Abstract
Introduction
SLE is increasingly recognized as an important risk factor for cardiovascular disease. Premature CAD and several other cardiac manifestations are resulting in significant morbidity and premature death among young and older adults. There is a considerable unmet need for developing specific guidelines towards the primary and secondary prevention of cardiovascular disease in SLE patients.
Areas covered
The authors describe the prevalence of various cardiovascular manifestations, associated with traditional and lupus specific risk factors.They summarize the evidence behind various nonpharmacological and pharmacological options such as cardiac medications, antimalarials, anti-inflammatory and immunosuppressant medications.
Expert opinion
There is considerable literature claiming that the traditional Framingam score used to calculate the risk in the general population would not clearly predict the 10-year risk among SLE patients as they don’t include lupus specific risk factors such as accelerated inflammation, immunometabolic changes, thrombosis, vasospasm, vasculitis and endothelial dysfunction into account. Identifying potential risk factors among SLE patients and treating hyperlipidemia regardless of their risk scores may be the first step in reducing mortality. Blocking lupus-specific inflammatory pathways by targeting validated biomarkers of pathogenesis have great future potential and more studies are needed on their cardiovascular benefits.
Keywords: Systemic Lupus erythematosus, cardiovascular disease, hydroxychloroquine, atherosclerosis, endothelial dysfunction
1. Introduction
Systemic Lupus Erythematosus (SLE) is a complex autoimmune disease, that has been recognized as one of the important risk factors for increased cardiovascular morbidity and mortality, among young and older adults.[1,2] Mortality in SLE follows a bimodal pattern, first peak caused by disease activity, infections and the second peak caused by cardiovascular disease (CVD).[3] A meta-analysis of observational studies done in SLE showed a standardized mortality ratio of 2.72 for CVD related death when compared with the general population.[4] Although the improvement in CVD mortality seen in the general population, a similar trend was not observed among SLE patients.[5] The etiology behind increased CVD in SLE patients is not completely elucidated, however, various pathophysiologic mechanisms like accelerated atherosclerosis, thromboembolism, arteritis, vasospasm, and abnormal coronary flow were proposed in the literature.[6] Understanding the spectrum of CVDs and the associated traditional and novel risk factors will help us to develop more targeted prevention and treatment strategies to reduce the high CVD burden. Accelerated atherosclerosis observed due to chronic inflammation in autoimmune diseases like SLE, RA and psoriasis thus American College of Cardiologists recommended considering SLE as one of the important risk factors in addition to the traditional ones.[7] Various treatment strategies like anti-inflammatory therapy, lipid-lowering therapy, anti-platelet agents were proposed to reduce morbidity from CVD. In this review, we evaluated the studies published over the last three of decades related to the pathogenesis and management of cardiovascular disease in SLE.
2. CVD types and their prevalence in SLE
The prevalence of cardiac diseases in SLE is reported to be higher than 50% of the SLE patients at some point in their life.[8] SLE increases the risk of a wide variety of cardiovascular diseases and is listing the different types below.
Coronary artery disease (CAD) - clinical and subclinical forms
Infectious and non-infectious endocarditis
Myocarditis, cardiomyopathy
Pericarditis, Pericardial effusion and tamponade
Cardiac arrhythmias (tachyarrhythmias and bradyarrhythmias)
Peripheral vascular disease
Cerebrovascular accident
Venous thromboembolism (deep vein thrombosis, pulmonary embolism, cerebral venous sinus thrombosis)
Pericarditis and pericardial effusion related to SLE are reported in nearly a quarter of the patients and the majority of the patients with pericardial effusion are asymptomatic.[9,10] Cardiac tamponade or hemopericardium also reported and very rarely they could be the initial presentation in SLE.[11–15] Autopsy studies done in SLE patients showed myocardial involvement around 40% patients, however, the prevalence of clinical myocarditis seemed to be around 10% or lower.[16–18] The natural progression of the myocarditis may vary from complete resolution to dilated cardiomyopathy, conduction abnormalities, tachyarrhythmias and heart failure, however majority of them the prognosis is positive.[19] Endocardial and valvular disease in lupus can occur with or without antiphospholipid syndrome (APS).[20,21] Symptomatic coronary artery disease occurs in 6–12 % of the SLE patients and asymptomatic subclinical disease occur in 40% of the patients.[22,23] Bhatt et al demonstrated a 28 % prevalence of PVD in 50 SLE patients they studied.[24] Secondary APS in SLE can also lead to various arterial and venous thromboembolic manifestations. CKD from lupus nephritis, drugs that are not commonly used like cyclosporine could cause hypertension or worsen pre-existing hypertension in SLE patients. SLE patients also suffer from pregnancy-related CVD like early-onset pre-eclampsia and other thrombotic microangiopathies.
3. Traditional, and SLE-specific risk factors for CAD
Risk factors associated with CAD in SLE could be broadly classified as traditional and lupus specific risk factors (Tables 1 and 2). The impact of the traditional risk factors for atherosclerosis in SLE patients is not clearly established as it for the general population. The Hopkins lupus cohort reported an 8 % cumulative incidence of CAD and identified an increased prevalence of at least 3 traditional risk factors in 53% of their cohort.[22] A Toronto cohort study of 250 women with SLE also identified an increased prevalence of diabetes, hypertension, hyperlipidemia, and early onset menopause.[25] A study demonstrated nearly 75.4 % of the patients developed hyperlipidemia within 3 years of diagnosis, 40.3% of has sustained elevation in their lipid levels and the best predictors for the sustained elevation of lipids were an age of onset of SLE >35 years, medications such as the presence of steroids, and absence of hydroxychloroquine.[26] CAD-related mortality observed in SLE patients was significantly higher than the prediction from Framingham 10 year prediction model.[27] There are various lupus specific factors that play a role in CAD and the important ones are inflammation with elevated pro-inflammatory cytokines, homocysteinemia, antiphospholipid antibodies, and renal disease and proteinuria increases the risk of atherosclerosis and thrombosis. [28] Arterial dysfunction markers such as increased systemic arterial stiffness diminished regional wall flexibility were demonstrated in SLE patients by showing increased aortic augmentation index, and increased carotid-radial pulse wave velocity.[29]
Table 1.
Traditional risk factors and therapeutic interventions
| Classical/Traditional CVD risk factors | Therapeutic interventions | References |
|---|---|---|
| Diabetes | Insulin and oral hypoglycemic drugs | [30] |
| Hypertension | Anti-hypertensives | [31] |
| Hyperlipidemia | Statins and other lipid lowering drugs | [32] |
| Obesity | Diet, exercise, FDA approved medications and surgery | [33] |
| Smoking | Smoking cessation | |
| Family history of CAD | Nothing specific other than controlling risk factors |
Table 2.
SLE-specific risk factors and therapeutic interventions.
| Lupus specific risk factors | Therapeutic interventions | References |
|---|---|---|
| Chronic inflammation with elevated pro-inflammatory cytokines | Hydroxychloroquine, anti-interferon therapy | [34–38] |
| Antiphospholipid antibodies | Anticoagulation, immunosuppression, hydroxychloroquine | [39] |
| Immune complex deposition with vasculitis | Immunosuppressants | [40] |
| Type 1 interferon pathway activation in plasmacytoid dendritic cells, neutrophils, and endothelium | Anti-interferon therapy | [41] |
| Lupus related renal disease, GN and IN | Immunosuppressants, Renal replacement therapy | [42] |
| Increased homocysteine and decreased mannose-binding lectin | Although no specific interventions, folic acid and vitamin B12 are beneficial in hyper-homocysteinemia patients | [43] |
| Drugs used in lupus treatment | Usually the benefits outweigh the risk, avoid when it is not | [44] |
| Oxidative stress, mTOR pathway activation | Rapamycin, NAC | [45–47] |
| Arthritis – diminished exercise tolerance due to pain | Treatment of arthritis, symptomatic therapy and physical therapy | [48,49] |
| Serositis: pericarditis, pleuritis | Immunosuppressants | [50] |
| Myocarditis, arrhythmia | Immunosuppressants, anti-arrhythmics | [51] |
4. Management of coronary artery disease disease in SLE
A combination of pharmacological and non-pharmacological approaches targeting the atherosclerosis, thrombosis and inflammation (Figure 1) with good compliance may contribute to an effective model of cardiovascular disease management in SLE. Few studies have evaluated the diet, exercise, lifestyle modifications, and behavioral modifications such as smoking cessation in SLE patients. To date, various cardiac medications, biologic and non-biologic immunosuppressants, vitamin and supplements are used to treat cardiovascular manifestations of SLE. The majority of the treatment decisions are not made from high-quality randomized control studies (RCT), rather supported by open label studies, case series, and cross-sectional studies. Although the risk of CAD is higher in SLE patients, we do not have any established guidelines that talk about prevention in this population. Management of cardiovascular disease will depend on the various cardiovascular manifestations and underlying pathology driving the disease. We are highlighting the treatment for each type of cardiovascular disease based on available literature.
Figure 1.
Pro-inflammatory cytokines in SLE like IFN alpha, IL-1, IL-6 and TNF-alpha promotes formation of foam cells, proliferation, platelet adherence, thrombosis and fibrosis. Smoking, hypertension in SLE results in endothelial injury. Impaired glycemic control, DM in SLE can results in endothelial injury and increased lipids. Obesity, sedentary life style in SLE increases LDL and decreases HDL. Pro-atherogenic lipoprotein particles such as LDL infiltrate into the intima of the arterial wall and undergo oxidative modification. Accumulation of these atherogenic particles attracts macrophages that engulf the cholesterol, causing formation of foam cells and subsequent fatty streaks. This process injures the epithelium of the arterial wall, promoting adherence of platelets, release of PDGF, and development of an advanced fibrocalcific lesion. Anti-atherogenic particles such as HDL and apo-A1 promote reverse cholesterol transport and cholesterol efflux from foam cells. They also help to prevent oxidation of LDL and other atherogenic particles. Targets 1 – 8 are proposed to consider for therapeutic interventions to manage cardiovascular disease in SLE.
1 – Drugs used to control inflammation in SLE like hydroxychloroquine, mycophenolate, azathioprine, rapamycin, belimumab, rituximab, cyclophosphamide, steroids, and methotrexate
2 – Anti cytokine therapy like anakinra, anifrolumab
3 – Smoking cessation
4, 5 – Effective treatment of hypertension, diabetes
6 – Diet, and exercise
7 – Statins
8 – Anti platelets and anticoagulants
Abbreviations : Interleukin (IL), Interferon (IFN), TNF – Tumor necrosis factor (TNF), diabetes mellitus (DM), PDGF – Platelet derived growth factor, LDL – low density lipoprotein, HDL – High density lipo protein, ApoA1 – apo lipoprotein A 1, LCAT – Lecithin cholesterol acyl transferase
5. Non-pharmacological measures in reducing CVD in SLE patients
An RCT evaluated the outcome of the low glycemic diet (n=11) and low-calorie index diet (n=12) in 23 SLE patients for six weeks and both diets resulted in significant initial weight reduction, improvement in fatigue and improved hip and waist circumferences.[52] Another RCT omega 3 polyunsaturated fatty acids supplementation in SLE patients who don’t have traditional CAD risk factors, not on prednisone showed significant improvement in endothelial function (evident by elevation in flow-mediated dilatation (FMD) of the brachial artery), disease activity (BILAG and SLAM-R) and reduction in the oxidative stress (demonstrated by a statistically significant reduction in platelet-8-isoprostanes) in the intervention group compared to the placebo group at 12 and 24 weeks.[53] Prospective non-randomized study with 38 SLE patients on the impact of physical exercise for 16 weeks showed statistically significant improvement in fatigue, aerobic capacity, quality of life without affecting the disease activity in the exercise group (n=18) compared to the control group (n=20).[49] A prospective study evaluating supervised exercise in SLE patients for 16 weeks demonstrated improvement in endothelial dysfunction[54,55] and aerobic capacity[49,55] without affecting the disease activity.[55] Prado et al demonstrated that 3 months of exercise improved cardiorespiratory capacity and autonomic function in SLE patients.[56] O’Neil et al demonstrated 47% of smoking cessation out of 308 patients followed for 3 years by adopting a protocol of risk stratification and counseling in SLE patients.[57]
6. Pharmacological management of clinical and subclinical atherosclerotic cardio vascular disease and thrombotic disease in SLE patients
6.1). Management of acute cardiovascular disease in SLE
The approach to treatement of symptomatic CAD in lupus patients depends on nature of the primary driving pathology. If it is acute coronary syndrome or stable angina related atheroscelerotic heart disease guideline directed therapy as per ACC/AHA guidelines recommended.[58,59] If there is an element of Prinzmetal angina from vasospasm vasodilators are used to treat.[60] If there is any evidence of small, medium or large vessel vasculitis steroids, immunosuppressants will be used to treat according to treatment protocol for the specific type of vascultis.[61] The management of acute stroke(AHA/ASA)[62], acute limb ischemia(AHA/ACC)[63], and acute venous thromboembolism manifestations(CHEST)[64] usually follows the respective society guidelines even if they are related to Lupus. As these guidelines are relatively well defined, reviewing them here will be beyond the scope of this review.
6.2). Primary and secondary prevention of cardiovascular disease in SLE
In the Toronto cohort group of SLE patients, treatment of hypertension, hyperlipidemia were compared among two different time periods. Although 88–96% of hypertensive patients received antihypertensive agents, only 21–28 % of hyperlipidemia patients received lipid-lowering agents.[65] One possible reason could be when risk stratifying for future cardiovascular disease SLE are typically not included by primary care physicians, as the traditional models didn’t include SLE. A study evaluating a randomized control prevention trial in reducing traditional CVD risk factors with aggressive use of preventive medications had difficulty in recruiting and retaining patients and also identified several barriers such as lack of enthusiasm in both patients and clinicians.[66] O’Neill et al evaluated the adaptation of a protocol mandating CVD 10-year risk score calculation in SLE patients in lupus clinic, did not alter the management in 96 % of the patients as these patients were already receiving appropriate treatment. [57]
6.1.1. Statins
Cardiovascular benefits of statins in auto immune diseases like lupus are not only limited to its lipid-lowering effect but also from its anti-inflammatory, anti-thrombotic, immunomodulatory and plaque stabilizing effect.[67] A study evaluated the use of Fluvastatin in renal transplant patients in SLE reduced major cardiac events by 73.4 % and LDL reduction by 29.2%.[68] A nationwide case-control study done at Taiwan evaluating 4095 patients with SLE and hyperlipidemia has shown the potential of statin therapy reducing the mortality in CAD and ESRD patients, furthermore high dose statin reduced the mortality in acute myocardial infarction and stroke.[69] A nonrandomized clinical trial evaluated the effect of 20 mg atorvastatin on 64 SLE patients [ CAD risk factors (n=33), without CAD risk factors (n=31)] by comparing the group without atorvastatin, showed improvement of FMD (primary outcome) from 4% to 7 % only in the treatment arm. [70] One year RCT with 60 patients with 40 mg atorvastatin daily (28 receiving atorvastatin 40 mg and placebo n=32) showed decreased coronary calcium score, lipid levels and CRP in the treatment group compared to placebo.[71]
However, two year-RCT with 200 SLE patients without prior CAD risk factors (atorvastatin 40 mg arm n= 99, placebo = 101) evaluated coronary calcium score (primary outcome) and didn’t find any statistically significant difference despite having a significant reduction in the LDL, Triglycerides (secondary outcome) in the treatment group.[72] A study that compared LDL, total cholesterol reduction with pravastatin on SLE and controls found similar reduction however the patients on corticosteroids, the mean reduction was lower.[73] Routine usage of atorvastatin for 3 years in young SLE patients didn’t show any significant reduction in subclinical atherosclerosis however subgroup analysis suggested a targeted group may have some benefits.[74] Few animal studies evaluated the immunomodulatory effects of atorvastatin, however, no survival benefits were noted.[75] The effect of statins on coronary calcium score is contradicting, and the effect of mortality is not clearly elucidated, further studies need to look in to this in the future. The lipid lowering effect of statin has been clearly demostrated in various studies, and benefits of controlling hyperlipidemia in SLE patients regardless of their risk score needs to be evaluated in future.
6.1.2. Anti-hypertensive medications
The pathogenesis of hypertension in SLE is complex, lupus specific risk factors inflammation, renal dysfunction, immune dysfunction and side effects of drugs may play a role.[76] With each 2 % rise in blood pressure, there is a considerably increased mortality risk for stroke (10%) and ischemic heart disease (7%).[77] Herlitz et al evaluated the effect of captopril in treating hypertension in 14 lupus nephritis patients and excellent blood pressure control (178 +/− 7 / 110 +/− 4 to 145 +/− 5/92 +/− 3 mm Hg) was achieved in at least 11/14 patients over 6 months period with varying degrees of renal dysfunction noted in at least 60 % of them.[78] Bursztyn et al. used nifedipine to treat hypertension in 8 patients and had an effective blood pressure control (151.9 +/− 10/103.7 +/− 8.6 mmHg to a mean of 130 +/− 14.1/87.5 +/− 5 mmHg) without altering renal function and other hematological, immunological indexes. [79] In summary patients with lupus have increased prevalence of hypertension due to higher prevalence of renal disease, arterial dysfunction and controlling their hypertension is an important step in reducing the overall cardiovascular risk.
6.1.3. Anti-platelets and anti-coagulant medications
A meta-analysis evaluating the efficacy of aspirin was published analyzing 11 studies (1 RCT and 10 observational studies) including 1208 patients with positive APL and 139 thrombotic events (460 asymptomatic APL antibodies positive, 440 SLE patients, 308 obstetric APS patients) showed a 50 % reduction (OR: 0.5) in who receive aspirin (n=601) compared to those who don’t (n=607).[80] Subgroup analysis revealed aspirin protects from arterial thrombosis (OR: 0.48 [95%CI: 0.28–0.82]) rather than venous thrombosis (OR: 0.58 [95% CI: 0.32–1.06]) in all groups such as asymptomatic APL positive (OR: 0.50 [0.25–0.99]), SLE (OR: 0.55 [0.31–0.98]) and obstetric APS (OR: 0.25 [0.10–0.62]) patients.[80] Individual patient data meta-analysis including 5 international cohort studies (497 patients and 79 thrombotic events) against showed the protective role against arterial thrombosis (HR: 0.43 [95%CI: 0.20–0.93]) but not against venous thrombosis (HR: 0.49 [95%CI: 0.22–1.11]) in SLE (HR: 0.43 [95%CI: 0.20–0.94]) patients and asymptomatic APL positive patients (HR: 0.43 [95%CI 0.20–0.93]). [81] A open RCT (n = 232) compared low dose aspirin (LDA) against LDA + warfarin in asymptomatic APL positive SLE and/or obstetric morbidity patients found no difference in preventing thrombosis and more episodes of bleeding observed in LDA + warfarin group.[82] A prospective cohort study over 5 years with 272 SLE patients evaluated the effectiveness of primary and secondary prevention of myocardial infarction and stroke. Patients were categorized in to 3 groups; SLE + APS (n=84), SLE + asymptomatic APL careers (n = 81) and SLE without APL antibodies (n= 107). Primary prophylaxis was used in 52 APL positive SLE patients (50 - aspirin, 1 - clopidogrel, 1 – warfarin), 79/84 patients with APS patients (23 - Coumadin, 17- LDA + Coumadin, 37 – LDA, 2 – clopidogrel) At the end of the study, in APL positive group, one patient on LDA and 2 patients without prophylaxis developed strokes and in the APS group, there were 2 myocardial infarction and 5 strokes. [83] In summary benefits of anti platelet therapy have been demonstrated in SLE with or without APL antibodies, however, future studies need to evaluate whether the bleeding risk associate with it would outweigh the overall benefits or not.
6.1.4. Hydroxychloroquine
To date, we have few studies showing the benefit of hydroxychloroquine in improving glycemic control and reducing thrombotic events in SLE patients.[34–38] Penn et al evaluated the relationship with glycemic control of nondiabetic women on hydroxychloroquine in SLE (n = 149) and rheumatoid arthritis (n=177) by using fasting blood glucose and insulin sensitivity as 2 indicators over the period of 16 years. The SLE patients on hydroxychloroquine had better glycemic control (fasting 87.5 mg/dL vs 91.5 mg/dL, p = 0.009) increased insulin resistance by homeostasis model assessment method; HOMA-IR (2.51 vs 2.87, p = 0.046) compared to the control group. Furthermore LDL levels also decreased in the treatment group compared to the control group (102 mg/dL vs 118 mg/dL, p=0.004).[35] A systematic review of English literature between 1982–2007 including 95 articles high-level evidence for increased survival and moderate evidence for protection against thrombosis.[34] Three other individual studies also demonstrated the benefit of antimalarials by showing a significant reduction in thrombotic events in SLE patients.[36–38] In summary the cardiovascular benefit of antimalarials is ovewhelming compared to the small risk of cardiomyopathy.
6.1.5. Belimumab
Belimumab is the first FDA approved biologic agent works by inhibiting B-lymphocyte stimulating protein. Parodis et al. surveyed the patients who received belimumab at a dose of 10 mg/kg in BLISS-52 and BLISS −76 trials and found cardiovascular damage had negative influence (OR: 0.13; 95% CI 0.02, 0.97; P = 0.047) on achieving remission (SLEDAI-2K = 0) along with limited or no steroid use.[84] Belimumab is widely used to treat SLE with mild to moderate SLE; future studies need to evaluate the long-term cardiovascular benefits.
7. Potential new treatments for atherosclerotic and thrombotic cardiovascular diseases in SLE
7.1. mTOR inhibitors.
The mechanistic target of rapamycin (mTOR) pathway is increasingly recognized as one of the key drivers of pro-inflammatory responses in autoimmune diseases like SLE.[85] The mTOR has been shown to be an effective target of therapy both in mice [86] and patients with SLE [87–89]. In cardiac and endothelial cells mTOR pathways are crucial for their growth, regulation of apoptosis and autophagy in oxidative stress, angiogenesis and tissue repair.[90] Chronic activation of mTOR can lead to cardiac and vascular dysfunction.[91] Long-term treatment with mTOR inhibitors has resulted in reduction of vasculopathy and improvement in coronary flow status post cardiac transplant.[92,93] Drug eluted stents that are placed during percutaneous coronary intervention also coated with mTOR inhibitor sirolimus to prevent restenosis.[94] Future trials need to evaluate role of mTOR inhibitors in the management of cardiovascular disease in Lupus. Given the newly uncovered role for mTOR in production of antiphospholipid antibodies outside[46] or within the context of SLE[47], their potential role in reducing thrombotic events also needs to be evaluated in future.
7.2. N-acetylcysteine (NAC)
The effectiveness of NAC has been demonstrated in lupus prone mice[95] and patients with SLE[45]. NAC therapy could result in matrix degradation; improve vascular stability in early and late stages of atherosclerosis.[96,97] The potential role of NAC in preventing cardiac myocyte injury in ischemia, reperfusion, preventing thrombosis through antiplatelet activity, activity against vasospasm, the ability to prevent and reverse nitrate tolerance has been demonstrated.[97–99] As there is considerable evidence of several significant benefits of NAC in SLE and cardiovascular disease, future trials need to focus its role in the management of cardiovascular disease in SLE.
7.3. Blockade of interferon signaling
Type 1 interferon plays a major pathogenic role in SLE and increased interferon signature demonstrated in peripheral blood and tissues in SLE patients.[100,101] Recent in-vitro studies have demonstrated the role of interferon alpha in promoting foam cell formation in atherosclerosis[102], increasing thrombosis[103] and may explain the role of interferon alpha in premature CVD associated with SLE. A recent study in a cohort of SLE patients without pre-existing CVD demonstrated that type 1 interferon activity associated with decreased FMD, increased carotid intima media thickness and coronary calcium score in SLE patients.[104] TULIP 1 trial (anifrolumab - anti interferon alpha receptor monoclonal antibody) did not meet its primary end point SRI 4 response, however, TULIP 2 trial met its primary end point BICLA and showed statistically and clinically meaningful increase in BICLA response [41] Future studies need to focus on the role of interferon blockade in managing cardiovascular disease in SLE patients.
7.4. PCSK9 inhibitors
Proprotein convertase subtilisin kexin 9 (PCSK9) is a protein that plays an important role in LDL metabolism by modulating LDL receptor degradation and recycling.[105] In experimental models, shear stress in vascular smooth muscle cells (VSMC) and endothelial cells up-regulated PCSK9 and its pro-inflammatory, pro-atherosclerotic effects were independent of LDL levels.[106,107] Oxidized LDL receptor LOX-1 is increased in VSMC during inflammation. PCSK9 increase LOX-1 transcription and in turn LOX-1 increases PCSK9 levels, resulting in accelerated atherosclerosis during inflammation.[106] In a LDL receptor dependent fashion, PCSK9 also favors the migration of inflammatory monocytes and macrophages.[106] PCSK9 inhibitors such as alirocumab, evolocumab were recently FDA approved to treat hyperlipidemia.[105] Their effect in controlling atherosclerosis in chronic inflammatory diseases needs to be evaluated with human studies.
8. Management of anti-phospholipid syndrome in SLE
Anti-phospholipid syndrome is an acquired pro-atherosclerotic and pro-thrombotic disease, which predisposes to premature CAD, thrombosis and obstetric complications. Following a thrombotic event, life-long anticoagulation is recommended, and the benefits outweigh the bleeding risks.[108] Studies have shown warfarin and enoxaparin are superior to direct anticoagulants.[39] RAPS a randomized, controlled, open label, phase 2/3, non-inferiority trial compared the use of direct oral anticoagulant (DOAC) rivaroxaban with warfarin in patients with thrombotic APS with or without SLE. The primary outcome, endogenous thrombin potential measured on day 42 from randomization with a non-inferiority set at less than 20 % difference from warfarin in mean percentage change, did not meet its non-inferiority threshold for rivaroxaban.[109] However, there was no increased risk of thrombosis or major bleeding observed on 210 days follow up compared to warfarin make us think whether it could be a safe alternative to warfarin.[109] Ordi-Ros J et al compared the rivoraxaban and warfarin in thrombotic APS patients and rivaroxaban didn’t show non-inferiority, in fact, depicted a non-statistically significant increase in recurrent thrombotic events.[110] TRAPS trial reported an increased risk of arterial thrombosis in triple positive APS patients given rivaroxaban compared to warfarin suggesting that DOAC drugs should be avoided in this situation.[111] The trial was stopped prematurely due to the excessive arterial thrombotic events in the rivaroxaban arm.[111] Currently ASTRO-APS trial is comparing apixaban with warfarin for thrombotic APS, and the investigators had to modify their protocol due to increased arterial events in one treatment arm.[112] The current consensus recommends warfarin over DOACs for thrombotic APS; however, emerging evidence may allow the use of DOACs in future in carefully selected individuals, especially lower risk APS group who is not triple positive. LDA and enoxaparin are used in obstetric APS to improve the outcome.[108]
Asymptomatic APL careers also have a higher risk of future CAD, thrombosis and obstetric complications compared to general population. In high-risk APL careers, LDA and hydroxychloroquine is recommended, and the benefit of anticoagulation doesn’t usually outweigh the bleeding risks.[82,108] Catastrophic antiphospholipid syndrome is managed with anticoagulation, steroids and plasmapheresis and in resistant cases B-cell depletion (rituximab) and anti-complement therapy (eculizumab) are recommended.[39]
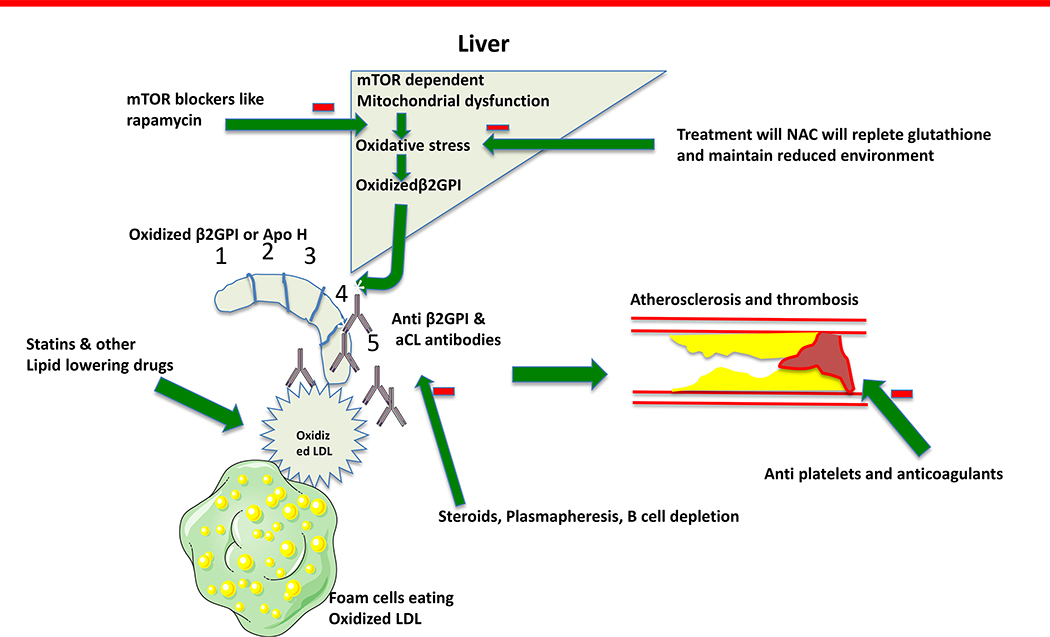
β2-glycoprotein- I (β2GPI) is recognized as the major driving antigen in APS. (Figure 2) It is produced in liver and exists in oxidized and non-oxidized form, studies in mice have shown mTOR dependent mitochondrial dysfunction and oxidative stress increases the oxidized B2GPI production.[47] Oxidized B2GPI with or without oxidized LDL/B2GPI complex binds with APL and increases the atherosclerosis and thrombosis.[113] Rapamycin, an mTOR inhibitor blocked aPL production along with clinical disease in mice; potential cardiovascular athero-thrombotic benefits should be evaluated in humans.[47] NAC helps to restore the reducing environment and controlled trial in SLE patients have shown mTOR blockade with rapamycin and NAC, resulted in reduction of APL antibody levels.[89,114]
Figure 2.
β2GPI is recognized as the primary antigen driving aPL production in APS. It is produced in liver. Only its oxidized form is antigenic. Its oxidization is promoted by mTOR dependent mitochondrial dysfunction. mTOR inhibitors, can be used to reduce the oxidative stress in liver and trials have shown they reduce the aPL production in SLE. Immunosuppressant medications and plasmapheresis are used to reduce the pathogenic antibodies in CAPS. Anticoagulation, and lipid lowering therapy like statin also benefits in thrombosis and atherosclerosis respectively. Abbreviations: aCL – anti cardiolipin, β2GPI – β 2 glycoprotein- I, CAPS – catastrophic anti phospholipid syndrome, mTOR – mammalian target of rapamycin, NAC – N-acetylcysteine, LDL – low density lipoprotein, aPL – Anti phospholipid antibodies, SLE – systemic lupus erythematosus.
9. Management of pericarditis and myocarditis in SLE patients
The first episode of mild to moderate pericarditis is usually treated with oral or parenteral steroids. FLOAT trial randomized 50 SLE patients who presented for mild to moderate SLE flare with pericarditis to receive either intramuscular triamcinolone 100 mg or oral methylprednisolone (Medrol dose-pack) and both groups did equally well at end of month.[115] However, the triamcinolone group had a quicker response compared to the medrol pack group.[115] Patients with recurrent pericarditis usually treated with steroid-sparing disease-modifying anti-rheumatic drugs (DMARDS) like azathioprine, methotrexate, mycophenolate mofetil.[8,116] Colchicine has shown good response in treating recurrent pericarditis especially in subgroups with post-procedural atrial fibrillation and post-pericardiotomy syndrome.[117] Although there are no specific trials in lupus, in general, refractory pericarditis has shown a good response to intravenous immunoglobulin therapy, which could be tried in refractory lupus pericarditis.[118] Anakinra, an interleukin-1 antagonist, has shown promising response in pericarditis in autoimmune/autoinflammatory diseases, however not described lupus, future clinical trials need to evaluate its use in refractory lupus pericarditis.[119–121] Cardiac tamponade or severe pericarditis usually considered as an organ/life-threatening manifestation of lupus and treated with pulse steroid therapy with 1 gram of methylprednisolone.[8] Cardiac tamponade with hemodynamic compromise also should involve cardiology and cardiothoracic surgery for evaluating the necessity for pericardiocentesis, pericardial window or stripping.[8]
Myocarditis is a fatal manifestation of SLE and the majority of the evidence derived from case reports, case-control and cohort studies. The majority of the patients with lupus myocarditis usually respond to treatment with high dose steroids and their cardiac function improves with time.[51,122] Severe cases of myocarditis result in poor cardiac function causing cardiogenic shock and those individual cases are treated with intravenous cyclophosphamide in the past.[123–125] Azathioprine could be used as steroid-sparing maintenance therapy.[126] A 10-year monocentric retrospective cohort study evaluated the use of rituximab in refractory lupus myocarditis in 3 SLE patients and shown improved outcomes in all.[127] There are individual case reports claiming the success of intravenous immunoglobulin therapy in severe lupus myocarditis.[128,129]
10. Expert opinion
There is considerable literature claiming that the traditional Framingham score used to calculate the risk in the general population would not clearly predict the 10-year risk among SLE patients as they don’t include lupus specific risk factors such as accelerated inflammation, thrombosis, vasospasm, vasculitis and endothelial dysfunction into account. Few studies have clearly demonstrated the benefits of diet, exercise and behavioural modifications in improving the cardiovascular health of lupus patients. Identifying potential risk factors such as hypertension, hyperlipidemia, smoking among SLE patients and treating hyperlipidemia regardless of their risk scores may be the first step in reducing mortality. Chronic kidney disease with or without lupus nephritis needs to be aggressively managed as preventing the progression of kidney disease will improve the cardiovascular morbidity and mortality in SLE patients.
Several studies have shown that statin therapy results in lowering lipids, contradicting effect on coronary calcium score and their overall benefit in cardiovascular mortality in SLE patients need to be studied further. The use of anti-platelet and anti-coagulants are beneficial in the primary prevention of thrombotic events in SLE with or without antiphospholipid syndrome. Steroids and other immuno-suppressants are mainstay in the management of lupus myocarditis and pericarditis and treatment tailored according to the severity of the disease. The cardiovascular benefit of hydroxychloroquine is overwhelming compared to the small risk of cardiomyopathy. NAC, mTOR inhibitors, anti-interferon therapy, PCSK 9 inhibitors have great future potential, albeit more studies needed with a clear focus on cardiovascular outcomes. Both the rheumatologists and primary care physicians should know the importance in assessing the cardiovascular risk in SLE patients and further management depends on their risk stratification.
Article Highlights.
SLE is increasingly recognized as an important risk factor for cardiovascular disease young and older adults.
SLE increases the risk of a wide variety of cardiovascular diseases, traditional models don’t include lupus specific risk factors.
Management of cardiovascular disease will depend on the various cardiovascular manifestations and underlying pathology driving the disease.
Todate, various pharmacological and non pharmacological methods were studied in atherosclerotic heart disease in SLE and we are reviewing evidence behind each in detail.
Although anti-platelet agents and statins are very effective secondary prevention of CAD, their effectiveness in primary prevention in SLE patients is controversial due to mixed results in past studies.
Hydroxychloroquine is effective in preventing in artherosclerotic heart disease and manifestations in anti-phospholipid syndrome. The effect of other anti-inflammatory therapies like mTOR inhibitors, belimumab and interferon blockade needs to be explored.
Warfarin is generally preferred over DOACs in antiphospholipid syndrome with or without SLE, however emerging evidence may favor their use in carefully selected individuals who in low risk group.
Acknowledgments
Funding:
This work was supported in part by grants AI072648, AI122176, AI141304, and AR068052 from the National Institutes of Health.
Abbreviations
- ACC
American College of Cardiology
- aCL
anti cardiolipin
- AHA
American Heart Association
- ApoA1
apolipoprotein A1
- aP
anti-phospholipid antibodies
- APS
anti phospholipid syndrome
- ASA
American Stroke Association
- β2GPI
β2 glycoprotein –I
- BILAG
British isles lupus assessment group
- BLISS
Belimumab in Subjects in SLE
- CAD
coronary artery disease
- CAPS
catastrophic APS
- CIMT
carotid intimal medial thickness
- CKD
chronic kidney disease
- CVD
cardiovascular disease
- CI
confidence interval
- DM
diabetes mellitus
- DMARDS
Disease modifying anti rheumatic drugs
- ESRD
end stage renal disease
- FDA
Food and Drug Administration
- FMD
Flow mediated dilatation
- FLOAT
Flares in lupus; outcome assessment trial
- HDL
high-density lipoprotein
- HOMA
homeostasis model assessment
- HR
hazard ratio
- IL
interleukin
- IFN
interferon
- LDL
low density lipoprotein
- LCAT
lecithin cholesterol acyl transferase
- LDA
low dose aspirin
- LOX 1
oxidized LDL receptor 1
- mTOR
mechanistic target of rapamycin
- NAC
N-acetylcysteine
- OR
odds ratio
- PCSK9
proprotein convertase subtilisin kexin 9
- PDGF
platelet-derived growth factor
- PVD
peripheral vascular disease
- RA
rheumatoid arthritis
- RCT
randomized control trial
- SLE
systemic lupus erythematosus
- SLAM R
systemic lupus activity measure revised
- TNF
tumor necrosis factor (TNF)
- TULIP
treatment of uncontrolled lupus via interferon pathway trial, VSMC – vascular smooth muscle cells
Footnotes
Declaration of Interest:
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures:
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Knight JS, Kaplan MJ. Cardiovascular disease in lupus: Insights and updates. Vol. 25, Current Opinion in Rheumatology. 2013. p. 597–605.*critical review
- 2.Amaya-Amaya J, Sarmiento-Monroy JC, Caro-Moreno J, Molano-González N, Mantilla RD, Rojas-Villarraga A, et al. Cardiovascular disease in Latin American patients with systemic lupus erythematosus: A cross-sectional study and a systematic review. Autoimmune Dis. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urowitz MB, Bookman AAM, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60(2):221–5. [DOI] [PubMed] [Google Scholar]
- 4.Yurkovich M, Vostretsova K, Chen W, Aviña-Zubieta JA. Overall and cause-specific mortality in patients with systemic lupus erythematosus: A meta-analysis of observational studies. Arthritis Care Res. 2014;66(4):608–16. [DOI] [PubMed] [Google Scholar]
- 5.Björnådal L, Yin L, Granath F, Klareskog L, Ekbom A. Cardiovascular Disease a Hazard Despite Improved Prognosis in Patients with Systemic Lupus Erythematosus: Results from a Swedish Population Based Study 1964–95. J Rheumatol. 2004. April;31(4):713–9. [PubMed] [Google Scholar]
- 6.Zeller C, Appenzeller S. Cardiovascular Disease in Systemic Lupus Erythematosus: The Role of Traditional and Lupus Related Risk Factors. Curr Cardiol Rev. 2008. May 1;4(2):116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whelton Seamus P, Freny Vaghaiwalla Mody, McEvoy John W., Seth Shay Martin RSB. Cardiovascular Risk Assessment and Allocation of Lipid-Lowering Therapy in Patients with Chronic Inflammatory Diseases [Internet]. American college of cardiology. 2017. Available from: https://www.acc.org/latest-in-cardiology/articles/2017/06/29/08/22/cv-risk-assessment-and-allocation-of-lipid-lowering-therapy-in-patients-with-chronic-inflammatory-diseases [Google Scholar]
- 8.Doria A, Iaccarino L, Sarzi-Puttini P, Atzeni F, Turriel M, Petri M. Cardiac involvement in systemic lupus erythematosus. Vol. 14, Lupus. 2005. p. 683–6. [DOI] [PubMed] [Google Scholar]
- 9.Sugiura T, Kumon Y, Kataoka H, Matsumura Y, Takeuchi H, Doi YL. Asymptomatic pericardial effusion in patients with systemic lupus erythematosus. Lupus. 2009. February;18(2):128–32. [DOI] [PubMed] [Google Scholar]
- 10.Cervera R, Font J, Pare C, Azqueta M, Perez-Villa F, Lopez-Soto A, et al. Cardiac disease in systemic lupus erythematosus: Prospective study of 70 patients. Ann Rheum Dis. 1992;51(2):156–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zashin SJ, Lipsky PE. Pericardial tamponade complicating systemic lupus erythematosus. Vol. 16, Journal of Rheumatology. 1989. p. 374–7. [PubMed] [Google Scholar]
- 12.Inase N, Enomoto N, Sakaino H, Shiigai T. Systemic lupus erythematosus presenting with pericardial tamponade and lupus pneumonitis. Jpn J Med [Internet]. 1989;28(3):362–5. Available from: https://www.ncbi.nlm.nih.gov/pubmed/2739145 [DOI] [PubMed] [Google Scholar]
- 13.Spodick DH. Hemopericardium in a patient with systemic lupus erythematosus. Circulation [Internet]. 1999. February 9;99(5):723–4. Available from: https://www.ncbi.nlm.nih.gov/pubmed/9950744 [PubMed] [Google Scholar]
- 14.Arabi MT, Malek EM, Fares MH, Itani MH. Cardiac tamponade as the first manifestation of systemic lupus erythematosus in children. BMJ Case Rep. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharda N Cardiac tamponade as an initial manifestation of systemic lupus erythematosus in a child. Cardiol Young. 2014;24(1):172–4. [DOI] [PubMed] [Google Scholar]
- 16.Doherty NE, Siegel RJ. Cardiovascular manifestations of systemic lupus erythematosus. Am Heart J [Internet]. 1985. December;110(6):1257–65. Available from: https://www.ncbi.nlm.nih.gov/pubmed/3907317 [DOI] [PubMed] [Google Scholar]
- 17.Harvey AMG, Shulman LE, Tumulty PA, Conley CL, Schoenrichf EH. Systemic lupus erythematosus: Review of the literature and clinical analysis of 138 cases. Vol. 33, Medicine (United States). 1954. p. 291–437. [PubMed] [Google Scholar]
- 18.Estes D, Christian CL. The natural history of systemic lupus erythematosus by prospective analysis1. Med (United States). 1971;50(2):85–95. [DOI] [PubMed] [Google Scholar]
- 19.Thomas G, Aubart FC, Chiche L, Haroche J, Hie M, Hervier B, et al. Lupus myocarditis: Initial presentation and longterm outcomes in a multicentric series of 29 patients. J Rheumatol. 2017. January 1;44(1):24–32. [DOI] [PubMed] [Google Scholar]
- 20.Asherson RA, Hughes GR. The expanding spectrum of Libman Sacks endocarditis: the role of antiphospholipid antibodies. Vol. 7, Clinical and experimental rheumatology. 1989. p. 225–8. [PubMed] [Google Scholar]
- 21.Gleason CB, Stoddard MF, Wagner SG, Longaker RA, Pierangeli S, Harris EN. A comparison of cardiac valvular involvement in the primary antiphospholipid syndrome versus anticardiolipin-negative systemic lupus erythematosus. Am Heart J. 1993;125(4):1123–9. [DOI] [PubMed] [Google Scholar]
- 22.Petri M Detection of coronary artery disease and the role of traditional risk factors in the Hopkins Lupus Cohort. Vol. 9, Lupus: Nature Publishing Group; 2000. p. 170–5. [DOI] [PubMed] [Google Scholar]
- 23.Abu-Shakra M, Codish S, Zeler L, Wolak T, Sukenik S. Atherosclerotic cardiovascular disease in systemic lupus erythematosus: The Beer Sheva experience. Isr Med Assoc J. 2008. January;10(1):43–4.* High interest study
- 24.Bhatt SP, Handa R, Gulati GS, Sharma S, Pandey RM, Aggarwal P, et al. Peripheral vascular disease in systemic lupus erythematosus. Lupus. 2007;16(9):720–3. [DOI] [PubMed] [Google Scholar]
- 25.Bruce IN, Urowitz MB, Gladman DD, Ibañez D, Steiner G. Risk Factors for Coronary Heart Disease in Women With Systemic Lupus Erythematosus: The Toronto Risk Factor Study. Arthritis Rheum. 2003. November;48(11):3159–67. [DOI] [PubMed] [Google Scholar]
- 26.Bruce IN, Urowitz MB, Gladman DD, Hallett DC. Natural history of hypercholesterolemia in systemic lupus erythematosus. J Rheumatol. 1999;26(10):2137–43. [PubMed] [Google Scholar]
- 27.Skamra C, Ramsey-Goldman R. Management of cardiovascular complications in systemic lupus erythematosus. Vol. 5, International Journal of Clinical Rheumatology. 2010. p. 75–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr Cardiol Rev [Internet]. 2013. February 1;9(1):15–9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23463953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cypiene A, Kovaite M, Venalis A, Dadoniene J, Rugiene R, Petrulioniene Z, et al. Arterial wall dysfunction in systemic lupus erythematosus. Lupus. 2009;18(6):522–9. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care [Internet]. 2019. January 1;42(Supplement 1):S90 LP–S102. Available from: http://care.diabetesjournals.org/content/42/Supplement_1/S90.abstract [DOI] [PubMed] [Google Scholar]
- 31.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J Am Coll Cardiol [Internet]. 2018 May 15;71(19):e127 LP–e248. Available from: http://www.onlinejacc.org/content/71/19/e127.abstract [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. J Am Coll Cardiol [Internet]. 2019 June 25;73(24):e285 LP–e350. Available from: http://www.onlinejacc.org/content/73/24/e285.abstract [DOI] [PubMed] [Google Scholar]
- 33.Michael J, Donna R, Caroline A, Jamy A, Anthony C, Karen D, et al. 2013. AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation [Internet]. 2014 June 24;129(25_suppl_2):S102–38. Available from: 10.1161/01.cir.0000437739.71477.ee [DOI] [Google Scholar]
- 34.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: A systematic review. Vol. 69, Annals of the Rheumatic Diseases. 2010. p. 20–8. [DOI] [PubMed] [Google Scholar]
- 35.Penn SK, Kao AH, Schott LL, Elliott JR, Toledo FGS, Kuller L, et al. Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J Rheumatol. 2010. June;37(6):1136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung H, Bobba R, Su J, Shariati-Sarabi Z, Gladman DD, Urowitz M, et al. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010. March;62(3):863–8. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: Results from a large, multi-ethnic cohort. Ann Rheum Dis. 2009. February;68(2):238–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruiz-Irastorza G, Egurbide MV, Pijoan JI, Garmendia M, Villar I, Martinez-Berriotxoa A, et al. Effect of antimalarials on thrombosis and survival in patients with systemic lupus erythematosus. Lupus. 2006;15(9):577–83. [DOI] [PubMed] [Google Scholar]
- 39.Limper M, Scirè CA, Talarico R, Amoura Z, Avcin T, Basile M, et al. Antiphospholipid syndrome: State of the art on clinical practice guidelines. Vol. 4, RMD Open. BMJ Publishing Group; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barile-Fabris L, Hernández-Cabrera MF, Barragan-Garfias JA. Vasculitis in systemic lupus erythematosus. Curr Rheumatol Rep. 2014;16(9). [DOI] [PubMed] [Google Scholar]
- 41.Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N Engl J Med. 2020. January 16;382(3):211–21. [DOI] [PubMed] [Google Scholar]
- 42.Almaani S, Meara A, Rovin BH. Update on lupus nephritis. Clin J Am Soc Nephrol. 2017;12(5):825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maron BA, Loscalzo J. The Treatment of Hyperhomocysteinemia . Annu Rev Med. 2009. February;60(1):39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rho YH, Oeser A, Chung CP, Morrow JD, Stein CM. Drugs to treat systemic lupus erythematosus: Relationship between current use and cardiovascular risk factors. Arch Drug Inf. 2008;1(1):23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64(9):2937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Canaud G, Bienaimé F, Tabarin F, Bataillon G, Seilhean D, Noël LH, et al. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. 2014;371(4):303–12. [DOI] [PubMed] [Google Scholar]
- 47.Oaks Z, Winans T, Caza T, Fernandez D, Liu Y, Landas SK, et al. Mitochondrial Dysfunction in the Liver and Antiphospholipid Antibody Production Precede Disease Onset and Respond to Rapamycin in Lupus-Prone Mice. Arthritis Rheumatol. 2016. November 1;68(11):2728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margiotta DPE, Basta F, Dolcini G, Batani V, Lo Vullo M, Vernuccio A, et al. Physical activity and sedentary behavior in patients with Systemic Lupus Erythematosus. PLoS One. 2018. March 1;13(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reis-Neto Silva, Pinheiro Sato. Supervised physical exercise improves fatigue, aerobic capacity and quality of life without worsening disease activity in patients with systemic lupus erythematosus. Lupus [Internet]. 2013;22(1):137 Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L71177090 [Google Scholar]
- 50.Man BL, Mok CC. Serositis related to systemic lupus erythematosus: Prevalence and outcome. Vol. 14, Lupus. 2005. p. 822–6. [DOI] [PubMed] [Google Scholar]
- 51.Appenzeller S, Pineau CA, Clarke AE. Acute lupus myocarditis: Clinical features and outcome. Lupus. 2011. August;20(9):981–8. [DOI] [PubMed] [Google Scholar]
- 52.Davies RJ, Lomer M, Yeo SI, Avloniti K, Sangle SR, D’Cruz DP. Weight loss and improvements in fatigue in systemic lupus erythematosus: A controlled trial of a low glycaemic index diet versus a calorie restricted diet in patients treated with corticosteroids. Lupus. 2012. May;21(6):649–55. [DOI] [PubMed] [Google Scholar]
- 53.Wright S, O’Prey FM, McHenry MT, Leahey WJ, Devine AB, Duffy EM, et al. A randomised interventional trial of ω−3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008. June;67(6):841–8. [DOI] [PubMed] [Google Scholar]
- 54.Neto Aline, Monteiro Camargo, Silva Pinheiro, et al. Physical exercise improves endothelial function in patients with systemic lupus erythematosus. Arthritis Rheum [Internet]. 2010;62:628 Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L70381622 [DOI] [PubMed] [Google Scholar]
- 55.dos Reis-Neto ET, da Silva AE, de C Monteiro CM, de Camargo LM, Sato EI. Supervised physical exercise improves endothelial function in patients with systemic lupus erythematosus. Rheumatol (United Kingdom). 2013. December;52(12):2187–95. [DOI] [PubMed] [Google Scholar]
- 56.Prado DM, Gualano B, Pinto ALS, Sallum AM, Perondi MB, Roschel H, et al. Exercise in a child with systemic lupus erythematosus and antiphospholipid syndrome. Med Sci Sports Exerc. 2011. December;43(12):2221–3. [DOI] [PubMed] [Google Scholar]
- 57.O’Neill SG, Pego-Reigosa JM, Hingorani AD, Bessant R, Isenberg DA, Rahman A. Use of a strategy based on calculated risk scores in managing cardiovascular risk factors in a large British cohort of patients with systemic lupus erythematosus. Rheumatology. 2009;48(5):573–5. [DOI] [PubMed] [Google Scholar]
- 58.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS Focused Update of the Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease. J Am Coll Cardiol [Internet]. 2014. November 4;64(18):1929 LP–1949. Available from: http://www.onlinejacc.org/content/64/18/1929.abstract [DOI] [PubMed] [Google Scholar]
- 59.Ezra A, Nanette W, Ralph B, Donald C, Theodore G, David H, et al. 2014. AHA/ACC Guideline for the Management of Patients With Non–ST-Elevation Acute Coronary Syndromes. Circulation [Internet]. 2014 December 1;130(25):e344–426. Available from: 10.1161/CIR.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 60.Hung MJ, Hu P, Hung MY. Coronary artery spasm: Review and update Vol. 11, International Journal of Medical Sciences. Ivyspring International Publisher; 2014. p. 1161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okazaki T, Shinagawa S, Mikage H. Vasculitis syndrome-diagnosis and therapy. J Gen Fam Med. 2017. April;18(2):72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.William P, Alejandro R, Teri A, Opeolu A, Nicholas B, Kyra B, et al. 2018. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke [Internet]. 2018 March 1;49(3):e46–99. Available from: 10.1161/STR.0000000000000158 [DOI] [Google Scholar]
- 63.Marie G-H, Heather G, Coletta B, Neal B, Matthew C, Douglas D, et al. 2016. AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation [Internet]. 2017 March 21;135(12):e686–725. Available from: 10.1161/CIR.0000000000000470 [DOI] [Google Scholar]
- 64.Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest [Internet]. 2016;149(2):315–52. Available from: http://www.sciencedirect.com/science/article/pii/S0012369215003359 [DOI] [PubMed] [Google Scholar]
- 65.Urowitz MB, Gladman DD, Ibanez D, Berliner Y. Modification of hypertension and hypercholesterolaemia in patients with systemic lupus erythematosus: A quality improvement study. Ann Rheum Dis. 2006. January;65(1):115–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Costenbader KH, Karlson EW, Gall V, De Pablo P, Finckh A, Lynch M, et al. Barriers to a trial of atherosclerosis prevention in systemic lupus erythematosus. Arthritis Care Res. 2005. October 15;53(5):718–23. [DOI] [PubMed] [Google Scholar]
- 67.Gurevich VS, Shovman O, Slutzky L, Meroni PL, Shoenfeld Y. Statins and autoimmune diseases Vol. 4, Autoimmunity Reviews. Elsevier B.V.; 2005. p. 123–9. [DOI] [PubMed] [Google Scholar]
- 68.Norby GE, Holme I, Fellström B, Jardine A, Cole E, Abedini S, et al. Effect of fluvastatin on cardiac outcomes in kidney transplant Patients with systemic lupus erythematosus a randomized placebo-controlled study. Arthritis Rheum. 2009. April;60(4):1060–4. [DOI] [PubMed] [Google Scholar]
- 69.Yu HH, Chen PC, Yang YH, Wang LC, Lee JH, Lin YT, et al. Statin reduces mortality and morbidity in systemic lupus erythematosus patients with hyperlipidemia: A nationwide population-based cohort study. Atherosclerosis. 2015. November 1;243(1):11–8. [DOI] [PubMed] [Google Scholar]
- 70.Ferreira GA, Navarro TP, Telles RW, Andrade LEC, Sato EI. Atorvastatin therapy improves endothelial-dependent vasodilation in patients with systemic lupus erythematosus: An 8 weeks controlled trial. Rheumatology. 2007. October;46(10):1560–5. [DOI] [PubMed] [Google Scholar]
- 71.Plazak W, Gryga K, Dziedzic H, Tomkiewicz-Pajak L, Konieczynska M, Podolec P, et al. Influence of atorvastatin on coronary calcifications and myocardial perfusion defects in systemic lupus erythematosus patients: A prospective, randomized, double-masked, placebo-controlled study. Arthritis Res Ther. 2011. July 20;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petri MA, Kiani AN, Post W, Christopher-Stine L, Magder LS. Lupus Atherosclerosis Prevention Study (LAPS). Ann Rheum Dis. 2011. May;70(5):760–5.** Important study
- 73.Costenbader KH, Liang MH, Chibnik LB, Aizer J, Kwon H, Gall V, et al. A pravastatin dose-escalation study in systemic lupus erythematosus. Rheumatol Int. 2007. September;27(11):1071–7. [DOI] [PubMed] [Google Scholar]
- 74.Schanberg LE, Sandborg C, Barnhart HX, Ardoin SP, Yow E, Evans GW, et al. Use of atorvastatin in systemic lupus erythematosus in children and adolescents. Arthritis Rheum. 2012. January;64(1):285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graham KL, Lee LY, Higgins JP, Steinman L, Utz PJ, Ho PP. Failure of oral atorvastatin to modulate a murine model of systemic lupus erythematosus. Arthritis Rheum. 2008. July;58(7):2098–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taylor EB, Ryan MJ. Understanding mechanisms of hypertension in systemic lupus erythematosus Vol. 11, Therapeutic Advances in Cardiovascular Disease. SAGE Publications Ltd; 2017. p. 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bakshi J, Rahman AID. Blood Pressure in SLE Patients. Rheumatology. 2015. April 20;54(1, 1 April 2015):i96. [Google Scholar]
- 78.Herlitz H, Edeno C, Mulec H, Westberg G, Aurell M. Captopril treatment of hypertension and renal failure in systemic Lupus erythematosus. Nephron. 1984;38(4):253–6. [DOI] [PubMed] [Google Scholar]
- 79.Bursztyn M, Many A, Rosenthal T. Nifedipine in the treatment of hypertension in systemic lupus erythematosus. Angiology [Internet]. 1987. May;38(5):359–62. Available from: https://www.ncbi.nlm.nih.gov/pubmed/3496026 [DOI] [PubMed] [Google Scholar]
- 80.Arnaud L, Mathian A, Ruffatti A, Erkan D, Tektonidou M, Cervera R, et al. Efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies: An international and collaborative meta-analysis. Vol. 13, Autoimmunity Reviews. 2014. p. 281–91. [DOI] [PubMed] [Google Scholar]
- 81.Arnaud L, Mathian A, Devilliers H, Ruffatti A, Tektonidou M, Forastiero R, et al. Patient-level analysis of five international cohorts further confirms the efficacy of aspirin for the primary prevention of thrombosis in patients with antiphospholipid antibodies Vol. 14, Autoimmunity Reviews. Elsevier; 2015. p. 192–200. [DOI] [PubMed] [Google Scholar]
- 82.Cuadrado MJ, Bertolaccini ML, Seed PT, Tektonidou MG, Aguirre A, Mico L, et al. Low-dose aspirin vs low-dose aspirin plus low-intensity warfarin in thromboprophylaxis: A prospective, multicentre, randomized, open, controlled trial in patients positive for antiphospholipid antibodies (ALIWAPAS). Rheumatol (United Kingdom). 2014. February;53(2):275–84. [DOI] [PubMed] [Google Scholar]
- 83.Tarr T, Lakos G, Bhattoa HP, Soltesz P, Shoenfeld Y, Szegedi G, et al. Clinical thrombotic manifestations in SLE patients with and without antiphospholipid antibodies: A 5-year follow-up. Clin Rev Allergy Immunol. 2007. April;32(2):131–7. [DOI] [PubMed] [Google Scholar]
- 84.Parodis I, Johansson P, Gomez A, Soukka S, Emamikia S, Chatzidionysiou K. Predictors of low disease activity and clinical remission following belimumab treatment in systemic lupus erythematosus. Rheumatology (Oxford) [Internet]. 2019. December 1;58(12):2170–6. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31157891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perl A Activation of mTOR (mechanistic target of rapamycin) in rheumatic diseases. Nat Rev Rheumatol [Internet]. 2016;12(3):169–82. Available from: 10.1038/nrrheum.2015.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caza TN, Fernandez DR, Talaber G, Oaks Z, Haas M, Madaio MP, et al. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann Rheum Dis. 2014;73(10):1888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fernandez D, Bonilla E, Mirza N, Niland B, Perl A. Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006. September;54(9):2983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lai Z-W, Borsuk R, Shadakshari A, Yu J, Dawood M, Garcia R, et al. Mechanistic Target of Rapamycin Activation Triggers IL-4 Production and Necrotic Death of Double-Negative T Cells in Patients with Systemic Lupus Erythematosus. J Immunol. 2013. September 1;191(5):2236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lai ZW, Kelly R, Winans T, Marchena I, Shadakshari A, Yu J, et al. Sirolimus in patients with clinically active systemic lupus erythematosus resistant to, or intolerant of, conventional medications: a single-arm, open-label, phase 1/2 trial. Lancet. 2018. March 24;391(10126):1186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chong ZZ, Shang YC, Maiese K. Cardiovascular Disease and mTOR Signaling. Vol. 21, Trends in Cardiovascular Medicine. 2011. p. 151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Popescu NI, Lupu C, Lupu F. Extracellular protein disulfide isomerase regulates coagulation on endothelial cells through modulation of phosphatidylserine exposure. Blood. 2010. August 12;116(6):993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mancini D, Pinney S, Burkhoff D, LaManca J, Itescu S, Burke E, et al. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003. July 8;108(1):48–53. [DOI] [PubMed] [Google Scholar]
- 93.Sinha SS, Pham MX, Vagelos RH, Perlroth MG, Hunt SA, Lee DP, et al. Effect of rapamycin therapy on coronary artery physiology early after cardiac transplantation. Am Heart J. 2008;155(5):889.e1–889.e6. [DOI] [PubMed] [Google Scholar]
- 94.Htay T, Liu MW. Drug-eluting stent: a review and update. Vol. 1, Vascular health and risk management. 2005. p. 263–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warner LM, Adams LM, Sehgal SN. Rapamycin Prolongs Survival and Arrests Pathophysiologic Changes in Murine Systemic Lupus Erythematosus. Arthritis Rheum. 1994;37(2):289–97. [DOI] [PubMed] [Google Scholar]
- 96.Galis ZS, Asanuma K, Godin D, Meng X. N-acetyl-cysteine decreases the matrix-degrading capacity of macrophage- derived foam cells: New target for antioxidant therapy? Circulation. 1998. June 23;97(24):2445–53. [DOI] [PubMed] [Google Scholar]
- 97.Talasaz AH, Khalili H, Fahimi F, Mojtaba S. Potential role of N -acetylcysteine in cardiovascular disorders. Therapy. 2011. May 1;8:237–45. [Google Scholar]
- 98.Andrews NP, Prasad A, Quyyumi AA. N-acetylcysteine improves coronary and peripheral vascular function. J Am Coll Cardiol. 2001;37(1):117–23. [DOI] [PubMed] [Google Scholar]
- 99.Boesgaard S, Aldershvile J, Poulsen HE. Preventive administration of intravenous N-acetylcysteine and development of tolerance to isosorbide dinitrate in patients with angina pectoris. Circulation. 1992;85(1):143–9. [DOI] [PubMed] [Google Scholar]
- 100.Banchereau J, Pascual V. Type I Interferon in Systemic Lupus Erythematosus and Other Autoimmune Diseases. Vol. 25, Immunity. 2006. p. 383–92. [DOI] [PubMed] [Google Scholar]
- 101.Obermoser G, Pascual V. The interferon-α signature of systemic lupus erythematosus. Vol. 19, Lupus. 2010. p. 1012–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li J, Fu Q, Cui H, Qu B, Pan W, Shen N, et al. Interferon-α priming promotes lipid uptake and macrophage-derived foam cell formation: A novel link between interferon-α and atherosclerosis in lupus. Arthritis Rheum. 2011. February;63(2):492–502. [DOI] [PubMed] [Google Scholar]
- 103.Lood C, Amisten S, Gullstrand B, Jönsen A, Allhorn M, Truedsson L, et al. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: Up-regulation of the type I interferon system is strongly associated with vascular disease. Blood. 2010. September 16;116(11):1951–7. [DOI] [PubMed] [Google Scholar]
- 104.Somers EC, Zhao W, Lewis EE, Wang L, Wing JJ, Sundaram B, et al. Type I interferons are associated with subclinical markers of cardiovascular disease in a cohort of systemic lupus erythematosus patients. PLoS One. 2012. May 14;7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bandyopadhyay D, Ashish K, Hajra A, Qureshi A, Ghosh RK. Cardiovascular Outcomes of PCSK9 Inhibitors: With Special Emphasis on Its Effect beyond LDL-Cholesterol Lowering. J Lipids. 2018;2018:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shapiro MD, Fazio S. PCSK9 and atherosclerosis - lipids and beyond Vol. 24, Journal of Atherosclerosis and Thrombosis. Japan Atherosclerosis Society; 2017. p. 462–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liberale L, Montecucco F, Camici GG, Dallegri F, Vecchie A, Carbone F, et al. Treatment with Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Inhibitors to Reduce Cardiovascular Inflammation and Outcomes. Curr Med Chem. 2017. March 6;24(14). [DOI] [PubMed] [Google Scholar]
- 108.Chighizola CB, Andreoli L, Gerosa M, Tincani A, Ruffatti A, Meroni PL. The treatment of anti-phospholipid syndrome: A comprehensive clinical approach. Vol. 90, Journal of Autoimmunity. Academic Press; 2018. p. 1–27. [DOI] [PubMed] [Google Scholar]
- 109.Cohen H, Hunt BJ, Efthymiou M, Arachchillage DRJ, Mackie IJ, Clawson S, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016. September 1;3(9):e426–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M, Vidal X, Riera-Mestre A, Castro-Salomó A, et al. Rivaroxaban versus Vitamin K antagonist in antiphospholipid syndrome a randomized noninferiority trial. Ann Intern Med. 2019. November 19;171(10):685–94. [DOI] [PubMed] [Google Scholar]
- 111.Pengo V, Denas G, Zoppellaro G, Jose SP, Hoxha A, Ruffatti A, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018. September 27;132(13):1365–71. [DOI] [PubMed] [Google Scholar]
- 112.Woller SC, Stevens SM, Kaplan DA, Rondina MT. Protocol Modification of Apixaban for the Secondary Prevention of Thrombosis Among Patients With Antiphospholipid Syndrome Study. Clin Appl Thromb. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Matsuura E, Shen L, Matsunami Y, Quan N, Makarova M, Geske FJ, et al. Pathophysiology of β2-glycoprotein i in antiphospholipid syndrome. Vol. 19, Lupus. 2010. p. 379–84. [DOI] [PubMed] [Google Scholar]
- 114.Winans T, Kelly R, Lai ZW, Faraone S, Phillips PE, Banki K, et al. mtorc1 blockade with rapamycin and n-acetylcysteine reduces anti-phospholipid antibody levels in controlled clinical trials of patients with SLE. Arthritis Rheumatol Conf Am Coll Rheumatol Rheumatol Heal Prof Annu Sci Meet ACR/ARHP. 2017;69(Supplement 10). [Google Scholar]
- 115.Danowski A, Magder L, Petri M. Flares in Lupus: Outcome Assessment Trial (FLOAT), a comparison between oral methylprednisolone and intramuscular triamcinolone. J Rheumatol. 2006. January;33(1):57–60. [PubMed] [Google Scholar]
- 116.Tincani A, Rebaioli CB, Taglietti M, Shoenfeld Y. Heart involvement in systemic lupus erythematosus, anti-phospholipid syndrome and neonatal lupus. Rheumatology. 2006. October;45(SUPPL. 4). [DOI] [PubMed] [Google Scholar]
- 117.Papageorgiou N, Briasoulis A, Lazaros G, Imazio M, Tousoulis D. Colchicine for prevention and treatment of cardiac diseases: A meta-analysis. Cardiovasc Ther. 2017. February 1;35(1):10–8. [DOI] [PubMed] [Google Scholar]
- 118.Imazio M, Lazaros G, Picardi E, Vasileiou P, Carraro M, Tousoulis D, et al. Intravenous human immunoglobulins for refractory recurrent pericarditis: A systematic review of all published cases. Vol. 17, Journal of Cardiovascular Medicine. Lippincott Williams and Wilkins; 2016. p. 263–9. [DOI] [PubMed] [Google Scholar]
- 119.Brucato A, Imazio M, Gattorno M, Lazaros G, Maestroni S, Carraro M, et al. Effect of anakinra on recurrent pericarditis among patients with colchicine resistance and corticosteroid dependence: The AIRTRIP randomized clinical trial. JAMA - J Am Med Assoc. 2016. November 8;316(18):1906–12. [DOI] [PubMed] [Google Scholar]
- 120.Finetti M, Insalaco A, Cantarini L, Meini A, Breda L, Alessio M, et al. Long-term efficacy of interleukin-1 receptor antagonist (Anakinra) in corticosteroid-dependent and colchicine-resistant recurrent pericarditis. J Pediatr. 2014;164(6). [DOI] [PubMed] [Google Scholar]
- 121.Jain S, Thongprayoon C, Espinosa RE, Hayes SN, Klarich KW, Cooper LT, et al. Effectiveness and safety of anakinra for management of refractory pericarditis. Am J Cardiol. 2015. October 15;116(8):1277–9. [DOI] [PubMed] [Google Scholar]
- 122.Zhang L, Zhu YL, Li MT, Gao N, You X, Wu QJ, et al. Lupus myocarditis: A case–control study from China. Chin Med J (Engl). 2015. January 10;128(19):2588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chan YK, Li EK, Tam LS, Chow LTC, Ng HK. Intravenous cyclophosphamide improves cardiac dysfunction in lupus myocarditis. Scand J Rheumatol. 2003;32(5):306–8. [DOI] [PubMed] [Google Scholar]
- 124.Disla E, Rhim HR, Reddy A, Ramaprasad S, Taranta A. Reversible cardiogenic shock in a patient with lupus myocarditis [9]. Vol. 20, Journal of Rheumatology. 1993. p. 2174. [PubMed] [Google Scholar]
- 125.Azzam Z, Maza I, Zeidan Shwiri T, Lorber M. Cyclophosphamide restores heart function in a patient with lupus myocarditis. Isr Med Assoc J. 2005. May 1;7:266–7. [PubMed] [Google Scholar]
- 126.Ashrafi R, Garg P, McKay E, Gosney J, Chuah S, Davis G. Aggressive cardiac involvement in systemic lupus erythematosus: A case report and a comprehensive literature review. Cardiol Res Pract. 2011;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang CR, Tsai YS, Li WT. Lupus myocarditis receiving the rituximab therapy—a monocentric retrospective study. Clin Rheumatol. 2018. June 1;37(6):1701–7. [DOI] [PubMed] [Google Scholar]
- 128.Suri V, Varma S, Joshi K, Malhotra P, Kumari S, Jain S. Lupus myocarditis: Marked improvement in cardiac function after intravenous immunoglobulin therapy. Rheumatol Int. 2010. September;30(11):1503–5. [DOI] [PubMed] [Google Scholar]
- 129.Barnado A, Kamen DL. Myocarditis successfully treated with intravenous immunoglobulin in a patient with systemic lupus erythematous and myositis. Am J Med Sci. 2014;347(3):256–7. [DOI] [PubMed] [Google Scholar]