the same expert pathologist in pancreatic diseases.
RESULTS
Four patients met the inclusion criteria and underwent curative parenchymal sparing surgery.
The final diagnosis of this developmental anomaly was based on surgical and pathological examinations in all four patients.
The study population (n = 4) included four women who initially presented with symptoms of recurrent acute pancreatitis. Patients had multiple (5–23) and some severe (1–3) episodes of acute pancreatitis over a mean 12.5 years (5–17). Mean age at the first episode of acute pancreatitis was 25 years old (16–32).
The radiological presentation of the developmental anomaly was similar in all cases, as described in Table 1. The presence of a duplicated pancreatic head was visible on both CT (Fig. 2A and B) and MRI (Fig. 2C). This accessory pancreatic parenchyma was connected to the pancreatic head on one side and a gastric duplication in the pyloric part of stomach on the other side (Fig. 1). It was seen as a small band, 1 cm in diameter, of pancreatic parenchyma with the same density or signal as the adjacent normal pancreas. The band was continuous with the anterior part of the head of the pancreas and then clearly separated, going toward the stomach in an anterior and cranial direction. A pancreatic accessory duct draining this duplicated pancreatic head and connected to the gastric duplication was seen in half of the cases on CT, and was always seen on MRCP (Fig. 2C).
Table 1.
Radiological presentation of the patients
| ‘Baseline’ radiological description of the developmental anomaly (with CECT and MRI were performed >3 months after last acute pancreatitis episodes) | N = 4 |
|---|---|
| Presence of both a duplicated pancreatic head and a cystic lesion in the antropyloric muscularis | 4/4 on CT and MRI |
| Visibility of a pancreatic accessory duct in the duplicated pancreatic head | 2/4 on CT 4/4 on MRCP |
| Visibility of the connection between the accessory duct and the gastric duplication | Two on CT and four on MRCP |
| Location of the gastric duplication | 4/4 gastric, beside the antropyloric region and surrounded by normal gastric muscularis |
| Signs of chronic pancreatitis (calcification, main or secondary duct abnormalities, anomaly in signal, shape or enhancement of the pancreas parenchyma) | 0 |
| MRI appearance of the gastric duplication on T1 sequence | Three homogeneous hypointense T1 and one heterogeneous hyperintense T1 |
| MRI appearance of the gastric duplication on T2 sequence | Homogeneous hyperintense T2 |
| Multi-layer appearance of the gastric duplication, after injection of intravenous contrast | Yes (4/4), presence of two layers, supposed to be mucosa and muscularis, both on CT or MRI |
| Signal of the external layer of the gastric duplication | 4/4 identical to gastric muscularis |
| Communication between gastric duplication and digestive lumen | 0/4 |
| Mean size (and range) of the gastric duplication (cystic part) | 2.4 cm (1.5–4.3) |
| Radiological presentation shortly (96 hours after onset of pain) after an acute pancreatitis episode | |
| Number of early pancreatitis imaging available (96 hours after onset of pain) | 6 |
| Type of acute pancreatitis (focal or diffuse) | Three diffuse and three focal |
| If focal pancreatitis, localization (groove, head, body and tail) | Groove and duplicated head |
| Presence of massive ascites | 1/6 |
| Presence of other cystic lesion: number of patients who presented a pseudocyst or walled-off necrosis | 2/4 patients |
| Formation of a pseudocyst few weeks after acute episode | 1/6 |
| Other noticeable radiological presentation | |
| Presence of a pancreas divisum on MRCP | 1/4 |
| Ansa pancreatica (e.g. Fig. 3B) | 2/4 |
Figure 2.
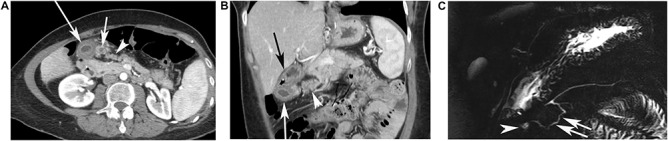
CT findings in a 49-year-old female patient who had 11 episodes of acute pancreatitis during 20 years. CECT performed 4 months after the occurrence of the last episode of pancreatitis showing an additional elongated pancreatic tissue, ahead of normal pancreas (arrowhead). (A) An additional elongated pancreatic tissue, ahead of normal pancreas (arrowhead) and two cysts are seen, under the pyloric part of stomach, very close to the heterotopic pancreas, the large cyst (long white arrow) was the gastric duplication, the small cyst (short white arrow) was a small abscess residue. (B) In this case, on this coronal view, the stomach was located over the gastric duplication (black arrow) and the duplicated pancreatic parenchyma (white arrow head), containing a duct, is clearly separated from the normal pancreatic parenchyma, associated with the gastric duplication cyst (long white arrow). (C) Magnetic Resonance Cholangio-Pancreatography (MRCP) points out the cystic lesion under the pyloric part of stomach (arrowhead), connected to a pancreatic accessory duct in a duplicated pancreatic head (white arrow), which is connected to the main pancreatic duct.
Imaging results, performed during episodes of acute pancreatitis, showed that half of the episodes were focal, focusing on the groove and the duplicated head of the pancreas.
Surgical treatment (Table 2, Fig. 3) included en bloc resection limited to the duplications that preserved both the orthotopic pancreatic head and the gastroduodenal continuity. Atypical pancreatectomy of the duplicated head alone was performed for the pancreatic duplication, with elective ligation of the accessory duct, while the gastric duplication was treated by short segmental resection on the antropyloric junction with transversal closure of the parietal defect to avoid stenosis (Fig. 3A). The procedure was well tolerated with mild complications (Clavien–Dindo Grade I).
Table 2.
Surgical management, histopathological analysis and outcome after surgery
| Surgical procedure | N = 4 |
|---|---|
| Number of patients undergoing surgery | 4/4 |
| Type of surgery | 4/4 atypical pancreatectomy (enucleation of the duplicated head) performed together with a partial gastrectomy of the antropyloric region (including the entire gastric duplication) |
| Mean hospitalization time (and range) after surgery | 22.5 days (16–35) |
| Occurrence of a pancreatic fistula | 4/4 patients presented a small pancreatic fistulas, each of them was self-limited within a few days, and did not needed additional treatment (biochemical leak according to ISGPS classification) |
| Clavien–Dindo classification | 4/4 patients were classified as Grade I |
| Histopathological analysis of the developmental anomaly | N = 4 |
| Mucosa type in the duplication | One duodenal; two gastric, one mixed (gastric + duodenal mucosa) |
| Connection duct between the duplicated pancreatic head and the duplication | Yes (4/4) |
| Communication between gastric duplication and gastric lumen | No (4/4) |
| Mean size (and range) of the duplication cyst | 3.75 (1.5–5.5) |
| Long time follow-up | N = 4 |
| Number of acute pancreatitis episode after surgery per patients | 0 |
| Development of incapacitating digestive symptoms or pancreatic insufficiency | 0 |
| Mean time (and range) of follow-up after surgery | 26.5 months (6–42) |
| Postoperative imaging available, showing complete resection of the duplication cyst and the duplicated pancreas (normal pancreas aspect) | 4/4 |
Figure 3.
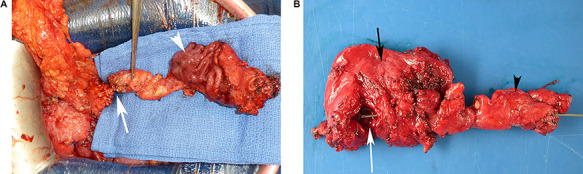
Gross examination of a surgical specimen. (A) Perioperative view of the complete resected tissue, showing the normal gastric mucosa (white arrowhead) and the duplicated head of pancreas (white arrow) before cutting the duct. (B) View of the complete specimen, including the gastric duplication (opened, white arrow), the gastric wall (side of the serosa, black arrow) and the duplicated head of pancreas (black arrowhead) containing a duplicated duct catheterized from the cut on pancreas to the gastric duplication with a metal probe.
Pathological analysis (Table 2) showed that the duplication cyst always communicated with an accessory pancreatic duct in the duplicated pancreatic head, but never communicated with the gastric lumen (Fig. 3B). The type of mucosa and the presence of a muscularis layer excluded the diagnosis of cystic dystrophy on an aberrant pancreas. The accessory parenchyma did not have any specific features except for the presence of different degrees of chronic pancreatitis.
Acute pancreatitis did not occur in any of the patients after surgery, and none of the patients developed incapacitating digestive symptoms or pancreatic insufficiency after a mean follow-up of 26.5 months (6–42).
DISCUSSION
Congenital anomalies are rarely considered to be a potential cause of recurrent pancreatitis in the adult population except for pancreas divisum, associated with the cystic fibrosis transmembrane regulator (CFTR) mutation [4]. Gastric duplications are uncommon and only account for 5% of all gastrointestinal duplications that are symptomatic in 80% of cases before the age of 2 [5]. They are even more rare when associated with a duplicated head of the pancreas in adults. To our knowledge, there are <10 published individual case reports of adults with this entity, and ours is the first series reporting identical conservative surgical treatment of this rare anomaly.
This congenital anomaly may be explained by the embryological model with three pancreatic anlages proposed by Takodoro et al. [6]. In this model, the ventral pancreatic anlage is initially paired, including a right and left ventral pancreas. The left ventral lobe disappears during normal development and the right ventral pancreatic anlage rotates 180° and fuses side by side with the dorsal anlage. We suggest that in our patients, the two ventral pancreatic anlages persisted and one moved toward the gastric antrum to connect to an associated gastric duplication.
This series includes four women who were all surgically cured. Although the gender predominance (100%) might be due to a sampling bias (small series) gastric duplications are also known to be more frequent in women (2:1 ratio), [7, 8].
Before the widespread use of CT or MRCP, patients with these anomalies underwent multiple exploratory surgeries before a correct diagnosis could be obtained [9]. More recent cases have described the use of endoscopic retrograde cholangiopancreatography to diagnose this rare variant [1, 10]. However, to our knowledge, this is the first series in which the preoperative diagnosis was made by non-invasive imaging alone, suspected on CT and confirmed on MRCP. The features of this entity were typical on MRCP and no additional invasive imaging techniques were needed.
The structural features of the cyst were specific in all patients: it was located in the gastric wall and had two layers that were connected and communicated with an accessory pancreatic duct.
In our series, patients had their first episode of acute pancreatitis a mean 12.5 years (5–17) before curative surgery, because this variant was not diagnosed, delaying referral to our institution.
One additional patient who was found to have this variant on MRCP was excluded from the series because she did not fulfill inclusion criteria. This 71-year-old woman did not require surgery because she had only had one episode of acute pancreatitis and her symptoms were limited. Further studies of this variant should be performed to determine the rate of complications.
Our review of published case reports [1] shows that patients with this variant underwent various types of surgery and 10/23 (43.5%) had more than one operation (1–4) before a cure was obtained. One patient even underwent pancreatico-duodenectomy, despite the significant morbidity of this technique [11]. Marsupialization of the duplication was found to be inadequate because it frequently required additional surgery [12]. Our series shows that patients can be cured by one-step surgery that preserves both the orthotopic pancreatic head and gastroduodenal continuity. We treated patients by combined en bloc excision of the gastric and pancreatic duplications (Fig. 3A). The procedure was well tolerated with mild complications and no recurrent pancreatitis.
Careful gross pathological examination should be performed to determine whether the gastric cyst communicates with the stomach and to clearly confirm that the main pancreatic duct of the duplicated pancreas communicates with the gastric cyst. The diagnoses of gastric diverticulum or cystic dystrophy on an aberrant pancreas must be excluded. Gastric diverticulum is connected to the main lumen of the stomach while cystic dystrophy develops on an aberrant pancreas, which is not connected to the pancreas.
Gastric duplications are composed of layers of complete gastric wall with gastric-type mucosae and muscularis.
In conclusion, congenital anomalies should be considered in the presence of recurrent pancreatitis in adult patients, including combined gastric and pancreatic duplications. Diagnosis of this rare anatomic variant may be made with non-invasive imaging, suspected on CT and confirmed by a typical ductal pattern on MRCP.
Parenchymal sparing surgery with combined en bloc excision of the gastric and pancreatic duplications provided a complete cure of the episodes of pancreatitis, with very low early morbidity and no delayed adverse effects.
CONFLICT OF INTEREST STATEMENT
None declared.
Funding
None.
Contributor Information
Edouard Hérin, Department of Radiology, Beaujon Hospital, Clichy, France.
Alain Sauvanet, Department of Surgery, Beaujon Hospital, Clichy, France; Paris 7-Rene Diderot University, Université de Paris, Paris, France.
Jérome Cros, Department of Pathology, Beaujon Hospital, Clichy, France; Paris 7-Rene Diderot University, Université de Paris, Paris, France.
Hasmik Koulakian, Department of Radiology, Cochin Hospital, Paris, France.
Philippe Lévy, Department of Pancreatology, Beaujon Hospital, Clichy, France; Paris 7-Rene Diderot University, Université de Paris, Paris, France.
Marie Pierre Vullierme, Department of Radiology, Beaujon Hospital, Clichy, France; Paris 7-Rene Diderot University, Université de Paris, Paris, France.
REFERENCES
- 1. Christians KK, Pappas S, Pilgrim C, Tsai S, Quebbeman E. Duplicate pancreas meets gastric duplication cyst: a tale of two anomalies. Int J Surg Case Rep 2013;4:735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161:584–91. [DOI] [PubMed] [Google Scholar]
- 4. Bertin C, Pelletier AL, Vullierme MP, Bienvenu T, Rebours V, Hentic O, et al. Pancreas divisum is not a cause of pancreatitis by itself but acts as a partner of genetic mutations. Am J Gastroenterol 2012;107:311–7. [DOI] [PubMed] [Google Scholar]
- 5. Stern LE, Warner BW. Gastrointestinal duplications. Semin Pediatr Surg 2000;9:135–40. [DOI] [PubMed] [Google Scholar]
- 6. Tadokoro H, Takase M, Nobukawa B. Development and congenital anomalies of the pancreas. Anat Res Int 2011;2011:351217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agha FP, Gabriele OF, Abdulla FH. Complete gastric duplication. Am J Roentgenol 1981;137:406–7. [DOI] [PubMed] [Google Scholar]
- 8. Macpherson RI. Gastrointestinal tract duplications: clinical, pathologic, etiologic, and radiologic considerations. Radiographics 1993;13:1063–80. [DOI] [PubMed] [Google Scholar]
- 9. Rizzo RJ, Szucs RA, Turner MA. Congenital abnormalities of the pancreas and biliary tree in adults. Radiographics 1995;15:49–68quiz 147-8. [DOI] [PubMed] [Google Scholar]
- 10. Oeda S, Otsuka T, Akiyama T, Ario K, Masuda M, Taguchi S, et al. Recurrent acute pancreatitis caused by a gastric duplication cyst communicating with an aberrant pancreatic duct. Intern Med (Tokyo, Japan) 2010;49:1371–5. [DOI] [PubMed] [Google Scholar]
- 11. Addeo P, Delpero JR, Paye F, Oussoultzoglou E, Fuchshuber PR, Sauvanet A, et al. Pancreatic fistula after a pancreaticoduodenectomy for ductal adenocarcinoma and its association with morbidity: a multicentre study of the French Surgical Association. HPB 2014;16:46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spence RK, Schnaufer L, Mahboubi S. Coexistant gastric duplication and accessory pancreas: clinical manifestations, embryogenesis, and treatment. J Pediatr Surg 1986;21:68–70. [DOI] [PubMed] [Google Scholar]


