Abstract
About nine months after the emergence of SARS-CoV-2, this special issue of the Biomedical Journal takes stock of its evolution into a pandemic. We acquire an elaborate overview of the history and virology of SARS-CoV-2, the epidemiology of COVID-19, and the development of therapies and vaccines, based on useful tools such as a pseudovirus system, artificial intelligence, and repurposing of existing drugs. Moreover, we learn about a potential link between COVID-19 and oral health, and some of the strategies that allowed Taiwan to handle the outbreak exceptionally well, including a COVID-19 biobank establishment, online tools for contact tracing, and the efficient management of emergency departments.
Keywords: COVID-19, SARS-CoV-2, Pseudovirus system, Repurposing drugs, Contact tracing
Spotlight on reviews
Pandemic number five – latest insights into the COVID-19 crisis
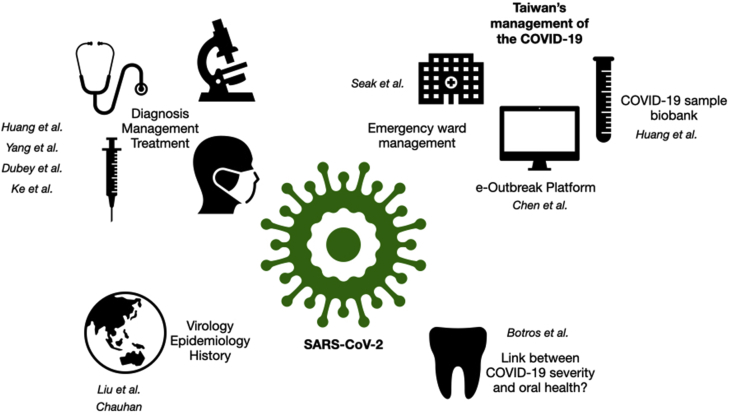
The current COVID-19 landscape is yet one of permanent transformation and uncertainty – the case and fatality numbers increase every day, articles are published by the hundreds, potential drug candidates come and go, and divergent crisis management strategies by different countries are hotly debated. Despite the ongoing evolution of the pandemic, two reviews in this special issue of the Biomedical Journal by Liu et al. and Chauhan comprehensively recapitulate the current state of the art regarding the origin and features of SARS-CoV-2, as well as its epidemiology, clinical manifestations, and the advancement of treatment options [1,2] [Fig. 1].
Fig. 1.
Overview of the many aspects of the COVID-19 pandemic covered in this issue. This special issue of the Biomedical Journal covers many facets of the current COVID-19 situation, including the evolution of the pandemic since its beginning, ongoing efforts to develop treatments and vaccines, and crisis management strategies with Taiwan as an example.
Both articles set forth by reminding the reader of the chronological milestones; from the onset of the disease in Wuhan, December 2019, to the official declaration of a pandemic by the World Health Organisation (WHO) on March 11th 2020 – rendering it the 5th registered pandemic since the 1918 Spanish influenza – and the process leading to the final nomenclature SARS-CoV-2. Subsequently, the virology, origin, and molecular features of the novel coronavirus are elucidated. In this respect, two elements are of particular interest, starting with the fairly recent increase in aggressive coronavirus cases [3]. Coronaviruses might have existed since millions of years, avian variants were officially identified in the 1930s, and four human types (HCoV) causing minor upper respiratory tract infection are known since 1965 [2,4]. Yet 2002 witnessed the first Severe Acute Respiratory Syndrome (SARS) outbreak, 2012 the Middle East Respiratory Syndrome (MERS) crisis, and 2019 the COVID-19 pandemic [5]. However, the important element is not particularly the culprits being coronaviruses, but rather zoonoses, that is to say diseases circulating in an animal reservoir and eventually acquiring animal-to-human, and sometimes human-to-human transmission [6]. For instance, all three dangerous coronaviruses stem from bats, with various intermediate animal hosts, such as civets (SARS), camels (MERS), and maybe pangolins in the case of COVID-19 [7]. On a more general level, zoonoses are the most threatening providers of epidemics and pandemics [6], and one thing is certain, COVID-19 was certainly not the last, nor the worst outbreak that mankind will have to face. In addition to the eruptions caused by the above-mentioned coronaviruses, the past two decades alone cumulate swine flu (H1N1), several threatening versions of avian flu (H5Nx), two massive Ebola crises, and the expansion of insect vector-mediated parasitic diseases such as Dengue, Zika, or Malaria. The reasons are rather obvious: increased human population and mobility, climate change, intense animal farming, human intrusion into every ecosystem, and displacement of both humans and animals as a consequence of global warming and ensuing political unrest [8].
On the other hand, in opposition to the broad characteristic of a zoonosis, the second SARS-CoV-2 feature which attracted much attention is the surface spike glycoprotein, or S protein, as it binds with high efficiency to the host cell angiotensin-converting enzyme 2 (ACE2), mainly present in the heart and the lungs. Moreover, it requires cleavage by the host serine protease TPRSS2 for internalisation [9]. As a direct consequence, much research has focused on the S protein in the role of a vaccine antigen or a target for blocking fusion with the host cell [10], a topic further elaborated by several articles in this issue of the Biomedical Journal [[11], [12], [13]].
Furthermore, the course of the pandemic worldwide so far is outlined. Most of us have certainly been consulting the official numbers on websites such as worldometers.info/coronavirus/ on a daily basis. Marked differences between countries, especially regarding the mortality rates, quickly became evident [14], and the pandemic has taken a highly political turn since, as governments chose different strategies, balancing the safety of the population against the security of their economy.
Liu et al. further address the question if SARS-CoV-2 has mutated, or is expected to, based on the known RNA virus proficiency to change or recombine. Although no crucial changes have been detected yet [15], the spread and continuous evolutionary pressure exerted on the virus keep increasing the risk for a scenario that could be disastrous. For these reasons, the authors call righteously for regular sequencings, the construction of a phylogenetic network, and the investigation on geographic patterns to stay ahead of unwelcome surprises [1].
Meanwhile, Chauhan provides a detailed account of the main clinical manifestations of COVID-19, including computerised tomography (CT) scan and haematological findings, as well as some preliminary biomarker candidates that might be able to predict the severity of the disease course. This leads straight to an overview of the standard diagnosis methods, headed up by real time PCR, and the priority guidelines for patient management. Concerning treatment options, the sobering statement up to now is that there are no proven therapies and treatments for COVID-19, despite massive universal efforts, and that potential vaccines have yet to prove their worth [2].
In conclusion, both reviews point out that a pandemic knows no borders, and thus that its outcome relies massively on the cooperation of all countries involved. The scientific community has without doubt already made some extraordinary progress since the first cases of avian influenza, and the immediate sharing of data regarding SARS-CoV-2 – from sequences to numbers – has been exemplary. On the other side of the coin, the crisis has highlighted countless flaws of modern societies: unequal access to health coverage and treatment, public hospitals already on the verge of collapse before the start of the pandemic, the complete unpreparedness of governments for this type of scenario, and the dangers of massive media-fuelled misinformation. We can but hope that the enormous amount of data generated by this period will be carefully analysed and evaluated, and that the right and responsible conclusions will be drawn for the future.
Spotlight on original articles
A pseudovirus for real experiments
Discovering a functioning treatment for COVID-19 as quickly as possible is imperative, and developing a safe and efficient vaccine probably even more important in the long run. Accordingly, research power worldwide has focused on the task at full tilt. For instance, at the moment of writing and according to the New York Times' Coronavirus Vaccine Tracker, over 165 vaccines against the coronavirus are at different stages of testing, 8 of them in phase III clinical trials and 2 approved for early or limited use.1 However, there is one considerable limitation to any SARS-CoV-2 related experimental research – the requirement of bio-safety level 3 (BSL-3) facilities, which are not available in every institution, and restrict the range of possible manipulations [16]. For this reason, Huang and colleagues from Taiwan advocate here for the multiple advantages of working with a pseudovirus system, and demonstrate its concrete usability for studying SARS-CoV(-2) as well as several influenza virus H5 subtypes [12]. The term “pseudovirus” was originally coined in 1967 after the discovery of a mouse DNA-polyomavirus hybrid.2 Nowadays it designates a recombinant viral particle made from a well-known backbone, like the human immunodeficiency virus (HIV-1) or the vesicular stomatitis virus (VSV), and envelope proteins from the virus to be investigated. Usually, an additional plasmid or viral vector, such as a packaging vector, is required to allow for the production of the pseudovirus, and the latter can replicate only once in the infected host cells. As a consequence, the system can be handled in BSL-2 laboratories, while being still a good model for viral entry and of great use for the study of virus–host cell interaction, drug screening, antibodies, and vaccines [16,17].
In order to emphasise the urgent need for drugs and vaccines, the authors lay the foundations of their story by reminding the reader of the long list of emerging and re-emerging viral diseases that caused a worrying number of epidemics and pandemics over the past two decades [8]. Subsequently, the design of their pseudovirus system is revealed: the classical VSV lentivirus vector system functions as the packaging vector, and is co-transfected with a RFP reporter plasmid and plasmids encoding either the full-length spike (S) protein sequence from SARS-CoV, SARS-CoV-2, or the haemagglutinin (HA) and neuraminidase (NA) genes from H5N2, H5N6, and H5N8 into 293T cells. The latter allow for the production of pseudotyped lentiviral particles, which are then used for the infection of chosen host cells.
First, proper expression of the S or HA proteins on the virions is assessed, revealing the presence of complete S proteins but the partial cleavage of HA proteins, probably due to the 293T cell-specific host proteases. Indeed, apart from the interaction of the S protein with the host ACE2 receptor, the requirement of the host cell serine protease TMPRSS2 to cleave it into S1 and S2 subunits for envelope fusion, has been considered another targeting hotspot for potential drugs [10], stressing how much the hijacking of the host cell mechanisms has to be taken into account when battling a viral infection. For instance, camostat mesylate, a TMPRSS2 inhibitor and drug used to treat chromic pancreatitis and reflux oesophagitis, is being tested for inhibition of SARS-CoV-2 fusion with host cells [11,18].
After proving efficient transduction of the SARS-CoV and SARS-CoV-2 pseudovirus particles into Vero-E6 cells, a simian epithelial cell line, and optimising the transduction conditions, the authors return indeed to the protease topic, and the question if transduction could be further enhanced by the addition of exogenous proteases. Yet, trypsin displays the opposite effect, leaving the question open of which enzyme would eventually help severing the S proteins in the given system.
Instead, Huang et al. focus on their main goal, demonstrating that their pseudovirus system can be used for the evaluation of neutralising antibodies and vaccine generation. Neutralisation readout consists in a reduction of RFP expression and thus transduction in the presence of antisera from mice immunised against SARS-CoV-2 S recombinant protein [12]. For one out of two antisera, neutralisation could be successfully quantified using the chimeric virions.
As for the expression of avian influenza surface proteins, the authors show satisfactory transduction into MDCK cells, a canine epithelial cell line, then use the system straight for the generation of antisera respectively against the three subtypes in mice. Neutralisation of the latter is confirmed again via interference with transduction, as well as a partial cross-reactivity of the antisera raised against different subtypes, a desirable feature when it comes to the development of broad spectrum vaccines.
Altogether, the described system reinforces the suitability of the lentiviral pseudovirus model for the safe study of dangerous pathogens and the much needed evaluation of antigenicity and immunogenicity during vaccine generation.
Also in this issue
Reviews
A race against time
Detect, treat, and cure – countless research groups and pharmaceutical companies rushed to fulfil this triad as soon as the severity of the COVID-19 crisis became clear, massive funds were allocated from governments and private institutes, and clinical trials initiated all over the world. Yet there is no validated treatment or vaccine on the market yet – with the yet controversial exception of “Sputnik V”, the Russian vaccine registered on August 11th before the end of the testing programme.3
In the meantime, Shih et al. sum up the recent state of affairs relating to diagnoses and treatment of COVID-19 in the present review [11]. They elaborate on the different levels of diagnosis starting with clinical symptoms and nucleic-acid-based identification methods, which are currently the most common tool for broad mass testing, as well as on the security issues with virus isolation and the challenges of antibody detection. Following this, the authors detail the therapeutic options under current investigation by their viral target site, plus some that could modulate the host immune system. Moreover, the option of SARS-CoV-2 specific neutralising antibodies is briefly discussed, which could be an efficient yet very expensive way to bridge the time until a safe vaccine is found [19]. Finally, different possible vaccine strategies are described with their respective advantages and risk factors [20], which should be of major interest given the recent developments.
Original articles
Picking the artificial brain
Beyond question, the best chance to track down an efficient treatment for COVID-19 in a close future relies on repurposing an already existent and approved drug, as this would considerably shorten the duration of clinical trials without jeopardising patient safety [21]. Before the era of computational approaches, this strategy would have relied uniquely on trial and error experiments [22], but lately, the process sped up greatly by machine learning. “Deep neural networks”, is the latest shibboleth somewhere between dystopian science-fiction and flying cars, and refers in brief to an algorithm's ability to recognise or predict novel elements based on a training dataset and permanent feedback loops.4 Incontestably, the method has already come a long way from its original playground made of the MNIST database of handwritten numbers, as proven here by Ke et al. and their machine learning approach to identify known drugs with potential to fight SARS-CoV-2 [23]. The authors trained their system both on a dataset consisting of compounds reported active against other RNA viruses, and another one made of known 3C-like protease inhibitors, and tested hits subsequently in vitro against the feline infectious peritonitis (FIP) virus. The results, plus more experimental data from the same institute also described in this issue [13], were fed back into the algorithm, leading to the identification of 13 promising candidates for further experimental validation.
Back to the roots
Or rather, back to all kinds of plant bits and pieces. Over the past decades, the de novo design of drugs and antibiotics has encountered a bottleneck. As a consequence, the interest in natural compounds has witnessed a huge comeback [24,25]. Notably polyphenols, such as flavonoids, have acquired a reputation of universal benefit against practically everything from obesity to cancer, although evidence stems so far mainly from culture dishes and animal models [[26], [27], [28]]. By way of illustration, narcissoside is a commercially available flavonoid found for example in Ginkgo leaves [29], enough to be pitched as possessing antioxidant and antiproliferative activities in cancer by providers.5 Here, Dubey et al. show that according to a computational docking model, the main protease of SARS-CoV-2 is bound by narcissoside with greater efficiency than the potent broad-spectrum non-covalent inhibitor X77. This suggests that the molecule could potentially act as a good inhibitor against the virus, and thus should be added on the list of compounds to be tested experimentally [22].
New tricks for old drugs
Researchers from the National Health Research Institutes in Taiwan combined old and new methods in order to hunt for existing drugs that could turn out to be of help in the fight against SARS-CoV-2: while Ke et al. trained artificial intelligence to skim through compound databases [23], Yang et al. experimentally screened 252 molecules for potential inhibition of the feline infectious peritonitis (FIP) coronavirus and the human coronavirus OC43 (HCoV-OC43) in a cell culture model [13]. This led to the identification of 15 compounds displaying activity against both viruses, and provided further feedback and training material to the abovementioned machine learning platform, thus demonstrating the fertile synergy of in silico methods and benchwork.
News and perspectives
Open your COVID account
Despite its geographic proximity to the epicentre of the COVID-19 outbreak and the significant amount of travel transit, Taiwan has handled the outbreak exceptionally well [30]. To date, the number of total cases amounts to only 481, with 7 deaths.6 By the end of April, the outbreak was already nearly over in the country [31]. A swift response by the government, a well-thought-out organisation of good quality medical facilities, contact tracing, and lessons learn from the 2003 SARS outbreak all contributed to the Taiwanese success, all of them being outlined in a series of short articles in this issue of the Biomedical Journal. In addition to the spotless management of the crisis, the National Health Research Institute (NHRI) set up a biobank for COVID-19 blood samples in record time with the goal to help with the urgent need for diagnosis and treatment tools, and to provide research material for data mining in the long term. Huang et al. describe the different features of the biobank project, including the facilities, legal framework, and standard operation procedures, as well as the possibilities provided by the available data [32].
Outbreak 2.0
Digital tools have been proven of great use at many levels during the COVID-19 pandemic: on the one hand, artificial intelligence has been recruited to screen known molecules for inhibitory potential against SARS-CoV-2 [23], on the other, phone applications and web interfaces have allowed for a bidirectional information flow between authorities and public. Taiwan's efficient containment of the crisis [31] partially relied on screening checkpoints and a tight follow-up of a patient's contact history through an epidemiological investigation questionnaire. Indeed, contact tracing is a powerful way to alleviate the strain on the health personnel in the early stages of any novel highly transmissible infectious disease. On these grounds, Chen et al. are in the process of developing an online questionnaire for COVID-19 and other diseases termed “e-Outbreak Platform”, allowing for individuals to enter all types of relevant information, such as source of exposure, contact history, and clinical features [33]. The authors briefly describe the platform framework, including the user-friendly graphic portal, flexibility of information input, compliance with data security, and correcting algorithms, leading altogether to the generation of knowledge graphs. Based on the accumulated statistics, authorities would then be able to quickly adjust counter-measures, individuals to avoid risk zones, and scientists to use it for data-mining, leading to an optimal exhaustion of shared information.
Karius, Bactus, and coronavirus
In 1949, the Norwegian playwright Thorbjørn Egner published the tale of “Karius and Bactus”, two minuscule creatures who wreak havoc inside the teeth of a little boy that values syrup over brushing his teeth. They have become since an iconic pedagogical tool to teach children the value of proper dental care.7 In addition to avoid the vividly depicted martyrs of toothache, it has recently become clear that the state of the oral cavity is a rather reliable readout of overall health, both as cause or consequence of systemic disease [34]. Indeed, pathogens that manage to establish themselves inside the mouth can relatively easily gain access to the respiratory and digestive system, as well as provide the organism with a continuous production of inflammatory stimuli. Reciprocally, an immune system weakened by infection displays higher permissiveness to periodontal disease [35]. Given the overlap between conditions known to increase the risk to develop serious COVID-19 symptoms and diseases correlating with poor oral health, Botros et al. argue that good mouth hygiene can't certainly do any wrong, and might even add some protective benefit against SARS-CoV-2 infection severity [36].
Brief communication
Healthcare workers are fighting at all fronts against SARS-CoV-2, battling not only the virus, but also work overload, psychological pressure, and the highest risk for infection [37]. Yet another pillar of Taiwan's successful handling of the COVID-19 crisis [31] has been the flawless organisation of hospitals for efficient patient admission and stratification, while keeping a zero-infection rate among the medical staff. For illustration, Seak et al. describe the key strategies implemented by the Linkou Chang Gung Memorial Hospital (LCGMH), housing one of the largest emergency departments worldwide. These comprise a clear flowchart for patient triage, strict regulation of manpower and medical equipment, spatiotemporal separation of well-trained teams, and robust standard procedures [30].
Conflicts of interest
The author declares no conflict of interests.
Footnotes
Peer review under responsibility of Chang Gung University.
An excellent broad-public approach on how neural networks actually work has been made by Grant Sanderson: https://www.youtube.com/watch?v=aircAruvnKk.
References
- 1.Liu Y.C., Kuo R.L., Shih S.R. COVID-19: the first documented coronavirus pandemic in history. Biomed J. 2020;43:328–333. doi: 10.1016/j.bj.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chauhan S. Comprehensive review of coronavirus disease 2019 (COVID-19) Biomed J. 2020;43:334–340. doi: 10.1016/j.bj.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntosh K. vol. 25. Springer; Berlin, Heidelberg: 1974. Coronaviruses: a comparative review; pp. 85–129. (Current topics in microbiology and immunology/Ergebnisse der mikrobiologie und immunitätsforschung). [Google Scholar]
- 5.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cross A.R., Baldwin V.M., Roy S., Essex-Lopresti A.E., Prior J.L., Harmer N.J. Zoonoses under our noses. Microbes Infect. 2019;21:10–19. doi: 10.1016/j.micinf.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J.H. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 8.Woolhouse M.E.J. Emerging diseases go global. Nature. 2008;451:898–899. doi: 10.1038/451898a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walls A.C., Park Y.-J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–286. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV — a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shih H.I., Wu C.J., Tu Y.F., Chi C.Y. Fighting COVID-19: a quick review of diagnoses, therapies, and vaccines. Biomed J. 2020;43:341–354. doi: 10.1016/j.bj.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S.W., Tai C.H., Hsu Y.M., Cheng D., Hung S.J., Chai K.M. Assessing the application of a pseudovirus system for emerging SARS-CoV-2 and re-emerging avian influenza virus H5 subtypes in vaccine development. Biomed J. 2020;43:375–387. doi: 10.1016/j.bj.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang C.W., Peng T.T., Hsu H.Y., Lee Y.Z., Wu S.H., Lin W.H. Repurposing old drugs as antiviral agents for coronaviruses. Biomed J. 2020;43:368–374. doi: 10.1016/j.bj.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto N., Bauer G. Apparent difference in fatalities between Central Europe and East Asia due to SARS-COV-2 and COVID-19: four hypotheses for possible explanation. Med Hypotheses. 2020;144:110160. doi: 10.1016/j.mehy.2020.110160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Dorp L., Acman M., Richard D., Shaw L.P., Ford C.E., Ormond L. Emergence of genomic diversity and recurrent mutations in SARS-CoV-2. Infect Genet Evol. 2020;83:104351. doi: 10.1016/j.meegid.2020.104351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q., Liu Q., Huang W., Li X., Wang Y. Current status on the development of pseudoviruses for enveloped viruses. Rev Med Virol. 2018;28 doi: 10.1002/rmv.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welch S.R., Guerrero L.W., Chakrabarti A.K., McMullan L.K., Flint M., Bluemling G.R. Lassa and Ebola virus inhibitors identified using minigenome and recombinant virus reporter systems. Antivir Res. 2016;136:9–18. doi: 10.1016/j.antiviral.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann M., Hofmann-Winkler H., Smith J.C., Krüger N., Sørensen L.K., Søgaard O.S. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. bioRxiv. 2020;12:233. doi: 10.1016/j.ebiom.2021.103255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ledford H. Antibody therapies could be a bridge to a coronavirus vaccine – but will the world benefit? Nature. 2020;584:333–334. doi: 10.1038/d41586-020-02360-y. [DOI] [PubMed] [Google Scholar]
- 20.Peeples L. News feature: avoiding pitfalls in the pursuit of a COVID-19 vaccine. Proc Natl Acad Sci U S A. 2020;117:8218–8221. doi: 10.1073/pnas.2005456117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riva L., Yuan S., Yin X., Martin-Sancho L., Matsunaga N., Pache L. Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 2020 doi: 10.1038/s41586-020-2577-1. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubey K., Dubey R. Computation screening of narcissoside a glycosyloxyflavone for potential novel coronavirus 2019 (COVID-19) inhibitor. Biomed J. 2020;43:363–367. doi: 10.1016/j.bj.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ke Y.Y., Peng T.T., Yeh T.K., Huang W.Z., Chang S.E., Wu S.H. Artificial intelligence approach fighting COVID-19 with repurposing drugs. Biomed J. 2020;43:355–362. doi: 10.1016/j.bj.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harvey A.L., Edrada-Ebel R., Quinn R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 25.Rossiter S.E., Fletcher M.H., Wuest W.M. Natural products as platforms to overcome antibiotic resistance. Chem Rev. 2017;117:12415–12474. doi: 10.1021/acs.chemrev.7b00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maleki S.J., Crespo J.F., Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124. doi: 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- 27.Guasch-Ferré M., Merino J., Sun Q., Fitó M., Salas-Salvadó J. Dietary polyphenols, Mediterranean diet, prediabetes, and type 2 diabetes: a narrative review of the evidence. Oxid Med Cell Longev. 2017;2017:6723931. doi: 10.1155/2017/6723931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alam M.N., Almoyad M., Huq F. Polyphenols in colorectal cancer: current state of knowledge including clinical trials and molecular mechanism of action. BioMed Res Int. 2018;2018:4154185. doi: 10.1155/2018/4154185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni J., Hao J., Jiang Z., Zhan X., Dong L., Yang X. NaCl induces flavonoid biosynthesis through a putative novel pathway in post-harvest ginkgo leaves. Front Plant Sci. 2017;8:920. doi: 10.3389/fpls.2017.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seak C.J., Liu Y.T., Ng C.J., investigators S.P.O.T. Rapid responses in the emergency department of Linkou Chang Gung Memorial Hospital, Taiwan effectively prevents spread of COVID-19 among healthcare workers of emergency department during outbreak: lessons learnt from SARS. Biomed J. 2020;43:388–391. doi: 10.1016/j.bj.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng H.Y., Li S.Y., Yang C.H. Initial rapid and proactive response for the COVID-19 outbreak — Taiwan's experience. J Formos Med Assoc. 2020;119:771–773. doi: 10.1016/j.jfma.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang S.F., Huang Y.C., Chang F.Y., Lin J.C., Chiu C.H., Chen C.W. Rapid establishment of a COVID-19 biobank in NHRI by National Biobank Consortium of Taiwan. Biomed J. 2020;43:314–317. doi: 10.1016/j.bj.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen W.J., Yang S.Y., Chang J.C., Cheng W.C., Lu T.P., Wang Y.N. Development of a semi-structured, multifaceted, computer-aided questionnaire for outbreak investigation: e-outbreak platform. Biomed J. 2020;43:318–324. doi: 10.1016/j.bj.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bui F.Q., Almeida-da-Silva C.L.C., Huynh B., Trinh A., Liu J., Woodward J. Association between periodontal pathogens and systemic disease. Biomed J. 2019;42:27–35. doi: 10.1016/j.bj.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y.M., Yan J., Ojcius D.M., Chen L.L., Gu Z.Y., Pan J.P. Correlation between infections with different genotypes of human cytomegalovirus and Epstein-Barr virus in subgingival samples and periodontal status of patients. J Clin Microbiol. 2007;45:3665–3670. doi: 10.1128/JCM.00374-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Botros N., Iyer P., Ojcius D.M. Is there an association between oral health and severity of COVID-19 complications? Biomed J. 2020;43:325–327. doi: 10.1016/j.bj.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Y., Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatr Res. 2020;288:112954. doi: 10.1016/j.psychres.2020.112954. [DOI] [PMC free article] [PubMed] [Google Scholar]