Abstract
Background
End stage renal disease (ESRD) is associated with elevated fibrinogen levels and fibrinolysis inhibition. However, there is a paucity of data on how renal transplantation impacts coagulation. we hypothesize that renal transplantation recipients with good functioning grafts will have improved fibrinolytic activity following surgery.
Methods
Kidney recipients were analyzed pre-operatively and on post-operative day 1(POD1) using three different TEG assays with and without two concentration of tissue-plasminogen activator (t-PA). TEG indices and percent reduction in creatinine from pre-op to POD1 were measured, with >50% defining “good” graft function. Follow up was done at 6, 12, and 24 months.
Results
Percent lysis(LY30) on POD1 the t-PA TEG was significantly correlated to change creatinine from pre-op to POD-1(p = 0.006). A LY30 ≥ 23% was associated with good early graft function, and lower creatinine at 24-months(p = 0.028) compared to recipients with low POD1 LY30.
Conclusions
Post-operative tPA-TEG LY30 is associated with favorable early and late outcomes in kidney transplant.
Keywords: Kidney transplant, Thromboelastography, TEG, Coagulation, Fibrinolysis, t-PA
Highlights
-
•
Thromboelastography (TEG) identifies coagulation changes in renal transplant.
-
•
Percent lysis on post-op t-PA TEG significantly correlates with graft function.
-
•
Post-op fibrinolysis plays a key role in early and late renal graft function.
Introduction
Kidney transplant is the definitive treatment for end stage renal disease (ESRD). Renal failure activates systemic inflammation and pro-coagulant factors, which drive the production of excess fibrinogen.1 The kidney is also the prominent source of urokinase, a plasminogen activator, and a key component in upregulation of fibrinolysis (fibrin clot degradation).2 Additionally, plasminogen activator inhibitor-I (PAI-I), which inhibits the fibrinolytic activity of tissue (t-PA) and urokinase plasminogen activator (uPA), is also elevated in renal failure.3 This combination of excess fibrinogen and impaired fibrinolysis sets this population up for a dangerous hypercoagulable state. Emerging data supports this hypothesis as the rate of deep vein thrombosis in patients with renal failure is 13 times higher than the general population.4 These hypercoagulable tendencies in the kidney transplant recipient are relevant as 30% of primary nonfunctioning kidneys from living donors (the most ideal donor) are due to thrombosis.5
There is a paucity of data on the perioperative changes of coagulation during kidney transplantation. Several small studies support that the prothrombotic state of kidney recipients persist after surgery.6 , 7 However, these studies did not evaluate the impact on fibrinolysis resistance during the perioperative period. Thrombelastography (TEG) is a point-of-care test that has been utilized widely in the fields of liver transplant 8, 9, 10, 11, cardiac surgery 12, 13, 14, and trauma15, 16, 17 to evaluate patients’ perioperative and intraoperative coagulation status. Unlike conventional laboratory tests of coagulation, TEG provides qualitative information throughout the entire “life-cycle” of a clot, from formation to lysis.18 Several studies using TEG for patients undergoing hemodialysis access surgery have demonstrated that these patients are both hyper and hypocoaguable, with prolong clotting time but increased clot strength and impaired fibrinolysis19 , 20. Given that a properly functioning renal graft should correct uremia and produce greater amounts of urokinase relative to the patient’s native diseased kidneys, one would expect an improvement in fibrinolytic activity following transplantation with a good functioning graft. Using TEG, we hypothesize that renal transplantation recipients with good functioning grafts will have improved fibrinolytic activity following surgery.
Methods
Patient population
Kidney transplant patients were enrolled in a Colorado Multi-Institutional Review Board study to prospectively collect blood samples before and after surgery. Enrollment criteria were adult (>18 years) and cadaveric or living donor recipients.
Blood samples for viscoelastic testing
Blood was collected and stored in a 3.5-ml tubes containing 3.2% citrate, and immediately transferred for analysis via a trained professional research assistant. All viscoelastic assays were completed within 2 h of blood draw. Serial blood samples were obtained before the surgical incision (pre-op) and on postoperative day 1 (POD1).
Thrombelastography
Blood samples were assayed with the TEG 5000 Hemostatic Analyzer (Haemonetics, Braintree, MA) according to manufacturer’s recommendations. The following measurements were recorded: R time (minutes), angle (α, degrees), maximum amplitude (MA, mm), and lysis 30 min after MA (LY30, %). Samples were run native, without any activator (n-TEG) in addition to two additional assays with tissue plasminogen activator TEG (t-PA TEG) at two different concentrations (low 75 ng/ml and high 150 ng/ml). The methods for these different concentrations of t-PA challenge TEGs has been previously described in trauma.21
Immunosuppression
A standard approach to perioperative immunosuppression was utilized for all patients. Patients were induced with 3 doses of Thymoglobulin (1.5 mg/kg). Post-operatively, patients received Myfortic (720 mg BID) and Tacrolimus titrated to therapeutic range (8–10 ng/ml).
Outcomes
The primary observation of interest is a 50% drop from pre-operative creatinine on POD 1 samples, which defined good graft function.22 Additional observations include percent change in creatinine from pre-op, post-operative day 2, and 3. Patients that underwent dialysis within the first three post-operative days were excluded from this analysis to eliminate the confounder of an artificially reduced creatinine. Other observations included creatinine at 6, 12, and 24 months following transplant, thrombotic complications (fistula thrombosis, deep vein thrombosis, pulmonary embolism, myocardial infarct, stroke), delayed graft function (dialysis within first week of transplant), dialysis within a year of transplant, and graft failure.
Statistical analysis
Statistical analysis was performed using SPSS 23 software (Microsoft, Armonk, NY). Normally distributed data were described as mean and standard deviation and non-normally distributed data were described as the median value with the 25th to 75th percentile values. All TEG variables pre- and post-op, including native, low and high dose t-PA TEG, were contrasted between patients with good graft function versus poor graft function. Coagulation variables were also correlated to changes in creatinine on POD1 with Spearman’s Rho. A receiver operating characteristic curve (ROC) was used to assess the inflection point for predicting good graft function with the TEG variable that had the highest association with good graft function. This coagulation variable cut point was used as a surrogate of good graft function. This cohort was contrasted to outcomes listed above using Mann Whitney U for continuous variables and chi square. These outcomes were also evaluated with patients with good graft function (POD-1 creatine decrease by > 50% from pre-op levels), and a combination score of good graft function (coagulation measures + creatinine decrease > 50% on POD-1).
Results
Patient population
Seventy-one renal transplant recipients were enrolled in this study from July 2017 to August 2019. The median age of the population was 53 years-old (45–62) with a pre-op creatinine of 6.7 (5.1–9.7). The most common cause for ESRD was hypertension/diabetes (42%), followed by autoimmune disease (25%) and polycystic kidney disease (15%). Donors were predominantly living (66%), followed by brain dead (27%) and donation after cardiac death (7%). One quarter of kidneys were placed on hypothermic perfusion prior to transplantation. In the overall cohort, 48% had good graft function, 8% had delayed graft function, and the remaining patients were somewhere in between.
Coagulation indices and correlation to good graft function
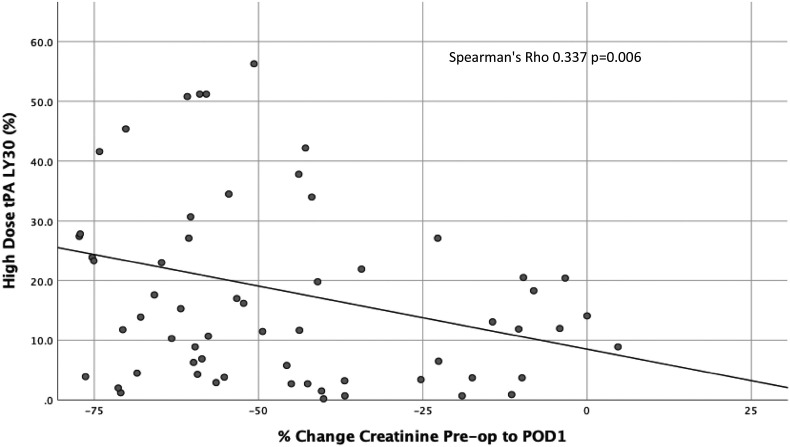
When analyzing the two different time points (pre-op, POD1) with three different assays (native, low dose, and high dose t-PA), each producing four indices of coagulation (R-time, angle, MA, and LY30), only one of the 26 coagulation measurements was significantly associated with good graft function on POD-1 (Table 1 ). This coagulation measurement was the fibrinolysis using the high dose t-PA assay, which demonstrated a higher median LY30 (17% vs 10% p = 0.007) in good versus poor graft function. High dose t-PA TEG LY30 had a significant correlation to change in pre-op creatinine to POD-1 (Spearman’s Rho 0.337 p = 0.006 Fig. 1 ). A receiver operating characteristic curve had an area under the curve of 0.695 (95%CI 0.567–0.823 p = 0.007) and the Youden index was identified at 23%.
Table 1.
Median values for TEG indices using 3 different assays measured preoperatively and on post-operative day 1 with 25th to 75th quartile values provided in parenthesis. Patients with good graft function were compared to patients with poor graft function for each index with each corresponding p-value provided. ∗ denotes statistical significance (p < 0.05).
| Time | tPA | R Good | R Poor | P | Angle Good | Angle Poor | P | MA Good | MA Poor | P | LY30 Good | LY30 Poor | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | 0 | 8.2 (6.5–9.5) | 7.6 (6.7–9.0) | 0.60 | 68 (60–70) | 67 (65–70) | 0.40 | 67 (63–71) | 68 (65–72) | 0.27 | 0.1 (0–1.1) | 0 (0–0.5) | 0.35 |
| Pre | 75 | 7.5 (6.3–8.9) | 7.5 (6.5–8.8) | 0.90 | 69 (65–71) | 67 (62–71) | 0.35 | 67 (60–71) | 67 (62–70) | 0.75 | 2.8 (0.7–4.6) | 1.2 (0.2–5.0) | 0.20 |
| Pre | 150 | 7.5 (6.1–9.1) | 7.1 (6.6–8.6) | 0.61 | 67 (62–71) | 68 (62–72) | 0.64 | 60 (55–68) | 63 (55–69) | 0.36 | 16 (4.9–29) | 12 (4.9–37) | 0.70 |
| POD1 | 0 | 7.8 (7.0–8.9) | 7.6 (6.5–8.7) | 0.43 | 69 (64–70) | 68 (64–71) | 0.85 | 67 (64–70) | 67 (65–71) | 0.86 | 0.3 (0–1.0) | 0.2 (0–0.8) | 0.80 |
| POD1 | 75 | 7.2 (6.2–8.1) | 6.5 (5.9–7.7) | 0.26 | 69 (63–72) | 69 (65–72) | 0.65 | 67 (63–69) | 66 (63–72) | 0.99 | 2.1 (1–6.6) | 1.8 (0.8–3.0) | 0.20 |
| POD1 | 150 | 6.9 (5.8–8.0) | 7.0 (5.8–8.5) | 0.68 | 71 (66–73) | 70 (64–72) | 0.53 | 65 (58–67) | 63 (60–70) | 0.68 | ∗17∗ (6.9–34) | 10 (2.7–20) | 0.007 |
Abbreviations: “Pre”, preoperative; “POD1”, post-operative day 1; “tPA”, tissue plasminogen activator (0 ng/ml, 75 ng/ml, 150 ng/ml); “R Good”, R-time (minutes) for patients with good graft function; “R Poor” R-time (minutes) for patients with poor graft function; “Angle Good”, angle (degrees) for patients with good graft function; “Angle Poor”, angle (degrees) for patients with poor graft function; “MA Good”, maximum amplitude (millimeters) for patients with good graft function; “MA Poor”, maximum amplitude (millimeters) for patients with poor graft function; “LY30 Good”, percent lysis 30 min after maximum amplitude for patients with good graft function; “LY30 Poor”, percent lysis 30 min after maximum amplitude for patients with poor graft function.
Fig. 1.
Correlation between graft function and lytic response in high dose tPA TEG on post-operative day 1 (POD1). Percent lysis 30 min after reaching maximum amplitude (LY30) on a TEG with 150 ng/ml of exogenous tPA (y-axis) is significantly correlated with the percent change in creatinine from pre-operative levels to POD1 (x-axis).
Characteristics with high dose t-PA LY30
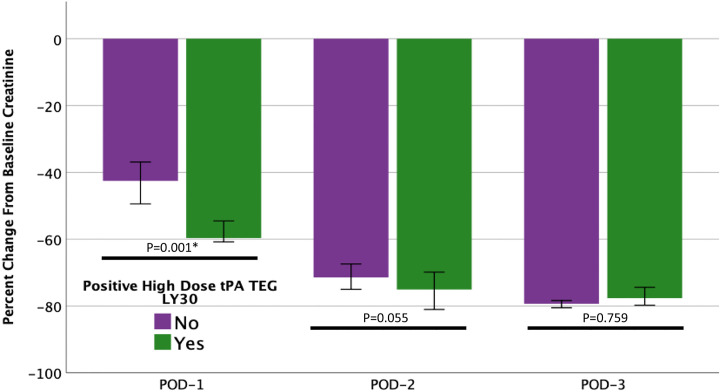
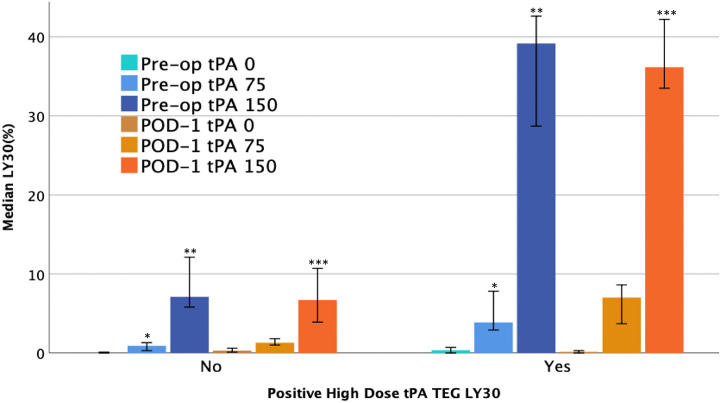
One quarter of patients had an elevated high dose t-PA TEG LY30 (150 ng/ml t-PA, LY30 > 23%) on POD1. As expected, these patients had a greater reduction in creatinine from pre-op to POD1 compared to patients with a lower LY30 (p = 0.001, Fig. 2 ), but the difference between cohorts decreased over time (POD2 p = 0.055 and POD3 p = 0.759). Donor characteristics did not have significant differences between cohorts. An elevated high dose t-PA TEG LY30 was present in 73% of living donors versus 63% of cadaveric donors (p = 0.565). Within DBD donors, the pump was used for 60% of grafts with an elevated high-dose t-PA TEG LY30 versus 76% with a non-elevated LY30 (p = 0.585) with no differences in warm [33 min (28–35) vs 30 min (27–39) p = 0.977] or cold [47 min (25–816) vs 97 min (28–599) p = 0.922] ischemia times. Table 2 demonstrates the difference between recipient factors. The high dose t-PA TEG LY30 group had a trend towards being younger (p = 0.083), had significantly lower platelet counts (p = 0.019), lower MA (p = 0.011), and significantly higher pre-op low dose t-PA TEG LY30 (p < 0.001) and high dose t-PA TEG LY30 (p < 0.001), which are depicted in Fig. 3 .
Fig. 2.
Elevated lytic activity on a post-op day-1 high dose tPA TEG is associated with a greater reduction in creatinine on post-op day-1. Patients that showed >23% lysis 30 min after maximum amplitude (LY30) on a TEG with 150 ng/ml of exogenous tPA (green bars) had a significantly greater percent change in creatinine from pre-op to post-op day 1 (POD-1) compared to patients with a LY30 < 23% (purple bars). The difference between cohorts narrowed and was not significant after POD-1. ∗denotes statistical significance (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Characteristics of kidney transplant recipients. Median values for each characteristic are provided with 25th to 75th quartile values in parenthesis. Patients were divided into two cohorts based on whether or not their high dose t-PA TEG on post-operative day-1 (POD-1) demonstrated elevated lysis (>or = 23%) 30 min after reaching maximum clot amplitude (LY30). For each characteristic, the two cohorts were compared, and p-values are provided.
| LY30 POD-1 = or <23% | LY30 POD-1 >23% | P | |
|---|---|---|---|
| Age | 54 (46–62) | 47 (38–57) | 0.083 |
| BMI | 26 (24–29) | 25 (24–27) | 0.336 |
| Female | 31% | 42% | 0.406 |
| Cause ESRD | 0.126 | ||
| DM/HTN | 42% | 42% | |
| AI | 18% | 42% | |
| PCKD | 18% | 5% | |
| Other | 22% | 10% | |
| Living Donor | 61% | 71% | 0.587 |
| Pre Creatinine | 7 (5-10) | 6 (5-8) | 0.255 |
| Pre Hct | 3624, 25, 26, 27, 28, 29 (24-29) | 25 (24-27) | 0.252 |
| Pre Plt | 219 (189–281) | 177 (161–243) | 0.019 |
| Pre INR | 1 (1–1.1) | 1.1 (1–1.1) | 0.298 |
| Pre R | 7 (5-10) | 65, 6, 7, 8 (5-8) | 0.277 |
| Pre Angle | 69 (64–70) | 66 (57–68) | 0.130 |
| Pre MA | 69 (65–72) | 64 (60–69) | 0.011 |
| Pre LY30 tPA 0 | 0 (0–0.5) | 0.4 (0–1.3) | 0.072 |
| Pre LY30 tPA 75 | 1.1 (0.2–3.7) | 3.8 (1.7–12) | <0.001 |
| Pre LY30 tPA 150 | 6.8 (3.9–18) | 39 (25–54) | <0.001 |
Abbreviations“BMI”, body mass index; “ESRD”, end stage renal disease; “DM/HTN”, diabetes/hypertension; “AI”, autoimmune disease; “PCKD”, polycystic kidney disease; “Pre”, preoperative; “Hct”, hematocrit; “Plt”, platelet count; “R”, R-time (minutes); “MA”, maximum amplitude (millimeters); “tPA 0”, native TEG assay with no exogenous tissue-plasminogen activator; “tPA 75”, low dose t-PA TEG assay with 75 ng/ml of exogenous t-PA; “tPA 150”, high dose t-PA TEG with 150 ng/ml of exogenous t-PA.
Fig. 3.
Elevated lytic activity on post-op day-1 high dose tPA TEG is associated in increased pre-operative sensitivity to tPA-mediated fibrinolysis. On post-op day-1, patients with > or = 23% lysis 30 min after maximum amplitude on a TEG with 150 ng/ml of exogenous tPA (“POD-1 tPA 150”, orange bars) were considered to have a “positive high dose tPA TEG LY30”. These patients demonstrated significantly more lysis in preoperative tPA challenge TEGs, with both high (150 ng/ml, pre-op tPA 150, purple bars) and low doses (75 ng/ml, “pre-op tPA 75”, blue bars) of exogenous tPA, compared to patients who had negative (<23% lysis) high dose tPA TEG LY30s on post-op day 1. No difference was observed between cohorts on pre-op native TEGs (“pre-op tPA 0”, teal bars), post-op native TEGs (“POD-1 tPA 0”, brown bars), and post-op low dose tPA TEGs (“POD-1 tPA 75”, gold bars). ∗, ∗∗, and ∗∗∗ denote statistical significance (p < 0.05). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Outcomes dichotomized high LY30, good graft function, and combination
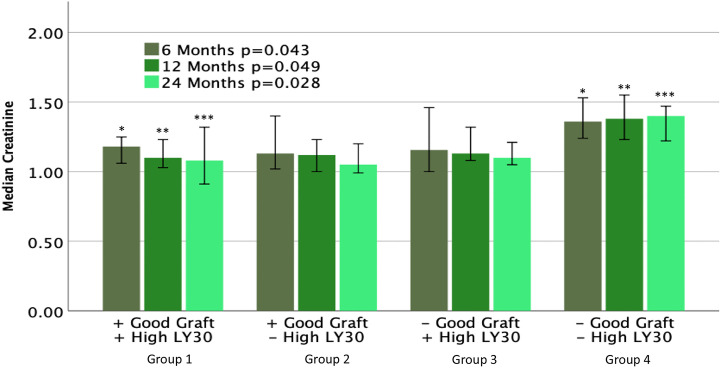
Patients were stratified into four groups based on their POD1 coagulation profile and graft function. Group 1 represents patients that had good graft function and elevated high dose t-PA TEG LY30. Group 2 represents patients with good graft function and non-elevated LY30. Group 3 represents patients with an elevated LY30 and poor graft function. Group 4 represents patients with non-elevated LY30 and poor graft function. The creatinine at 6 months (p = 0.043) 12 months (p = 0.049) and 24 months (p = 0.028) are depicted in Fig. 4 . Group 4 had the highest creatinine for all follow up lab draws which was elevated compared to the other groups at 24 months following transplant. The percent of adverse outcomes is depicted in Table 3 . Patients with good graft function had had a significantly lower rate of dialysis after transplant. Group 3 had a 50% rate of dialysis, but these dialysis episodes were all limited to within the first 7 days post-transplant, which is consistent with delayed graft function. Rejection rates were similar between groups, and the incidence of graft failure was too low to draw any significant conclusions. The thrombotic complication rate, while not significant, reached 30% in Group 4, with 0% in Group 3.
Fig. 4.
Immediate post-operative graft function and systemic fibrinolytic status are predictive of longer term graft function. Kidney recipients were divided into 4 cohorts based post-operative day 1 (POD1) creatine levels and lytic response on a high dose tPA (150 ng/ml) TEG. Patients with a >50% reduction in creatinine from pre-op to POD1 were considered to have a good graft function (+Good Graft). Recipients with > or = 23% lysis 30 min after reaching maximum clot amplitude (LY30) on a POD1 high dose tPA TEG were considered to have an elevated LY30 (+High LY30). Group 1 had significantly lower creatinine levels at 6, 12, and 24 months compared to Group 4. ∗, ∗∗, and ∗∗∗ denote statistical significance (p < 0.05).
Table 3.
Adverse outcomes among kidney transplant recipients. Patients were divided into 4 groups based on immediate post-operative graft function and percent lysis on a post-operative day 1 high dose t-PA TEG.
| Group 1 +Good Graft + High LY30 N = 15 |
Group 2 + Good Graft - High LY30 N = 20 |
Group 3 - Good Graft + High LY30 N = 7 |
Group 4 - Good Graft - High LY30 N = 29 |
p | |
|---|---|---|---|---|---|
| Dialysis 12 months | 7% (0 DGF) | 0% (0DGF) | 42% (100% DGF) | 25% (42% DGF) | 0.006 |
| Rejection | 13% | 10% | 14% | 17% | 0.957 |
| Thrombotic | 7% | 15% | 0% | 30% | 0.171 |
| Graft Loss | 0% | 5% | 0% | 7% | 0.847 |
Abbreviations: “+ Good Graft”, good graft function (>50% drop in pre-operative creatinine on post-operative day 1); “- Good Graft”, poor graft function (<50% drop in creatinine); “+ High LY30”, >23% lysis 30 min after maximum amplitude on a postoperative day 1 high dose t-PA TEG; “- High LY30” <23% lysis 30 min after maximum amplitude on a postoperative day 1 high dose t-PA TEG; “DGF”, delayed graft function (dialysis within 7 days post-transplant); “Thrombotic”, any of the following events: fistula thrombosis, deep vein thrombosis, pulmonary embolism, myocardial infarct, stroke.
Discussion
Evaluation of a large number of coagulation measurements during the perioperative period of renal transplantation showed that only high dose t-PA TEG LY30 on POD-1 was associated with good early graft function (>50% drop in creatinine from pre-op level). Donor variables were not associated with this favorable coagulation profile on POD-1. Rather, the recipient’s pre-operative coagulation properties were associated with this favorable POD-1 coagulation status, in which they had lower platelet counts, lower clot strength, and high LY30s using the low and high dose t-PA challenge. When combining this favorable coagulation group with good graft function on POD-1, long-term outcomes were favorable with 2-year creatinine significantly lower than patients with poor function and lower high dose t-PA TEG LY30s. Another concerning finding was that this poor early graft function group with lower high dose t-PA TEG LY30s had a 30% rate of thrombotic complications within 2 years post-transplantation.
Previous work has shown that intrarenal fibrin deposition occurs in response to ischemia.23, 24, 25 While most of these studies rely on animal models, Turunen et al.‘s work with human DBD renal transplantation concluded that much of the fibrin deposition occurred during organ procurement and cold storage.25 As such, it appears that the recipient’s ability to degrade fibrin clots within the renal microvasculature may be important for insuring adequate perfusion and maintaining long term good graft function. This is supported by the fact that patients with good graft function and high lytic activity on POD1 had significantly lower creatinine levels 2 years after transplantation compared to patients with poor graft function and low lytic activity. One possible physiologic explanation for this relationship is that good functioning grafts produce a greater amount of uPA relative to poor functioning grafts as the actions of uPA and tPA are complementary and synergistic. The activation of three different fibrin-bound plasminogens is required for intravascular fibrinolysis.26 The first is activated by tPA and initiates lysis.27 This initial lysis creates two new plasminogen binding sites, both of which are activated by uPA. At high doses, it possible for tPA alone to activate at all three plasminogens, but the resulting lysis is poor relative to that achieved by a combination of tPA and uPA.28, 29, 30
These findings of this study have clinical implications with regards to organ allocation and perioperative management. The Kidney Allocation System (KAS) in the United States relies on the Kidney Donor Profile Index (KDPI) and Estimated Post-Transplant Survival (EPTS) Score to estimate the quality of deceased donor kidneys and predict long-term graft survival, respectively.31 , 32 The EPTS is the recipient-specific score that is calculated based on the recipient’s age, duration on dialysis, history of diabetes, and history of prior solid organ transplantation. Currently, the EPTS is only considered in the allocation of highest quality kidneys (KDPI < 20%), in which candidates with the lowest EPTS (<20%) score are given priority over candidates with higher scores.32 Our data support that coagulation factors could be a variable with significant impact on graft function following transplant. Specifically, pre-transplant platelet count, MA, low dose t-PA TEG LY30, and high dose t-PA TEG LY30. Ongoing investigation in a larger study with longer term follow up is needed to see if assessment of coagulation factors can add to the performance of the EPST score.
In addition to improving performance, incorporating personalized, and possibly modifiable, coagulation data into the EPST could also improve patient satisfaction. As previously mentioned, there are currently only four EPST criteria, all of which are non-modifiable. According to Tong et al. when patients are waitlisted for an extended period of time, they become suspicious of inequality and many resign themselves to never getting an organ and opt to simply remain on dialysis.33 With the median wait time of 7.6 years to deceased-donor kidney transplantation, it is understandable why many patients may feel discouraged.34 Adding personalized coagulation measurements could provide patients a better explanation of rank on the waitlist and hope that they have some ability to change their chances to receive an organ.
Alternatively, these data bring up another clinical question about the potential role of reducing fibrinolytic resistance or activating fibrinolysis in the perioperative period to improve graft function. This treatment concept has shown promise in DCD liver transplantation as patients whose grafts were flushed with t-PA had significantly decreased ischemic biliary injuries and re-transplantation rate, as well as, better one-year graft survival.35 The COVID-19 pandemic has demonstrated that patients with a low fibrinolytic activity on TEG can harbor an extremely high rate of renal failure requiring dialysis.36 Autopsy series have demonstrated microvascular clots in organs of patients that have died from COVID-19 from multiple organ failure.37 Animal work supports that thrombin generation alone does not cause kidney fibrin deposition, but the combination of thrombin generation and medical inhibition of fibrinolysis is a potent combination for renal failure with fibrin plugging.38 Renal fibrin deposition has been reported to be present in up to 10% of cadaveric donors that get biopsied.39 In this study the rate of delayed graft function was 49% in patients with renal fibrin compared to 39% in the non-fibrin deposition cohort. This study did not evaluate the coagulation status of the recipient, which would play a role in degradation of these clots. Ongoing work in this area is crucial to identify a recipient with low fibrinolysis pre-transplant would further increase the risk of delayed graft function if they received a kidney with micro thrombi. The cost of delayed graft function is approximately $15,000 for the first year following transplant40 which is a fraction of the cost of two TEGs. Therefore, this could be a cost-effective strategy in the future, but requires more research before clinical implementation.
Our study has several limitations. The TEGs were run at two time points, pre-operatively and on POD1, without a post-discharge evaluation of coagulation, when systemic inflammation from surgery has mostly resolved. Surgery in itself is known to cause inhibition of fibrinolysis, that improves over time.41 Measuring patients’ two-week post-operative blood samples (or later) may unmask more subtle changes in coagulation that occur with good graft function following transplantation. The present study’s follow up from transplant is currently limited to only 2 years when approximately 93% of grafts from living donors are anticipated to still have good graft function.42 We will continue to follow this cohort for the next 5- and 10-years post-transplant during which, we anticipate more graft failures to occur. The 12- and 24-month follow-up data are missing for nine recipients (8 DBD and 1 DCD) who had yet to be 1-year post-transplant by the time of publication. While these data would be of value, they would not change the study’s major findings which are based on early graft function. Lastly, immunologic status, which has been shown to influence the coagulation system,43 , 44 was not considered in the analysis. However, the fact that there was no difference in rejection rates suggests that immunologic differences did not play a major role in the results. Despite these limitations, the results of this study clearly show that increased perioperative fibrinolytic capacity is associated with improved early and longer-term renal graft function.
Conclusion
The coagulation measurement of LY30 on a high dose t-PA TEG correlated to good graft function on POD1 following renal transplantation. This is the first paper to demonstrate an association between a post-operative TEG-based coagulation measurement and favorable short- and long-term outcomes. These results support the utilization of recipient coagulation measurements to optimize donor matching, improve the patient experience, and may also have a therapeutic role to increase graft longevity in those patients with less favorable coagulation profiles.
Declaration of competing interest
HM has share intellectual property with Haemonetics related to the tPA TEG.
Footnotes
This study was supported in part by National Heart, Lung, and Blood Institute: K99HL151887 and American Society of Transplant Surgeons Veloxis Fellowship Award, and University of Colorado Academic Enrichment Fund.
References
- 1.Shlipak M.G., Fried L.F., Crump C. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation. 2003;107(1):87–92. doi: 10.1161/01.cir.0000042700.48769.59. [DOI] [PubMed] [Google Scholar]
- 2.Pannell R., Black J., Gurewich V. Complementary modes of action of tissue-type plasminogen activator and pro-urokinase by which their synergistic effect on clot lysis may be explained. J Clin Invest. 1988;81(3):853–859. doi: 10.1172/JCI113394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tay K.H., Lip G.Y. What "drives" the link between the renin-angiotensin-aldosterone system and the prothrombotic state in hypertension? Am J Hypertens. 2008;21(12):1278–1279. doi: 10.1038/ajh.2008.315. [DOI] [PubMed] [Google Scholar]
- 4.Lu H.Y., Liao K.M. Increased risk of deep vein thrombosis in end-stage renal disease patients. BMC Nephrol. 2018;19(1):204. doi: 10.1186/s12882-018-0989-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verbesey J., Thomas A.G., Ronin M. Early graft losses in paired kidney exchange: experience from 10 years of the national kidney registry. Am J Transplant. 2020 doi: 10.1111/ajt.15778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reissell E., Lalla M., Hockerstedt K., Lindgren L. Coagulation abnormalities in diabetic patients undergoing renal transplantation. Ann Chir Gynaecol. 1994;83(3):251–255. [PubMed] [Google Scholar]
- 7.Ballow A., Gader A.M., Huraib S., Mitwalli A., Al-Suleimani F., Al-Wakeel J. Successful kidney transplantation does not reverse the coagulopathy in patients with chronic renal failure on either hemo or peritoneal dialysis. Saudi J Kidney Dis Transpl. 2007;18(2):177–185. [PubMed] [Google Scholar]
- 8.Mallett S.V. Clinical utility of viscoelastic tests of coagulation (TEG/ROTEM) in patients with liver disease and during liver transplantation. Semin Thromb Hemost. 2015;41(5):527–537. doi: 10.1055/s-0035-1550434. [DOI] [PubMed] [Google Scholar]
- 9.Krzanicki D., Sugavanam A., Mallett S. Intraoperative hypercoagulability during liver transplantation as demonstrated by thromboelastography. Liver Transplant. 2013;19(8):852–861. doi: 10.1002/lt.23668. [DOI] [PubMed] [Google Scholar]
- 10.Cerutti E., Stratta C., Romagnoli R. Thromboelastogram monitoring in the perioperative period of hepatectomy for adult living liver donation. Liver Transplant. 2004;10(2):289–294. doi: 10.1002/lt.20078. [DOI] [PubMed] [Google Scholar]
- 11.Hawkins R.B., Raymond S.L., Hartjes T. Review: the perioperative use of thromboelastography for liver transplant patients. Transplant Proc. 2018;50(10):3552–3558. doi: 10.1016/j.transproceed.2018.07.032. [DOI] [PubMed] [Google Scholar]
- 12.Deppe A.C., Weber C., Zimmermann J. Point-of-care thromboelastography/thromboelastometry-based coagulation management in cardiac surgery: a meta-analysis of 8332 patients. J Surg Res. 2016;203(2):424–433. doi: 10.1016/j.jss.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Johansson P.I., Solbeck S., Genet G., Stensballe J., Ostrowski S.R. Coagulopathy and hemostatic monitoring in cardiac surgery: an update. Scand Cardiovasc J. 2012;46(4):194–202. doi: 10.3109/14017431.2012.671487. [DOI] [PubMed] [Google Scholar]
- 14.Westbrook A.J., Olsen J., Bailey M., Bates J., Scully M., Salamonsen R.F. Protocol based on thromboelastograph (TEG) out-performs physician preference using laboratory coagulation tests to guide blood replacement during and after cardiac surgery: a pilot study. Heart Lung Circ. 2009;18(4):277–288. doi: 10.1016/j.hlc.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 15.Hunt H., Stanworth S., Curry N. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma induced coagulopathy in adult trauma patients with bleeding. Cochrane Database Syst Rev. 2015;(2) doi: 10.1002/14651858.CD010438.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez E., Moore E.E., Moore H.B. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann Surg. 2016;263(6):1051–1059. doi: 10.1097/SLA.0000000000001608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez E., Moore E.E., Moore H.B. Management of trauma-induced coagulopathy with thrombelastography. Crit Care Clin. 2017;33(1):119–134. doi: 10.1016/j.ccc.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen A., Teruya J. Global hemostasis testing thromboelastography: old technology, new applications. Clin Lab Med. 2009;29(2):391–407. doi: 10.1016/j.cll.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Nunns G.R., Moore E.E., Chapman M.P. The hypercoagulability paradox of chronic kidney disease: the role of fibrinogen. Am J Surg. 2017;214(6):1215–1218. doi: 10.1016/j.amjsurg.2017.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman M.P., Moore E.E., Burneikis D. Thrombelastographic pattern recognition in renal disease and trauma. J Surg Res. 2015;194(1):1–7. doi: 10.1016/j.jss.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore H.B., Moore E.E., Chapman M.P. Viscoelastic tissue plasminogen activator challenge predicts massive transfusion in 15 minutes. J Am Coll Surg. 2017;225(1):138–147. doi: 10.1016/j.jamcollsurg.2017.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Govani M.V., Kwon O., Batiuk T.D., Milgrom M.L., Filo R.S. Creatinine reduction ratio and 24-hour creatinine excretion on posttransplant day two: simple and objective tools to define graft function. J Am Soc Nephrol. 2002;13(6):1645–1649. doi: 10.1097/01.asn.0000014253.40506.f6. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen-Zender I., Rong S., Susnik N. Role of fibrinogen in acute ischemic kidney injury. Am J Physiol Ren Physiol. 2013;305(5):F777–F785. doi: 10.1152/ajprenal.00418.2012. [DOI] [PubMed] [Google Scholar]
- 24.Enestrom S., Druid H., Rammer L. Fibrin deposition in the kidney in post-ischaemic renal damage. Br J Exp Pathol. 1988;69(3):387–394. [PMC free article] [PubMed] [Google Scholar]
- 25.Turunen A.J., Lindgren L., Salmela K.T., Kyllonen L.E., Petaja J., Pesonen E.J. Intragraft coagulation events and delayed graft function in clinical renal transplantation. Transplantation. 2008;85(5):693–699. doi: 10.1097/TP.0b013e31816615d8. [DOI] [PubMed] [Google Scholar]
- 26.Suenson E., Lutzen O., Thorsen S. Initial plasmin-degradation of fibrin as the basis of a positive feed-back mechanism in fibrinolysis. Eur J Biochem. 1984;140(3):513–522. doi: 10.1111/j.1432-1033.1984.tb08132.x. [DOI] [PubMed] [Google Scholar]
- 27.Hoylaerts M., Rijken D.C., Lijnen H.R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982;257(6):2912–2919. [PubMed] [Google Scholar]
- 28.Liu J.N., Gurewich V. Fragment E-2 from fibrin substantially enhances pro-urokinase-induced Glu-plasminogen activation. A kinetic study using the plasmin-resistant mutant pro-urokinase Ala-158-rpro-UK. Biochemistry. 1992;31(27):6311–6317. doi: 10.1021/bi00142a021. [DOI] [PubMed] [Google Scholar]
- 29.Petersen L.C. Kinetics of reciprocal pro-urokinase/plasminogen activation--stimulation by a template formed by the urokinase receptor bound to poly(D-lysine) Eur J Biochem. 1997;245(2):316–323. doi: 10.1111/j.1432-1033.1997.00316.x. [DOI] [PubMed] [Google Scholar]
- 30.Harpel P.C., Chang T.S., Verderber E. Tissue plasminogen activator and urokinase mediate the binding of Glu-plasminogen to plasma fibrin I. Evidence for new binding sites in plasmin-degraded fibrin I. J Biol Chem. 1985;260(7):4432–4440. [PubMed] [Google Scholar]
- 31.Rao P.S., Schaubel D.E., Guidinger M.K. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–236. doi: 10.1097/TP.0b013e3181ac620b. [DOI] [PubMed] [Google Scholar]
- 32.Clayton P.A., McDonald S.P., Snyder J.J., Salkowski N., Chadban S.J. External validation of the estimated posttransplant survival score for allocation of deceased donor kidneys in the United States. Am J Transplant. 2014;14(8):1922–1926. doi: 10.1111/ajt.12761. [DOI] [PubMed] [Google Scholar]
- 33.Tong A., Hanson C.S., Chapman J.R. ’Suspended in a paradox’-patient attitudes to wait-listing for kidney transplantation: systematic review and thematic synthesis of qualitative studies. Transpl Int. 2015;28(7):771–787. doi: 10.1111/tri.12575. [DOI] [PubMed] [Google Scholar]
- 34.Bae S., Massie A.B., Thomas A.G. Who can tolerate a marginal kidney? Predicting survival after deceased donor kidney transplant by donor-recipient combination. Am J Transplant. 2019;19(2):425–433. doi: 10.1111/ajt.14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayant K., Reccia I., Virdis F., Shapiro A.M.J. Systematic review and meta-analysis on the impact of thrombolytic therapy in liver transplantation following donation after circulatory death. J Clin Med. 2018;7(11) doi: 10.3390/jcm7110425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright F.L.V.T, Moore E.E, Moore H.B. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J Am Coll Surg. 2020;231(2):193–203. doi: 10.1016/j.jamcollsurg.2020.05.007. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yao X.H., Li T.Y., He Z.C. [A pathological report of three COVID-19 cases by minimally invasive autopsies] Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 38.Margaretten W., Zunker H.O., McKay D.G. Production of the generalized shwartzman reaction in pregnant rats by intravenous infusion of thrombin. Lab Invest. 1964;13:552–559. [PubMed] [Google Scholar]
- 39.Batra R.K., Heilman R.L., Smith M.L. Rapid resolution of donor-derived glomerular fibrin thrombi after deceased donor kidney transplantation. Am J Transplant. 2016;16(3):1015–1020. doi: 10.1111/ajt.13561. [DOI] [PubMed] [Google Scholar]
- 40.Helantera I., Isola T., Lehtonen T.K., Aberg F., Lempinen M., Isoniemi H. Association of clinical factors with the costs of kidney transplantation in the current era. Ann Transplant. 2019;24:393–400. doi: 10.12659/AOT.915352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chakrabarti R., Hocking E.D., Fearnley G.R. Reaction pattern to three stresses--electroplexy, surgery, and myocardial infarction--of fibrinolysis and plasma fibrinogen. J Clin Pathol. 1969;22(6):659–662. doi: 10.1136/jcp.22.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andreoni K.A., Brayman K.L., Guidinger M.K., Sommers C.M., Sung R.S. Kidney and pancreas transplantation in the United States, 1996-2005. Am J Transplant. 2007;7(5 Pt 2):1359–1375. doi: 10.1111/j.1600-6143.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- 43.Siedlecki A., Irish W., Brennan D.C. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11(11):2279–2296. doi: 10.1111/j.1600-6143.2011.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Faulk W.P., Gargiulo P., McIntyre J.A., Bang N.U. Hemostasis and fibrinolysis in renal transplantation. Semin Thromb Hemost. 1989;15(1):88–98. doi: 10.1055/s-2007-1002691. [DOI] [PubMed] [Google Scholar]