Abstract
The coronavirus disease 2019 (COVID-19) pandemic is redefining the world we live in, and scientists are struggling to find the best severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) diagnostic tool. Routine testing is currently performed using real-time reverse transcription PCR (RT-PCR) of upper or lower respiratory tract secretions. We sought to demonstrate the importance of conducting RT-PCR using deep sampling when initial upper respiratory testing is negative in cases of high index of suspicion for COVID-19. We present the case of a 47-year-old man admitted for fever and bilateral pneumonia diagnosed via chest computed tomographic scan amidst the early peak of the COVID-19 pandemic, suggesting a SARS-CoV-2 infection. Two RT-PCR results from nasopharyngeal swab samples were negative. A bronchoscopy was then performed, and RT-PCR testing on bronchoalveolar lavage samples yielded positive results, confirming the diagnosis of COVID-19 pneumonia. RT-PCR samples of the lower respiratory tract likely contain a higher virus load and thus retain a higher sensitivity for SARS-CoV-2 detection.
Keywords: Coronavirus, COVID-19, pneumonia, polymerase chain reaction, SARS-CoV-2
Introduction
The number of confirmed coronavirus disease 2019 (COVID-19) cases around the globe is growing dramatically by the minute. Although the reason behind this uptick is chiefly due to the high contagiousness of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it also is a result of increased testing. The reference standard diagnosis is currently based on real-time reverse transcription PCR (RT-PCR) of respiratory specimens, most commonly using nasopharyngeal swabs. However, as of this writing, no diagnostic method has yet exhibited perfect sensitivity, with testing often leading to false-negative results and delayed diagnosis [1,2].
Case report
We report the case of a 47-year-old man with no relevant medical history who sought care at our tertiary-care hospital with spiking fever. Onset of symptoms was a week earlier, with the patient's fever temperature ranging 37.5°C to 39.5°C. He reported myalgia and asthenia but denied having any upper or lower respiratory symptoms. However, the patient had been in contact with a confirmed COVID-19 case 10 days before his hospitalization, although his household remained asymptomatic.
At admission the patient had a temperature of 40°C and an oxygen saturation of 97% on ambient air without any need for oxygen supplementation; there was no dyspnoea, cough or haemodynamic instability. His physical examination revealed bibasilar crackles.
Initial laboratory results showed no significant alterations except for an elevated C-reactive protein at 30.6 mg/L and procalcitonin at 0.14 μg/L. Leucocyte count and differential were within normal limits (Table 1).
Table 1.
Initial laboratory results at admission
| Test | Unit | Value at admission | Reference value |
|---|---|---|---|
| Haemoglobin | g/dL | 15.7 | 13–17 |
| White blood cells | μL of blood | 6100 | 4000–9000 |
| White blood cell differential | % | 62.3% neutrophils, 32.1% lymphocytes | 50–78% neutrophils, 20–45% lymphocytes |
| Platelets | μL of blood | 135 000a | 150 000–400 000 |
| Creatinine | μmol/L | 104 | 58–110 |
| Blood urea nitrogen | mmol/L | 10.3a | 2.5–7.5 |
| C-reactive protein | mg/L | 30.6a | <3.5 |
| Procalcitonin | μg/L | 0.14a | <0.1 |
| Ferritin | μg/L | 753 | 30–400 |
| Lactase dehydrogenase | U/L | 309a | 120–246 |
| Lipase | U/L | 230 | 23–300 |
Abnormal value.
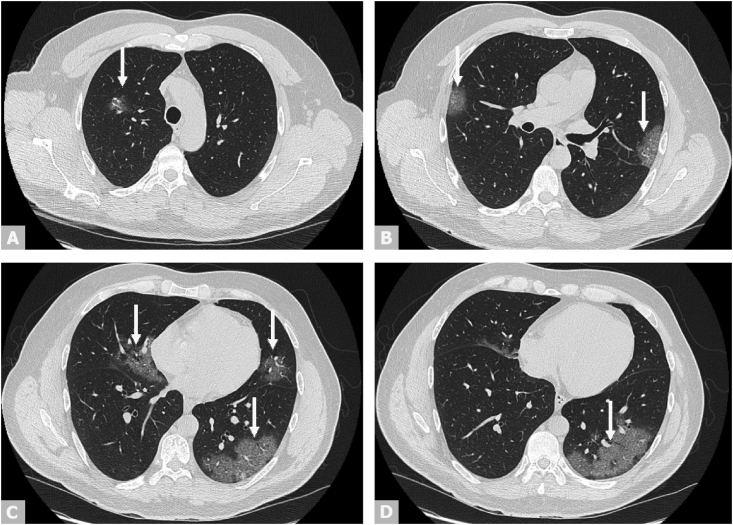
A chest computed tomographic scan revealed several well-circumscribed central and peripheral ground-glass opacities associated with vascular ectasia, highly suggestive of a viral bilateral pneumonia, and particularly a COVID-19–related pneumonia, in view of the raging pandemic [3,4]. The index of severity was estimated at 5/25 [5]. There was no pleural or pericardiac effusion. A few lymph nodes less than 1 cm in size were noted in the mediastinum (Fig. 1).
Fig. 1.
Axial view of chest CT scan revealing bilateral ground-glass opacities (arrows) indicating COVID-19 pneumonia. COVID-19, coronavirus disease 2019; CT, computed tomography.
Therapy with hydroxychloroquine and azithromycin was then empirically initiated while awaiting the SARS-CoV-2 RT-PCR results conducted using nasopharyngeal swabs.
The results of the first nasopharyngeal SARS-CoV-2 RT-PCR testing [6] conducted on day 7 of symptoms were negative. The test was repeated 2 days later with the same result.
Given the high index of suspicion of COVID-19 despite the two negative initial nasopharyngeal RT-PCR results, a bronchoscopy was performed the following day (day 10 after symptom onset); RT-PCR testing was done on the bronchoalveolar lavage sample. The latter was positive for SARS-CoV-2, confirming our suspected diagnosis of COVID-19 pneumonia.
Multiplex RT-PCR testing using the BioFire FilmArray technique [7] on the bronchoalveolar lavage sample was negative for other common respiratory viruses (wild coronavirus subtypes HKU1, NL63, 229E and OC43), adenovirus, rhinovirus, enterovirus, influenza A (H1, H3, H1-2009), influenza B, parainfluenza 1/2/3/4, human metapneumovirus and respiratory syncytial virus and bacteria (Bordetella pertussis, Chlamydophila pneumoniae, Mycoplasma pneumoniae). Culturing the bronchoalveolar lavage sample yielded no microbial growth.
Lopinavir and ritonavir were then added to the patient's treatment regimen following local institutional COVID-19 treatment guidelines.
The patient's disease course progressed clinically well without the need for mechanical ventilation. However, his C-reactive protein increased to 53.8 mg/L during his hospitalization, and ceftriaxone was added to treat a likely bacterial secondary infection. Only one fever spike (temperature 39.5°C) occurred the following day; he remained apyretic for the rest of his hospital stay.
The patient's hospitalization in our COVID-19 unit was uncomplicated, and he was discharged on day 7 of hospitalization with instructions to remain in strict home confinement. The SARS-CoV-2 RT-PCR using nasopharyngeal swab was repeated on days 24 and 29 after symptom onset, following the US Centers for Disease Control and Prevention's guidelines on discontinuation of isolation [8]. Both test results were negative.
Discussion
Daily medical practice commonly focuses on expertise and clinical judgement to guide diagnosis and treatment. With the coronavirus pandemic hitting hospitals around the globe, physicians must remain alert and maintain a high index of suspicion when it comes to COVID-19 [9].
Studies have found RT-PCR to have an imperfect sensitivity, estimated to be around 63% for nasopharyngeal sampling [10]. The disparity in results could be due to low virus shedding in the upper respiratory tract early on, damaged specimen (as with heat exposure) [11] or inappropriate sample collection. A combination of clinical presentation and radiologic evidence can help dodge underdiagnosis [12]. Chest computed tomographic scan has a higher sensitivity in detecting COVID-19 compared to RT-PCR [13], even in paucisymptomatic patients, but it has low specificity [14], as the imaging pattern could be similar in non–coronavirus-associated viral pneumonia.
Because virologic evidence of SARS-CoV-2 is required to confirm diagnosis of COVID-19, repeat testing using RT-PCR is indicated, preferably via deeper specimen collection, such as sputum or bronchoalveolar lavage [10,15], if initial results are negative in cases of high clinical, epidemiologic or radiologic suspicion.
Conclusion
When a clinician strongly suspects a COVID-19–related infection but faces negative RT-PCR results, it is critical to perform repeat testing for SARS-CoV-2, preferably using deep respiratory specimens, such as bronchoalveolar lavage. While RT-PCR remains the reference standard for diagnosis, a negative result should be interpreted with a grain of salt. Its negative predictive value is based on pretest probability. Combining several diagnostic tests would increase the diagnostic accuracy. When in doubt, testing should be repeated, especially when a high index of suspicion for SARS-CoV-2 persists.
Conflict of interest
None declared.
References
- 1.Wang X., Tan L., Wang X., Liu W., Lu Y., Cheng L. Comparison of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 detection in 353 patients received tests with both specimens simultaneously. Int J Infect Dis. 2020;94:107–109. doi: 10.1016/j.ijid.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zitek T. The appropriate use of testing for COVID-19. West J Emerg Med. 2020;21:470–472. doi: 10.5811/westjem.2020.4.47370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou S., Wang Y., Zhu T., Xia L. CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol. 2020;214:1287–1294. doi: 10.2214/AJR.20.22975. [DOI] [PubMed] [Google Scholar]
- 4.Simpson S., Kay F.U., Abbara S., Bhalla S., Chung J.H., Chung M. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. J Thorac Imaging. 2020;35:219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao W., Zhong Z., Xie X., Yu Q., Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 6.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.bioMérieux BioFire FilmArray product information. https://www.biomerieux-usa.com/clinical/biofire-film-array Available at:
- 8.US Centers for Disease Control and Prevention (CDC) Coronavirus disease 2019 (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html Available at:
- 9.Kokkinakis I., Selby K., Favrat B., Genton B., Cornuz J. COVID-19 diagnosis: clinical recommendations and performance of nasopharyngeal swab-PCR. Rev Med Suisse. 2020;16:699–701. [PubMed] [Google Scholar]
- 10.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan Y., Long L., Zhang D., Yuan T., Cui S., Yang P. Potential false-negative nucleic acid testing results for severe acute respiratory syndrome coronavirus 2 from thermal inactivation of samples with low viral loads. Clin Chem. 2020 01;66:794–801. doi: 10.1093/clinchem/hvaa091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y., Hou H., Wang W., Wang W. Combination of CT and RTPCR in the screening or diagnosis of COVID-19. J Glob Health. 2020;10 doi: 10.7189/jogh.10.010347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020;296:E32–E40. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waller J.V., Kaur P., Tucker A., Lin K.K., Diaz M.J., Henry T.S. Diagnostic tools for coronavirus disease (COVID-19): comparing CT and RT-PCR viral nucleic acid testing. AJR Am J Roentgenol. 2020 doi: 10.2214/AJR.20.23418. [DOI] [PubMed] [Google Scholar]
- 15.Hornuss D., Laubner K., Monasterio C., Thimme R., Wagner D. COVID-19 associated pneumonia despite repeatedly negative PCR-analysis from oropharyngeal swabs. Dtsch Med Wochenschr. 2020;145:844–849. doi: 10.1055/a-1170-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]