INTRODUCTION
The RET gene can be oncogenically activated by point mutations, in-frame deletions, and chromosomal rearrangements,1,2 gain-of-function events that render the RET tyrosine kinase constitutively active.3-5 In pediatric and young adult patients, RET gene fusions have been reported in 22% to 45% of papillary thyroid carcinomas (PTCs)6-9 and less frequently in pediatric and young adult patients with glioma,10 lipofibromatosis,11 inflammatory myofibroblastic tumor,12 and infantile myofibromatosis.13 In addition, activating point mutations of RET have been reported in 40% to 50% of sporadic medullary thyroid cancers (MTCs).14,15 If they are constitutional, such mutations lead to the hereditary autosomal-dominant cancer syndrome called multiple endocrine neoplasia type 2 (MEN2), one characteristic of which is predisposition to MTC.16,17 Currently, no highly specific RET-targeted agents are approved for the treatment of patients with RET-altered cancers.
Selpercatinib (LOXO-292) is a potent, adenosine triphosphate–competitive, highly selective small molecule RET inhibitor with nanomolar potency against diverse RET alterations (including anticipated acquired gatekeeper resistance mutations).18,19 Preliminary results for selpercatinib in a phase I/II trial (LIBRETTO-001; ClinicalTrials.gov identifier: NCT03157128) are highly encouraging, showing that it is generally well tolerated and has marked antitumor activity in adolescent and adult patients with RET-altered cancers, including those with brain metastases and those with tumors resistant to previous multitargeted kinase inhibitors.20,21 We report the clinical activity of selpercatinib in five pediatric patients with tumors harboring RET alterations, four of whom were ineligible for the selpercatinib clinical trial open at the time their treatment was started because of their young age (younger than 12 years).
METHODS
Patients
Given the lack of other treatment options, access to selpercatinib for the four patients ineligible for an ongoing clinical trial was enabled by single patient protocols that were allowed by country-specific regulatory agencies and approved by institutional review boards. All four patients received selpercatinib (capsule or liquid formulation) orally in continuous 28-day cycles at a starting dose of 90 mg/m2 twice per day. This dose was intended to deliver exposure equivalent to the recommended adult phase II dose of 160 mg twice per day. One patient was enrolled to the 80-mg cohort of the ongoing selpercatinib phase I/II trial (ClinicalTrials.gov identifier: NCT03157128) and underwent intrapatient dose escalation to 160 mg per protocol.
Pharmacokinetic Analysis
Serial blood samples were collected for pharmacokinetic analyses. Plasma concentrations of selpercatinib were determined by liquid chromatography with detection by mass spectrometry. Pharmacokinetic parameters were calculated using Microsoft Excel (Microsoft, Redmond, WA)
RESULTS
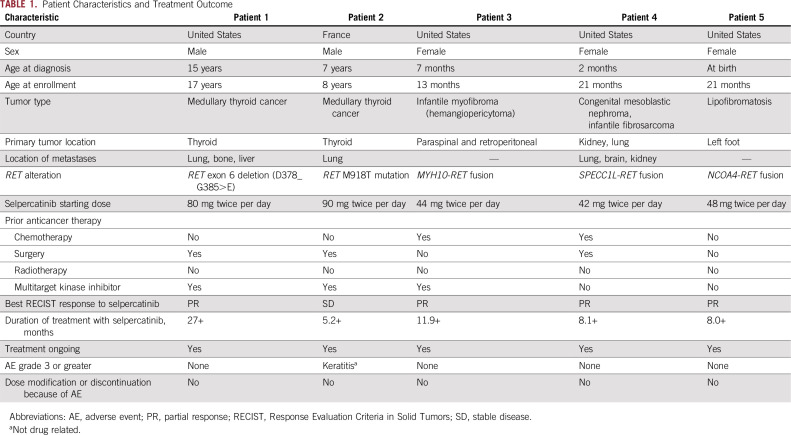
The analysis cutoff date was October 1, 2019. Two patients had thyroid cancers and three had soft-tissue sarcomas (Table 1).
TABLE 1.
Patient Characteristics and Treatment Outcome
Thyroid Cancers
Patient 1.
A 15-year-old boy presented with symptoms of night sweats, flushing, cramping, and weight loss. A biopsy revealed the presence of an MTC, with metastases detected in the lung, bone, and liver. The patient underwent total thyroidectomy and bilateral neck dissection. Molecular analysis showed that the tumor harbored an RET exon 6 deletion (d378_G685>E), and the patient was treated sequentially with four multitargeted kinase inhibitors: vandetanib, sunitinib, cabozantinib, and lenvatinib, which were each discontinued because of either adverse events or lack of efficacy. The patient was subsequently enrolled on the LIBRETTO-001 trial, and selpercatinib was initiated at 80 mg twice per day. After 8 weeks of treatment, a partial response was demonstrated, with a maximum tumor reduction of 86% at 40 weeks (Fig 1A-D). The patient remains in response and in excellent health after 25 cycles of treatment with no adverse events reported. Calcitonin was 53,125 pg/mL at the onset of therapy and is now 555 pg/mL; carcinoembryonic antigen was 1,850 ng/mL at treatment cycle 1 and is now 287 ng/mL.
FIG 1.
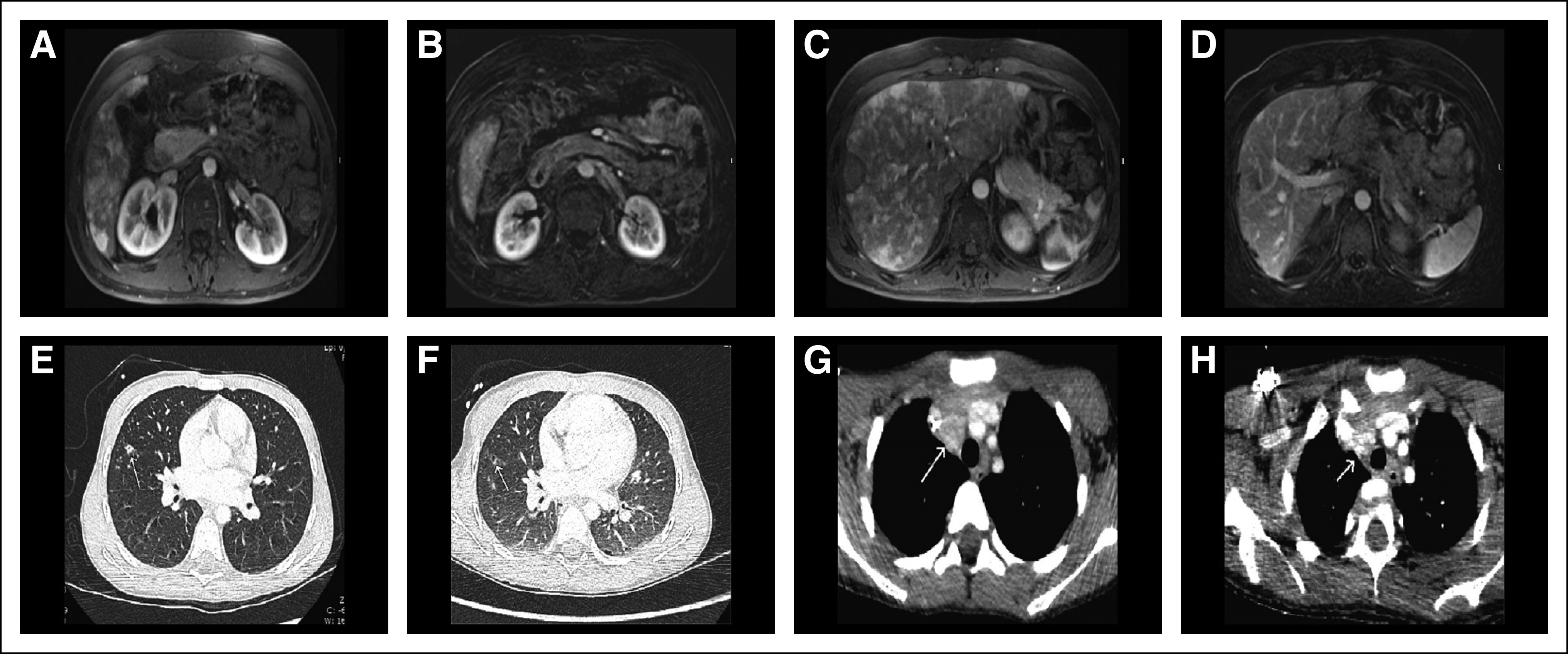
Selpercatinib activity in patients with medullary thyroid cancer. Patient 1: magnetic resonance imaging scans of (A) right lateral and (B) left anterior liver metastatic lesions at baseline and after 22 months of treatment with selpercatinib of (C) right lateral and (D) left anterior in a heavily pretreated patient with RET-mutated medullary thyroid cancer. A rapid improvement in symptoms and a partial response were reported after two cycles of treatment, which deepened over time. Patient 2: computed tomography scans at baseline and after 4 months of treatment with selpercatinib of thorax (E, F, respectively) and mediastinum (G, H, respectively) showing metastatic disease in a patient with RET-mutated medullary thyroid cancer. Early disease control was achieved after two cycles of treatment.
Patient 2.
A 7-year-old boy with neonatal hypotonia, abdominal pain, hollow feet, and unexplained laryngeal spasms was referred for diagnostic exome sequencing of genomic DNA (SureSelect XT Clinical Research Exome, Agilent, Santa Clara, CA). This revealed a constitutional de novo RET M918T mutation, a pathogenic variant associated with MEN2B that confers the highest risk of early onset MTC.16 The patient was subsequently diagnosed with an MTC and underwent thyroidectomy with tracheostomy followed by vandetanib therapy, which was discontinued because of the onset of grade 3 colitis. Selpercatinib was subsequently initiated at 90 mg twice per day. Treatment-related adverse events included grade 1 vomiting and diarrhea. After two cycles of treatment, stable disease was observed (Fig 1E-H).
Soft-Tissue Sarcomas
Patient 3.
A 7-month-old girl presented with lower extremity paraplegia. Magnetic resonance imaging showed a mass infiltrating the spinal canal, retroperitoneum, and pelvis. Initial pathology revealed clusters of CD56+ spindle cells consistent with neuroblastoma. Because of its location, the primary tumor was deemed unresectable.
The patient was started emergently on chemotherapy with cyclophosphamide and topotecan. Treatment was complicated by Pseudomonas-associated ecthyma gangrenosum, pneumonia, and bacteremia. Final pathology resulted in a definitive diagnosis of infantile myofibroma/hemangiopericytoma. Magnetic resonance imaging demonstrated new lung nodules indicative of metastatic disease.
An RNA-based next-generation sequencing (NGS) fusion assay (Solid Fusion Assay V2; ArcherDx, Boulder, CO) indicated that the tumor harbored an MYH10-RET gene fusion. The patient was started on vandetanib and achieved a partial tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1.22 However, after 4 months of treatment, she developed disease progression at the primary site. Treatment with selpercatinib was subsequently initiated. After 23 days, imaging demonstrated a partial response, which was confirmed 3 weeks later. Repeat imaging after 6 months of treatment showed complete resolution of the paraspinal mass and a 50% decrease in the size of the retroperitoneal, pelvic, and lumbosacral mass (Fig 2A-D). Together with tumor response, the patient regained normal sensation and muscle movement in her lower extremities and was able to stand and walk with support. No adverse events were reported.
FIG 2.
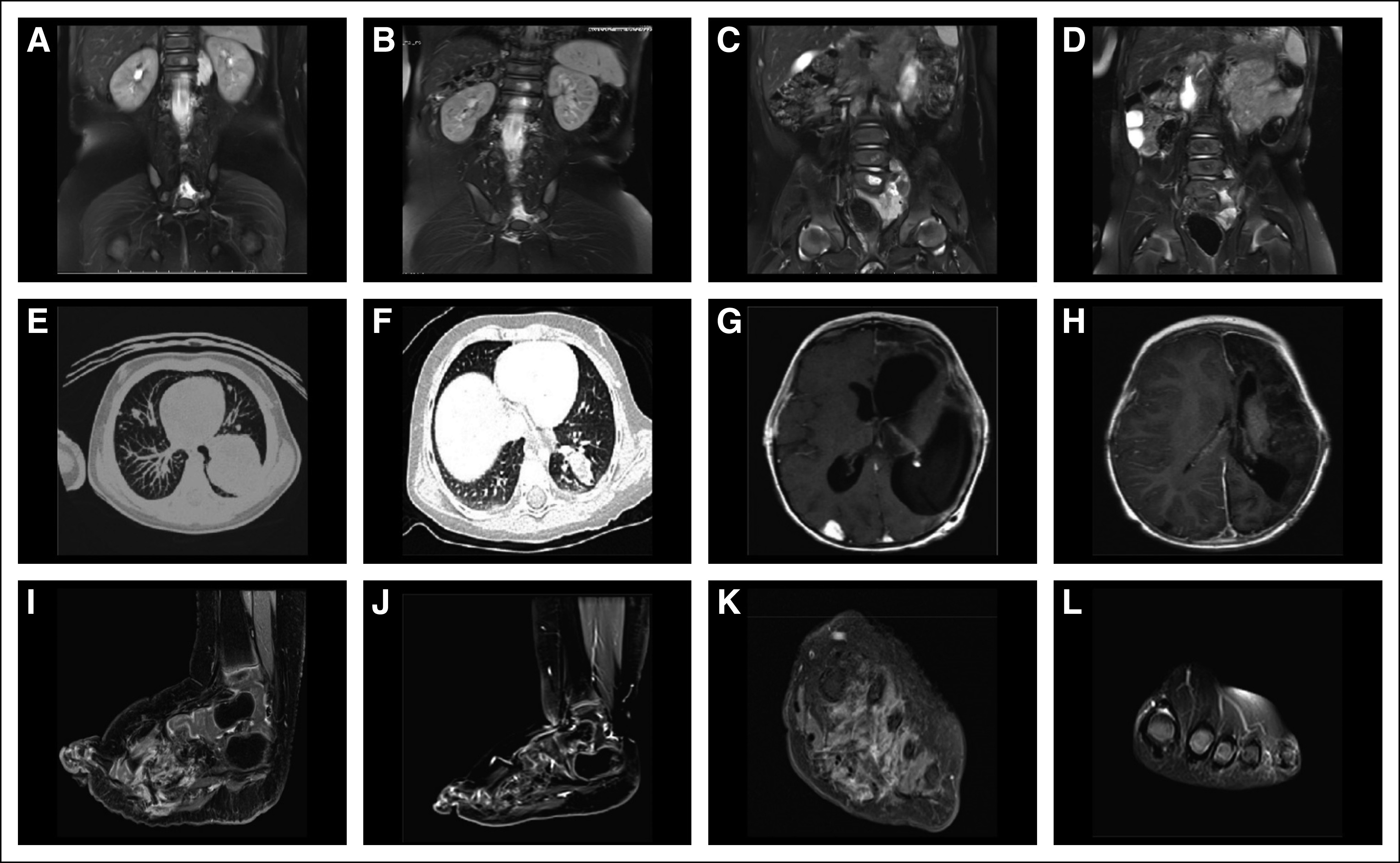
Selpercatinib activity in patients with soft-tissue sarcoma. Patient 3: (A, C) computed tomography (CT) scans of the abdomen at baseline and (B, D) after 6 months of treatment with selpercatinib, revealing multiple paraspinal retroperitoneal and pelvic lesions in a patient with infantile myofibroma/hemangiopericytoma harboring an MYH10-RET fusion. A partial response was observed after one cycle of selpercatinib; after six cycles, the paraspinal lesion had completely resolved, and the patient regained lower extremity neurologic function. Patient 4: CT scans at baseline of (E) the lungs and (G) brain and after 8 months of treatment with selpercatinib of (F) the lungs and (H) brain in a patient with an SPECC1L-RET fusion–positive congenital mesoblastic nephroma and infantile fibrosarcoma. After two cycles of selpercatinib, a partial response was observed with a 41% tumor reduction, which deepened to 66% by cycle 8. Patient 5: CT scans (I, K) at baseline and (J, L) after 2 months of treatment with selpercatinib of the left foot in a patient with an NCOA4-RET fusion–positive lipofibromatosis. Selpercatinib treatment resulted in a significant decrease in tumor burden leading to improvements in gait and locomotion.
Patient 4.
During repair of congenital bilateral inguinal hernias, a 2-month-old girl was found to have a right renal mass, and she underwent a right nephrectomy. Pathology identified mesoblastic nephroma (cellular type) with positive surgical margins. The patient initially did well without systemic therapy. She subsequently presented with the sudden onset of inconsolable crying and refusal to bear weight. Abdominal ultrasound and chest x-ray identified a mass in the left kidney and a large left lower lung mass; computed tomography imaging confirmed these lesions and identified additional, multiple small lung lesions. Brain imaging identified a large left-sided cerebral vascular accident and multifocal right posterior brain lesions. Biopsy of a lung mass revealed high-grade spindle cell sarcoma consistent with an infantile fibrosarcoma; pathology review of the original right nephrectomy specimen confirmed the initial diagnosis of mesoblastic nephroma. She received one cycle of actinomycin and vincristine followed by vincristine, dactinomycin, and cyclophosphamide. A subsequent ultrasound scan showed the renal mass to be enlarging. The patient received additional chemotherapy with cyclophosphamide and topotecan, but treatment was complicated by febrile neutropenia and Enterococcus faecalis ventriculitis.
DNA-based NGS (using Memorial Sloan Kettering integrated mutation profiling of actionable cancer targets [MSK-IMPACT]) of the tumor from the renal and lung masses identified the same SPECC1L-RET gene fusion, and the patient initiated treatment with selpercatinib. After two cycles, a partial response was observed with a 41% tumor reduction, (Fig 2E-H). A selpercatinib concentration of 4.5 ng/mL was achieved in cerebrospinal fluid at a dose level of 48 mg twice per day (90 mg/m2 per dose). Upon detection and resection of an isolated metastasis at the right posterior temporal occipital junction, the dose of selpercatinib was increased to 94 mg (180 mg/m2) twice per day, raising the concentration of the drug to 16 ng/mL in the cerebrospinal fluid. Responses deepened to 66% by cycle 6, and no treatment-related adverse events were reported.
Patient 5.
An otherwise healthy 21-month-old girl had been diagnosed with lipofibromatosis of her left foot at birth. This had progressively increased in size and affected her ability to ambulate. She was evaluated by oncologists and surgeons who recommended amputation. NGS analysis of a biopsy specimen (FoundationOne CDx; Foundation Medicine, Cambridge, MA) identified an NCOA4-RET fusion, which was confirmed by whole genome and transcriptome analysis. Selpercatinib was initiated, and imaging after 2 months revealed a partial response by RECIST 1.1, with a 59% reduction in tumor volume and resolution of tumor infiltration of the metatarsals (Fig 2I-L). No adverse events were reported.
Pharmacokinetic analysis.
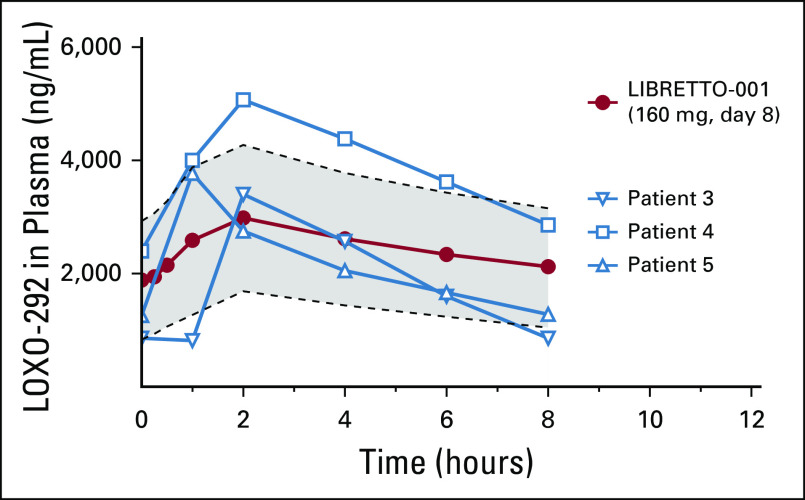
Selpercatinib pharmacokinetic data are available for three pediatric patients (patients 2, 3, and 4). The estimated steady-state maximum serum concentration (Cmax) and area under the serum concentration-time curve for 24 hours (AUC0-24) in these patients was similar to that of adults treated with selpercatinib 160 mg twice per day and consistent with significant (plasma concentrations greater than the concentration that inhibits 90% [IC90]) calculated RET target inhibition (Fig 3; data on file, Loxo Oncology, Stamford, CT).23
FIG 3.
Pharmacokinetics of selpercatinib in children. Plasma samples from three patients revealed that adequate plasma concentrations of selpercatinib were achieved (greater than RET wild-type concentration that inhibits 90% [IC90]), which were within the range seen in adult patients treated with the recommended dose of 160 mg in the LIBRETTO-001 trial. The gray area represents the 95% CIs for the median plasma concentrations observed in patients treated in the LIBRETTO-001 trial (red circles).
DISCUSSION
RET alterations are actionable oncogenic drivers that occur commonly in MTCs,24 pediatric PTCs,6-9 and rarely in other pediatric cancers.10-13,25 The multitargeted kinase inhibitors cabozantinib, vandetanib, and lenvatinib have demonstrated modest antitumor activity in adult and pediatric patients with MTC,26-29 and adult patients with RET fusion–positive cancers.4,30,31 The clinical activity of these agents is limited by suboptimal RET inhibition and significant toxicity, most likely because of strong inhibition of other kinases such as KDR and VEGFR2.5,32
Selpercatinib has demonstrated durable tumor responses and high tolerability in adolescents and adults with RET-altered cancers, with response rates of 68% reported in patients with RET fusion–positive platinum-pretreated non–small-cell lung cancer,20 and 56% in patients with RET mutation–positive cabozantinib and/or vandetanib-pretreated MTC.21 The results described here in patients with limited treatment options indicate that selpercatinib is also effective and safe in pediatric patients whose tumors harbor RET alterations. A phase I/II pediatric trial for patients with advanced RET-altered solid or primary CNS tumors is ongoing (LIBRETTO-121; ClinicalTrials.gov identifier: NCT03899792).
ACKNOWLEDGMENTS
We thank the patients and their families and contributing clinical staff across all sites. We also thank Alturas Analytics for providing real-time bioanalytical assessments. Medical writing services were provided by Jim Heighway of Cancer Communications and Consultancy, Knutsford, United Kingdom, and were funded by Loxo Oncology, Stamford, CT.
Presented as a poster at the 55th Annual Meeting of the American Society for Clinical Oncology, Chicago, IL, May 31-June 4, 2019, and as a poster at the 51st Congress of the Société Internationale d’Oncologie Pédiatrique, Lyon, France, October 23-26, 2019.
SUPPORT
Supported by Loxo Oncology (Stamford, CT), by Cannonball Kids’ Cancer (M.V.O.), by family and friends of Caroline Bhatt, by Grants No. K12CA184746 and P30CA008748 from the National Cancer Institute (M.V.O. and J.G.B.), and in part by the American Lebanese Syrian Associated Charities.
AUTHOR CONTRIBUTIONS
Conception and design: Steve Smith, Michael C. Cox, Stéphanie Proust, Julia Glade Bender, A. Lindsay Frazier, Peter Anderson, Alberto S. Pappo
Provision of study materials or patients: All authors
Collection and assembly of data: Michael V. Ortiz, Ulrike Gerdemann, Sandya Govinda Raju, Dahlia Henry, Stéphanie Proust, Peter Anderson, Alberto S. Pappo
Data analysis and interpretation: Sandya Govinda Raju, Steve Smith, S. Michael Rothenberg, Michael C. Cox, Stéphanie Proust, Julia Glade Bender, A. Lindsay Frazier, Peter Anderson, Alberto S. Pappo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST AND DATA AVAILABILITY STATEMENT
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Sandya Govinda Raju
Employment: Loxo Oncology
Dahlia Henry
Employment: Loxo Oncology
Stock and Other Ownership Interests: Loxo Oncology, Allergan, Axovant Sciences, Palatin Technologies
Steve Smith
Consulting or Advisory Role: Various, Loxo Oncology
Patents, Royalties, Other Intellectual Property: Various patents and applications
Travel, Accommodations, Expenses: Various
S. Michael Rothenberg
Employment: Loxo Oncology
Stock and Other Ownership Interests: Loxo Oncology
Michael C. Cox
Employment: Bayer, Loxo Oncology, Merck KGaA, Amgen, Day One Biopharmaceuticals
Stock and Other Ownership Interests: Loxo Oncology, Bayer, Merck KGaA, Amgen, Day One Biopharmaceuticals
Patents, Royalties, Other Intellectual Property: US patent 62/318,041 issued to Loxo Oncology (Inst)
Julia Glade Bender
Consulting or Advisory Role: AbbVie (Inst)
Research Funding: Celgene (Inst), Merck (Inst), Pfizer (Inst), Amgen (Inst), Ignyta (Inst), Bristol-Myers Squibb (Inst), Eisai (Inst), Novartis (Inst), Eli Lilly (Inst), Loxo Oncology (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: Novartis, Amgen, Merck, Bayer, Genentech
Uncompensated Relationships: SpringWorks Therapeutics, Bristol-Myers Squibb
A. Lindsay Frazier
Stock and Other Ownership Interests: Decibel Therapeutics
Consulting or Advisory Role: Decibel Therapeutics
Peter Anderson
Stock and Other Ownership Interests: Healios
Consulting or Advisory Role: Enlivity
Patents, Royalties, Other Intellectual Property: Patent for glutamine and trehalose compositions; priority date 13 September 2013; App 14/470, 545, filed August 27, 2014; patent 61/878,084 issued December 26, 2017
Other Relationship: Enlivity
Alberto S. Pappo
Honoraria: Bayer, Roche
Consulting or Advisory Role: Merck, Loxo Oncology/Bayer, EUSA Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Kato S, Subbiah V, Marchlik E, et al. RET aberrations in diverse cancers: Next-generation sequencing of 4,871 patients. Clin Cancer Res. 2017;23:1988–1997. doi: 10.1158/1078-0432.CCR-16-1679. [DOI] [PubMed] [Google Scholar]
- 2.Ceccherini I, Pasini B, Pacini F, et al. Somatic in frame deletions not involving juxtamembranous cysteine residues strongly activate the RET proto-oncogene. Oncogene. 1997;14:2609–2612. doi: 10.1038/sj.onc.1201079. [DOI] [PubMed] [Google Scholar]
- 3.Prescott JD, Zeiger MA. The RET oncogene in papillary thyroid carcinoma. Cancer. 2015;121:2137–2146. doi: 10.1002/cncr.29044. [DOI] [PubMed] [Google Scholar]
- 4.Paratala BS, Chung JH, Williams CB, et al. RET rearrangements are actionable alterations in breast cancer. Nat Commun. 2018;9:4821. doi: 10.1038/s41467-018-07341-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara R, Auger N, Auclin E, et al. Clinical and translational implications of RET rearrangements in non-small cell lung cancer. J Thorac Oncol. 2018;13:27–45. doi: 10.1016/j.jtho.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Cordioli MI, Moraes L, Bastos AU, et al. Fusion oncogenes are the main genetic events found in sporadic papillary thyroid carcinomas from children. Thyroid. 2017;27:182–188. doi: 10.1089/thy.2016.0387. [DOI] [PubMed] [Google Scholar]
- 7.Fenton CL, Lukes Y, Nicholson D, et al. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000;85:1170–1175. doi: 10.1210/jcem.85.3.6472. [DOI] [PubMed] [Google Scholar]
- 8.Prasad ML, Vyas M, Horne MJ, et al. NTRK fusion oncogenes in pediatric papillary thyroid carcinoma in northeast United States. Cancer. 2016;122:1097–1107. doi: 10.1002/cncr.29887. [DOI] [PubMed] [Google Scholar]
- 9.Vanden Borre P, Schrock AB, Anderson PM, et al. Pediatric, adolescent, and young adult thyroid carcinoma harbors frequent and diverse targetable genomic alterations, including kinase fusions. Oncologist. 2017;22:255–263. doi: 10.1634/theoncologist.2016-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho D, Mackay A, Bjerke L, et al. The prognostic role of intragenic copy number breakpoints and identification of novel fusion genes in paediatric high grade glioma. Acta Neuropathol Commun. 2014;2:23. doi: 10.1186/2051-5960-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Ibraheemi A, Folpe AL, Perez-Atayde AR, et al. Aberrant receptor tyrosine kinase signaling in lipofibromatosis: A clinicopathological and molecular genetic study of 20 cases. Mod Pathol. 2019;32:423–434. doi: 10.1038/s41379-018-0150-3. [DOI] [PubMed] [Google Scholar]
- 12.Antonescu CR, Suurmeijer AJ, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol. 2015;39:957–967. doi: 10.1097/PAS.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenzweig M, Ali SM, Wong V, et al. A case of advanced infantile myofibromatosis harboring a novel MYH10-RET fusion. Pediatr Blood Cancer. 2017;64:e26377. doi: 10.1002/pbc.26377. [DOI] [PubMed] [Google Scholar]
- 14.Dvorakova S, Vaclavikova E, Sykorova V, et al. Somatic mutations in the RET proto-oncogene in sporadic medullary thyroid carcinomas. Mol Cell Endocrinol. 2008;284:21–27. doi: 10.1016/j.mce.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Elisei R, Cosci B, Romei C, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: A 10-year follow-up study. J Clin Endocrinol Metab. 2008;93:682–687. doi: 10.1210/jc.2007-1714. [DOI] [PubMed] [Google Scholar]
- 16.Raue F, Frank-Raue K. Update on multiple endocrine neoplasia type 2: Focus on medullary thyroid carcinoma. J Endocr Soc. 2018;2:933–943. doi: 10.1210/js.2018-00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latteyer S, Klein-Hitpass L, Khandanpour C, et al. A 6-base pair in frame germline deletion in exon 7 of RET leads to increased RET phosphorylation, ERK activation, and MEN2A. J Clin Endocrinol Metab. 2016;101:1016–1022. doi: 10.1210/jc.2015-2948. [DOI] [PubMed] [Google Scholar]
- 18.Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29:1869–1876. doi: 10.1093/annonc/mdy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirth LJ, Kohno T, Udagawa H, et al. Emergence and targeting of acquired and hereditary resistance to multikinase RET inhibition in patients with RET-altered cancer. JCO Precis Oncol. doi: 10.1200/PO.19.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drilon A, Oxnard G, Wirth L, et al. Registrational results of LIBRETTO-001: A phase 1/2 trial of LOXO-292 in patients with RET fusion-positive lung cancers. J Thorac Oncol. 2019;14:S6–S7. (suppl; abstr PL02.08) [Google Scholar]
- 21.Wirth LJ, Sherman E, Drilon A, et al. Registrational results of LOXO-292 in patients with RET-altered thyroid cancers. Ann Oncol. 2018;30:v851–v934. (suppl_5; abstr LBA93) [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Drilon AE, Subbiah V, Oxnard GR, et al. A phase 1 study of LOXO-292, a potent and highly selective RET inhibitor, in patients with RET-altered cancers. J Clin Oncol. 2018;36 (suppl; abstr 102) [Google Scholar]
- 24.Chernock RD, Hagemann IS. Molecular pathology of hereditary and sporadic medullary thyroid carcinomas. Am J Clin Pathol. 2015;143:768–777. doi: 10.1309/AJCPHWACTTUYJ7DD. [DOI] [PubMed] [Google Scholar]
- 25.Antonescu CR, Dickson BC, Swanson D, et al. Spindle cell tumors with RET gene fusions exhibit a morphologic spectrum akin to tumors with NTRK gene fusions. Am J Surg Pathol. 2019;43:1384–1391. doi: 10.1097/PAS.0000000000001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox E, Widemann BC, Chuk MK, et al. Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res. 2013;19:4239–4248. doi: 10.1158/1078-0432.CCR-13-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: A randomized, double-blind phase III trial. J Clin Oncol. 2012;30:134–141. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol. 2013;31:3639–3646. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlumberger M, Jarzab B, Cabanillas ME, et al. A phase II trial of the multitargeted tyrosine kinase inhibitor lenvatinib (E7080) in advanced medullary thyroid cancer. Clin Cancer Res. 2016;22:44–53. doi: 10.1158/1078-0432.CCR-15-1127. [DOI] [PubMed] [Google Scholar]
- 30.Gautschi O, Milia J, Filleron T, et al. Targeting RET in patients with RET-rearranged lung cancers: Results from the Global, Multicenter RET Registry. J Clin Oncol. 2017;35:1403–1410. doi: 10.1200/JCO.2016.70.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoh K, Seto T, Satouchi M, et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): An open-label, multicentre phase 2 trial. Lancet Respir Med. 2017;5:42–50. doi: 10.1016/S2213-2600(16)30322-8. [DOI] [PubMed] [Google Scholar]
- 32.Drilon A, Hu ZI, Lai GGY, et al. Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15:151–167. doi: 10.1038/nrclinonc.2017.175. [DOI] [PMC free article] [PubMed] [Google Scholar]