Abstract
Background
The COVID-19 pandemic has greatly altered the practice of cardiac electrophysiology around the world for the foreseeable future. Professional organizations have provided guidance for practitioners, but real-world examples of the consults and responsibilities cardiac electrophysiologists face during a surge of COVID-19 patients is lacking.
Methods
In this observational case series we report on 29 consecutive inpatient electrophysiology consultations at a major academic medical center in New York City, the epicenter of the pandemic in the United States, during a 2 week period from March 30-April 12, 2020, when 80% of hospital beds were occupied by COVID-19 patients, and the New York City metropolitan area accounted for 10% of COVID-19 cases worldwide.
Results
Reasons for consultation included: Atrial tachyarrhythmia (31%), cardiac implantable electronic device management (28%), bradycardia (14%), QTc prolongation (10%), ventricular arrhythmia (7%), post-transcatheter aortic valve replacement conduction abnormality (3.5%), ventricular pre-excitation (3.5%), and paroxysmal supraventricular tachycardia (3.5%). Twenty-four patients (86%) were positive for COVID-19 by nasopharyngeal swab. All elective procedures were canceled, and only one urgent device implantation was performed. Thirteen patients (45%) required in-person evaluation and the remainder were managed remotely.
Conclusion
Our experience shows that the application of a massive alteration in workflow and personnel forced by the pandemic allowed our team to efficiently address the intersection of COVID-19 with a range of electrophysiology issues. This experience will prove useful as guidance for emerging hot spots or areas affected by future waves of the pandemic.
Keywords: Electrophysiology, Arrhythmia, Coronavirus, COVID-19
Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; IRB, Institutional review board; PPE, Personal protective equipment; CIED, Cardiac implantable electronic device; AF, Atrial fibrillation; AAD, Antiarrhythmic drug; ICD, Implantable cardioverter defibrillator; CRT-D, Cardiac resynchronization therapy defibrillator
1. Introduction
COVID-19, the disease caused by the SARS-CoV-2 novel coronavirus, causes a range of clinical symptoms, from asymptomatic infection to acute respiratory failure, cytokine storm, and death, and has multiple implications for the cardiovascular specialist [[1], [2], [3], [4], [5]]. The global pandemic has impacted every aspect of society during this current phase including the practice of cardiac electrophysiology, requiring electrophysiologists to ramp down standard clinical practice, and, in the case of our hospital in New York City, one of the hardest hit epicenters of the global pandemic (with 174,709 confirmed cases in New York City as of May 6, 2020, and the New York City metropolitan area representing one-third of cases in the United States and over 10% of the global case burden), pivoting workflow to meet the demands of the pandemic [[6], [7], [8], [9], [10]].
In recent months, a taskforce from the Heart Rhythm Society, in accordance with the American College of Cardiology and American Heart Association, issued guidance and an update for the practice of electrophysiology during the pandemic [11,12]. These documents provided welcomed guidance on the complex interplay between our missions to deliver the highest quality patient-centered care, balanced with the constraints of the pandemic, including triage of patients, minimization of patient and staff exposure, and conservation of resources. However, real-world experience with this guidance, especially regarding the specific challenges to delivering care in areas with high prevalence of COVID-19, is lacking. This represents an important knowledge gap in the field that may become especially relevant as other hotspots or future waves of the pandemic emerge. In this manuscript, we present an observational series of our real-world experience on the inpatient electrophysiology service from the epicenter at the height of the current phase of the pandemic. We hypothesized that despite requisite alteration in workflow and personnel, we would be able to deliver high quality guideline-directed electrophysiology care at the center of the pandemic.
2. Methods
2.1. Study population
In this analysis we present a retrospective observational case series of 29 consecutive cardiac electrophysiology consultations at a single center, the New York-Presbyterian/Columbia University Irving Medical Center, an academic tertiary care center in New York City, during a two-week period from March 30, 2020 to April 12, 2020. These dates coincided with the local peak in COVID-19 cases, and during the study period the hospital was inundated with COVID-19 inpatients, facing 60–100 new COVID-19 confirmed inpatient admissions per day, with 34% of hospitalized COVID-19 patients requiring ventilator support. Inpatient bed utilization peaked at 84% with 621 of 738 beds occupied by COVID-19 patients, on April 14, 2020. Approval for this study was included in Human Subjects protocol IRB-AAAS9622 (COVID-Care: A Continuous Quality Improvement (CQI) Project to Improve Care of Patient's With COVID-19 at the NYP-West Campus), which was approved by the Institutional Review Board at Columbia University Medical Center. Informed consent was waived.
2.2. Workflow
Electrophysiology consultations were initiated by formal verbal or electronic order from the primary service. Thus, arrhythmia issues managed by the primary team, such as a cardiologist or intensive care physician, were not included in this analysis.
Prior to COVID-19, inpatient electrophysiology consultation was performed by a general cardiology fellow or specialized nurse practitioner and subsequently staffed with an attending electrophysiologist. The average volume on new consultations was 8–12 new encounters per day, and these focused on a range of complex arrhythmia issues ranging from more routine SVT, AF, and CIED management, to complex arrhythmias related to advanced heart failure therapies, cardiac surgery, and congenital heart disease. During the COVID-19 crisis, the electrophysiology service was restructured, details of which have been previously published [9], in accordance with the state of emergency declaration by New York State [13]. In brief, beginning March 16, 2020, elective procedures were canceled, outpatient visits converted to virtual visits, and essential members of our team redeployed to care for critically ill patients in the intensive care units and emergency departments. Electrophysiology consultations were managed by an electrophysiology fellow and attending. Consults were triaged in order to maximize patient care, minimize unnecessary exposures and preserve PPE, and we adopted a policy of foregoing in-person patient encounters including a physical examination when reasonable. If at any point it was determined that patient care would benefit from an in-person encounter then either the fellow or the attending performed direct patient examination. We also attempted to perform remote device interrogations via “in hospital” remote monitoring with CareLink (Medtronic, Minneapolis, MN), Latitude (Boston Scientific, Marlborough, MA), and Merlin (Abbott Cardiac Arrhythmias, Plymouth, MN), or utilizing magnets to disable ICDs during surgery or at end of life, bundled with routine nursing care. Electrophysiology lab team was redeployed to other areas of the hospital, but we maintained an on-call for emergency procedures.
2.3. Data collection
Baseline characteristics and clinical course was reviewed for all patients including reason for consultation, hospital location and level of care, hospital day of consultation, disposition, and whether in-person examination was required. Also, SARS-CoV-2 reverse transcription polymerase chain reaction (RT PCR) testing status, level of respiratory support, paralysis, proning, mechanical circulatory support, renal replacement therapy, inpatient medications, laboratory studies, and EKG rhythm, QRS and QTc intervals.
3. Results
3.1. Consult service population
During the study period, twenty-nine consultations were performed in twenty-eight patients (age 71 ± 14 years, 67% male, 29% Hispanic and 25% Black) (Table 1). One patient had two encounters for different reasons. Twenty-four patients (86%) were SARS-CoV-2 positive. Sixteen of 29 encounters (55%) were managed remotely.
Table 1.
Baseline characteristics, n = 28 patients.
| SARS-CoV-2 Positive (n = 24) | SARS-CoV-2 Negative (n = 4) | |
|---|---|---|
| Age, years ± standard deviation | 71 ± 15 | 70 ± 7 |
| Male | 16/24 (67%) | 3/4 (75%) |
| Ethnicity/Race | ||
|
8 (33%) | 0 (0%) |
|
7 (29%) | 0 (0%) |
|
2 (8%) | 2 (50%) |
|
2 (8%) | 0 (0%) |
|
0 (0%) | 1 (25%) |
|
5 (21%) | 1 (25%) |
| Body mass index, kg/m2 | 29.6 ± 8.3 | 22.7 ± 3.0 |
| Diabetes mellitus | 13/24 (54%) | 1/4 (25%) |
| Hypertension | 19/24 (79%) | 3/4 (75%) |
| Chronic kidney disease, stage III or worse | 4/20 (20%) | 0/1 (0%) |
|
2 | 0 |
| Prior stroke | 3/24 (13%) | 0/4 (0%) |
| Dementia | 3/24 (13%) | 0/4 (0%) |
| Chronic obstructive pulmonary disease | 3/24 (13%) | 0/4 (0%) |
| Prior coronary artery disease | 7/24 (29%) | 1/4 (25%) |
| Prior congestive heart failure | 10/24 (42%) | 2/4 (50%) |
|
1 | 0 |
| Prior atrial fibrillation | 3/24 (13%) | 1/4 (25%) |
|
3 | 1 |
|
0 | 0 |
|
0 | 0 |
|
0 | 0 |
| Prior cardiac implantable electronic device | 7/24 (29%) | 2/4 (50%) |
|
2 | 0 |
|
5 | 2 |
There was a high prevalence of comorbidities including diabetes mellitus (50%), hypertension (79%), and prior congestive heart failure (36%). Four patients (14%) had prior paroxysmal AF. No patients had persistent or chronic AF, prior AF ablation, prior cardioversion, or prior AAD use. Nine patients (32%) had a prior CIED implant (2 pacemakers, 5 ICDs, 2 CRT-Ds).
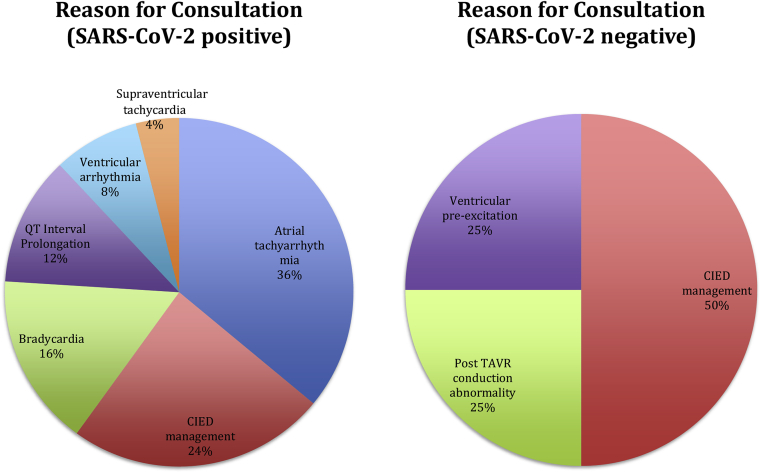
The most frequent reasons for consultation were atrial tachyarrhythmia in 9 patients (31%) and CIED management in 8 patients (28% of encounters) (Fig. 1). Two of the patients with atrial tachyarrhythmia also had a CIED and required device management at the time of consultation. The remainder of consultations were for bradycardia in 4 patients, QTc interval prolongation in the context of hydroxychloroquine usage in 3 patients, ventricular arrhythmias in 2 patients, and in 1 patient each paroxysmal supraventricular tachycardia, ventricular pre-excitation, and post-TAVR conduction abnormality (Table 2).
Fig. 1.
Reason for Electrophysiology Consultation During Two-Week Study Period Stratified by SARS-CoV-2 Status. Left panel – SARS-CoV-2 Positive Patients (n = 25). Right panel – SARS-CoV-2 Negative Patients (n = 4).
Table 2.
Inpatient encounter characteristics, n = 29.
| Total Encounters | In-Person Encounters | |
| Reason for consultation | ||
|
9 (31%) | 3/9 |
| 8 (28%) | 8/8 | |
|
4 (14%) | 1/4 |
|
3 (10%) | 0/3 |
|
2 (7%) | 0/2 |
|
1 (3%) | 1/1 |
|
1 (3%) | 0/1 |
|
1 (3%) | 0/1 |
| Total | 29 | 13/29 (45%) |
| Location | ||
|
14 | |
|
7 | |
|
2 | |
|
1 | |
|
4 | |
|
4 | |
|
4 | |
TAVR = Transcatheter aortic valve replacement.
CCL = Cardiac catheterization laboratory.
CIED management was performed for two additional patients with primary reason for consultation Atrial fibrillation or flutter/RVR.
In one patient, a second separate encounter for CIED management was performed for a different indication later in the hospitalization.
Electrophysiology consults were requested an average of 5 (±4.7) days into the patient's hospitalization (median 3 days). Fourteen patients were located on a medical, surgical, or telemetry floor, 7 patients in an ICU, 4 in the emergency department, and 4 in a procedural area (cardiac catheterization laboratory, perioperative holding, MRI suite).
Of the 24 SARS-CoV-2 positive patients, regarding respiratory status, 7 patients (29%) were on a ventilator and 11 patients (49%) were on a non-rebreather mask. Four (17%) were on renal replacement therapy. None (0%) were on temporary mechanical circulatory support (Table 3).
Table 3.
Inpatient characteristics stratified by SARS-CoV-2 status, n = 28 patients.
| SARS-CoV-2 Positive (n = 24) | SARS-CoV-2 Negative (n = 4) | |
|---|---|---|
| Respiratory Support | ||
|
2 | 4 |
|
4 | 0 |
|
11 | 0 |
|
0 | 0 |
|
1 | 0 |
|
6 | 0 |
|
5 | 0 |
|
1 | 0 |
| Mechanical circulatory support | ||
|
0 | 0 |
|
1 | 0 |
| Renal replacement therapy | ||
|
2 | 0 |
|
2 | 0 |
Five patients (21%) received vasoactive medications. Seven (29%) received amiodarone. Twenty (83%) received prophylactic or therapeutic anticoagulation. Antibiotics and anti-inflammatory medications varied considerably (Table 4).
Table 4.
Inpatient medications stratified by SARS-CoV-2 status, n = 28 patients.
| SARS-CoV-2 Positive (n = 24) | SARS-CoV-2 Negative (n = 4) | SARS-CoV-2 Positive (n = 24) | SARS-CoV-2 Negative (n = 4) | ||
|---|---|---|---|---|---|
| Vasoactive medications | Antibiotics and anti-inflammatory drugs | ||||
| Norepinephrine | 3 | 0 | HCQa (full course) | 12 | 0 |
| Vasopressin | 2 | 0 | HCQa (partial course) | 2 | 0 |
| Dopamine | 2 | 0 |
|
||
|
6 | 0 | |||
|
2 | 0 | |||
| Dobutamine | 2 | 0 | Doxycycline | 3 | 0 |
| Antiarrhythmic drugs | 0 | Levofloxacina | 1 | 0 | |
| Amiodarone | 7 | 0 | Ceftriaxone, cefepime, or piperacillin-tazobactam | 16 | 0 |
| Other AAD (Ic, III) | 0 | 0 | Corticosteroids | 4 | 0 |
| Anticoagulants | Sedation medications | ||||
| Coumadin | 1 | 0 | Propofol | 5 | 0 |
| NOAC | 3 | 0 | Dexmedetomidine | 0 | 0 |
| Unfractionated heparin | 2 | 0 | Fentanyl | 7 | 0 |
| LMWH, 1 mg/kg twice daily | 0 | 0 | Midazolam | 6 | 0 |
| LMWH, 1 mg/kg daily or less | 14 | 0 | Seroquela | 2 | 0 |
| Other cardiac medications | Other medications | ||||
| Metoprolol | 12 | 2 | Insulin | 8 | 0 |
| Diltiazem | 5 | 0 | |||
| Digoxin | 2 | 0 | |||
| ACEi/ARB | 2 | 1 | |||
| Statin | 9 | 1 | |||
| Aspirin | 10 | 1 | |||
NOAC: Novel Oral Anticoagulant; LMWH: Low Molecular Weight Heparin; ACEi: Angiotensin Converting Enzyme Inhibitor; ARB Angiotensin Receptor Blocker; HCQ: Hydroxychloroquine.
Risk of QT interval prolongation.
3.2. Atrial tachyarrhythmia
Of the 9 patients with atrial tachyarrhythmia, 8 had AF and 1 had typical-appearing atrial flutter. All patients 9 of 9 (100%) were SARS-CoV-2 positive and all had either hemodynamic instability or rapid ventricular response refractory to ≥2 rate control medications. In these 9 patients with SARS-CoV-2 and atrial tachyarrhythmia, two of the patients had prior known paroxysmal atrial fibrillation, and the atrial tachyarrhythmia was de novo in the remaining 7 patients. CHA2DS2VASc scores ranged from 3 to 7, median 5, mean 4.9 (±1.6). Two patients were already on anticoagulation, 1 patient had a contraindication to anticoagulation (major bleeding requiring transfusion this admission), and anticoagulation was initiated for the other patients. Four patients had AF of >48 h or unknown duration. Assessment for LAA thrombus with trans-esophageal echocardiogram or cardiac computed tomography was not performed in any patient. Emergent cardioversion was performed at bedside in 2 patients due to hypotension with systolic blood pressure less than 70 mmHg refractory to medical therapy. Otherwise, cardioversion was deferred and aggressive rate control was instituted with intravenous digoxin and beta-blockade, which resulted in rate control within 24 h in one patient and 48–72 h in the other. Excessive bradycardia was not reported in either patient. Amiodarone was utilized in 7 patients. In one patient, amiodarone usage was discontinued due to multifactorial acute liver injury. Of note, despite the mandate to limit patient interaction, physical examination was deemed necessary for medical decision making in 3 of 9 patients with AF, 2 of which required simultaneous management of a CIED and one with a challenging volume status where physical examination impacted management decisions.
3.3. Cardiac implantable electronic device management
Of the 8 encounters for CIED management, and 2 additional instances of CIED management performed in patients with primary reason for consultation atrial tachyarrhythmia, all 10 encounters required in-person evaluation and/or programming, despite attempts to implement remote interrogation practices. Eight of the 10 encounters were in SARS-CoV-2 positive patients. Two encounters were for peri-MRI device management. Four encounters were to deactivate tachycardia therapies in severely ill COVID-19 patients who were transitioning to comfort care (Table 5). Use of a magnet to deactivate tachycardia therapies was not deemed appropriate because of lack of familiarity of the patient and ward staff with utilizing the magnet over a longer term, ie. hours-to-days. Since the 4 patients were all in acute respiratory distress and suffering from encephalopathy, and facing possible transfer to another unit with different staffing norms, manual interrogation was considered the most durable, reliable, and least burdensome means of deactivating therapies. Of note, these 4 patients were all relatively well compensated prior to admission, and one patient was implanted as recently as January 2020 for primary prevention in NYHA class II CHF. Other indications for interrogation were pace-termination of atrial flutter, evaluation of an LVAD patient with VT, a patient with complex telemetry findings due to PAC, PVC, and AV search mode, and peri-MRI reprogramming.
Table 5.
Details of CIED interrogation encounters.
| Reason for Interrogation | Type of CIED | Clinical Indication | In- Person? | SARS-CoV-2 | Location | Notes | Disposition | |
|---|---|---|---|---|---|---|---|---|
| 1 | Peri-MRI programming | Single chamber ICD | Brain MRI for surgical planning | Yes | Negative | MRI Suite | None | Discharged |
| 2 | Peri-MRI programming | Single chamber ICD | Brain MRI for AMS | Yes | Negative | MRI Suite | None | Discharged |
| 3 | Other clinical indication | Dual chamber PPM | Complex findings on telemetry | Yes | Positive | Floor | Telemetry findings due to frequent PACs, PVCs, and normal AV search mode | Expired |
| 4 | Other clinical indicationa | Dual chamber PPM | Pace termination of AFL | Yes | Positive | ER | Successful pace termination of atrial flutter | Admitted, later expired |
| 5 | LVAD patient with VT | Single chamber ICD | Normal ICD function | Yes | Positive | Floor | Slow VT in LVAD patient due to dehydration and suction events | Discharged |
| 6 | ICD shockab | CRT-D | Inappropriate shock for AF | Yes | Positive | ER | Made VF zone more conservative; added VT zone for SVT discrimination | Admitted, later expired |
| 7 | Disable tachytherapies | Single chamber ICD | DNR/DNI | Yes | Positive | Floor | NRB; recently implanted January 2020 | Expired |
| 8 | Disable tachytherapies | Single chamber ICD | DNR/DNI | Yes | Positive | Floor | NRB | Discharged to NH |
| 9 | Disable tachytherapiesb | CRT-D | DNR/DNI | Yes | Positive | Floor | NRB | Expired |
| 10 | Disable tachytherapies | CRT-D | DNR/DNI | Yes | Positive | Floor | NRB | Expired |
Primary reason for encounter 4 and 6 was atrial fibrillation or flutter with RVR.
Encounters 6 and 9 were on the same patient for different indications at different points during the hospitalization.
3.4. QT interval prolongation and other issues
Three consultations for QT interval prolongation were managed with discontinuation of hydroxychloroquine use per protocol. One consultation for asymptomatic ventricular pre-excitation in the peri-operative setting was scheduled for outpatient follow up since the benefits of urgent cancer surgery outweighed the potential risks from the accessory pathway. Bradycardia was encountered in 4 patients. The mechanism was sinus bradycardia in 2 patients and sinus arrest with junctional and intermittent wide complex escape in 1 patient, which responded to monitoring and beta-1-agonist therapy. Another patient presented to the emergency department in transient high-grade atrio-ventricular block in the setting of COVID-19 respiratory failure and electrolyte disarray, which initially responded to intubation, correction of electrolytes, and intravenous beta-1-agonist therapy, although the patient ultimately succumbed to COVID-19 multi-organ failure. Two patients that had refractory ventricular arrhythmias (one ventricular tachycardia and one ventricular fibrillation) expired in the context of advanced COVID-19 multi-organ failure. One patient, who was SARS-CoV-2 negative, underwent urgent transcatheter aortic valve replacement on an outpatient basis. After valve deployment of a self-expanding valve, the patient had a new left bundle branch block and first degree AV block, and we elected to implant a dual chamber pacemaker immediately after the TAVR. The patient was discharged home the same day with instructions to perform a remote device interrogation, send a photograph of the incision, and follow up virtually. This was the only invasive procedure performed during the study period.
Of 28 patients seen during the study period, as of April 21, 2020, 5 patients had been discharged, 12 had expired during the hospitalization, 4 remained in ICU, and 7 remained on the hospital floor.
4. Discussion
This observational case series of 29 consecutive electrophysiology inpatient consultations during a two-week period at the peak of the COVID-19 pandemic highlights real-world clinical experience of arrhythmia specialists at the epicenter of the pandemic in the United States. Consult volume during the COVID-19 peak was overall lower than during a comparable pre-COVID two-week period, when we would have anticipated 80–100 new inpatient encounters. All elective procedures had been canceled, and only one invasive procedure, a permanent pacemaker implant, was performed during the entire two-week study period. There were no temporary pacemakers or ablations performed. More than half of the encounters were managed remotely, and when an examination was performed only one member of the team entered the patient's bedside to preserve PPE and mitigate risk. The hospital had >80% COVID-19 positive patients, and the profile of electrophysiology consultations mirrored that ratio. The two most common reasons for consultation in COVID-19 patients were atrial tachyarrhythmias and CIED management. In the few patients without COVID-19 undergoing consultation, the principle reason was CIED management prior to emergent surgery or MRI. As the hospital was inundated, the patients were located throughout the hospital complex increasing risk of navigating patient and staff exposure.
The HRS guidance for electrophysiology practice during the pandemic provides an excellent framework for guiding clinical practice during the pandemic [11]. It addresses management of non-urgent/emergent procedures, protocols for performing procedures on COVID-19 patients, advocates for virtual visits, and, in the hospital setting, emphasizes social distancing, conservation of PPE, and minimization of face-to-face interaction when possible. Our experience shows the application of this proposed alteration in personnel and daily workflow that has been forced upon us by the pandemic, and also highlights the application of guideline-based principles to patient management within these constraints.
In terms of AF, in contrast to our typical AF population, none of the patients with atrial tachyarrhythmia in this series had a history of cardiac surgery, ablation, cardioversion, or AAD use. In fact, of the 9 patients for which we were consulted for AF in the settings of COVID-19 illness, only 2 had known prior PAF, and the atrial tachyarrhythmia in remaining 7 patients was de novo. Given the high prevalence of comorbidities in this population, this may represent unmasking of pre-existing substrate due to the inflammation and physiologic stress of COVID-19. Despite the differences from routine clinical practice, these patients were managed per standard guidelines [14]. CHA2DS2VASc scores for AF patients ranged from 3 to 7 and all those without contraindications were recommended to either initiate or continue anticoagulation. Although there is evidence for hypercoagulability in COVID-19 [15], thought to be related to endothelial injury and systemic inflammatory state, and data reporting increased venous and arterial events in COVID-19 patients, including higher incidence of pulmonary embolism in ICU patients compared to two matched cohorts [16] and a series of young patients presenting with CVA [17], empiric full dose anticoagulation remains a controversial topic and the subject of ongoing debate and clinical trials. The role of full dose anticoagulation in COVID-19 patients with AF and CHADSVASc score 0 or 1 will require further research to determine.
Routine use of trans-esophageal echocardiography (TEE) was not available for assessing for LAA thrombus due to risk of virus aerosolization. Although cardiac computed tomography with delayed contrast imaging is comparable to TEE for detecting LAA thrombus [18], there were logistical challenges to implementing this technique during the study period due to the requirement for IV contrast, the remote location of the CT scanner in unstable patients, and the burden of terminal cleaning of the CT area. Outside of an emergent setting, electrical cardioversion was utilized sparingly due to the considerable resources required, including anesthesiology, nursing, and potential bed transfer to a negative pressure environment. Amiodarone was the AAD of choice when appropriate. Of 9 patients with AF, 5 expired and 4 remained hospitalized at the time of writing, suggesting that AF is a poor prognostic indicator, although this observation requires further study with a comparison arm to determine an association.
Despite current guidance and attempts in our series to utilize “in hospital” remote monitoring for bedside interrogation or magnet use bundled with routine nursing cares, all CIED consultations required in-person bedside interrogation, accounting for the largest indication for interaction with COVID-19 patients. Appropriate PPE was donned and the interrogators were meticulously cleaned with disinfectant wipes before and after entry to patient rooms. The telemetry wand was placed in a sleeve when possible.
Electrophysiology service was consulted for QTc prolongation in the context of hydroxychloroquine use for assistance with interpretation of QTc interval and risks and benefits of continuation of hydroxychloroquine therapy in borderline cases. Hydroxychloroquine, though inexpensive and widely used for decades in the outpatient setting for chronic treatment of inflammatory conditions and malaria, is known to cause mild QTc prolongation through inhibition of IKr and several case reports in this population report an association with torsades de pointes [19]. Due to elevated risk for ventricular arrhythmias owing to acute inflammatory state, electrolyte abnormalities, possibility of myocarditis, and other factors, use of hydroxychloroquine to treat COVID-19 remains controversial at best with the United States Food and Drug Administration recommending against its use outside a hospital setting or clinical trial [20]. A small randomized controlled pilot study from China showed no clinical benefit for hydroxychloroquine in COVID-19 [21], and an observational study from our institution showed no difference in a composite endpoint of intubation and deaths compared with propensity-matched controls [22]. While it is clear that further study is necessary, this medication is being utilized in the real world hospitalized patient and the risk of ventricular arrhythmias needs to be mitigated. At our institution, we adopted and operationalized an algorithmic approach to QTc risk management with hydroxychloroquine based on guidance from the American College of Cardiology [23]. During the study period, we had low volume of consultations on this issue, likely due to implementation of the algorithm.
The remainder of consultations, on a wide range of electrophysiology issues, were managed according to guidelines with a few key alterations in workflow and logistics. For example, in the age of COVID-19, outpatient procedures will require attention to SARS-CoV-2 status, including testing of asymptomatic patients, and appreciation of how SARS-CoV-2 status may impact delivery of care and management of potential complications or adjunctive procedures. In the case of our post-TAVR pacemaker, the risk of heart block was foreseen and informed consent for possible pacemaker was obtained prior to TAVR. Where our typical practice would be to reassess a patient with post-TAVR conduction abnormality after 24 h with a temporary pacemaker left in place [24], in order to shorten length of stay and reduce risk of infectious exposure to staff and the patient, we elected to implant a dual chamber pacemaker after the TAVR. Consideration of SARS-CoV-2 status and the implication for resuming semi-urgent and elective procedures is also relevant as we attempt to ramp up outpatient electrophysiology procedures.
While this study opens a window for electrophysiologists into the real-world experience at the heart of the pandemic, this study also has several limitations. This study is retrospective, and the patient population selected for referral was likely more severely ill than the typical arrhythmia patient, and it does not represent the full depth of arrhythmia issues managed independently, for example, by cardiologists and intensive care physicians, in our hospital. While the experience of providing inpatient consultative electrophysiology care will vary based on local and regional variations in both practice patterns and COVID-19 severity, we believe the workflow and staffing considerations surrounding COVID-19 and the issues encountered on our service will be common and are widely applicable.
5. Conclusion
Our experience shows that despite massive alteration in personnel and workflow forced by the pandemic and a dramatically shifted patient population that was >80% COVID-19 positive, our team was able to provide guideline-based and evidence-based recommendations on a range of electrophysiology issues. This experience will prove useful as guidance for emerging hot spots and areas affected by future waves of the pandemic.
Sources of funding
None.
Declaration of competing interest
The authors have no relevant conflicts of interest to report.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Bikdeli B. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020:27284. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driggin E. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020:27204. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.-j. New England Journal of Medicine; 2020. Clinical characteristics of coronavirus disease 2019 in China. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6."World Health Organization Coronavirus disease 2019 (COVID-19) situation report - 92. 21 April. 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200421-sitrep-92-covid-19.pdf?sfvrsn=38e6b06d_6 Available at:, April 21, 2020.
- 7.United States Centers for Disease Control Coronavirus disease 2019 (COVID-19) cases in the US. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fcases-in-us.html.
- 8.New York State Department of Health An update for NYS healthcare providers on COVID-19. April 23, 2020. Available at: https://coronavirus.health.ny.gov/information-healthcare-providers.
- 9.Rubin G.A. Publish Ahead of Print; 2020. Restructuring electrophysiology during the COVID-19 pandemic: a practical guide from a New York city hospital network. (Critical pathways in cardiology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.New York City Covid-19 Daily Data Summary May 6, 2020. https://www1.nyc.gov/site/doh/covid/covid-19-data.page Available at:
- 11.D. R. Lakkireddy et al., “Guidance for cardiac electrophysiology during the coronavirus (COVID-19) pandemic from the heart rhythm society COVID-19 task force; electrophysiology section of the American College of cardiology; and the electrocardiography and arrhythmias committee of the council on clinical cardiology, American heart association,” Circulation, vol. 0, no. 0. [DOI] [PMC free article] [PubMed]
- 12.Varma N. HRS/EHRA/APHRS/LAHRS/ACC/AHA worldwide practice update for telehealth and arrhythmia monitoring during and after a pandemic. Heart Rhythm. 2020 Apr 1;17(9):e233–e241. doi: 10.1016/j.hrthm.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Executive order declaring an emergency in the state of New York. 2020. March 7. Available at: https://www.governor.ny.gov/news/no-202-declaring-disaster-emergency-state-new-york. [Google Scholar]
- 14.January C.T. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140(2):e125–e151. doi: 10.1161/CIR.0000000000000665. 2019. [DOI] [PubMed] [Google Scholar]
- 15.Bowles L. Lupus anticoagulant and abnormal coagulation tests in patients with covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMc2013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.J. Poissy et al., "Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence," Circulation, vol. 0, no. 0. [DOI] [PubMed]
- 17.Oxley T.J. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med. 2020:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero J., Husain S.A., Kelesidis I., Sanz J., Medina H.M., Garcia M.J. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation. Circulation: Cardiovasc Imag. 2013;6(2):185–194. doi: 10.1161/CIRCIMAGING.112.000153. [DOI] [PubMed] [Google Scholar]
- 19.Chen C.-Y., Wang F.-L., Lin C.-C. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol. 2006;44(2):173–175. doi: 10.1080/15563650500514558. 2006/01/01. [DOI] [PubMed] [Google Scholar]
- 20.FDA Drug safety communication. 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or April 24, Available at:
- 21.Chen Jun L.D., Liu Li, Liu Ping, Xu Qingnian, Lu X.I.A., Yun L.I.N.G., Huang Dan, Song Shuli, Zhang Dandan, Qian Zhiping, Tao L.I., Shen Yinzhong, Lu Hongzhou. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ. 2020;49(1) doi: 10.3785/j.issn.1008-9292.2020.03.03. 0-0. 2020-03-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geleris J. Observational study of hydroxychloroquine in hospitalized patients with covid-19. N Engl J Med. 2020 doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simpson T., Kovacs R.J., Stecker E.C. Ventricular arrhythmia risk due to hydroxychloroquine-azithromycin treatment for COVID-19. Am Coll Cardiol Mag. 2020;29:2020. March. [Google Scholar]
- 24.Rodés-Cabau J. Management of conduction disturbances associated with transcatheter aortic valve replacement. Sci Exp Panel. 2019;74(8):1086–1106. doi: 10.1016/j.jacc.2019.07.014. [DOI] [PubMed] [Google Scholar]