Abstract
Purpose
Coronavirus Disease 2019 (COVID-19) has caused suffering and death around the world. Careful selection of facial protection is paramount for preventing virus spread among healthcare workers and preserving mask and N95 respirator supplies.
Methods
This paper is a comprehensive review of literature written in English and available on Pubmed comparing the risk of viral respiratory infections when wearing masks and N95 respirators. Current international oral and maxillofacial surgery guidelines for mask and N95 respirator use, patient COVID-19 disease status, aerosol producing procedures were also collected and incorporated into a workflow for selecting appropriate facial protection for oral and maxillofacial surgery procedures during the current pandemic.
Results
Most studies suggest N95 respirators and masks are equally protective against respiratory viruses. Some evidence favors N95 respirators, which are preferred for high-risk procedures when aerosol production is likely or when the COVID-19 status of a patient is positive or unknown. N95 respirators may also be used for multiple patients or reused depending on the type of procedure and condition of the respirator after each patient encounter.
Conclusion
N95 respirators are preferred over masks against viral respiratory pathogens, especially during aerosol-generating procedures or when a patient’s COVID-19 status is positive or unknown.
Introduction
The primary route for the spread of COVID-19 is through aerosolized droplets that are expelled during coughing, sneezing, or breathing. Healthcare workers (HCWs) caring for patients with COVID-19 are at high risk for nosocomial transmission, especially during various aerosol-generating procedures. Meanwhile, the shortage of personal protective equipment (PPE) has made it difficult to continue working safely and to reduce the risk of exposure to virus particles and infection among HCWs. Oral and maxillofacial surgeons (OMSs) are particularly vulnerable as they perform surgeries in and around the mouth and face and commonly perform in-office anesthesia via IV sedation and general anesthetics. As a result, OMSs accept great risk caring for patients and require deliberate and thoughtful PPE selection, particularly when choosing mouth and nose coverings.
The purpose of this study was to compare the protective effects of masks and N95 respirators against SARS-CoV-2 and similar viruses. The investigators hypothesized that aerosol-generating procedures with COVID-19 positive or unknown patients would require respirators and that nonaerosol-generating procedures and asymptomatic patients may be safely treated with medical or surgical masks. The specific aims were: 1) compare the protective effects of masks and N95 respirators against SARS-CoV-2 and similar viruses, 2) compare recommendations for masks and N95 respirators in low- and high-risk oral-maxillofacial surgery procedures, 3) apply N95 extended use and reuse policies to oral-maxillofacial surgery, and 4) create a workflow for selecting facial PPE based on oral-maxillofacial surgery procedure type, patient risk, and reusability of N95 respirators.
Methods
The study was designed to make a comprehensive review of the efficacy of PPE for mouth and nose protection, especially in oral-maxillofacial surgery. N95 respirators and surgical masks, which are the most common forms of facial PPE, were selected as the predictors. To address the research purpose, the investigators designed and implemented a comprehensive review modeled after the Cochrane Collaboration’s recommendations for systematic reviews. Publication searching was conducted using PubMed, and the study population was composed of all publications on the topic of “Coronavirus”, “COVID-19”, “SARS-CoV-2”, “Aerosol and droplet transmission”, “N95 respirators”, “Surgical mask”; “Personal Protective Equipment (PPE)”; “Maxillofacial procedures”, “Oral and Maxillofacial Surgeons (OMFSs)” between January 1, 2000 and July 7, 2020). Peer-reviewed articles or followed clinical trial results were included in the study sample. We also checked government websites (CDC) and hospital websites for policies regarding COVID-19 protection. Publications were excluded from analysis if studies were focused on laboratory exposure simulations, nonhealthcare workers, other types of respirators and surgical masks, and written languages other than English. Laboratory-confirmed respiratory infection or influenza-like illness were screened as the outcome for review and analysis. A descriptive summary and table of the reviewed publications were made, as shown in the result part.
Results
For this review, 8 studies were included to assess the effectiveness of surgical masks versus N95 respirators in protecting against viral respiratory infection. Of the 8 studies, 5 were RCTs,1, 2, 3, 4, 5, 6 1 was a cohort study,7 and 2 were case-control studies8 , 9 (Table 1 ). Six studies were extracted from a previously published meta-analysis,10 and the results showed no significant difference between N95 respirators and surgical masks in the associated risk of laboratory-confirmed respiratory infection (RCTs: OR 0.89, 0.64–1.24; cohort study: OR 0.43, 0.03–6.41; case–control studies: OR 0.91, 0.25–3.36) and influenza-like illness (RCTs: OR 0.51, 0.19–1.41). In addition, a large randomized clinical trial (RCT) performed in China5 was included in Table 1, which showed that rates of all outcomes of infection were lower in the N95 groups, while another large RCT performed in the US showed there was no significant difference in the incidence of laboratory-confirmed influenza.6
Table 1.
Characteristics of Studies of Comparison of N95 Respirators and Medical masks in Reducing the Risk of Infection
| Study | Country/Area | Research Type | Participants | Interventions | Outcome | Results | |
|---|---|---|---|---|---|---|---|
| 1∗ | Loeb et al., 200912 | 8 hospitals in Ontario | RCT | 446 |
∗Targeted use, fit-tested N95 respirator ∗Targeted use, surgical mask |
Laboratory-confirmed respiratory infection, influenza-like illness |
|
| 2∗ | MacIntyre et al., 2011/20142,3 | 15 hospitals in Beijing | RCT | 1441 |
∗Continual use, fit-tested N95 respirator ∗Continual use, non–fit-tested N95 respirator ∗Continual use, surgical mask |
Laboratory-confirmed respiratory infection, influenza-like illness |
|
| 3∗ | MacIntyre et al., 20134 | 19 hospitals in Beijing | RCT | 1669 |
∗Continual use, fit-tested N95 respirator ∗Targeted use, fit-tested N95 respirator ∗Control: continual use, surgical mask |
Laboratory-confirmed respiratory infection, influenza-like illness |
|
| 4∗ | Loeb et al., 20047 | 2 hospitals in Ontario | Cohort study | 43 |
∗N95 respirator ∗Surgical mask |
Laboratory-confirmed respiratory infection |
|
| 5∗ | Seto et al., 20038 | 5 hospitals in Hong Kong | Case–control studies | 13 infected 241 noninfected |
∗N95 respirator ∗Surgical mask ∗Paper mask |
Laboratory-confirmed respiratory infection |
|
| 6∗ | Zhang et al., 20139 | 25 hospitals in Beijing | Case–control studies | 51 infected 204 noninfected |
∗N95 respirator ∗Surgical mask ∗Cloth mask |
Laboratory-confirmed respiratory infection |
|
| 7 | MacIntyre et al., 20175 | 9 hospitals in Beijing | RCT | 3591 |
∗Continuous N95 respirator use ∗Targeted N95 respirator use ∗Medical mask use ∗Control arm. |
Laboratory confirmed viral respiratory infection |
|
| 8 | Radonovich et al., 20196 | 7 hospitals in US | RCT | 4051 |
∗N95 respirators ∗Medical masks |
Laboratory-confirmed influenza; |
|
Abbreviations: CRI (credible interval), HCP (healthcare personnel), HR (hazard rate), OR (odds ratio), RCT (Randomized controlled trial), RR (relative risk), SARS (severe acute respiratory syndrome.
1-6 were included in the meta-analysis: Effectiveness of N95 respirators versus surgical masks in protecting healthcare workers from acute respiratory infection: a systematic review and meta-analysis.10
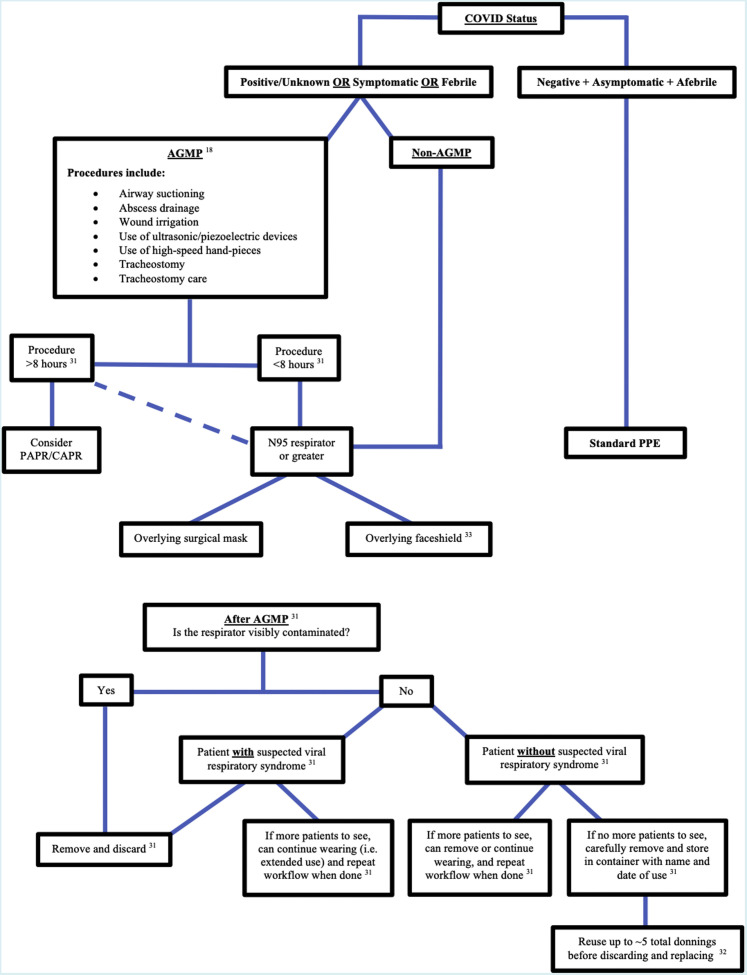
The WHO’s recommendations about when to use a surgical mask versus an N95 respirator based on pathogens and situational risks were used to create Table 2 .11, 12 Table 3 converts the information from Table 1 and Table 2 into recommendations for OMSs and anesthesiologists based on the dichotomy of a patient’s fever status charted against the type of patient encounter area. International guidelines on mask and respirator use during COVID-19 from oral-maxillofacial surgery associations, journals, and relevant government websites cited in the oral-maxillofacial surgery literature are represented in Table 4 .13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 The mask or respirator most frequently cited for each scenario is represented by the highest tally of checkmarks associated with each scenario. Overall, N95 respirators were the favorite in all scenarios, except when performing nonaerosol-generating medical procedures (non-AGMPs) on symptomatic patients, which favored surgical masks, and when performing aerosol-generating medical procedures (AGMPs) on COVID positive patients, which favored N99 respirators. Table 4 also collates global oral-maxillofacial surgery recommendations pertaining to facial PPE during COVID-19, with a comparison of mask/respirator rating systems used in the United States and Europe, as seen in the right two columns.15 , 16 , 21 , 22 , 26 , 27 Tables 2 through Table 3, Table 4, and supplementary literature on facial PPE,23 , 34, 35, 36 helped form the workflow seen in Figure 1 for mask or respirator selection prior to an AGMP or non-AGMP. It also provided a workflow following AGMPs when deciding eligibility for respirator re-use, extended use, or replacement. The workflow for mask or respirator selection starts with patient COVID status, then assesses procedure type (AGMP or non-AGMP), followed by procedure length, and secondary layers of facial protection. Following an AGMP, there is then the question of visible mask contamination, which then leads to disposal if present, potential extended use if the patients have respiratory symptoms, or re-use if the patients are symptom-free.
Table 2.
WHO recommendation for PPE based on risk of situation
| PPE choice11 | Surgical Masks11 | N95 Respirators11 |
|---|---|---|
| Rationale11 | Large droplets (>5 μm) in short distance (<2m) | Infectious aerosols (<5 μm) over a long distance (>2m) |
| Pathogens11 | Febrile acute respiratory disease, RSV, adenovirus, and influenza | Pulmonary tuberculosis, measles, SARS, novel or unknown organism causing acute respiratory diseases |
| Risk situations12 |
|
|
Data from World Health Organization (WHO): Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in healthcare, 2014.11
Abbreviations: AGP, aerosol-generating procedures; PPE, personal protective equipment; RSV, respiratory syncytial viral; SARS, severe acute respiratory syndrome.
Table 3.
Surgical Mask or N95 respirator for OMSs and Anesthesiologists
| Status of Patients Classification of area |
Without Fever | With Fever |
|---|---|---|
| OR | Surgical Mask | N95 respirator or PAPR/CAPR |
| Regular Ward | Surgical Mask | N95 respirator |
| Clinic | Surgical Mask | N95 respirator |
| Fever Clinic/ER | N95 respirator | N95 respirator |
Abbreviations: CAPR (controlled air-purifying respirator); ER (emergency room); OMSs (Oral and Maxillofacial Surgeons); OR (operation room); PAPR (powered air-purifying respirator); “Ward” represents a standard hospital floor occupied by patients admitted for medical or surgical reasons.
Table 4.
Current OMFS literature and guidelines on the surgical mask or respirator use During the COVID-19 pandemic
| NonAerosol-Generating Medical Procedures (non-AGMP) | Aerosol-Generating Medical Procedures (AGMP) | USA Mask/Respirator Levels |
European Mask/Respirator Levels |
|||||
|---|---|---|---|---|---|---|---|---|
| COVID-19 Negative | Standard PPE |  |
✓✓ 13,14 |
PAPR/CAPR15 Use HEPA or ULPA filters Filtration efficiency: • 99.97% @ 0.3 micron |
PAPR/CAPR16 Use HEPA or ULPA filters Filtration efficiency: •≥ 99.95% @ 0.3 micron |
|||
 |
✓ 17 |
|||||||
| Unknown COVID-19 status | No symptoms - or - Infection unlikely |
 |
✓✓ 18,19 (no valve, with overlying surgical mask or visor) |
 |
✓✓ 14,20 |
N10021 Filtration efficiency: •≥ 99.97% @ 0.3 micron |
RESPIRATORS | |
 |
✓✓✓✓✓✓✓ 18, ∗ 17 [6/12 (50%) sources recommend FFP2 mask or equivalent] |
 |
✓✓✓ 19 (withouta valve and with overlying surgical mask or visor),18,20 |
N9921 Filtration efficiency: •≥ 99% @ 0.3 micron |
FFP322 Filtration efficiency: •≥ 99% @ 0.3 micron |
|||
 |
✓ 23(outpatient exam) |
 |
✓✓✓✓✓✓✓✓✓✓✓✓ 24,25 (if “risk of exposure is high”), 18,∗ 17 [8/12 (67%) sources recommend FFP2 mask or equivalent (change after each patient)], 20 (if PAPR or FFP3 not available) |
N9521 Filtration efficiency: •≥ 95% @ 0.3 micron |
FFP222 Filtration efficiency: •≥ 94% @ 0.3 micron |
|||
 |
✓✓✓✓✓ 17,19,24,25 (exam only),23 (outpatient exam) |
 |
✓ 25 |
FFP122 Filtration efficiency: • 80% @ 0.3 microns |
||||
| Symptoms - or -Infection likely |
 |
✓ 18 |
 |
✓✓✓ 14,17,20 |
ASTM Level 326 Filtration efficiency: •BFE ≥ 98% @ 3 microns •PFE ≥ 98% @ 0.1 micron High fluid resistance: 160 mmHg |
Type IIR27 Filtration efficiency: •BFE ≥ 98% @ 3 microns |
MASKS | |
 |
✓✓ 23 (outpatient clinical exam),18 |
 |
✓✓ 18,20 |
ASTM Level 226 Filtration efficiency: •BFE ≥ 98% @ 3 microns •PFE ≥ 98% @ 0.1 micron Mod. fluid resistance: 160 mmHg |
Type II27 Filtration efficiency: •BFE ≥ 98% @ 3 microns |
|||
 |
✓✓✓ 17,24,25 |
 |
✓✓✓✓✓✓✓ 18,20,24 (if does not have PAPR or FFP3),28 (if PAPR not available),17 (N95 with surgical mask over it if PAPR unavailable),25 (consider Hazmat suit if the risk of exposure is high),29 |
|||||
| COVID-19 Positive |  |
✓ 18 |
 |
✓✓ 14,17 |
ASTM Level 126 Filtration efficiency: •BFE ≥ 95% @ 3 microns •PFE ≥ 95% @ 0.1 micron Low fluid resistance: 80 mmHg |
Type I27 Filtration efficiency: •BFE ≥ 95% @ 3 microns |
||
 |
✓✓✓✓✓✓✓✓✓✓✓✓ 23,24 (inpatient exam room with patient contact, or outpatient exam), 18, ∗ 17 [9/12 (75%) sources recommend use of FFP2 mask or equivalent] |
 |
✓✓✓✓ 23(In the patient room/exam room/negative pressure operating room), ∗17 [3/12 (25%) sources suggest the use of FFP3 masks or equivalent if available.] |
|||||
 |
✓ 23 (inpatient exam room without patient contact) |
 |
✓✓✓✓✓✓✓✓✓✓✓✓ 17 (with a surgical mask over it), ∗ 17 [10/12 (83%) sources recommend the use of an FFP2 mask or equivalent (changed after each patient)], 18 |
Low performance26 Physical barrier only |
||||
 |
✓✓ 17,23 (inpatient exam room without patient contact) |
 |
✓✓✓ 18,24,29 |
|||||
Note: medical masks and surgical masks are the same in this table; AGMPs were described slightly differently in each paper, but generally involve operating room procedures or the use of drills or ultrasonic instruments.
Abbreviations: AGMP, aerosol-generating medical procedure; ASTM, American Society for Testing and Materials; BFE, bacterial filtration efficiency; CAPR, controlled air-purifying respirator; PAPR, powered air-purifying respirator; PFE, particle filtration efficiency.
✓ = tally of references recommending each type of PPE.
Cochrane review of the national recommendations for the re-structuring and reopening of dental services from 11 countries with 12 guidance documents (produced between March 18 and May 5, 2020).30
Figure 1.
Preprocedure workflow (above) and OMSs AGMP procedure workflow (below) for Mask/Respirator Selection. Solid lines are primary pathway and dotted line represents alternative options.
Discussion
Aerosol Transmission
For viruses causing acute respiratory diseases (ARD), the main mode of transmission is by contact, droplets, and aerosols or airborne particles. Droplet transmission refers to large particles (>5 μm) that have a very low risk of transmission beyond 1 to 2 m and sink rapidly in the air. In contrast, the airborne transmission allows for relatively long-distance travel over 2m by aerosols of multiple different sizes. Aerosols can vary in size and include small droplets and droplet nuclei. Aerosols <5 to 10 μm in diameter follow airflow streamlines, and transmission may be over a short- or long-range. Small aerosols (<5 μm) can reach the alveolar spaces. Large aerosols (<10 μm) can penetrate below the glottis,28 , 37 while those >20 μm fall mostly under the influence of gravity without the following airflow streamline.37
Aerosols are produced during everyday activities such as breathing, coughing, sneezing, or talking. Healthcare workers are often exposed to higher aerosol levels during AGMP,38 which are various, but include the following: (1) bronchoscopy, cardiopulmonary resuscitation, manual ventilation, tracheal intubation, sputum induction suctioning, and nebulizer treatment; (2) noninvasive ventilation such as bilevel positive airway pressure (BiPAP) therapy, continuous positive airway pressure (CPAP) therapy, and high-frequency oscillatory ventilation (HFOV); (3) oral-maxillofacial surgeries that utilize lasers, or pneumatic or electric tools, such as rotary drills and saws. These procedures can either mechanically create and disperse aerosols or provoke patients to produce aerosols and are recognized as essential sources of respiratory virus transmission in hospitals.39
The risk of aerosol exposure lies in the potential they have to carry infectious organisms, mainly viruses. The family of Coronaviridae contains viruses that are known to be transmitted between humans routinely through an aerosol route, such as Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). During the SARS outbreak in 2003, many HCWs suffered severe illness and death, suggesting nosocomial transmission of Coronavirus is significantly associated with AGMPs.39 , 40 Therefore, reducing exposure to aerosol production is vitally important to the safety of healthcare workers.
Masks and Respirators
More than 1,700 HCWs had confirmed COVID-19 in China due to lack of self-protection as of February 11, 2020.41 Meanwhile, one case report described 41 HCWs (85% wearing surgical masks, and the rest wearing N95 respirators) who were exposed for at least 10 minutes during AGMPs, including intubation, extubation, and noninvasive ventilation. After 2 weeks of quarantine, it was reported that no one developed symptoms, and all COVID-19 Polymerase chain reaction (PCR) tests were negative.42 This raises the question as to what the safety efficiency is for the use of surgical masks combined with other standard procedures compared with using respirators during COVID-19 pandemics. Masks and respirators are recommended for diseases spread by droplet transmission and aerosol transmission, but recommendations and terminology differ among the various different guidelines.43
A surgical mask prevents aerosol produced by the wearer from spreading to the patient or into the environment, which is the original design purpose. At the same time, it can be used as a liquid barrier to prevent the wearer from being contaminated by blood and large droplets.44 The N95 respirator is a National Institute for Occupational Safety and Health (NIOSH) certified respiratory protection device designed to reduce aerosol exposure. The term “N95” indicates that the respirator blocks at least 95 percent of test particles of 300 nm. Laboratory studies have shown that the most penetrating particle size (MPPS) of N95 respirators is 0.03-0.1 μm, and of surgical masks, it is approximately up to 0.3 μm.45 Surgical masks may not provide substantial protection from aerosol of at least up to 0.5 μm.45 Furthermore, it has been shown that for nano-sized airborne viral agents, the blocking ability of some N95 respirators may be less than 95%, which was even lower for surgical masks.44 Both SARS-CoV-2 and SARS-CoV are about 85 nm in size,46 and it can be inferred that the new Coronavirus that causes COVID-19 is of similar size. Table 2 shows the risk situations described in influenza guidelines12 and recommendations according to the World Health Organization (WHO).11 It is recommended for HCWs in direct contact with infected patients to wear N95 respirators, not surgical masks, during the current COVID-19 epidemic.
There have been several clinical studies comparing the effectiveness of N95 respirators and medical masks on the protection of the virus’s infection. As shown in Table 1, 6 studies included in meta-analysis10 showed no significant difference between N95 respirators and surgical masks in terms of protective effect. At the same time, this review collected 23 surrogate exposure studies, which showed that N95 respirators were associated with less filter penetration, less face-seal leakage, and less total inward leakage under experimental laboratory conditions. Similarly, the remaining 2 studies from Table 1 indicated that the N95 was superior in 1 situation5 and equal to the surgical mask in the other one.6 The mixed results of these studies fail to identify the superior choice for facial PPE among N95 respirators and surgical masks, and thus, indicate that more RCTs are needed to make a clinical conclusion. However, some sources advocate for the use of respirators in some risk situations, as shown in Table 2.12 In addition to using masks and respirators, successful prevention of disease spread also relies on education programs, user compliance, and other preventative hygiene protocols.
There are few studies analyzing the cost-effectiveness of masks. A study conducted during the influenza season in China showed that the cost of wearing an N95 in order to prevent a single case of a clinical respiratory illness (CRI) was US $490 to $1,230 more than if only medical masks were worn. In a high incidence period, the incremental cost can even be much lower, which suggests continuously using respirators may be a cost-effective choice when there is a pandemic like COVID-19.47 This information provides new evidence for effective allocation of medical resources and medical decision making at the present time.
Mask and Respirator Extended Use/Reuse Guidelines
Mass General Brigham (MGB) is the largest healthcare system in Massachusetts, with 12 hospitals and more than 75,000 employees. In March 2020, extended use and reuse policies for masks and respirators have been adopted from the CDC published guidelines.34 , 48 Universal masking of all HCWs and patients with surgical masks at MGB was associated with a significantly lower rate of SARS-CoV-2 positivity among HCWs. This association may be related to a decrease in transmission between patients and HCWs and among HCWs.49 Extended use of N95 respirators is allowed after AGMP in patients with presumed viral respiratory symptoms such that they can continue to be worn to see other patients, but once removed, must be discarded and not redonned or reused (see Fig 1). If AGMP is done in patients with no symptomatology of a viral respiratory illness, then the N95 respirator may be redonned and reused after it has been doffed. Given that extended use is preferred over reuse, it is highly recommended to avoid the removal of N95 respirators as much as possible between patient encounters. At our institution, designated receptacles (eg labeled storage containers such as a paper tray, paper bag, emesis basin) are used to store all N95 still eligible for reuse (based on the above criteria). The American Dental Association (ADA) and NIOSH recommend limiting the N95 reuse to 5 times,35 and N95 are believed to provide protection as designed for 8 hours of continuous or intermittent use.34 With some oral-maxillofacial surgeries extending beyond 8 hours, particularly extensive orthognathic or craniofacial surgeries, resections, and reconstructions, and for surgeons with longer average operating times, it may be more appropriate to wear a powered air-purifying respirator (PAPR) or controlled air-purifying respirator (CAPR). Additionally, it has also been found that SARS-CoV-2 can last up to 72 hours on plastic, cardboard, and stainless steel, suggesting that donning and doffing of used, but nonsoiled, respirators requires great caution so as not to contaminate the inside of the mask or oneself.50
Both N95 and surgical masks that are not soiled or damaged after use in clinical settings should be limited to only one work shift. In situations when N95 respirators are used in patients with viral respiratory symptoms, extended use guidelines should be applied, which dictate that the N95 respirator be discarded the next time it is removed.34
Face Shields and Overlying Masks
The CDC reports that the benefit of face shields in preventing viral spread is not completely known, and as such, they advocate for cloth masks over face shields as the primary source of mouth and nose protection for the general public.51 However, face shields worn by OMSs may serve as a second line of defense against aerosols and splatter when worn over masks or respirators. One notable consideration with adding a face shield over a mask or respirator is that many OMSs wear loupes to perform procedures, which often stick out and prevent face shields from folding down completely. Some oral-maxillofacial researchers have looked into more appropriate designs that provide protection in a more customized way with the help of 3D printed face shields.52 Properly designed face shields can ultimately help extend the usefulness of respirators, especially when in short supply. The protective effect of the face shield is especially important for AGMPs in patients without respiratory symptoms, where contaminating the mask would otherwise relegate it to be discarded the next time it is doffed, as opposed to being reusable if not visibly soiled. One study of influenza-laden cough aerosols found that face shields worn by providers reduce the surface contamination of a respirator by 97% when the provider is about 18 inches from the patient.36 This benefit could lead to greater respirator reuse over time, and overall greater PPE efficiency, especially since face shields can be cleaned and continually reused. Another benefit is that face shields create a barrier preventing inadvertent or subconscious urge to scratch or touch one’s own face. In a similar fashion, wearing a surgical mask over an N95 respirator for AGMP can help prevent direct contamination of the respirator to extend its use, especially since they are reusable and more difficult to manufacture than more simple surgical masks.
Effects of Prolonged Respirator use on Healthcare Providers
Wearing masks for extended periods of time can be uncomfortable. Wearing an N95 respirator for extended periods of time can lead to nausea, shortness of breath, complaints of visual challenges, headache, lightheadedness, and difficulty with communication.53
It is also important to consider provider fatigue and barriers to compliance. One study found that wearing an N95 with an overlying surgical mask resulted in greater blood CO2 levels as compared to wearing an N95 alone. Although these levels of CO2 never met the definition of clinical hypercapnia (ie 45 mmHg or greater arterial CO2 levels), 25% of the time, respirators needed to be removed due to discomfort over a 12-hour shift.53 The CDC recommends taking scheduled breaks where providers can remove their respirators in a safe area.54 Given the preference for extended use of respirators over reuse, we recommend avoiding respirator removal between AGMP and blocking scheduled AGMP patients in order to conserve supplies. Also, for longer procedures, such as in the OR, PAPR, and CAPR may be more appropriate choices over N95 respirators.
Another issue is the discomfort experienced by the prolonged use of masks with ear loops. This has motivated some to use ear guards or ear relief caps for face masks with ear loops55 or alternative mask designs such as those that have two sets of strings that tie around the back of one’s head. Greater mask/respirator comfort theoretically reduces the need to adjust the mask leading to fewer opportunities for contamination of oneself or others and for greater overall compliance.
Role of Cloth Masks
As OMSs and other healthcare providers struggled to acquire mask supplies during some of the busiest periods of the pandemic, community members were also asked to take precautions by wearing masks. However, with masks and respirators in short supply, many have sought out homemade or cloth masks. Although cloth masks are not recommended for healthcare providers during direct patient care, the general public is encouraged to wear some type of mask, regardless of the type.56 Cloth masks, in particular, should be washed daily with soap and water.57 Oral-maxillofacial surgery patients presenting to the clinic or hospital should, at a minimum, wear a mask, either a disposable facemask or cloth mask. Doing so provides a barrier to protect patients from one another when in the same waiting room and clinical areas.
Mouth Rinse
A recent study by Bidra et al58 showed that povidone-iodine (PVP-I) oral antiseptic rinse of various concentrations can inactivate SARS-CoV-2 within 15 seconds, suggesting potential utility as an adjuvant to existing treatment algorithms for treating dental and oral-maxillofacial surgery patients with known or unknown COVID-19 status. By reducing the infectivity of oral secretions, the clinician will be afforded greater protection during AGMP in the oral-maxillofacial surgery clinic or operating room and will ultimately reduce the burden on masks and N95 respirators as the sole mechanism of preventing SARS-CoV-2 transmission. In addition, it could also reduce the infectivity of the smaller aerosols that unknowingly contaminate the provider’s mask.
Choices for Oral and Maxillofacial Surgeons and Anesthesiologists in Routine, Daily Activities During COVID-19 Pandemic
In the midst of COVID-19, the daily work of the OMS must continue to be carried out in a routine and orderly fashion. The question remains of how to choose among different forms of mouth and nose protection and how to then use them properly to adequately protect HCWs. Other options for mouth and nose protection are also available, such as PAPR, CAPR, and elastomer half-face respirators (EHFRs) for OMSs and anesthesiologists, and barrier enclosures with/without negative pressure for patients.59 These high-level PPE are often available in limited quantities and require complicated don/doff procedures, and are, therefore, only used in select situations. For OMSs and anesthesiologists in most countries and patient care settings, surgical masks and N95 respirators are the most commonly used daily mouth and nose protection equipment. There are two major considerations when making a choice: first, the classification of the patient, whether he/she is with or without fever, or with suspected or diagnosed COVID-19; and second, whether the procedure is in a low- or high-risk situation (Table 2). For OMSs and anesthesiologists, AGMPs are experienced each day in the operating room (OR) and clinic, during bronchoscopy, cardiopulmonary resuscitation, manual ventilation, tracheal intubation, and extubation, suctioning, and surgeries (especially using drills and ultrasonic instruments). In most cases, the patient who is undergoing surgery has been graded for risk before being admitted to the operating room. In the operating room, some of the most high-risk or aerosol producing moments involve anesthesia induction and intubation, patient awakening and extubation, and during aerosol generation from surgical instrumentation.60 Since OMSs are often aiding the anesthesiologist with routine or difficult intubations by holding jaw thrust or holding the tongue forward, they are also susceptible to aerosol generated from patient coughing. Aerosol production from coughing is especially likely during awake fiberoptic intubations for difficult airways such as for severe odontogenic infections. As a result, anesthesia clinicians have developed intubation hoods that are draped over the patient during traditional endotracheal intubation and extubation, and most recently, for fiberoptic intubation.61 For oral-maxillofacial surgical trainees and attendings, having a barrier during intubation and extubation may help augment the protection provided by wearing a respirator. Regarding AGMP from surgical instrumentation in the operating room, it is advantageous to supplement N95 respirator use with a face shield if possible, or at least an overlying surgical mask to protect respirators from direct contamination that may compromise their filtering capacity (Fig 1). Patients that have tested negative for COVID-19, or are afebrile and asymptomatic, can be treated with standard PPE inside the operating room as was done pre-COVID.
At the same time, OMSs and anesthesiologists are also involved in medical procedures outside the OR, such as outpatient clinics, patient transport, and care at the bedside, which do not involve a significant risk of AGMPs. However, in areas outside the OR, there exists a large number of patients and people with unknown nosocomial transmission risk. As such, it is necessary to assess the risk of each location and respond with appropriate PPE.62 For example, the emergency room and fever clinics are more likely to have COVID-19 patients than routine outpatient clinics and wards. Table 3 summarizes the recommendations for the choice of a surgical mask and N95 respirator in the daily work of OMSs and anesthesiologists, and Table 4 organizes the current literature guiding OMSs on the mask or respirator use using the two aforementioned PPE selection criteria (ie patient COVID-19 risk and AGMP risk).
In summary, aerosol production during many AGMPs poses a significant risk of COVID-19 transmission. AGMPs are routinely experienced in the daily work of OMSs and anesthesiologists. We have briefly reviewed the comparative protective efficiency between masks and respirators and have synthesized that information into our Tables and workflows. Comprehensive assessment of the risks and protective efficiency of surgical masks and N95 respirators is vital as PPE is in short supply. A large number of procedures performed by OMSs and anesthesiologists are AGMPs, with other procedures having an unknown nosocomial transmission risk, thereby making the decision on how to properly and efficiently choose PPE for mouth and nose protection critically important. At this point, COVID-19 has spread to more than 200 countries, and given the difference in medical resources and personnel found at various hospitals, adjustments will have to be made to these recommendations based on what is available and feasible. Timely communication and prompt review of protocols around PPE will continue to be extremely important in optimally protecting HCWs.
Footnotes
Mingzhu Zhang and Andrew Robert Emery equally contributed to this article.
Conflict of Interest Disclosures: None of the authors have any relevant financial relationship(s) with a commercial interest.
References
- 1.Loeb M., Dafoe N., Mahony J., et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: A randomized trial. JAMA. 2009;302:1865. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 2.MacIntyre C.R., Wang Q., Cauchemez S., et al. A cluster randomized clinical trial comparing fit-tested and non-fit-tested N95 respirators to medical masks to prevent respiratory virus infection in health care workers. Influenza Other Respi Viruses. 2011;5:170. doi: 10.1111/j.1750-2659.2011.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacIntyre C.R., Wang Q., Rahman B., et al. Efficacy of face masks and respirators in preventing upper respiratory tract bacterial colonization and co-infection in hospital healthcare workers. Prev Med (Baltim) 2014;62:1. doi: 10.1016/j.ypmed.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacIntyre C.R., Wang Q., Seale H., et al. A randomized clinical trial of three options for N95 respirators and medical masks in health workers. Am J Respir Crit Care Med. 2013;187:960. doi: 10.1164/rccm.201207-1164OC. [DOI] [PubMed] [Google Scholar]
- 5.MacIntyre C.R., Chughtai A.A., Rahman B., et al. The efficacy of medical masks and respirators against respiratory infection in healthcare workers. Influenza Other Respi Viruses. 2017;11:511. doi: 10.1111/irv.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radonovich L.J., Simberkoff M.S., Bessesen M.T., et al. N95 respirators vs medical masks for preventing influenza among health care personnel: A randomized clinical trial. JAMA. 2019;322:824. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loeb M., McGeer A., Henry B., et al. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10:251. doi: 10.3201/eid1002.030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seto W.H., Tsang D., Yung R.W.H., et al. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet. 2003;361:1519. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Seale H., Yang P., et al. Factors associated with the transmission of pandemic (H1N1) 2009 among hospital healthcare workers in Beijing, China. Influenza Other Respi Viruses. 2013;7:466. doi: 10.1111/irv.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith J.D., Macdougall C.C., Johnstone J., et al. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: A systematic review and meta-analysis. Can Med Assoc J. 2016;188:567. doi: 10.1503/cmaj.150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) 2014. Infection Prevention and Control of Epidemic- and Pandemic-Prone Acute Respiratory Infections in Health Care. Available at: https://www.who.int/csr/bioriskreduction/infection_control/publication/en. Accessed June 12, 2020. [PubMed] [Google Scholar]
- 12.Chughtai A.A., Seale H., Macintyre C.R. Availability, consistency and evidence-base of policies and guidelines on the use of mask and respirator to protect hospital health care workers: A global analysis. BMC Res Notes. 2013;6:1. doi: 10.1186/1756-0500-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barca I., Cordaro R., Kallaverja E., et al. Management in oral and maxillofacial surgery during the COVID-19 pandemic: Our experience. Br J Oral Maxillofac Surg. 2020 doi: 10.1016/j.bjoms.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh T.-Y., Dedhia R.D., Chiao W., et al. A guide to facial trauma triage and precautions in the COVID-19 pandemic. Facial Plast Surg Aesthet Med. 2020;22:164. doi: 10.1089/fpsam.2020.0185. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Department of Energy (DOE) DOE-STD-3025-2007, Quality Assurance Inspection and Testing of HEPA Filters. https://www.standards.doe.gov/standards-documents/3000/3025-astd-2007/@@images/file Available at: Accessed June 12, 2020.
- 16.EMW filtertechnik GmbH ISO 29463 - New test standard for HEPA Filters. https://www.emw.de/en/filter-campus/iso29463.html Available at:
- 17.Panesar K., Dodson T., Lynch J., Bryson-Cahn C., Chew L., Dillon J. Evolution of COVID-19 guidelines for University of Washington oral and maxillofacial surgery patient care. J Oral Maxillofac Surg. 2020;78:1136. doi: 10.1016/j.joms.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominiak M., Rózyło-Kalinowska I., Gedrange T., et al. COVID-19 and professional dental practice. The Polish Dental Association working group recommendations for procedures in dental office during an increased epidemiological risk. J Stomatol. 2020;73:1. [Google Scholar]
- 19.Magennis P., Coulthard P. 2020. FFP3 Masks with Valves Should Be Avoided to Reduce Risk to Patients during Close Interactions when a Clinician Is Unknowingly COVID Positive. [Google Scholar]
- 20.Grant M., Schramm A., Strong B., et al. 2020. AO CMF International Task Force Recommendations on Best Practices for Maxillofacial Procedures during COVID-19 Pandemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The National Personal Protective Technology Laboratory (NPPTL) 42 CFR Part 84 respiratory protective devices. https://www.cdc.gov/niosh/npptl/topics/respirators/pt84abs2.html Available at:
- 22.Lee S.-A., Hwang D.-C., Li H.-Y., et al. In: J Healthc Eng. Affatato S., editor. 2016. Particle size-selective assessment of protection of European standard FFP respirators and surgical masks against particles-tested with human subjects; p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmermann M., Nkenke E. Approaches to the management of patients in oral and maxillofacial surgery during COVID-19 pandemic. J Cranio-maxillo-facial Surg. 2020;48:521. doi: 10.1016/j.jcms.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Société Française de Stomatologie, Chirurgie Maxillo-Faciale et Chirurgie Orale (SFSCMFCO) Practitioners specialized in oral health and coronavirus disease 2019: Professional guidelines from the French society of stomatology, maxillofacial surgery and or. J Stomatol Oral Maxillofac Surg. 2020;121:155. doi: 10.1016/j.jormas.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y., Soh H.Y., Cai Z.G., et al. Experience of diagnosing and managing patients in oral maxillofacial surgery during the prevention and control period of the new coronavirus pneumonia. Chin J Dent Res. 2020;23:57. doi: 10.3290/j.cjdr.a44339. [DOI] [PubMed] [Google Scholar]
- 26.ASTM International Astm F2100 - 19e1 standard specification for performance of materials used in medical face masks. https://www.astm.org/Standards/F2100.htm Available at:
- 27.Crosstex: The right mask for the right task! https://cdn.vivarep.com/contrib/va/documents/al_lib_44.2015112134294585.pdf Available at:
- 28.Institute of Medicine, Preventing Transmission of Pandemic Influenza and Other Viral Respiratory Diseases: Personal Protective Equipment for Healthcare Personnels. Update 2010. Washington, DC: The National Academies Press; 2011 [PubMed]
- 29.Bali R.K., Chaudhry K. Maxillofacial surgery and COVID-19, the pandemic!! J Maxillofac Oral Surg. 2020;19:159. doi: 10.1007/s12663-020-01361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarkson J., Ramsay C., Aceves M., et al. 2020. Recommendations for the re-opening of dental services: A rapid review of international sources | COVID-19 dental services evidence review (CoDER) working group; p. 1. [Google Scholar]
- 31.OSHA Assigned protection factors for the revised respiratory protection standard. 2009. https://www.osha.gov/Publications/3352-APF-respirators.pdf Available at:
- 32.3MTM health care particulate respirator and surgical mask 1860, N95 120 EA/Case | 3M United States. https://www.3m.com/3M/en_US/company-us/all-3m-products/∼/3M-Health-Care-Particulate-Respirator-and-Surgical-Mask-1860-N95-120-EA-Case/?N=5002385+3294795990&preselect=3293786499&rt=rud Available at:
- 33.3MTM earloop procedure face mask 1820 | 3M United States. https://www.3m.com/3M/en_US/company-us/all-3m-products/∼/3M-Earloop-Procedure-Face-Mask-1820/?N=5002385+3294796142&rt=rud Available at:
- 34.Recommended guidance for extended use and limited reuse of N95 filtering facepiece respirators in healthcare settings | NIOSH | CDC. https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html Available at:
- 35.ADA . 2020. Extending the Use of N95 Masks. [Google Scholar]
- 36.Lindsley W.G., Noti J.D., Blachere F.M., et al. Efficacy of face shields against cough aerosol droplets from a cough simulator. J Occup Environ Hyg. 2014;11:509. doi: 10.1080/15459624.2013.877591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tellier R., Li Y., Cowling B.J., Tang J.W. Recognition of aerosol transmission of infectious agents: A commentary. BMC Infect Dis. 2019;19:1. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies A., Thomson G., Walker J., Bennett A. A review of the risks and disease transmission associated with aerosol generating medical procedures. J Infect Prev. 2009;10:122. [Google Scholar]
- 39.Judson S.D., Munster V.J. Nosocomial transmission of emerging viruses via aerosol-generating medical procedures. Viruses. 2019;11:940. doi: 10.3390/v11100940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran K., Cimon K., Severn M., et al. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: A systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng L., Qiu H., Wan L., et al. Intubation and ventilation amid the COVID-19 outbreak: Wuhan’s experience. Anesthesiology. 2020:1317. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng K., Poon B.H., Kiat Puar T.H., et al. COVID-19 and the risk to health care workers: A case report. Ann Intern Med. 2020;172:766. doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacIntyre C.R., Chughtai A.A. Facemasks for the prevention of infection in healthcare and community settings. BMJ. 2015;350:1. doi: 10.1136/bmj.h694. [DOI] [PubMed] [Google Scholar]
- 44.Balazy A., Toivola M., Adhikari A., et al. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am J Infect Control. 2006;34:51. doi: 10.1016/j.ajic.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 45.He X., Reponen T., McKay R.T., Grinshpun S.A. Effect of particle size on the performance of an N95 filtering facepiece respirator and a surgical mask at various breathing conditions. Physiol Behav. 2013;47:1180. doi: 10.1080/02786826.2013.829209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yi Y., Lagniton P.N.P., Ye S., et al. COVID-19: What has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mukerji S., MacIntyre C.R., Seale H., et al. Cost-effectiveness analysis of N95 respirators and medical masks to protect healthcare workers in China from respiratory infections. BMC Infect Dis. 2017;17:1. doi: 10.1186/s12879-017-2564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massachusetts General Hospital - extended use, reuse, and conservation of personal protective equipment policy. 2020. [Google Scholar]
- 49.Wang X., Ferro E.G., Zhou G., Hashimoto D., Bhatt D.L. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. JAMA. 2020;324:703. doi: 10.1001/jama.2020.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doremalen N van, Bushmaker T., Morris D.H., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.COVID-19: Considerations for wearing cloth face coverings | CDC. 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/cloth-face-cover-guidance.html Available at:
- 52.Amin D., Nguyen N., Roser S.M., Abramowicz S. 3D printing of face shields during COVID-19 pandemic: A technical note. J Oral Maxillofac Surg. 2020;78:1275. doi: 10.1016/j.joms.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rebmann T., Carrico R., Wang J. Physiologic and other effects and compliance with long-term respirator use among medical intensive care unit nurses. Am J Infect Control. 2013;41:1218. doi: 10.1016/j.ajic.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.The physiological burden of prolonged PPE use on healthcare workers during long shifts | | Blogs | CDC. https://blogs.cdc.gov/niosh-science-blog/2020/06/10/ppe-burden/ Available at:
- 55.If COVID-19 face masks hurt your ears, try these solutions | Miami Herald. https://www.miamiherald.com/news/coronavirus/article242196196.html Available at:
- 56.Advice on the Use of Masks in the Context of COVID-19: Interim Guidance. WHO; 2020. [Google Scholar]
- 57.Chughtai A.A., Seale H., Macintyre C.R. Effectiveness of cloth masks for protection against severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26 doi: 10.3201/eid2610.200948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bidra A.S., Pelletier J.S., Westover J.B., et al. Rapid in-vitro inactivation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse. J Prosthodont. 2020;2:1. doi: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Canelli R., Connor C.W., Gonzalez M., et al. Barrier enclosure during endotracheal intubation. N Engl J Med. 2020;382:1957. doi: 10.1056/NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chanpong B, Tang M, Rosenczweig A, Lok P, Tang R: Aerosol-generating procedures and simulated cough in dental anesthesia [e-pub ahead of print]. Anesth Prog. 10.2344/anpr-67-03-04, accessed September 20, 2008 [DOI] [PMC free article] [PubMed]
- 61.Emery A.R., Saniukovich O., Lang A.L., et al. A novel approach to fiberoptic intubation in patients with coronavirus disease 2019. J Oral Maxillofac Surg. 2020;78:2182. doi: 10.1016/j.joms.2020.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M., Zheng H., Wang J. Strategy of using personal protective equipment during aerosol generating medical procedures with COVID-19. J Clin Anesth. 2020;66 doi: 10.1016/j.jclinane.2020.109911. [DOI] [PMC free article] [PubMed] [Google Scholar]