Abstract
Ticks are the most important vectors of human pathogens, leading to increased public health burdens worldwide. Tick-borne pathogens include viruses (e.g. tick-borne encephalitis and Powassan); bacteria, such as the causative agents of Lyme disease, spotted fever rickettsiosis and human anaplasmosis; and malaria-like protozoan parasites causing babesiosis. Tick-borne diseases are emerging due to the geographical expansion of their tick vectors, especially in the northern hemisphere. Two examples of this phenomenon are Ixodes scapularis and Amblyomma americanum, which have expanded their ranges in the USA in recent decades and are responsible for the continuous emergence of Lyme disease and human ehrlichiosis, respectively. This phenomenon is also occurring worldwide and is reflected by the increasing number of tick-borne encephalitis and haemorrhagic fever cases in Europe and Asia. In this review, we provide a concise synopsis of the most medically important tick-borne pathogen worldwide, with a particular emphasis on emerging public health threats.
Keywords: tick-borne diseases, Lyme disease, Ixodes, Haemaphysalis longicornis, arboviruses
Introduction
Pathogens transmitted by ticks are responsible for the majority of the vector-borne diseases in temperate North America, Europe and Asia. In the USA, ticks are responsible for over 95 % of vector-borne disease cases [1]. Lyme disease is, by far, the most prevalent tick-borne disease in the northern hemisphere. According to two studies, approximately 300 000 cases of Lyme disease are diagnosed annually in the USA alone, about 10-fold higher than the number of reported cases [2, 3]. Based on these estimates, Lyme disease may be among the most common infectious diseases in the USA. Additionally, around 10 000 cases of other tick-borne diseases are reported annually [1], although the actual number of cases is probably significantly higher [4, 5].
The economic impact of tick-borne diseases is significant and increases every year. In the USA, the reported cost per patient diagnosed with Lyme disease totalled USD $8172 in 2002 [6], equal to USD $11 838 in 2019 (CPI inflation calculator, https://data.bls.gov/cgi-bin/cpicalc.pl). A conservative approximation based on 42 743 cases reported to the Centers for Disease Control and Prevention (CDC) in 2017 would result in a cost estimate of over USD $500 million annually. These costs might be even higher for patients with post-treatment Lyme disease syndrome [7]. The societal burden of Lyme disease can be very considerable, with one-quarter of the patients receiving public support or disability benefits [8]. Additional economic costs that are substantial, but difficult to quantify, are imposed on the hospitality and tourism industry in the endemic areas [9]. Public health burdens due to Lyme disease are not unique to the USA. A recent comprehensive review found Lyme disease-associated expenditures of tens of millions of euros in several European countries [10]. The combined public health impact of all tick-borne diseases remains mostly unquantified.
Although the main focus of this review is tick-borne pathogens of humans, the impact of veterinary tick-borne diseases can also be staggering, especially in developing countries [11]. Tick-borne diseases affect ~80 % of the world’s cattle population, with the estimated cost of between USD $13.9 billion and USD $18.7 billion [12]. For countries such as Tanzania, where the economic losses due to tick-borne disease have been quantified, the impacts were estimated at USD $364 million, with an estimated mortality of 1.3 million cattle due mostly to theileriosis (68 %), anaplasmosis (13 %) and babesiosis (13 %) [13]. Theileriosis occurs only sporadically in the USA, but this situation could change rapidly with the recent introduction of the Asian longhorned tick, Haemaphysalis longicornis [14, 15]. This tick species is a competent vector of Theileria orientalis [16, 17], which has recently been detected on cattle farms in Virginia in the USA [18].
The expansion of tick populations, as well as the increasing incidence of tick-borne diseases, is bringing ticks to the attention of a broader range of public health professionals. The purpose of this review is to summarize the most recent advances in tick-borne diseases, with a particular emphasis on newly described or emerging diseases worldwide that present a public health threat. In this review, tick-borne pathogens are organized according to their causative agents into different taxonomic groups (Table 1). This review provides a short synopsis of a much broader topic that has been covered in depth by recent publications [1, 5, 19] for those who are interested in more details on tick-borne diseases and their vectors.
Table 1.
Main groups of tick-borne pathogens and diseases. See the main text for references
|
Disease or condition |
Pathogen [main vector]* |
Geography |
Prevalence† and comments |
|---|---|---|---|
|
Viral | |||
|
Flaviviridae / Flavivirus (tick-borne encephalitis group) | |||
|
Tick-borne encephalitis (TBE) |
TBE virus complex (I. ricinus, I. persulcatus) |
Europe, Asia, Middle East |
Common and widespread |
|
Powassan encephalitis (POW) |
POW virus (I. scapularis, I. cookei) |
Northeastern USA/ adjacent Canada, Russian Far East |
Rare, increasing |
|
Other TBEs: Omsk haemorrhagic fever (OHF), Kyasanur Forest Disease (KFD), louping ill, others |
OHF, KFD, other viruses (Ixodes, Dermacentor, Haemophysalis sp.) |
Europe, Russia, China, Japan, India, Southeast Asia, Middle East |
Rare to common within localized range; some increasing |
|
Bunyavirales/ Orthonairovirus | |||
|
Crimean–Congo haemorrhagic fever (CCHF) |
CCHF virus (Hyalomma marginatum, other tick sp.) |
Europe, Central Asia, India, Africa |
Common and widespread; increasing |
|
Bunyavirales/ Phlebovirus | |||
|
Severe fever with thrombocytopenia syndrome (SFTS) |
SFTS virus (H. longicornis and R. microplus) |
China, Korea, Japan |
Uncommon, increasing |
|
Heartland virus |
(A. americanum) |
Mid-western and southern USA |
Rare |
|
Bhanja virus |
(Dermacentor, Haemophysalis sp.) |
Africa, Central Asia, southern Europe |
Rare |
|
Orthomyxoviridae / Thogotovirus | |||
|
Thogoto (THOV), Dhori (DHOV) and Bourbon virus |
(Hyalomma, Amblyomma, Rhipicephalus sp.) |
Africa, Asia, Europe (THOV and DHOV), USA (Bourbon) |
Rare; Bourbon virus isolated from A. americanum |
|
Reoviridae / Coltivirus |
|
|
|
|
Colorado tick fever (CTF) |
CTF virus (D. andersoni) |
Western USA and Canada |
Rare |
|
Eyach virus |
(I. ricinus) |
Central Europe |
Rare |
|
Bacterial | |||
|
Spirochaetales/Borrelia spirochetes (borreliosis and relapsing fever) | |||
|
Lyme disease |
Borrelia burgdorferi (I. ricinus complex) |
Temperate North America, Europe, Asia |
Common, widespread |
|
Relapsing fever borreliosis |
B.miyamotoi (I. ricinus complex) |
Temperate North America, Europe, Asia? |
Rare |
|
Relapsing fever borreliosis – tick-borne relapsing fever (TBRF) |
Relapsing fever Borrelia (Ornithodoros and Carios sp.) |
Worldwide (except Australia), mostly tropical and desert regions |
Rare to common locally |
|
Southern tick-associated rash illness (STARI) |
Unknown (A. americanum) |
Southern and eastern USA |
Rare |
|
Rickettsiales/Rickettsia (spotted fever and tick typhus) | |||
|
Rocky Mountain spotted fever (RMSF) |
(D. variabilis, D. andersoni, R. sanguineus) |
Western hemisphere |
Common |
|
Rickettsiosis |
R.parkeri (A. maculatum) |
Southern and mid-Atlantic USA, South America |
Rare, emerging in South America |
|
Pacific Coast tick fever (PCTF) |
R. philipii
(D. occidentalis) |
California, Pacific coast |
Rare |
|
Mediterranean spotted fever |
R. conorii complex (Rhipicephalus sanguineus) |
Europe, Africa, Middle East, Asia |
Uncommon, imported |
|
African tick bite fever |
R. africae (Amblyomma sp.) |
Africa, West Indies, Oceania |
Uncommon, imported |
|
Rickettsiales/ Anaplasmataceae (anaplasmosis and ehrlichiosis) | |||
|
Human granulocytic anaplasmosis (HGA) |
(I. scapularis, other sp.) |
Northeastern and central USA |
Common; emerging in Europe and Asia |
|
Human ehrlichiosis |
Ehrlichia chaffeensis , E. ewingii (A. americanum) |
Eastern USA |
Common |
|
Human ehrlichiosis |
(I. scapularis) |
Minnesota and Wisconsin |
Rare |
|
Neoehrlichiosis |
Ca. N. mikurensis (Ixodes sp.) |
Europe, Asia |
Rare |
|
Other bacterial | |||
|
Tularaemia |
Francisella tularensis (Amblyomma, Dermacentor Ixodes and Haemaphysalis) |
North America, Europe, Asia |
Uncommon by tick bite; other routes of exposure |
|
Parasites (Protists) | |||
|
Human babesiosis |
Babesia microti, B. divergens, (I. ricinus complex) |
Northeastern and Midwestern USA Europe, China |
Common; emerging in Europe and Asia |
|
Human babesiosis |
B. duncani (WA-1 type parasite) (D. albipictus, I. pacificus?) |
Pacific Coast of North America |
Emerging |
|
Tick bite associated (non-pathogenic) | |||
|
Alpha-gal syndrome (red meat allergy) |
(A americanum, H. longicornis, Ixodes sp.) |
Worldwide |
Uncommon, increasing |
|
Tick paralysis |
Various species |
USA, Australia, other countries |
Rare |
*Generic tick names abbreviated in (): A., Amblyomma; D., Dermacentor; H., Haemophysalis; I., Ixodes; R., Rhipicephalus.
†Prevalence, reported annual number of human cases (approximate): rare, <10; uncommon, >10–1000; common, >1000; common and widespread, ≈several thousands.
The Vectors
Tick-borne pathogens circulate in enzootic cycles, alternating between ticks and suitable animal hosts, mostly rodents [20]. Both the tick vector and the vertebrate host are needed to maintain tick-borne pathogens in enzootic cycles. An exception to this general rule are those pathogens that have an efficient transovarial transmission for which the tick serves as a vector as well as a host [21]. Infected ticks can transmit pathogens to humans; however, with very few exceptions (i.e. tick-borne relapsing fever caused by Borrelia duttonii in East Africa [22]), humans are dead-end hosts and do not play any role in the transmission and maintenance of tick-borne pathogens in enzootic cycles.
Ticks are divided into two main families, Argasidae or soft ticks and Ixodidae or hard ticks, that differ in their ecology and public health impact [20, 23]. In contrast to hard ticks, soft ticks of the family Argasidae feed quickly (from minutes to an hour), and can take several blood meals per stage [24]. Soft ticks have several developmental stages per moulting and typically inhabit wildlife nests, burrows,and caves. Thus, they have a more restricted habitat compared to hard ticks [25, 26]. Fewer human pathogens are transmitted by soft ticks compared to hard ticks, probably due to their behavioural traits, including short feeding times and host-seeking strategies [20].
Hard ticks, members of the Ixodidae family, are cosmopolitan and can be found in different natural habitats and suburban and even urban areas [25]. Hard ticks have different host-seeking behaviours and typically feed for extended periods, ranging from 3–12 days, depending on the species and tick stage [23]. Prolonged contact between ticks and their hosts facilitates the transmission of pathogens, especially bacterial pathogens that generally require over 24 h to be transmitted efficiently [27].
Hard ticks have three active feeding stages in their life cycle – larva, nymph and adult. Typically, the immature tick stages, larvae and nymphs feed on small mammals, often rodents, which are suitable animal reservoirs for many tick-borne pathogens (see Table 2 in Parola and Raoult [20] for details). Tick-borne pathogens are acquired by the vector while feeding on an infected host . These pathogens are well adapted to their vector and persist throughout moulting. This is known as transstadial transmission and is necessary for pathogen survival and transmission. Some pathogens, particularly Rickettsia species (see Parola and Raoult [20] for a full list), are also transmitted transovarially, from the female tick to her offspring [21].
Nymph and adult ticks are the most important stages in the transmission of human pathogens, whereas larvae only play a role in the transmission of transovarial pathogens. The most dangerous tick stage for humans are nymphs since, in contrast to adults, they are small, hard to detect and active during spring and summer, a period that concurs with the highest human outdoor activity in temperate climates. After the bite of an infected tick and a short incubation period, the initial course of infection usually presents itself as an unspecific febrile illness with symptoms including chills, sweating, headaches, myalgias, arthralgias, malaise, nausea and vomiting [28]. The severity of the illness varies among tick-borne pathogens and depends on the immune status of the patient. Some tick-borne diseases are self-limited, while others are life-threatening. For example, the course of infection for human babesiosis is often subclinical in healthy individuals but can be much more severe in immunocompromised patients [29]. While Lyme disease is not typically fatal, it may lead to debilitating and persistent conditions [30]. On the other hand, tick-borne encephalitis (TBE) or Powasan virus can be fatal or leave the patient with permanent cognitive impairment [31]. Accurate identification of ticks and their stages are essential for diagnosis, treatment and prophylaxis of tick-borne diseases [32]. Larval stages of most tick species pose little to no risk of pathogen transmission to humans. Some tick species, such as Ixodes scapularis, may harbour numerous pathogens, whereas other commonly encountered species may typically be associated with one major pathogen (Table 1) [33]. Successful prophylaxis of Lyme disease with preventative antibiotic treatment requires not only accurate tick species identification, but also the determination of the duration of tick feeding [32, 34].
The Pathogens
Viral
Tick-borne viruses are the most taxonomically diverse group, representing four viral families or orders – Flaviviridae, Bunyavirales (formerly family Bunyaviridae), Orthomyxoviridae and Reoviridae (Table 1). Tick-borne flaviviruses represent a large and important group of viruses, including TBE virus, louping ill, the Asian Omsk haemorrhagic fever virus, Kyasanur Forest disease (KFD) and Powassan virus (POW). These viruses are notable for causing encephalitis and haemorrhagic symptoms [35] and are responsible for more than 10 000 cases that require hospitalization per year in Europe, Russia, China and Japan (Table 1, Fig. 1) [36]. TBE virus is expanding in Europe as a result of climate and land use changes [37]. The Asian Omsk haemorrhagic fever virus causes a febrile disease that often displays severe haemorrhagic symptoms [38]. KFD is present in the Indian subcontinent and causes encephalitis accompanied by haemorrhages [39]. The most recent and remarkable trends are the increase of TBE viruses outside of the typical temperate Eurasian range [40]. The rise of POW is an example of the current trend observed in different members of the TBE group. Once an obscure pathogen in northeastern North America, the number of human cases of POW has increased in the last decade and now is considered an emerging disease [31, 41]. The range of POW encompasses North America and Eurasia, where I. scapularis and Ixodes ricinus are widely distributed [42]. Symptomatic patients infected with POW develop neurological signs and symptoms, including encephalitis, meningitis, loss of coordination and speech difficulties, after an incubation period of 1–4 weeks, with high fever and headaches [43]. Approximately 10 % of POW encephalitis cases are fatal or severe, with long-lasting neurological sequelae [41]. Another member of the TBE complex, KFD, is on the rise on the Indian subcontinent [37, 44].
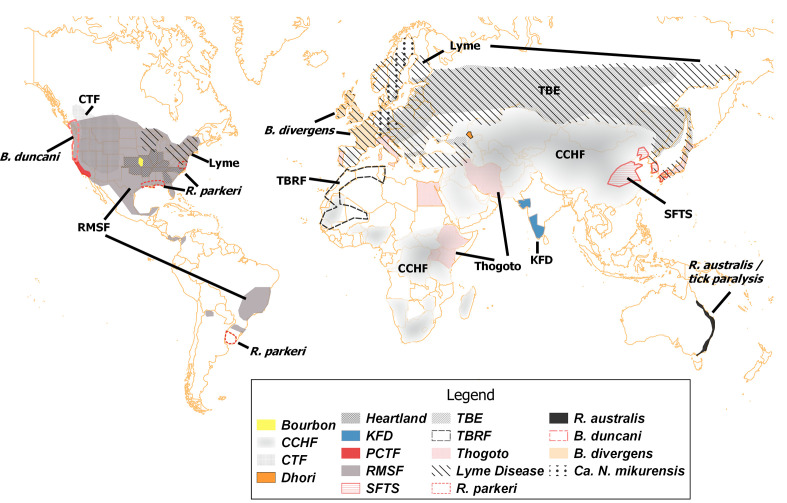
Fig. 1.
Geographical distribution of selected tick-borne pathogens and diseases. All geographical boundaries are generalized and approximate. Abbreviations and descriptions are shown in Table 1. For some pathogens (Heartland virus, Thogoto virus, B. divergens, Ca. N. mikurensis), only country or state-level data were available. Only partial ranges are shown for some worldwide diseases and conditions – TBRF (tick-borne relapsing fever) in northwestern Africa, tick paralysis in Australia. Modified from previously published records and maps [29, 37, 44, 51, 53, 64, 66, 72, 83, 94, 105, 149–155]
TBE arboviruses are typically transmitted by members of the I. ricinus complex ticks, including I. ricinus L. in Europe, Ixodes persulcatus in Russia and China, and I. scapularis or Ixodes pacificus in North America [35]. Recently, Dermacentor species have been implicated in the transmission of TBE in those areas devoid of Ixodes species in East Asia [45], while Haemaphysalis spinigera has been proposed as the primary vector of KFD in the Indian subcontinent [44].
The order Bunyavirales (formerly family Bunyaviridae) is another crucial arboviral group and perhaps the most daunting among the emerging tick-borne viruses (Table 1, Fig. 1). This group includes the Crimean–Congo haemorrhagic fever virus (CCHFV), which has a wide range of hosts and tick vectors in Eurasia and Africa [46–48]. The transmission and life cycle of CCHFV is well-characterized; nonetheless, it remains a critical emerging pathogen over much of its range, with various modes of transmission, including tick-borne and directly from infected livestock [37, 40]. CCHFV affects the nervous system and causes neurological manifestations that precede more dramatic haemorrhagic symptoms [46]. CCHFV has a short incubation period, followed by a rapid onset characterized by severe headache, dizziness, neck pain and vomiting, with high mortality rates in untreated or nosocomial infections [46, 47, 49]. CCHFV has been isolated from over 30 tick species, including Ixodidae and Argasidae ticks [50, 51]. However, the major CCHFV vectors belong to the genus Hyalomma, including H. truncatum in Africa and H. marginatum in southern Europe and western Asia [46, 48, 50].
Members of the genus Phlebovirus (Buyanvirales) that are closely related to CCHFV are among the newly recognized emerging pathogens, such as the severe fever with thrombocytopenia syndrome virus (SFTSV). The SFTSV was first described in 2009 and is associated with fever, thrombocytopenia, leukocytopenia and gastrointestinal symptoms [52–54]. The distribution of SFTSV is currently restricted to Asia (Fig. 1); PR China accounts for approximately 1000 cases per year with a mortality rate that ranges from 6 to 30 %, while Japan and he Republic of Korea have sporadic cases [52, 53] The primary vector for the SFTSV is the Asian longhorned tick, Haemaphysalis longicornis Neumann [54]. The distribution of the Asian longhorned tick encompasses its original distribution in East Asia (PR China, Republic of Korea, Japan) as well as Australia, New Zealand and some Pacific Islands, where it was introduced in the past century [55]. Recently, the Asian longhorned tick was found in New Jersey [14] and neighbouring states in the USA after subsequent surveillance efforts. The Asian longhorned tick, according to suitable habitat modelling based on climatic factors, can spread throughout the eastern USA and parts of the West Coast [15]. The Midwest of the USA, which is an endemic area for the heartland virus, a novel Phlebovirus that is closely related to SFTSV, is a particularly suitable habitat for this tick species. The heartland virus was isolated in 2009 from two patients in Missouri, USA [56]. Since then, several more cases have been detected in nearby states [57]. The clinical manifestations of the heartland virus are similar to those of other tick-borne diseases, but it can also disseminate rapidly, causing a severe shock and multisystemic organ failure that accounts for its high mortality rate [58, 59]. The heartland virus has been isolated from lone-star ticks (Amblyomma americanum L.), which are considered to be its primary vector based on field isolations and laboratory studies [57, 60, 61]. Closely related to SFTSV and heartland virus are Bhanja serogroup Phleboviruses that are widespread in the old world. They are likely transmitted by Haemaphysalis ticks (Europe and Asia) or other tick species, including Amblyomma, Dermacentor, Rhipicephalus and Hyalomma ticks in Africa [62]. Reported human disease was febrile and mild [63].
The lone-star tick, Amblyomma americanum, likely serves as the vector for another emerging pathogen, the Bourbon virus, from the family Orthomyxoviridae genus Thogotovirus (Table 1, Fig. 1). The Bourbon virus was isolated from multiple pools of A. americanum collected in Missouri, USA [64]. Although Bourbon virus infections are rare, they can be fatal [65]. This virus is the first Thogotovirus to be found in North America that infects humans, but other viruses such as Thogoto and Dhori are emerging in parts of Europe and Asia. In southern Russia, the Dhori virus was isolated from Hyalomma marginatum, and the seroprevalence in humans ranges from 4–6 % [66]. Similarly, these viruses were isolated from Hyalomma ticks in Egypt [67]. In Japan, the recently identified Thogoto virus is likely vectored by H. longicornis [68]. Thogotoviruses and influenza viruses are related, and both belong to the same family, Orthomyxoviridae. Thus, Thogotoviruses can also be transmitted by other routes, such as aerosol [69]. Although in most patients Thogotovirus infection courses as a febrile illness, severe cases present encephalitis and other neurological disorders [70].
Similarly, Colorado tick fever (western North America, Fig. 1) and Eyach (Western Europe) viruses from the genus Coltivirus (family Reoviridae) present a self-limited flu-like illness; complications are rare but can be severe when they affect the central nervous system [71]. While Colorado tick fever is transmitted by Dermacentor andersoni [5, 72], the closely related Eyach virus is transmitted by Ixodes ventalloi and I. ricinus [71, 73].
Bacterial
Over 90 % of bacterial species transmitted by ticks belong to two orders, Spirochaetales and Rickettsiales (Table 1) [20]. Lyme borreliosis, caused by a spirochete, is the most common tick infection, with approximately 300 000 (240 000 – 444 000) annual cases in the USA [1–3] and 85 000 cases in Europe [74] (Table 1, Fig. 1). Lyme disease is caused by different genetic species (genospecies) within the Borrelia burgdorferi sensu lato complex [1, 40, 75] and is transmitted by the same I. ricinus complex involved in TBE virus cycle – I. ricinus (Europe), I. persulcatus (Russia and Asia) and I. scapularis or I. pacificus (North America) [76–78]. The pathogenic genospecies include B. burgdorferi sensu stricto, B. garinii , B. afzelii , B. spilmanii, B. bavariensis and B. mayonii, which was recently isolated from patients, I. scapularis ticks, and rodents in the upper Midwest of the USA [79, 80].
The genus Borrelia includes other human pathogenic species that cause relapsing fever [81]. Relapsing fever is characterized by recurring febrile episodes that last around 3 days and are separated by a nonfebrile period. During these episodes, patients commonly experience high fever, headache, myalgia, chills and nausea [82]. In contrast to Lyme disease Borrelia , most relapsing fever spirochetes are transmitted by soft ticks from the genus Ornithodoros, which are present in Africa, Eurasia and the Americas [81, 83–85]. In western North America, Ornithodoros ticks are found in the desert and scrub regions from Texas to British Columbia [81, 84]. A notable exception in the tick-borne relapsing fever group is Borrelia miyamotoi , which is vectored by the same I. ricinus complex species that transmit Lyme borreliosis [86–88]. B. miyamotoi has been isolated from I. ricinus (Europe), I. persulcatus (Russia) and I. scapularis ticks [88]. In contrast to other relapsing fever Borrelia species, B. miyamotoi is not frequently recognized in patients, since it presents a limited febrile illness that is often subclinical. Patients display mild symptoms that are similar to those from influenza infection with high fever (98 %), fatigue (98 %), headache (89 %), myalgia (59 %), chills (35 %) and nausea (30 %), occasionally followed by a relapse about a week later in approximately 10 % of patients [87, 88].
Tick-borne rickettsial diseases, such as spotted fever group rickettsioses (SFGR), are a group of diseases caused by members of the genus Rickettsia . The SFGR encompass a large number of emerging and established diseases that have a worldwide distribution (Table 1, Fig. 1) [39, 89, 90]. In the USA, Rocky Mountain spotted fever (RMSF) is vectored by Dermacentor andersoni Styles, the Rocky Mountain wood tick, Dermacentor variabilis Say, the American dog tick, and the brown dog tick (Rhipicephalus sanguineus Latreille) in the southwestern USA and Mexico,and is is the most severe rickettsiosis and frequently requires hospitalization [91]. A delay in diagnosis or treatment can result in complications, including amputation, cognitive disabilities, paralysis, hearing loss and even death [5, 92]. There are other forms of rickettsiosis in the USA that are caused by Rickettsia parkeri, which is transmitted by the Gulf Coast tick Amblyomma maculatum Koch and is also emerging in South America [93], and Rickettsia philipii, transmitted by the Pacific Coast tick Dermacentor occidentalis Marx. These two rickettsia species cause a less severe form of rickettsiosis with fever, diarrhoea, nausea, vomiting and an eschar, i.e., cutaneous necrosis at the site of the tick bite [92, 94].
Within the same order Rickettsiales , the family Anaplasmataceae includes emerging pathogens in the genera Anaplasma and Ehrlichia . The most important public health pathogen within this group is Anaplasma phagocytophilum, which is responsible for approximately 6000 cases of human granulocytic anaplasmosis (HGA) per year in the USA [95]. The vectors of HGA in the USA are I. scapularis and I. pacificus on the East and West Coasts of the USA, respectively. The onset of HGA is accompanied by unspecific symptoms, including fever, chills, malaise, headache and myalgias; appropriate treatment is essential to prevent life-threatening complications [95, 96]. HGA has also been reported in northern Europe, and Southeast Asia, including China, Mongolia and Korea [97].
Human ehrlichiosis is caused by Ehrlichia chaffeensis or Ehrlichia ewingii , which are transmitted by the American lone star tick, A. americanum. The symptoms are similar to those described for human anaplasmosis, and its prognosis depends on early diagnosis and treatment to avoid further complications that could potentially be fatal [98]. Human ehrlichiosis caused by E. chaffeensis has been detected in North America, Europe and Asia [99]. More recently, a new pathogen, Ehrlichia muris eauclairensis, was detected in patients from Minnesota and Wisconsin in the USA [100, 101]. Interestingly, these Ehrlichia species are transmitted by I. scapularis in contrast to other Ehrlichia species in the USA. Another, closely related Ehrlichia bacterium, Candidatus Neoehrlichia mikurensis, is also transmitted by members of the I. ricinus complex, I. ricinus and I. persulcatus. It is an emerging pathogen in Europe and Asia, occasionally causing life-threatening conditions in immunocompromised patients [102–105].
Another bacterial disease, tularaemia, caused by the intracellular Gram-negative coccobacillus Francisella tularensis , can be transmitted by ticks. However, it is not limited to this mode of transmission and can also be acquired by many different routes, such as inhalation of contaminated aerosols, ingestion, direct contact, through deer fly, or potentially, mosquito bite [106–109]. Tularaemia is found in countries throughout the northern hemisphere in America, Europe and Asia [110].
Protozoan
Human babesiosis is an emerging tick-borne disease caused by members of the genus Babesia, protozoans that share many clinical features with Plasmodium malaria parasites, including reproduction within mammalian red blood cells [29, 40, 75, 111]. In North America, B. microti is the most common agent of human babesiosis and is present in the northeast and upper Midwest of the USA, where its vector, I. scapularis, is widely distributed. The course of human babesiosis is generally similar to a flu-like subclinical infection in healthy individuals. Older people, as well as immunocompromised and splenectomized patients, are at higher risk of developing severe symptoms and complications [29]. Human babesiosis can also be acquired through blood transfusions, a problem exacerbated by the lack of a licensed test to screen for B. microti in donated blood, making it the most common blood transfusion-transmitted pathogen reported in the USA [112].
Recently, a second species, Babesia duncani, was detected in patients along the Pacific coast of the USA and Canada [29, 113]. Until recently, the recognized vectors for B. duncani were Ixodes species, including I. scapularis and I. pacificus. A new study provided strong support for the winter tick, Dermacentor albipictus, and the mule deer as the respective vector and host species of B. duncani, suggesting that the pathogen might have a wider geographical distribution [114]. Lastly, a third species close to Babesia divergens has been detected sporadically in patients in the USA [113, 115, 116]. In Europe, B. divergens is transmitted by I. ricinus and is the leading cause of human babesiosis (Fig. 1) [29, 113, 117–119]. Other species vectored by I. ricinus, such as Babesia venatorum and B. microti, have been associated with human cases in Europe [120]. In South Asia, B. microti is transmitted by Ixodes ovatus Neumann, while Babesia crassa and B. venatorum cases have been reported from northern China, likely vectored by I. persulcatus [120, 121].
Tick bite-associated complications
Tick bites may cause an allergy to red meat, or alpha-gal syndrome. Alpha-gal syndrome is mediated by an IgE antibody response against an epitope present in the mammalian oligosaccharide, galactose-alpha-1,3-galactose (alpha-gal). This allergic reaction is attributed to the bite of certain tick species and is considered to be an emerging condition around the world [122, 123]. Several hundred to several thousand cases have been documented in North America, Europe, Australia and Japan; single cases have been reported from South America and Africa [124]. Some of the typical symptoms associated with the alpha-gal syndrome include urticarial or anaphylactic reactions that are generally manifested a few hours after eating red meat, including pork, lamb, beef, or kangaroo. In the USA, the lone star tick (A. americanum) has been often associated with reports of delayed urticaria angioedema, a painful and pruritic urticarial rash, abdominal pain, diarrhoea and sore throat [125, 126]. The bite of the Asian longhorned tick (H. longicornis), which was recently identified in the USA, is the most probable cause of red meat allergy in Japan [127].
Tick paralysis is a neuromuscular paralysis caused by salivary neurotoxins secreted by female hard ticks while feeding [128–131]. Worldwide, over 40 species of ticks have been implicated in tick paralysis, among them D. andersoni and D. variabilis in North America [128, 130]. The majority of reported cases have occurred in southeastern and western USA and southwestern Canada in young girls (<8 years old) with long hair where ticks are attached unnoticed [128, 129]. In Australia, tick paralysis, a rare but potentially fatal condition, is transmitted by Ixodes holocyclus, the aptly named Australian paralysis tick, the cause of paralysis to humans, domestic animals and wildlife [131, 132].
Tick and tick-borne disease management and control
The US Centers for Disease Control and Prevention (CDC) recommends personal protection as the best defence against tick-borne disease [133], in addition to landscaping modifications and acaricide applications to individual properties [133–136]. There is very little evidence that any of these measures are effective in reducing human–tick encounters, exposure to ticks, or disease incidence [137–139].
A prerequisite for effective personal protection is lowering tick population numbers or pathogen prevalence area-wide in highly endemic regions [140]. The only available area-wide control options to reduce tick populations target deer or mice as hosts for either adult or immature tick stages, respectively [141]. Deer population reduction was proposed as the cornerstone of integrated tick management [142], as treatments of deer and mice with topical pesticides have failed to produce a sustainable reduction in tick populations or human disease incidence [143–146].
The reasons for the failure of conventional tick control as opposed to more successful mosquito control are diverse [147]. It is clear that control of Lyme and other tick-borne diseases will require a paradigm shift emphasizing measures to reduce tick and host populations and a substantial research and development effort. At present, clinical assessment and treatment remain the most critical tools in reducing the burden of tick-borne diseases.
Conclusions
The resurgence of tick-borne diseases is driven by the expansion of tick populations, particularly in northern America, Europe and Asia. Much of North America is endemic for a confounding variety of tick-borne pathogens, including viruses, bacteria and protozoa. In addition, tick bites can also lead to severe allergic reactions. In high-risk areas, public health practitioners are at the frontline of tick-borne diseases and should be familiar with the different tick species present in the area as well as the pathogens they transmit. Personal protection, vector control and accurate diagnosis and treatment will remain the cornerstones of coping with tick-borne diseases in the foreseeable future. While vaccine development may provide some relief, in general, relying solely on a vaccine does not work well in the multi-pathogen multi-vector systems [148] that are found in most endemic tick-borne disease areas (Fig. 1). The recent introduction of vector-borne pathogens such as West Nile virus and a new tick species, the Asian longhorned tick (Haemaphysalis longicornis), underscores the risk of increased expansions by vectors and pathogens in the increasingly mobile and interconnected world.
Funding information
A. T. is supported by NIH grant AI‐125806‐01.
Author contributions
I. R. and A. T. conceptualized the study and wrote the original draft.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: CCHFV, Crimean–Congo hemorrhagic fever virus; CDC, Centers for Disease Control and Prevention; KFD, Kyasanur Forest disease; POW, Powassan virus; RMSF, Rocky Mountain spotted fever; SFGR, Spotted fever group rickettsioses; SFTSV, Severe fever with thrombocytopenia syndrome virus; TBE, Tick-borne encephalites.
References
- 1.Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, et al. Vital Signs : Trends in Reported Vectorborne Disease Cases — United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep. 2018;67:496–501. doi: 10.15585/mmwr.mm6717e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, et al. Incidence of Clinician-Diagnosed Lyme disease, United States, 2005-2010. Emerg Infect Dis. 2015;21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, et al. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis. 2014;59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egizi A, Fefferman NH, Jordan RA. Relative risk for ehrlichiosis and Lyme disease in an area where vectors for both are sympatric, new Jersey, USA. Emerg Infect Dis. 2017;23:1080–6059. doi: 10.3201/eid2306.160528. [DOI] [PubMed] [Google Scholar]
- 5.Eisen RJ, Kugeler KJ, Eisen L, Beard CB, Paddock CD. Tick-Borne zoonoses in the United States: persistent and emerging threats to human health. ILARJ. 2017;58:319–335. doi: 10.1093/ilar/ilx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Meltzer MI, Peña CA, Hopkins AB, Wroth L, et al. Economic impact of Lyme disease. Emerg Infect Dis. 2006;12:653–660. doi: 10.3201/eid1204.050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adrion ER, Aucott J, Lemke KW, Weiner JP. Health care costs, utilization and patterns of care following Lyme disease. PLoS ONE. 2015;10:e0116767. doi: 10.1371/journal.pone.0116767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson L, Aylward A, Stricker RB. Healthcare access and burden of care for patients with Lyme disease: a large United States survey. Health Policy. 2011;102:64–71. doi: 10.1016/j.healthpol.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Donohoe H, Pennington-Gray L, Omodior O. Lyme disease: current issues, implications, and recommendations for tourism management. Tour Manag. 2015;46:408–418. doi: 10.1016/j.tourman.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mac S, da Silva SR, Sander B. The economic burden of Lyme disease and the cost-effectiveness of Lyme disease interventions: a scoping review. Plos One. 2019;14:e0210280. doi: 10.1371/journal.pone.0210280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estrada-Peña A, Salman M. Current limitations in the control and spread of ticks that affect livestock: a review. Agriculture. 2013;3:221–235. [Google Scholar]
- 12.de Castro JJ, James AD, Minjauw B, Di Giulio GU, Permin A, et al. Long-Term studies on the economic impact of ticks on Sanga cattle in Zambia. Exp Appl Acarol. 1997;21:3–19. [PubMed] [Google Scholar]
- 13.Kivaria FM. Estimated direct economic costs associated with tick-borne diseases on cattle in Tanzania. Trop Anim Health Prod. 2006;38:291–299. doi: 10.1007/s11250-006-4181-2. [DOI] [PubMed] [Google Scholar]
- 14.Rainey T, Occi JL, Robbins RG, Egizi A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) Parasitizing a Sheep in New Jersey, United States. J Med Entomol. 2018;55:757–759. doi: 10.1093/jme/tjy006. [DOI] [PubMed] [Google Scholar]
- 15.Rochlin I. Modeling the Asian Longhorned tick (Acari: Ixodidae) suitable habitat in North America. J Med Entomol. 2019;56:384–391. doi: 10.1093/jme/tjy210. [DOI] [PubMed] [Google Scholar]
- 16.Fujisaki K, Kawazu S, Kamio T. The taxonomy of the bovine Theileria spp. Parasitol Today. 1994;10:31–33. doi: 10.1016/0169-4758(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 17.Hammer JF, Emery D, Bogema DR, Jenkins C. Detection of Theileria orientalis genotypes in Haemaphysalis longicornis ticks from southern Australia. Parasit Vectors. 2015;8:229. doi: 10.1186/s13071-015-0839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oakes VJ, Yabsley MJ, Schwartz D, LeRoith T, Bissett C, et al. Theileria orientalis Ikeda Genotype in Cattle, Virginia, USA. Emerg Infect Dis. 2019;25:1653–1659. doi: 10.3201/eid2509.190088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paddock CD, Branch MRZ. Changing Paradigms for Tick-Borne Diseases in the Americas. Global Health Impacts of Vector-Borne Diseases: Workshop Summary. National Academies Press; 2016. [PubMed] [Google Scholar]
- 20.Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. doi: 10.1086/319347. [DOI] [PubMed] [Google Scholar]
- 21.Socolovschi C, Mediannikov O, Raoult D, Parola P. The relationship between spotted fever group Rickettsiae and Ixodid ticks. Vet Res. 2009;40:34. doi: 10.1051/vetres/2009017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCall PJ, Hume JCC, Motshegwa K, Pignatelli P, Talbert A, et al. Does tick-borne relapsing fever have an animal reservoir in East Africa? Vector Borne Zoonotic Dis. 2007;7:659–666. doi: 10.1089/vbz.2007.0151. [DOI] [PubMed] [Google Scholar]
- 23.Sonenshine DE, RMichael R. Biology of Ticks. 2nd ed. Oxford University Press; 2014. [Google Scholar]
- 24.Vial L. Biological and ecological characteristics of soft ticks (Ixodida: Argasidae) and their impact for predicting tick and associated disease distribution. Parasite. 2009;16:191–202. doi: 10.1051/parasite/2009163191. [DOI] [PubMed] [Google Scholar]
- 25.Sonenshine DE. The biology of tick vectors of human disease. in. 2005.
- 26.Uspensky I. Encyclopedia of Entomology. Dordrecht: Springer Netherlands; 2005. Argasid (Soft) Ticks (Acari: Ixodida: Argasidae) pp. 195–198. [Google Scholar]
- 27.Eisen L. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Vol. 9, Ticks and Tick-borne Diseases. Vol. 9. Urban & Fischer; 2018. pp. 535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dennis DT, Piesman JF. Overview of tick-borne infections of humans. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-Borne Diseases of Humans. Vol. 401. Washington, DC: ASM Press; 2005. [Google Scholar]
- 29.Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–2407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 30.Halperin JJ. Lyme Disease: An Evidence-Based Approach. 2nd edn. Wallingford, UK: CABI; 2018. [Google Scholar]
- 31.Hermance ME, Thangamani S. Powassan virus: an emerging arbovirus of public health concern in North America. Vector-Borne Zoonotic Dis. 2017;17:453–462. doi: 10.1089/vbz.2017.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the infectious diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez-Vicente S, Tagliafierro T, Coleman JL, Benach JL, Tokarz R. Polymicrobial nature of tick-borne diseases. mBio. 2019;10 doi: 10.1128/mBio.02055-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falco RC, Daniels TJ, Vinci V, McKenna D, Scavarda C, et al. Assessment of Duration of Tick Feeding by the Scutal Index Reduces Need for Antibiotic Prophylaxis After Ixodes scapularis Tick Bites. Clin Infect Dis. 2018;67:614–616. doi: 10.1093/cid/ciy221. [DOI] [PubMed] [Google Scholar]
- 35.Nuttall PA, Labuda M. Tick-borne encephalitis. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-Borne Diseases of Humans. Washington, D.C: ASM Press; 2005. [Google Scholar]
- 36.Heinz FX, Holzmann H. Tick-borne encephalitis. In: Service MW, editor. The Encyclopedia of Arthropod-Transmitted Infections. Wallingford, UK: CABI Publishing; 2001. pp. 507–512. editor. [Google Scholar]
- 37.Mansfield KL, Jizhou L, Phipps LP, Johnson N. Emerging tick-borne viruses in the twenty-first century. Front Cell Infect Microbiol. 2017;7:298. doi: 10.3389/fcimb.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gavrilovskaya IN. Omsk haemorrhagic fever. In: Service MW, editor. The Encyclopedia of Arthropod-Transmitted Infections. Wallingford, UK: CABI Publishing; 2001. pp. 370–374. editor. [Google Scholar]
- 39.Brown RN, Lane RS, Dennis DT. Geographic distributions of tick-borne diseases and their vectors. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-Borne Diseases of Humans. Washington, D.C: ASM Press; 2005. pp. 363–391. [Google Scholar]
- 40.Hartemink N, Takken W. Trends in tick population dynamics and pathogen transmission in emerging tick-borne pathogens in Europe: an introduction. Exp Appl Acarol. 2016;68:269–278. doi: 10.1007/s10493-015-0003-4. [DOI] [PubMed] [Google Scholar]
- 41.Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol. 2010;55:95–110. doi: 10.1146/annurev-ento-112408-085446. [DOI] [PubMed] [Google Scholar]
- 42.Deardorff ER, Nofchissey RA, Cook JA, Hope AG, Tsvetkova A, et al. Powassan virus in mammals, Alaska and new Mexico, U.S.A., and Russia, 2004-2007. Emerg Infect Dis. 2013;19:2012–2016. doi: 10.3201/eid1912.130319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birge J, Sonnesyn S, Encephalitis PV, Minnesota USA. Emerg Infect Dis. 2012;18:1669–1671. doi: 10.3201/eid1810.120621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah SZ, Jabbar B, Ahmed N, Rehman A, Nasir H, et al. Epidemiology, pathogenesis, and control of a tick-borne disease- Kyasanur forest disease: current status and future directions. Front Cell Infect Microbiol. 2018;8:149. doi: 10.3389/fcimb.2018.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kholodilov I, Belova O, Burenkova L, Korotkov Y, Romanova L, et al. Ixodid ticks and tick-borne encephalitis virus prevalence in the South Asian part of Russia (Republic of Tuva). Ticks Tick-Borne Dis. 2019 doi: 10.1016/j.ttbdis.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Hoogstraal H. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 1979;15:307–417. doi: 10.1093/jmedent/15.4.307. [DOI] [PubMed] [Google Scholar]
- 47.Nuttall PA. Crimean-Congo haemorrhagic fever. In: Service MW, editor. The encyclopedia of arthropod-transmitted infections. Wallingford, UK: CABI Publishing; 2001. pp. 126–132. editor. [Google Scholar]
- 48.Whitehouse CA. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004;64:145–160. doi: 10.1016/j.antiviral.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 49.Naderi H, Sheybani F, Bojdi A, Khosravi N, Mostafavi I. Fatal nosocomial spread of Crimean-Congo hemorrhagic fever with very short incubation period. Am J Trop Med Hyg. 2013;88:469–471. doi: 10.4269/ajtmh.2012.12-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burt FJ, Swanepoel R. Tick-Borne Diseases of Humans. American Society of Microbiology; 2005. Crimean-Congo Hemorrhagic Fever. pp. 164–175. [Google Scholar]
- 51.Whitehouse CA. Crimean–Congo hemorrhagic fever. Antiviral Res. 2004;64:145–160. doi: 10.1016/j.antiviral.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Liu K, Zhou H, Sun R-X, Yao H-W, Li Y, et al. A national assessment of the epidemiology of severe fever with thrombocytopenia syndrome, China. Sci Rep. 2015;5:9679. doi: 10.1038/srep09679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu X-J, Liang M-F, Zhang S-Y, Liu Y, Li J-D, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011;364:1523–1532. doi: 10.1056/NEJMoa1010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo L-M, Zhao L, Wen H-L, Zhang Z-T, Liu J-W, et al. Haemaphysalis longicornis ticks as reservoir and vector of severe fever with thrombocytopenia syndrome virus in China. Emerg Infect Dis. 2015;21:1770. doi: 10.3201/eid2110.150126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoogstraal H, Roberts FHS, Kohls GM, Tipton VJ. Review of Haemaphysalis (Kaiseriana) longicornis Neumann (resurrected) of Australia, New Zealand, New Caledonia, Fiji, Japan, Korea, and northeastern China and USSR, and its parthenogenetic and bisexual populations (Ixodoidea, Ixodidae) J Parasitol. 1968:1197–1213. [PubMed] [Google Scholar]
- 56.McMullan LK, Folk SM, Kelly AJ, MacNeil A, Goldsmith CS, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med. 2012;367:834–841. doi: 10.1056/NEJMoa1203378. [DOI] [PubMed] [Google Scholar]
- 57.Pastula DM, Turabelidze G, Yates KF, Jones TF, Lambert AJ, et al. Notes from the field: Heartland virus disease-United states, 2012-2013. MMWR Morb Mortal Wkly Rep. 2014;63:270–271. [PMC free article] [PubMed] [Google Scholar]
- 58.Muehlenbachs A, Fata CR, Lambert AJ, Paddock CD, Velez JO, et al. Heartland virus–associated death in Tennessee. Clin Infect Dis. 2014;59:845–850. doi: 10.1093/cid/ciu434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fill M-MA, Compton ML, McDonald EC, Moncayo AC, Dunn JR, et al. Novel clinical and pathologic findings in a Heartland virus–associated death. Clin Infect Dis. 2017;64:510–512. doi: 10.1093/cid/ciw766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savage HM, Godsey MS, Lambert A, Panella NA, Burkhalter KL, et al. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg. 2013;89:445–452. doi: 10.4269/ajtmh.13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Godsey MS, Savage HM, Burkhalter KL, Bosco-Lauth AM, Delorey MJ. Transmission of Heartland Virus (Bunyaviridae: Phlebovirus) by Experimentally Infected Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 2016;53:1226–1233. doi: 10.1093/jme/tjw080. [DOI] [PubMed] [Google Scholar]
- 62.Matsuno K, Weisend C, Travassos da Rosa APA, Anzick SL, Dahlstrom E, et al. Characterization of the Bhanja Serogroup Viruses (Bunyaviridae): a Novel Species of the Genus Phlebovirus and Its Relationship with Other Emerging Tick-Borne Phleboviruses. J Virol. 2013;87:3719–3728. doi: 10.1128/JVI.02845-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calisher CH, Goodpasture HC. Human infection with Bhanja virus. Am J Trop Med Hyg. 1975;24:1040–1042. doi: 10.4269/ajtmh.1975.24.1040. [DOI] [PubMed] [Google Scholar]
- 64.Savage HM, Burkhalter KL, Godsey MS, Panella NA, Ashley DC, et al. Bourbon virus in field-collected ticks, Missouri, USA. Emerg Infect Dis. 2017;23:2017–2022. doi: 10.3201/eid2312.170532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kosoy OI, Lambert AJ, Hawkinson DJ, Pastula DM, Goldsmith CS, et al. Novel Thogotovirus associated with febrile illness and death, United States, 2014. Emerg Infect Dis. 2015;21 doi: 10.3201/eid2105.150150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smirnova SE, Karan LS, Kolyasnikova NM, Rubkin VS, Platonov AE. Prevalence of Batken/Dhori virus in the Crimean-Congo hemorrhagic fever-endemic Astrakhan region of the Russian Federation [Russian] Epidemiol Infect Dis Russ. 2011;1:12–19. [Google Scholar]
- 67.Williams RE, Hoogstraal H, Casals J, Kaiser MN, Moussa MI. Isolation of Wanowrie, Thogoto, and Dhori Viruses from Hyalomma Ticks Infesting Camels in Egypt1. J Med Entomol. 1973;10:143–146. doi: 10.1093/jmedent/10.2.143. [DOI] [PubMed] [Google Scholar]
- 68.Talactac MR, Yoshii K, Hernandez EP, Kusakisako K, Galay RL, et al. Vector competence of Haemaphysalis longicornis ticks for a Japanese isolate of the Thogoto virus. Sci Rep. 2018;8:9300. doi: 10.1038/s41598-018-27483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butenko AM, Leshchinskaia E, V, Semashko I, V, Donets MA, Mart’ianova LI. Dhori virus--a causative agent of human disease. 5 cases of laboratory infection [Russian] Vopr Virusol. 1987;32:724–729. [PubMed] [Google Scholar]
- 70.Dobler G. Arboviruses causing neurological disorders in the central nervous system. Arch Virol Suppl. 1996;11:33–40. doi: 10.1007/978-3-7091-7482-1_4. [DOI] [PubMed] [Google Scholar]
- 71.Attoui H, Jaafar FM, de Micco P, Lamballerie de., X Coltiviruses and Seadornaviruses in North America, Europe, and Asia. Emerg Infect Dis. 2005;11:1673–1679. doi: 10.3201/eid1111.050868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marfin AA, Campbell GL. Colorado tick fever and related Coltivirus infections. in: tick-borne diseases of humans. American Society of Microbiology. 2005:143–149. p. [Google Scholar]
- 73.Chastel C, Main AJ, Couatarmanac’h A, Le Lay G, Knudson DL, et al. Isolation of Eyach virus (Reoviridae, Colorado tick fever group) from Ixodes ricinus and I. ventalloi ticks in France. Arch Virol. 1984;82:161–171. doi: 10.1007/BF01311160. [DOI] [PubMed] [Google Scholar]
- 74.Lindgren E, Jaenson TGT. Lyme borreliosis in Europe: influences of climate and climate change, epidemiology, ecology and adaptation measures. WHO Reg Off Eur. 2006 [Google Scholar]
- 75.Fang L-Q, Liu K, Li X-L LS, Yang Y, Yao H-W, et al. Emerging tick-borne infections in mainland China: an increasing public health threat. Lancet Infect Dis. 2015;15:1467–1479. doi: 10.1016/S1473-3099(15)00177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lane RS, Piesman J, Burgdorfer W. Lyme borreliosis: relation of its causative agent to its vectors and hosts in North America and Europe. Annu Rev Entomol. 1991;36:587–609. doi: 10.1146/annurev.en.36.010191.003103. [DOI] [PubMed] [Google Scholar]
- 77.Piesman J, Gray JS. Lyme disease/Lyme borreliosis. Ecol Dyn Tick-Borne Zoonoses Sonenshine TN Mather Eds. Oxf Univ Press Inc N Y; 1994. pp. 327–350. [Google Scholar]
- 78.Coburn J, Steere AC, Glickstein L. Lyme Borreliosis. In: Tick-Borne Diseases of Humans. American Society of Microbiology. 2005. pp. 176–206. pp. [Google Scholar]
- 79.Pritt BS, Mead PS, Johnson DKH, Neitzel DF, Respicio-Kingry LB, et al. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis. 2016;16:556–564. doi: 10.1016/S1473-3099(15)00464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson TL, Graham CB, Hojgaard A, Breuner NE, Maes SE, et al. Isolation of the Lyme Disease spirochete Borrelia mayonii from naturally infected rodents in Minnesota. J Med Entomol. 2017;54:1088–1092. doi: 10.1093/jme/tjx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barbour AG. Relapsing Fever. In: Tick-Borne Diseases of Humans. American Society of Microbiology. 2005. pp. 268–291. pp. [Google Scholar]
- 82.Dworkin MS, Anderson JDE, Schwan TG, Shoemaker PC, Banerjee SN, et al. Tick‐Borne relapsing fever in the northwestern United States and southwestern Canada. Clin Infect Dis. 1998;26:122–131. doi: 10.1086/516273. [DOI] [PubMed] [Google Scholar]
- 83.Trape J-F, Diatta G, Arnathau C, Bitam I, Sarih M, et al. The Epidemiology and Geographic Distribution of Relapsing Fever Borreliosis in West and North Africa, with a Review of the Ornithodoros erraticus Complex (Acari: Ixodida). Bergström S, editor. PLoS ONE. 2013;8:e78473. doi: 10.1371/journal.pone.0078473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dworkin MS, Schwan TG, Anderson Jr DE, Borchardt SM. Tick-Borne relapsing fever. Infect Dis Clin North Am. 2008;22:449–viii. doi: 10.1016/j.idc.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Assous MV, Wilamowski A. Relapsing fever borreliosis in Eurasia—forgotten, but certainly not gone! Clin Microbiol Infect. 2009;15:407–414. doi: 10.1111/j.1469-0691.2009.02767.x. [DOI] [PubMed] [Google Scholar]
- 86.Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, et al. Human Borrelia miyamotoi infection in the United States. N Engl J Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Platonov AE, Karan LS, Kolyasnikova NM, Makhneva NA, Toporkova MG, et al. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg Infect Dis. 2011;17:1816. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wagemakers A, Staarink PJ, Sprong H, Hovius JWR. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015;31:260–269. doi: 10.1016/j.pt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 89.Parola P, Raoult D. Tick-borne typhuses. In: Service MW, editor. The Encyclopedia of Arthropod-Transmitted Infections. Wallingford, UK: CABI Publishing; 2001. pp. 516–524. editor. [Google Scholar]
- 90.Dobler G, Wölfel R. Typhus and other rickettsioses: emerging infections in Germany. Dtsch Ärztebl Int. 2009;106:348. doi: 10.3238/arztebl.2009.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]
- 92.Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, et al. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- 93.Labruna MB. Ecology of Rickettsia in South America. Ann N Y Acad Sci. 2009;1166:156–166. doi: 10.1111/j.1749-6632.2009.04516.x. [DOI] [PubMed] [Google Scholar]
- 94.Padgett KA, Bonilla D, Eremeeva ME, Glaser C, Lane RS, et al. The eco-epidemiology of Pacific coast tick fever in California. PLoS Negl Trop Dis. 2016;10:e0005020. doi: 10.1371/journal.pntd.0005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015;29:341–355. doi: 10.1016/j.idc.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dahlgren FS, Mandel EJ, Krebs JW, Massung RF, McQuiston JH. Increasing incidence of Ehrlichia chaffeensis and Anaplasma phagocytophilum in the United States, 2000–2007. Am J Trop Med Hyg. 2011;85:124–131. doi: 10.4269/ajtmh.2011.10-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Guzman N, Beidas SO. Anaplasma phagocytophilum (Anaplasmosis) StatPearls: StatPearls Publishing; 2018. [PubMed] [Google Scholar]
- 98.Ismail N, Bloch KC, McBride JW. Human ehrlichiosis and anaplasmosis. Clin Lab Med. 2010;30:261–292. doi: 10.1016/j.cll.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ganguly S, Mukhopadhayay SK. Tick-borne ehrlichiosis infection in human beings. J Vector Borne Dis. 2008;45:273–280. [PubMed] [Google Scholar]
- 100.Pritt BS, Allerdice MEJ, Sloan LM, Paddock CD, Munderloh UG, et al. Proposal to reclassify Ehrlichia muris as Ehrlichia muris subsp. muris subsp. nov. and description of Ehrlichia muris subsp. eauclairensis subsp. nov., a newly recognized tick-borne pathogen of humans. Int J Syst Evol Microbiol. 2017;67:2121–2126. doi: 10.1099/ijsem.0.001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnson DKH, Schiffman EK, Davis JP, Neitzel DF, Sloan LM, et al. Human infection with Ehrlichia muris-like pathogen, United States, 2007-2013(1) Emerg Infect Dis. 2015;21:1794–1799. doi: 10.3201/eid2110.150143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fehr JS, Bloemberg GV, Ritter C, Hombach M, Lüscher TF, et al. Septicemia caused by tick-borne bacterial pathogen Candidatus Neoehrlichia mikurensis. Emerg Infect Dis. 2010;16:1127–1129. doi: 10.3201/eid1607.091907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li H, Jiang J-F, Liu W, Zheng Y-C, Huo Q-B, et al. Human infection with Candidatus Neoehrlichia mikurensis, China. Emerg Infect Dis. 2012;18:1636–1639. doi: 10.3201/eid1810.120594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wennerås C. Infections with the tick-borne bacterium Candidatus Neoehrlichia mikurensis. Clin Microbiol Infect. 2015;21:621–630. doi: 10.1016/j.cmi.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 105.Portillo A, Santibz P, Palomar AM, Santibz S, Oteo JA, et al. Candidatus Neoehrlichia mikurensis in Europe. New Microbes and New Infections. 2018;22:30–36. doi: 10.1016/j.nmni.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maurin M, Gyuranecz M. Tularaemia: clinical aspects in Europe. Lancet Infect Dis. 2016;16:113–124. doi: 10.1016/S1473-3099(15)00355-2. [DOI] [PubMed] [Google Scholar]
- 107.Hirschmann JV. From squirrels to biological weapons: the early history of tularemia. Am J Med Sci. 2018;356:319–328. doi: 10.1016/j.amjms.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Eliasson H, Lindbäck J, Nuorti JP, Arneborn M, Giesecke J, et al. The 2000 tularemia outbreak: a case-control study of risk factors in Disease-Endemic and emergent areas, Sweden. Emerg Infect Dis. 2002;8:956–960. doi: 10.3201/eid0809.020051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.T Yu K, Popov VP, Mokrievich AN, Pakskina ND, Kholin AV, et al. Tularemia: relevant issues and forecast of epidemic situation in the territory of the Russian Federation in 2018. Probl Osobo Opasnykh Infektsii Probl Part Danger Infect Russ. 2018;1:22–29. [Google Scholar]
- 110.Gürcan Ş. Epidemiology of tularemia. Balk Med J. 2014;31:3–10. doi: 10.5152/balkanmedj.2014.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pritt BS. Manual of Clinical Microbiology. 11th ed. American Society of Microbiology; 2015. Plasmodium and Babesia; pp. 2338–2356. [Google Scholar]
- 112.Moritz ED, Winton CS, Tonnetti L, Townsend RL, Berardi VP, et al. Screening for Babesia microti in the U.S. Blood Supply. N Engl J Med. 2016;375:2236–2245. doi: 10.1056/NEJMoa1600897. [DOI] [PubMed] [Google Scholar]
- 113.Herwaldt BL, de Bruyn G, Pieniazek NJ, Homer M, Lofy KH, et al. Babesia divergens–like infection, Washington State. Emerg Infect Dis. 2004;10:622–629. doi: 10.3201/eid1004.030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Swei A, O'Connor KE, Couper LI, Thekkiniath J, Conrad PA, et al. Evidence for transmission of the zoonotic apicomplexan parasite Babesia duncani by the tick Dermacentor albipictus. Int J Parasitol. 2019;49:95–103. doi: 10.1016/j.ijpara.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burgess MJ, Rosenbaum ER, Pritt BS, Haselow DT, Ferren KM, et al. Possible transfusion-transmitted Babesia divergens-like/MO-1 infection in an Arkansas patient. Clin Infect Dis. 2017;64:1622–1625. doi: 10.1093/cid/cix216. [DOI] [PubMed] [Google Scholar]
- 116.Herc E, Pritt B, Huizenga T, Douce R, Hysell M, et al. Probable Locally Acquired Babesia divergens– Like Infection in Woman, Michigan, USA. Emerg Infect Dis. 2018;24:1558–1560. doi: 10.3201/eid2408.180309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Herwaldt BL, et al. A fatal case of babesiosis in Missouri: identification of another Piroplasm that infects humans. Ann Intern Med. 1996;124:643. doi: 10.7326/0003-4819-124-7-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 118.Beattie JF, Michelson ML, Holman PJ. Acute babesiosis caused by Babesia divergens in a resident of Kentucky. N Engl J Med. 2002;347:697–698. doi: 10.1056/NEJM200208293470921. [DOI] [PubMed] [Google Scholar]
- 119.Gray J, Zintl A, Hildebrandt A, Hunfeld K-P, Weiss L. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010;1:3–10. doi: 10.1016/j.ttbdis.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 120.Krause PJ. Human babesiosis. Int J Parasitol. 2019;49:165–174. doi: 10.1016/j.ijpara.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 121.Jia N, Zheng Y-C, Jiang J-F, Jiang R-R, Jiang B-G, et al. Human babesiosis caused by a Babesia crassa-Like pathogen: a case series. Clin Infect Dis. 2018;67:1110–1119. doi: 10.1093/cid/ciy212. [DOI] [PubMed] [Google Scholar]
- 122.Commins SP, Platts-Mills TAE. Tick bites and red meat allergy. Curr Opin Allergy Clin Immunol. 2013;13:354–359. doi: 10.1097/ACI.0b013e3283624560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kwak M, Somerville C, van Nunen S. A novel Australian tick Ixodes (Endopalpiger) australiensis inducing mammalian meat allergy after tick bite. Asia Pac Allergy. 2018;8:e31. doi: 10.5415/apallergy.2018.8.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.de la Fuente J, Pacheco I, Villar M, Cabezas-Cruz A. The alpha-Gal syndrome: new insights into the tick-host conflict and cooperation. Parasit Vectors. 2019;12:154. doi: 10.1186/s13071-019-3413-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jackson WL. Mammalian meat allergy following a tick bite: a case report. Oxf Med Case Reports. 2018;2018:omx098. doi: 10.1093/omcr/omx098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaplan AC, Carson MP. Diagnosing meat allergy after tick bite without delay. J Am Board Fam Med. 2018;31:650–652. doi: 10.3122/jabfm.2018.04.170425. [DOI] [PubMed] [Google Scholar]
- 127.Chinuki Y, Ishiwata K, Yamaji K, Takahashi H, Morita E. Haemaphysalis longicornis tick bites are a possible cause of red meat allergy in Japan. Allergy. 2016;71:421–425. doi: 10.1111/all.12804. [DOI] [PubMed] [Google Scholar]
- 128.Diaz JH. A 60-year meta-analysis of tick paralysis in the United States: a predictable, preventable, and often misdiagnosed poisoning. J Med Toxicol. 2010;6:15–21. doi: 10.1007/s13181-010-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Felz MW, Smith CD, Swift TR. A six-year-old girl with tick paralysis. N Engl J Med. 2000;342:90–94. doi: 10.1056/NEJM200001133420204. [DOI] [PubMed] [Google Scholar]
- 130.Morshed M, Li L, Lee M-K, Fernando K, Lo T, et al. A retrospective cohort study of tick paralysis in British Columbia. Vector Borne Zoonotic Dis. 2017;17:821–824. doi: 10.1089/vbz.2017.2168. [DOI] [PubMed] [Google Scholar]
- 131.Hall-Mendelin S, Craig SB, Hall RA, O'Donoghue P, Atwell RB, et al. Tick paralysis in Australia caused by Ixodes holocyclus Neumann. Ann Trop Med Parasitol. 2011;105:95–106. doi: 10.1179/136485911X12899838413628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barker SC, Walker AR, Australia Tof. The species that infest domestic animals and humans. Zootaxa. 2014;3816:1–144. doi: 10.11646/zootaxa.3816.1.1. [DOI] [PubMed] [Google Scholar]
- 133.Beard CB, Strickman D. Federal Initiative: Tick-Borne Disease Integrated Pest Management White Paper. Washington, DC: Federal Tick-Borne Disease Integrated Pest Management Workgroup; 2014. [Google Scholar]
- 134.Ginsberg HS, Stafford III KC. Management of ticks and tick-borne diseases. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-borne diseases of humans. Washington, DC: American Society for Microbiology; 2005. pp. 65–86. [Google Scholar]
- 135.Clark RP, Hu LT. Prevention of Lyme disease and other tick-borne infections. Infect Dis Clin North Am. 2008;22:381–396. doi: 10.1016/j.idc.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hook SA, Nelson CA, Mead PS. U.S. public's experience with ticks and tick-borne diseases: results from national healthstyles surveys. Ticks Tick Borne Dis. 2015;6:483–488. doi: 10.1016/j.ttbdis.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hinckley AF, Meek JI, Ray JAE, Niesobecki SA, Connally NP, et al. Effectiveness of residential acaricides to prevent Lyme and other tick-borne diseases in humans. J Infect Dis. 2016;214:182–188. doi: 10.1093/infdis/jiv775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Malouin R, Winch P, Leontsini E, Glass G, Simon D, et al. Longitudinal evaluation of an educational intervention for preventing tick bites in an area with endemic Lyme disease in Baltimore County, Maryland. Am J Epidemiol. 2003;157:1039–1051. doi: 10.1093/aje/kwg076. [DOI] [PubMed] [Google Scholar]
- 139.Connally NP, Durante AJ, Yousey-Hindes KM, Meek JI, Nelson RS, et al. Peridomestic Lyme disease prevention: results of a population-based case-control study. Am J Prev Med. 2009;37:201–206. doi: 10.1016/j.amepre.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 140.Ginsberg HS. Transmission risk of Lyme disease and implications for tick management. Am J Epidemiol. 1993;138:65–73. doi: 10.1093/oxfordjournals.aje.a116778. [DOI] [PubMed] [Google Scholar]
- 141.Eisen RJ, Piesman J, Zielinski-Gutierrez E, Eisen L. What do we need to know about disease ecology to prevent Lyme disease in the northeastern United States? J Med Entomol. 2012;49:11–22. doi: 10.1603/ME11138. [DOI] [PubMed] [Google Scholar]
- 142.Telford SR. Deer reduction is a cornerstone of integrated deer tick management. J Integr Pest Manag. 2017;8:1–5. doi: 10.1093/jipm/pmx024. [DOI] [Google Scholar]
- 143.Brei B, Brownstein JS, George JE, Pound JM, Miller JA, et al. Evaluation of the United States department of agriculture northeast Area-wide tick control project by meta-analysis. Vector Borne Zoonotic Dis. 2009;9:423–430. doi: 10.1089/vbz.2008.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grear JS, Koethe R, Hoskins B, Hillger R, Dapsis L, et al. The effectiveness of permethrin-treated deer stations for control of the Lyme disease vector Ixodes scapularis on Cape cod and the islands: a five-year experiment. Parasit Vectors. 2014;7:292. doi: 10.1186/1756-3305-7-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Daniels TJ, Fish D, Falco RC. Evaluation of host-targeted acaricide for reducing risk of Lyme disease in southern New York state. J Med Entomol. 1991;28:537–543. doi: 10.1093/jmedent/28.4.537. [DOI] [PubMed] [Google Scholar]
- 146.Stafford KC. Effectiveness of host-targeted permethrin in the control of Ixodes dammini (Acari: Ixodidae) J Med Entomol. 1991;28:611–617. doi: 10.1093/jmedent/28.5.611. [DOI] [PubMed] [Google Scholar]
- 147.Rochlin I, Ninivaggi DV, Benach JL. Malaria and Lyme disease - the largest vector-borne US epidemics in the last 100 years: success and failure of public health. BMC Public Health. 2019;19:804. doi: 10.1186/s12889-019-7069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Uspensky I. Ticks as the main target of human tick-borne disease control: Russian practical experience and its lessons. J Vector Ecol. 1999;24:40–53. [PubMed] [Google Scholar]
- 149.Choi SJ, Park S-W, Bae I-G, Kim S-H, Ryu SY, et al. Severe fever with thrombocytopenia syndrome in South Korea, 2013-2015. PLoS Negl Trop Dis. 2016;10:e0005264. doi: 10.1371/journal.pntd.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dantas-Torres F. Rocky Mountain spotted fever. Lancet Infect Dis. 2007;7:724–732. doi: 10.1016/S1473-3099(07)70261-X. [DOI] [PubMed] [Google Scholar]
- 151.Takahashi T, Maeda K, Suzuki T, Ishido A, Shigeoka T, et al. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J Infect Dis. 2014;209:816–827. doi: 10.1093/infdis/jit603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yoshii K, Okamoto N, Nakao R, Klaus Hofstetter R, Yabu T, et al. Isolation of the Thogoto virus from a Haemaphysalis longicornis in Kyoto City, Japan. J Gen Virol. 2015;96:2099–2103. doi: 10.1099/vir.0.000177. [DOI] [PubMed] [Google Scholar]
- 153.Woodall JP. Thogoto virus. In: Service MW, editor. Encyclopedia of Arthropod-Transmitted Infections of Man and Domesticated Animals. Wallingford, UK: CABI Publishing; 2001. pp. 504–507. editor. [Google Scholar]
- 154.Kurtenbach K. Lyme borreliosis. In: Service MW, editor. Encyclopedia of Arthropod-Transmitted Infections of Man and Domesticated Animals. Wallingford, UK: CABI Publishing; 2001. pp. 299–305. editor. [Google Scholar]
- 155.CDC Heartland virus [Internet]. 2019. Available from: https://www.cdc.gov/heartland-virus/statistics/index.html