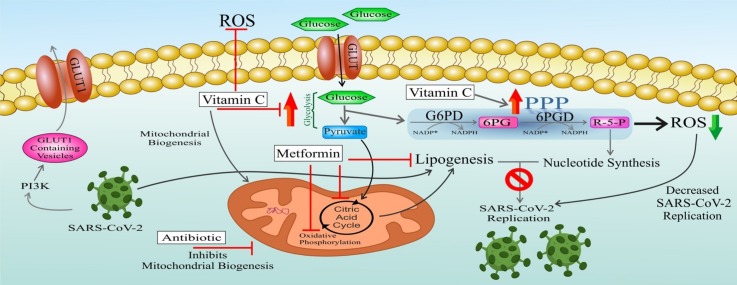
Graphical abstract
Keywords: SARS-CoV-2, Cellular metabolism, Glycolysis, PPP, TCA cycle
Abstract
As a process entailing a high turnover of the host cell molecules, viral replication is required for a successful viral infection and requests virus capacity to acquire the macromolecules required for its propagation. To this end, viruses have adopted several strategies to harness cellular metabolism in accordance with their specific demands. Most viruses upregulate specific cellular anabolic pathways and are largely dependent on such alterations. RNA viruses, for example, upregulate both glycolysisand glycogenolysis providing TCA cycle intermediates essential for anabolic lipogenesis. Also, these infections usually induce the PPP, leading to increased nucleotide levels supporting viral replication. SARS-CoV-2 (the cause of COVID-19)that has so far spread from China throughout the world is also an RNA virus. Owing to the more metabolic plasticity of uninfected cells, a promising approach for specific antiviral therapy, which has drawn a lot of attention in the recent years,
would be the targeting of metabolic changes induced by viruses. In the current review, we first summarize some of virus-induced metabolic adaptations and then based on these information as well as SARS-CoV-2 pathogenesis, propose a potential therapeutic modality for this calamitous world-spreading virus with the hope of employing this strategy for near-future clinical application.
1. Introduction
A Severe respiratory illness was lately reported in Wuhan, Hubei province, China. Metagenomic RNA sequencing of bronchoalveolar lavage fluids identified a novel virus strain belonging to the family Coronaviridae, named as SARS-CoV-2, and the resultant disease was termed Coronavirus Disease 2019 (COVID‐19). By conducting phylogenetic analysis on the complete viral genome, the virus strain was shown to be most closely (75–80 % nucleotide similarity) related to a group of severe acute respiratory syndrome (SARS)-like coronaviruses (subgenus Sarbecovirus, genus Betacoronavirus) previously found in bats in China [1,2].
According to the COVID-19 Situation Report-209 published by the World Health Organization (WHO), a total of 8 21,294,845 confirmed cases and 761,779 deaths have been identified globally until 16 August 2020 [3]. However, there is still no antiviral drug proven effective for definitive treatment of COVID-19, underpining the need for further studies to find an effective and safe treatment for the disease.
Eukaryotic viruses have been shown to induce large-scale changes in host cellular metabolism. Most viruses evaluated to date trigger aerobic glycolysis, which is also known as the Warburg effect. Numerous tested viruses also induce glutaminolysis and fatty acid (FA) synthesis. These alterations of carbon source usage by infected cells can provide specific cellular substrates for viral particles, enhance available energy for viral replication and virion production, thereby creating viral replication niche, while augmenting infected cell survival [4].
A better appreciation of the metabolic alterations required for each virus replication may provide the basis for developing novel therapeutic strategies aimed at targeted inhibition of specific metabolic pathways.
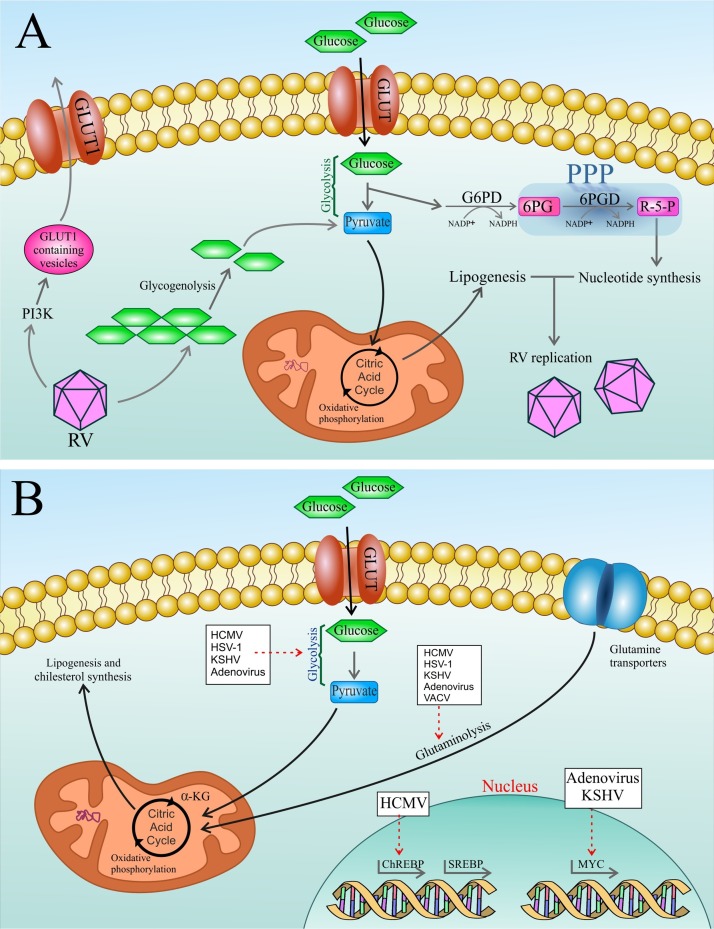
RNA virus (RV) infection induces anabolic reprogramming of the host cell metabolism by 1) Inducing PI3K-mediated trafficking of glucose transporter 1 (GLUT1)-containing vesicles to the host cell membrane, thereby increasing glucose uptake. Also, overexpression of GLUT1 has been found to give rise to increased PPP intermediates 2) Upregulating both glycolysis and glycogenolysis which provide TCA cycle intermediates required for anabolic lipogenesis. 3) Activating the PPP that results in enhanced levels of nucleotides supporting viral replication [5].
Since SARS-CoV-2 is also an RV [6], it is therefore expected to induce the same metabolic reprogramming as other RVs for replication in the host cell. Thus, targeting these metabolic pathways could be applied to treat this infection. There is evidence reflecting upregulated glycolysis, PPP and TCA cycle following coronavirus infection [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16]]. Therefore, in the current review, we first summarize some of the virus-induced metabolic adaptations and then, based on this information as well as SARS-CoV-2 pathogenesis, propose a potential therapeutic modality for this calamitous world-spreading virus with the hope of employing this strategy for near-future clinical application.
2. Viruses harness host cell metabolism
A plethora of strategies have been adopted by viruses to ensure the undistributed supply of macromolecules and suit the host cell metabolism according to their specific demands. The high turnover of biomolecules linked with virion production as well as simultaneous activation of cellular defense mechanisms brings about a highly anabolic state that is often associated with upregulated uptake of extracellular carbon sources (like glucose and glutamine) as well as their redirection to metabolic pathways vital for viral replication including lipogenesis and nucleotide synthesis [5].
It has been demonstrated that viruses employ strategies as diverse as the activation of cytosolic signaling, including PI3K [17] and CaMKK1/AMPK [18,19] or transcriptional regulation like activation of Myc [20,21], SREBP [16,[22], [23], [24], [25]] and ChREBP [26]. The current data point to a dichotomy between RNA and DNA viruses when delving into their respective strategies of host cell manipulation. That is to say, while DNA viruses have been found to control key metabolic pathways at the transcription level [20,21,26], RNA viruses seem to shape host-cell metabolism through post-transcriptional modifications [17], that are in concordance with the pace of the respective replication cycles (Fig. 1 ).
Fig. 1.
a) Rhinovirus, as an RNA virus (RV), post-transcriptionally modulates the host cell metabolism. Infection with this virus induces anabolic reprogramming of the host cell metabolism by 1) Inducing PI3K-mediated trafficking of GLUT1-containing vesicles to the host cell membrane thereby increasing glucose uptake. Also, overexpression of GLUT1 has been found to result in increased PPP intermediates 2) Upregulating both glycolysis and glycogenolysis which provides TCA cycle intermediates required for anabolic lipogenesis 3) Activating the PPP which results in enhanced levels of nucleotide that support viral replication. RV: rhinovirus; PI3K: phosphatidylinositol 3-kinase; GLUT: glucose transporter; TCA: tricarboxylic acid cycle. b) Schematic overview representing metabolic targets of some DNA viruses. Various DNA viruses activate particular anabolic metabolic programs following infecting the host cells, to finally support viral replication and virion maturation. Dashed arrows show a virus induced activation of the respective metabolic pathway or a transcription factor activation. TCA: tricarboxylic acid cycle; α-KG: α-ketoglutarate; SREBP: sterol regulatory element-binding protein; ChREBP: carbohydrate-response element-binding protein; GLUT: glucose transporter; HCMV: human cytomegalovirus; HSV-1: herpes simplex virus-1; KSHV: Kaposi's sarcoma-associated herpesvirus; VACV: vaccinia virus.
Host cell FA synthesis machinery has proven substantial for viral genome replication, virion production, and morphogenesis. Numerous viruses instigate the formation of phosphatidylinositol 4-phosphate/cholesterol-enriched membranes to create viral replication complexes (VRCs) at the interface of the host endoplasmic reticulum (ER). Generation of secluded membranes that protect viral nucleic acids from immune surveillance (for example, cytosolic pattern recognition receptors) and encompass an optimal environment for viral replication, requires the accumulation of sterols at the VRCs of RNA viruses [27,28]. VRC formation critically relies on the host cell's sterol synthesis reprogramming by recruiting the phosphatidylinositol-4 kinase III beta and oxysterol-binding protein (PI4KB–OSBP) axis. Thus, perturbation of cellular cholesterol homeostasis dampens viral replication [[29], [30], [31], [32], [33]].
This information highlights the pivotal role of lipogenesis for sustained viral propagation and that targeting of lipogenesis could pave the way for effective inhibition of viral replication.
There is evidence reflecting upregulated glycolysis, PPP, and TCA cycle following coronavirus infection, including:
HCoV-229E could be regarded as a model coronavirus for comprehensive characterization of the host cell lipid response following coronavirus infection. Glycerophospholipids and FAs of HCoV-229E-infected cells are significantly elevated and the linoleic acid to arachidonic acid metabolism axis is notably perturbed [34]. In the mitochondria, FAs are synthesized from the precursor molecules acetyl-CoA, malonyl-CoA, and malonate, and their elongation into FAs requires ATP and NADPH [35,36]. Proteomic analysis conducted on infectious bronchitis virus (IBV) coronavirus particles has identified some proteins involved in the glycolytic pathway including glyceraldehyde-3-phosphate dehydrogenase (GAPDH), aldehyde dehydrogenase 9 family, member A1 (ALDH9A1) and alpha-enolase, which had been previously identified in other viral particles including HIV-1, human cytomegalovirus (HCMV), moloney murine leukemia virus (MMLV), Kaposi's sarcoma-associated herpesvirus (KSHV) and avian influenza virus (AIV) [[37], [38], [39], [40], [41], [42]]. Lipidomic analysis has also extended our understanding of the metabolic reprogramming following HCoV [34] and MERS-CoV infections [16,34]. Yan at al. found a considerable rearrangement of the cellular lipid profile as indicated by the accumulation of phospholipids and FAs (saturated and unsaturated) upon HCoV infection. These researchers claimed that the Coronaviridae specifically fine-tunes the host lipid profile to accomplish optimal viral replication [34]. These findings were confirmed by a recent report on the pharmacologic targeting of sterol regulatory element-binding proteins (SREBP) by AM580 (as a specific inhibitor) as a promising strategy to inhibit MERS-CoV infection [16]. Impeding the proteolytic processing of SREBP using AM580 resulted in the inhibition of multiple post-viral-entry steps, including decreased intracellular lipid droplet formation, reduced palmitoylation of viral proteins as well as reduced double-membrane vesicle (DMV) formation [16].
It has been demonstrated that PI3K/AKT/mTOR and ERK/MAPK signaling pathways have important roles in MERS-CoV infection and might represent new drug targets for therapeutic intervention [43]. PI3K-dependent trafficking of GLUT1-containing vesicles to the cell membrane, leads to increased glucose uptake and metabolism [5].
3. Metabolic interventions in COVID-19
Coronaviruses are pandemic viruses able to cause lethal lung injuries and death from acute respiratory distress syndrome (ARDS) [44]. ARDS characterized by severe hypoxemia, is generally accompanied by oxidative injury, uncontrolled inflammation, and damage to the alveolar-capillary barrier [45,46]. Enhanced oxidative stress is a key insult in pulmonary injury including ARDS, as one clinical manifestation of acute respiratory failure with considerably high morbidity and mortality [47]. Therefore, oxidative stress is a dangerous aspect of this infection. Increased extracellular oxidative stress, as a consequence of “cytokine storm”, results in ARDS which is the key pathologic cause for the high mortality rate of this pandemic infection.
On such a basis, metabolic intervention in COVID-19 is suggested to follow two main goals: 1) Inhibition of virus replication 2) Inhibition of oxidative stress.
A possible strategy for achieving the first goal is: "targeting lipogenesis" that is required for virus packaging. This goal could be achieved in 3 ways a) downregulating glycolysis to inhibit pyruvate production (in a way other than inhibiting GLUT activity like GLUT1, because they are required to keep the PPP active) and entry into the TCA cycle thereby decreasing lipogenesis. Normally, glycolysis is needed to provide additional intermediates of TCA cycle for anabolic lipogenesis; b) mitochondrial targeting to decrease TCA flux; c) direct inhibition of cellular lipogenesis.
Possible strategies for achieving the second goal include: a) Upregulating the PPP and b) scavenging of extracellular ROS produced during infection (especially at alveolar spaces)
Regarding a) upregulating the PPP;
Intracellular oxidative stress contributes to coronavirus infectivity and increases its replication in lung epithelial cells. As demonstrated by recent studies, glucose-6-phosphate dehydrogenase (G6PD)-deficient or G6PD-knockout cells (with decreased intracellular redox power as a result of decreased NADPH) exhibit a higher susceptibility to coronavirus infection. Indeed, intracellular oxidative stress provides a proper milieu for coronavirus replication [48]. Also, these cells show a lower capacity to mount antiviral responses [49]. In fact, another reason convincing us to propose targeting of oxidative stress as a therapeutic strategy for coronavirus infection, relates to the previously published reports on the association of G6PD deficiency with coronavirus infectivity (HCoV-229E). As stated before, RNA viruses, in general, upregulate PPP which provides adequate nucleotides levels and redox power (NADPH) for sustained viral replication. Several studies, notwithstanding, made us look at this pathway (in the case of coronaviruses) somehow different and take our general view away of total beneficial effects of this pathway for coronavirus replication. As it is well-established, oxidative phase of PPP, whose key and rate-limiting enzyme is G6PD, plays a pivotal role in providing cells with sufficient redox power via NADPH production. Thus, cells lacking this key PPP enzyme have lower capacities to counteract oxidative stress. On the other hand, it has been shown that G6PD-deficient cells have a higher susceptibility to HCoV-229E (causative coronavirus of SARS) infection. In a study carried out by Wu et al. in 2008, G6PD-deficient and G6PD-knockdown cells showed much higher viral gene expression as well as viral particle production upon infection with HCoV-229E. These phenomena were associated with a higher production of oxidants, representing oxidative stress in host cells as an important factor for coronavirus infectivity. Furthermore, these researchers demonstrated that antioxidant agents could ameliorate increased viral infection of G6PD-deficient cells. This study provides the evidence that redox status of host cells plays an important role in viral infectivity [48]. Concordant with this report, these researchers in 2015 tried to delineate the underlying mechanism of this interesting phenomenon. They showed that in normal cells, viral infection triggers IκB degradation and hence NF-κB translocation that promotes antiviral responses and inhibits viral replication. But, regarding G6PD-deficient cells, they found a fall in the NADPH/NADP+ ratio, which results in the upregulation of HSCARG protein as an NADPH sensor. This protein negatively affects the NF-κB signaling pathway, which is responsible for the expression of the antiviral genes: tumor necrosis factor-alpha (TNF-α) and GTPase myxovirus resistance 1 (MX1). Therefore, its upregulation in G6PD-deficient cells results in impaired antiviral response and, as a consequence, enhanced HCoV-229E replication [49]. PPP is the prime cellular antioxidant defense system [50]. PPP, derived from glycolysis at the first committed step of glucose metabolism, is indispensable for ribonucleotides synthesis and is also a major source of NADPH. NADPH is required for efficient scavenging of ROS. Indeed, increased PPP can be considered as a cellular mechanism to cope with intracellular oxidative stress as a result of elevated ROS levels. Therefore, upregulating this pathway and keeping it active in this infection seems essential to reduce the detrimental effects of ROS.
Another evidence indicating the advantage of increased PPP activity in this infection is related to the autophagy process. Autophagy is suggested to play a key role in the replication of coronaviruses [51]. RNA replication of the coronavirus mouse hepatitis virus (MHV) in the host cell cytoplasm is performed on DMVs. Prentice E et al. reported that autophagy is required for the formation of DMV-bound replication complexes in MHV-infected cells; and that DMV formation markedly increased the efficiency of replication [51]. Besides, an increased autophagy has been attributed to a reduced intracellular redox power. G6PD inhibition has been shown to induce ER stress, which is responsible for autophagy flux deregulation. G6PD blockade is shown to result in a constant increase in autophagosome formation independently from mTOR status [52]. Concordantly, PPP inhibition is suggested to lead to autophagy induction. Thus, keeping this pathway active or upregulating it, as suggested in our paper, may play a role in reducing autophagy to prevent viral replication.In addition, chloroquine phosphate, which has apparent efficacy against non-severe COVID-19, restrains virus replication via blocking autophagy [53]. Chloroquine increases the pH in host cell lysosomes, and this way copes with viruses’ attempts for acidifying the lysosomes. Lysosome acidification is required to form autophagosomes that cells use to eat themselves [54]. Chloroquine can restrain virus replication via blocking autophagy [55].
Nevertheless, we should keep in mind that increased NADPH as a result of upregulated PPP may be a double-deck sword as NADPH has a dual activity. In addition to serving as a fuel for the antioxidant system, NADPH can exert pro-oxidant effects by acting as a substrate for NADPH oxidases (NOXs) thereby causing lung injuries and favoring SARS-CoV-2 infectivity. On the other hand, polymorphonuclear leukocytes (PMNs) and macrophages, upon infiltrating the inflamed regions through the microvascular blood vessels, can secrete cytotoxic factors including proinflammatory cytokines and ROS. These mediators contribute to the endothelial and epithelial dysfunction leading to fluids leakage from the circulation into the interstitial space and alveoli [56]. Studies have shown that an excess of glucose entry can be diverted through the PPP, which provides additional substrates for NOX thereby resulting in a pro-oxidant environment that exacerbates inflammation [57]. Thus, increased PPP flux, through providing additional substrates for NOX enzymes, can bring about a pro-oxidant environment aggravating inflammation. Also, NOX1 and NOX2 deletion gives rise to a dramatic decrease in ROS production by macrophages [56]. Accordingly, targeting of NOX represents a proper strategy to not only decrease oxidative stress in the host cells but also mitigate the capacity of macrophages and other inflammatory cell,s for ROS production. On such a basis, it is recommended to utilize a medication that not only upregulates PPP and provides high NADPH levels to counteract oxidative stress but also concomitantly prevents NADPH entry into the mentioned pro-oxidant pathway via NOX inhibition.
As mentioned above, PPP inhibition leads to autophagy induction. Thus, keeping this pathway active or upregulating it, as suggested in this study, may play a role in reducing autophagy to prevent viral replication.
3.1. Glycolysis intervention in COVID-19
As stated, glycolysis intervention aims at lipogenesis downregulation. Lipogenesis is required for virus packaging. Glycolysis intervention inhibits pyruvate production and entry into the TCA cycle, thereby decreasing lipogenesis. Normally, glycolysis is needed to provide additional intermediates of the TCA cycle for anabolic lipogenesis;
As in other viruses, lipids have critical roles in the life cycle of coronaviruses [34]. Lipids play crucial roles at different stages of the virus life cycle. First, lipids can act as direct receptors or entry cofactors for non-enveloped and enveloped viruses at the cell surface or the endosomes [58,59]. Second, lipids and lipid synthesis have key roles in both the formation and function of VRCs [60,61]. Third, lipid metabolism can produce the required energy for efficient viral replication [62]. Furthermore, lipids can dictate the appropriate cellular distribution of viral proteins as well as the trafficking, packaging, and release of virus particles [63,64]. Thus, the host lipid biogenesis metabolic pathways play essential roles in modulating virus propagation.
3.1.1. Glycolysis inhibitors
As outlined in Table 1 [18,[65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117], [118], [119], [120], [121], [122], [123], [124], [125], [126], [127], [128], [129], [130], [131], [132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146]], our knowledge on the particular changes induced by a given virus has led to numerous strategies for targeting viral replication in cell culture and in vivo models. Therefore, given its very well-established and favorable side effect profile, ascorbic acid (vitamin C) in particular seems to be a promising compound.
Table 1.
Glycolysis inhibitors.
| Inhibitor | Target | virus |
|---|---|---|
| 2-DG | Phosphoglucose-isomerase | RV HIV HCMV |
| STO-609 Compound C AICAR |
CaMKK AMPK AMPK |
HCMV |
| Oxamate | Lactate-dehydrogenase | KSHV |
| VU0359595 | PLD-1 | HIV |
| Phloretin | GLUT1 | Zika virus (ZIKV) |
| Quercetin | GLUT1 | H5N1, hepatitis C virus (HCV), HBV, influenza A virus (IAV) H1N1, DENV-2, HSV-1, polio-virus type 1, Pf-3, RSV, ZIKV, EV71, HIV |
| STF31 | GLUT1 | – |
| WZB117 | GLUT1 | – |
| Fasentin | GLUT1 | – |
| Apigenin | GLUT1 | ASFV, EV71, HSV-1 and HSV-2, influenza, hepatitis C virus, virus, hand, foot, and mouth disease virus |
| Genistein | GLUT1 | B virus, HSV-1, Arenavirus, H1N1, H9N2, ASFV, human immunodeficiency type 1 virus |
| Oxime-based GLUT1 inhibitors | GLUT1 | – |
| Pyrrolidinone derived GLUT1 inhibitors | GLUT1 | – |
| DNA-damaging anticancer agents | GLUT3 | – |
| GSK-3 inhibitors | GLUT3 | – |
| Ritonavir | GLUT4 | HIV/AIDS |
| Silibinin | GLUT4 | hepatitis C virus, HSV-2, HBV, dengue virus, influenza virus, togaviruses (Chikungunya virus and Mayaro virus) |
| 3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one] 3PO | PFKFB3 | – |
| N4A | PFK2 | – |
| YZ9 | PFK2 | – |
| PGMI-004A | PGAM1 | – |
| MJE3 | PGAM1 | – |
| TT-232 | PKM2 | – |
| Shikonin/alkannin | PKM2 | HIV type 1, AdV3, H1N1 |
| FX11 | LDHA | – |
| 3-bromopyruvate (3BP) | hexokinase II | – |
| Dichloroacetate (DCA) | PDHK1 | – |
| Diarylsulfonamide (DASA-58) (DASA) | PDHK1 | – |
| Oxamic acid | LDH | – |
| NHI-1 | LDH A | – |
| PFK158 | PFKFB3 | – |
| 2-deoxyglucose (2-DG) | HK | HSV-1 |
| Sodium fluoride (NaF) | enolase | influenza virus A/PR8/34 (H0N1), poliomyelitis virus |
| Acidification of blood combined with the addition of NaF and EDTA | enolase | – |
| sodium fluoride–potassium oxalate (NaF–KOx) | enolase | – |
| arsenate compounds | glyceraldehyde-3-phosphate dehydrogenase | – |
| Sorbinil | aldose reductase inhibitor | – |
| Galloflavin | lactate dehydrogenase inhibitor | – |
| Lonidamine | mitochondrial HK2 | – |
| combination 3-BrOP and rapamycin | hexokinase II | – |
| combination MGCD265 and erlotinib | hexokinase II | Reactivation of hepatitis B virus after withdrawal of erlotinib |
| Dihydroartemisinin | pyruvate kinase M2 | – |
| AZD8055 | mTOR | – |
| Ethanol | hexokinase and alpha-glycerophosphate dehydrogenase | flu virus, the common cold virus, and HIV |
| Arenaemycin (pentalenolactone) | glyceraldehyde 3-phosphate dehydrogenase | – |
| Sorafenib | multikinase | Rift Valley Fever virus, HCV virus, Sindbis virus and chikungunya virus, EEEV, hepatitis B virus |
| 1-methyl-tryptophan | Indoleamine 2,3-dioxygenase | MHV-A59, HIV, HBV, HCV, herpes, CMV |
| Iodoacetate | glyceraldehyde-3-phosphate dehydrogenase | Sendai virus, progeny virus, potato virus X |
| Iodoacetamide | glyceraldehyde-3-phosphate dehydrogenase | bovine leukemia virus, tobacco mosaic virus, Rauscher leukemia virus, RS virus, poliovirus, psittacosis virus, Cricket paralysis virus |
| Ascorbic Acid | blocked the energy flux | * |
| LY294002 | PI3K | hepatitis C virus |
| Pt3glc and LY294002 | PI3K | – |
| mannoheptulose | glucokinase inhibitor | – |
| iodoacetic acid | glyceraldehyde-3-phosphate dehydrogenase | VSV, Sendai virus, HSV-1 |
| Malonate | succinate dehydrogenase | influenza and herpes viruses, measles virus |
| FTS | HIF1α expression | EMC-D virus |
| Lactate | PI3K | – |
| FK866 | NAMPT | – |
| 6-AN | G6PD | vaccinia virus |
| Oxythiamine | TKTL1 | – |
| pentalenolactone | glyceraldehyde-3-phosphate dehydrogenase | HSV-1, HSV-2, Vac-IHD, Vac-DIE, NDV, VSV, WEE |
| Compound C | AMP-activated protein kinase | HCMV |
| FUT-175 | complement inhibitor | – |
| Luteolin | HEK2 | dengue virus, influenza A virus, HIV-1, Hepatitis B virus, pseudorabies virus, Epstein-Barr virus, Japanese encephalitis virus, Chikungunya virus |
| Quinoline 3-sulfonamides | LDHA | – |
3.1.1.1. Vitamin C (ascorbic acid)
Vitamin C or ascorbic acid at high doses has shown alterations in metabolic pathways involving increased upstream glycolysis and PPP metabolites and decreased downstream glycolysis metabolites. Thus, this vitamin can be considered as a glycolysis inhibitor [147].
Vitamin C, by lowering viral infectivity, can be used as an inactivating agent for DNA and RNA viruses [148,149]. Additionally, ascorbic acid can detoxify viral products that cause pain and inflammation [150]. It has been shown that high dose intravenous (IV) injection of vitamin C is effective against viral infections including the common cold (rhinovirus) [151]; influenza [152,153]; zika [154]; and chikungunya [155,156]. Oral supplementation with vitamin C (at doses over 3 g) also appears to be capable of both preventing and treating respiratory and systemic infections [157].
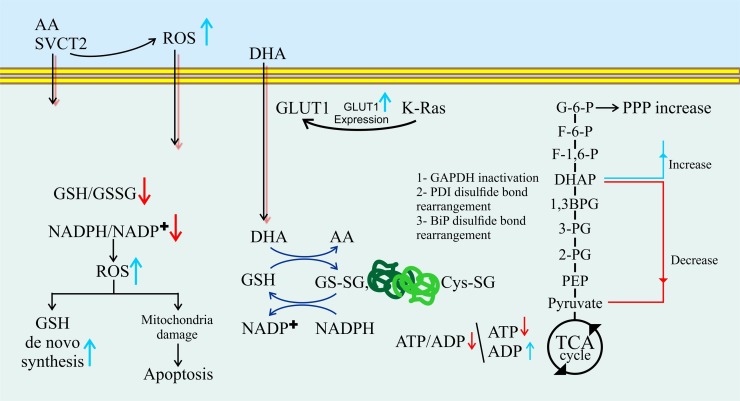
Two families of transport proteins, including GLUTs and sodium-dependent vitamin C transporters (SVCTs) 1 and 2, mediate vitamin C uptake. GLUTs, mainly GLUT1 and GLUT3, transport dehydroascorbic acid (DHA) into cells while SVCTs transport reduced vitamin C directly into the cells (Fig. 2 ) [158,159].
Fig. 2.
Effects of vitamin C on intracellular redox metabolism and glucose metabolism.
3.2. TCA intervention in COVID-19
The TCA cycle provides the fuel acetyl-CoA for the process of lipogenesis [46] that is crucial for sustained viral propagation. Therefore, targeting lipogenesis could pave the way for effective inhibition of viral replication.
3.2.1. TCA inhibitors
As shown in Table 2 [21,[160], [161], [162], [163], [164], [165], [166], [167], [168], [169], [170], [171], [172], [173], [174], [175], [176], [177], [178], [179], [180], [181], [182], [183], [184], [185], [186], [187], [188], [189], [190], [191], [192], [193], [194], [195], [196], [197], [198], [199], [200], [201], [202], [203], [204], [205], [206], [207]], there are many Krebs cycle inhibitors with antiviral properties. Here, metformin, according to its well-established and favorable side effect profile and antiviral activity [[208], [209], [210], [211], [212], [213], [214], [215]], seems to be a promising compound.
Table 2.
TCA inhibitors.
| Inhibitor | Enzymes | VIROUS |
|---|---|---|
| H2O2 | Aconitase, α-Ketoglutarate dehydrogenase | rabies virus, plant virus,, influenza A and B, rhinoviruses 1A, 1B, and type 7, adenovirus types 3 and 6, adenoassociated virus type 4, myxoviruses, respiratory syncytial virus strain Long, and coronavirus strain 229E |
| AG-120 (Ivosidenib) | IDH1 | – |
| AG-221 | IDH2 | – |
| Novartis-530 | IDH1 | – |
| FX 11 | LDH-A | – |
| Dichloroacetate (DCA) | PDK | – |
| miR-26a | PDHX | Influenza A virus, Feline Herpesvirus 1, respiratory syndrome virus |
| miR-146b-5p | PDHB | human papilloma virus 16, dengue virus (DENV) |
| miR-370 | PDHB | hepatitis B virus, Japanese encephalitis virus |
| miR-137 | ASCT2 | – |
| miR-183 | IDH2 | vesicular stomatitis virus (VSV) |
| miR-181a | IDH1 | LCMV |
| fumarate | PHD2 | – |
| succinate | PHD2 | – |
| 2-hydroxyglutarate | α-KG-dependent dioxygenases | – |
| Alloxan | mitochondrial aconitase, Succinic dehydrogenase | – |
| Thioredoxin | succinate dehydrogenase and fumarase & ATP-citrate lyase | H9N2 avian influenza virus |
| 6-diazo-5-oxo-l-norleucine | glutaminolysis | mumps and vesicular stomatitis viruses, human parainfluenza virus type 2 (HPIV-2), NSV |
| CB-839 | GLS | adenovirus, HSV-1, and influenza A |
| CPI-613 | PDH and KGDHC | – |
| enasidenib (AG-221) | IDH2 | – |
| AG-881 | IDH1 and IDH2 | – |
| AG-221 | IDH2-R140 and IDH2-R172 | – |
| Oxalomalate | oxoglutarate dehydrogenase, aconitate hydratase and isocitrate dehydrogenase | – |
| gamma-hydroxy-alpha-oxoglutarate | oxoglutarate dehydrogenase, aconitate hydratase and isocitrate dehydrogenase | – |
| glyoxylic acid | pyruvic oxidase and tentatively as α-oxoglutaric oxidase and succinic oxidase | – |
| Fluoroacetate | aconitase | influenza virus |
| 3-BrPA | isocitrate dehydrogenase, α-ketoglutarate dehydrogenase and succinate dehydrogenase | – |
| Sodium malonate | succinate dehydrogenase | – |
| Sodium arsenite | pyruvate dehydrogenase | WT or NS1 mutant viruses, PEDV |
| Metformin and phenformin | mitochondrial complex 1 | * |
| D-malate | Fumarase | – |
| Citrate | Fumarase | – |
| D-tartrate | Fumarase | hepatitis C virus NS5A |
| L,a-hydroxy-beta-sulfopropionate | Fumarase | – |
| Maleate | Fumarase | Influenza Viruses, Dengue virus |
| mesaconate | Fumarase | – |
| Transaconitate | Fumarase | – |
| Succinate | Fumarase | – |
| Malonate | Fumarase | – |
| Adipate | Fumarase | – |
| Glutarate | Fumarase | – |
| Glycine | Fumarase | – |
| Arsenoso compounds | Pyruvic oxidase, choline dehydrogenase, succinic dehydrogenase | – |
| Hematin | Succinic dehydrogenase | – |
| Cyanide | succinate dehydrogenase | – |
| Copper ions | Succinic dehydrogenase | influenza A virus |
| Maleic acid | Succinic dehydrogenase | aphthous fever virus |
| Sodium diethyldithiocarbamate (DDC) | Succinic dehydrogenase | HIV and AIDS |
3.2.1.1. Metformin
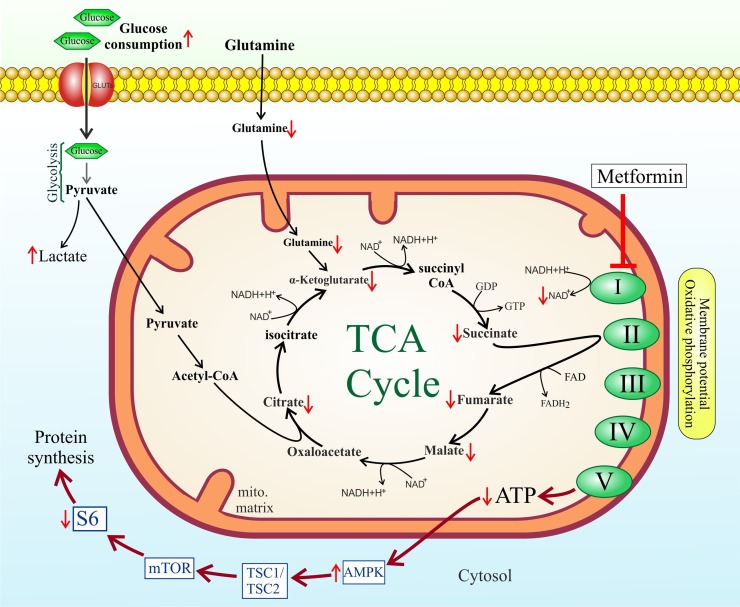
As a drug with pleiotropic effects, metformin participates in glucose homeostasis, mostly through inhibiting liver glucose production [216]. Also, 0ne study found that metformin was significantly associated with reduced mortality from COVID-19 [217]. Metformin, by lowering the flow of glucose- and glutamine-derived metabolic intermediates into the TCA cycle leads to decreased citrate production and de novo lipid biosynthesis. Metformin acts directly on mitochondria to restrain TCA cycle activity and oxidative phosphorylation, leading cells to accept less glucose-derived carbon that favors lactic acid production [218]. Metformin brings about energetic stress in cells by inhibiting the complex I of the electron transport chain in mitochondria. This causes decreased NADH oxidation and TCA flux resulting in low levels of TCA metabolites. Therefore, metformin indirectly inhibits cellular lipogenesis (Fig. 3 ) [219].
Fig. 3.
TCA cycle intervention by metformin.
On the other hand, metformin also directly hinders lipogenesis. Direct anti-lipogenic activities of metformin are mediated through inhibition of key metabolic enzymes including ATP citrate lyase (ACLY), acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS) complex, 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA) [220]. Of note, metformin hinders ROS production by suppressing NOX activity [221]. This is a key characteristic with respect to our proposed treatment protocol for COVID-19, as high cellular NADPH levels (as a result of upregulated PPP), by acting as NOX substrate, not only can result in oxidative stress and promote viral replication in the infected cell but also potentiates ROS production by the recruited inflammatory cells at the alveolar spaces contributing to ARDS.
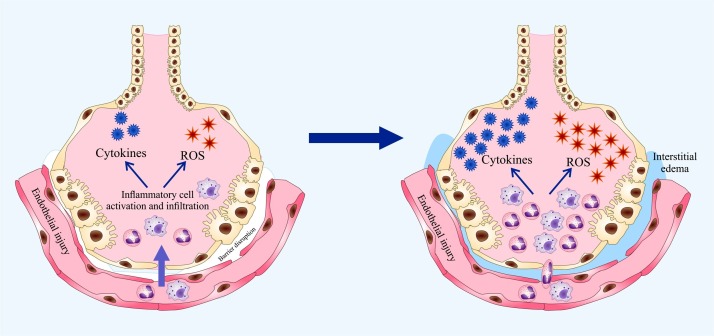
4. Inhibition of COVID-19 related oxidative stress
Acute organ failure, particularly pulmonary failure (ARDS), is the major mechanism for COVID-19 related fatality [222,223]. Substantially increased oxidative stress owing to the rapid release of cytokines and free radicals, etc. is the hallmark of ARDS that results in cellular injury, organ failure, and death [44]. Therefore, early intake of high dose antioxidants, especially vitamin C, plays a crucial role in the management of such patients. Hence, in the case of coronavirus epidemic, all those in the leadership and those providing direct assistance patients are recommended to rapidly and bravely apply high dose IV vitamin C.
Increased oxidative stress, as a consequence of “cytokine storm,” results in ARDS as the key pathologic cause for the high mortality rate of this pandemic viral infection. Cytokine storm-induced ARDS is the key pathoenic event responsible for the death of these patients [44]. IV vitamin C is suggested to effectively cope with cytokine storm-induced oxidative stress (Fig. 4 ) [224].Vitamin C is a pivotal component of the antioxidant system in cellular response [225].As a matter of fact, high dose IV vitamin C has been applied clinically successfully in viral ARDS as well as in influenza. Fowler et al. reported a 26-year-old woman developing viral ARDS due to rhinovirus and enterovirus-D68 [226] Hemila et al. reported that vitamin C makes ICU stay shorter according to their 2019 meta-analysis of 18 clinical studies published in the journal Nutrients [227]. In their report, vitamin C shortened ICU stay by 97.8 % in a subgroup of 1766 patients. Marik and colleagues reported their use of IV vitamin C in 47 sepsis ICU cases. They found a considerable decrease in the mortality rate in the group of patients receiving IV vitamin C [228].
Fig. 4.
Impaired function of alveolar epithelium and microvascular endothelium in ARDS. Microvascular blood vessels allow elevated infiltration of polymorphonuclear leukocytes (PMNs) and macrophages into the inflamed region followed by increased release of cytotoxic factors including proinflammatory cytokines and ROS. These released mediators contribute to the endothelial and epithelial dysfunction leading to fluids leakage from the circulation into the interstitial space and alveoli. Combined, these events result in pulmonary edema and impaired gas exchange.
In patients receiving mechanical ventilation, dietary antioxidants (vitamin C and sulforaphane) have been shown to decrease oxidative stress-induced acute inflammatory lung injury [229]. Other antioxidants (like curcumin) have also shown promising anti-inflammatory potentials in pneumonia [230].
Based on these reports, targeting the recalcitrant COVID-19 related oxidative stress with high dose antioxidants (like vitamin C) seems a logical step in the immediate management of this catastrophic pandemic, without the lengthy waiting for pathogen-specific drugs and vaccines. Also, metformin has been shown to decrease ROS and nitric oxide (NO) levels and increase the antioxidant system, such as superoxide dismutase (SOD) [231].
Vitamin C serves as a mild pro-oxidant capable of producing free radicals and, as a result, induces mitochondrial biogenesis. Mitochondrial biogenesis enhances the metabolic enzymes needed for glycolysis and oxidative phosphorylation, and hence creates a greater mitochondrial metabolic capacity. As shown by a study exploring the effects of azithromycin and doxycycline on the mitochondrial biogenesis, low doses of these antibiotics were able to inhibit mitochondrial biogenesis [232]. Also, azithromycin and doxycycline exhibit antiviral activities [[233], [234], [235], [236], [237], [238], [239], [240], [241]].
5. Conclusion
The pandemic outbreak of the novel coronavirus SARS-CoV-2 in 2020 vigorously necessitates as urgent finding of effective therapeutic agents as possible [242]. At present, there is no definite and effective treatment for SARS-CoV-2 infection. Acute organ failure, especially pulmonary failure (acute respiratory distress syndrome or ARDS), is the key pathogenic mechanism governing SARS-CoV-2 fatality. Significantly increased oxidative stress due to the rapid release of free radicals and cytokines, etc. is the hallmark of ARDS, resulting in cellular injury, organ failure, and finally death [222,223]. Also, according to the recent publications concerning the higher susceptibility of G6PD-deficient cells to coronavirus (HCoV-229E) infectivity, oxidative stress in host cells provides a proper milieu for coronavirus replication [48]. Therefore, reducing ROS levels in patients with COVID-19 seems a therapeutic strategy against this disease. Another therapeutic strategy is to prevent virus replication. A therapeutic approach incorporating both of these aspects can be considered optimal treatment.
Vitamin C, with its antioxidant properties, is known to reduce ROS. But can it stop the virus replication?
We propose the use of vitamin C for COVID-19 treatment to not only directly counteract the deleterious and dangerous effects of augmented oxidative stress responsible for ARDS in the extracellular space (alveolar space), but also hinder glycolysis and instead upregulate PPP and intracellular NADPH for creating a higher cellular redox status with the aim of lowering SARS-CoV-2 infectivity, as oxidative stress has been elucidated to promote coronavirus infectivity. Also, due to the very recent report on the autophagy-inducing effect of G6PD blockade that causes increased viral replication, we assume that PPP upregulation is likely to cope with SARS-CoV-2 replication through this autophagy-mediated mechanism, too. But further researches in the future remain to fully shed light on this facet of coronavirus infection. Early use of high dose antioxidants, especially vitamin C, therefore, plays a key role in the management of these patients. In a study, modest quantities of supplemental vitamin C (200 mg of vitamin C daily) led to an 80 % reduction in death among severely ill, hospitalized respiratory disease patients [227]. Infants with viral pneumonia administered with vitamin C have shown reduced mortality [243]. In a subgroup of 1766 patients, moderate doses of vitamin C shortened ICU stay by 97 % [227]. The major danger with SARS-CoV-2 infection is disease progression to SARS and pneumonia. Since the 1940s, medical doctors have successfully used vitamin C against viral pneumonia [244].
To replicate, RNA viruses upregulate both glycolysis and glycogenolysis, providing their host cells with TCA intermediates required for anabolic lipogenesis. The novel emerged coronavirus, SARS-CoV-2, which has thus far spread from China throughout the world with fatal outcomes, is an RNA virus as well [6]. Also, evidence points to a higher PPP flux following RNA virus infections [245], as is the case in the rhinovirus infection, which results in the production of higher nucleotide levels supporting viral replication. On such a basis, inhibition/modulation of these metabolic pathways could contribute to the inhibition of viral replication. But, concerning coronaviruses, direct and clear data on the exact status of this metabolic pathway in the infected cell is scarce. Rather, recent data highlights the contributing role of intracellular oxidative stress to coronavirus infectivity, further bringing into question the exact role/status of this pathway during coronavirus infection. From this point of view, PPP upregulation, by providing higher NADPH levels and potentiating intracellular redox power, is suggested to impair coronavirus replication. But how does SARS-CoV-2 itself manipulate this pathway in the lung epithelial cells? The response hides behind the meticulous evaluation of the PPP following SARS-CoV-2 infection warranting more in-depth investigations in this regard. However, based on the reported pathogenic role oxidative stress plays in promoting coronavirus infection, we suggest upregulated PPP in the infected host cells as a therapeutic mechanism.
Vitamin C inhibits glycolysis, which is a metabolic pathway used by cells to convert glucose into energy. As stated, RNA viruses for sustained replication increase glycolysis and glycogenolysis, thereby providing TCA intermediates required for anabolic lipogenesis [245]. Thus, despite the blockade of the glycolysis pathway by vitamin C, other pathways linked with the TCA cycle (like catabolism of some amino acids) are still open. Therefore, in addition to vitamin C (which indirectly downregulates the TCA cycle), inhibition of viral replication requires another substance that can block the TCA cycle.
We propose upregulated PPP could exert antiviral effects in SARS-CoV-2 infection. Since increased levels of ROS play an important role in this viral infection, an increased flux of PPP in this infection is regarded as a therapeutic strategy. RNA virus-infected cells immediately upregulate glucose uptake in a PI3K-dependent manner. In parallel, infected cells augment the expression of the PI3K-regulated GLUT1 [17]. The point to consider in blocking the glycolysis pathway is to use an inhibitor that does not inhibit GLUT1, because we need this transporter to keep the PPP active. 2-deoxyglucose (2-DG) appears to be a promising compound in this regard, given its well-established and favorable side effect profile and antiviral properties. 2-DG has no antioxidant property compared to vitamin C. Rather, it induces oxidative stress [246] Therefore, vitamin C as an antioxidant and an inhibitor of the glycolysis pathway with concomitant effects on PPP upregulation, could be a good choice for the treatment of this viral infection.
As the PPP upregulates, the production of ribonucleotides required for virus replication increases. Since the virus relies on host cell lipogenesis for its replication, targeting lipogenesis is speculated to inhibit virus replication even in the presence of large amounts of ribonucleotides. In this regard, inhibition of lipogenesis via Krebs cycle blockade seems conceivable. As mentioned above, TCA cycle blockade is required for the inhibition of viral replication. Metformin is introduced for this purpose. Metformin, not only decreases mitochondrial TCA cycle intermediates but also directly inhibits lipogenesis [219,220]. Since metformin suppresses NOX activity, its intake would cicumvent the potential hazardous impacts of high intracellular NADPH levels (due to upregulated PPP) in terms of generating higher ROS (contributing to the detrimental function of proinflammatory cells) and creating intracellular oxidative stress (contributing to increased SARS-CoV-2 infectivity in the host cell), as well. On the other hand, vitamin C is a mild pro-oxidant. Therefore, it can produce free radicals and, as a result, induces mitochondrial biogenesis. This can act in favor of SARS-CoV-2 replication by providing the cell with a higher number of factories in charge of TCA cycle flux and consequently lead to increased lipogenesis. To avoid the effect, we propose a strategy to both inhibit mitochondrial biogenesis and hamper mitochondrial protein translation. The use of azithromycin or doxycycline (or both) at low doses (that spares antibiotic resistance) has been shown to prevent mitochondrial biogenesis. Alsoas off-target side effects, azithromycin and doxycycline can inhibit large and small mitochondrial ribosomes, respectively [232]. Additionally, these antibiotics also possess antiviral activities [[233], [234], [235], [236], [237], [238], [239], [240], [241]].
A combination of vitamin C, metformin and doxycycline/azithromycin, is suggested to serve as an effective treatment for this infection. As these compounds are non-toxic, we hope that this therapeutic strategy can be applied with minimal side effects. It is worth mentioning that, applying this strategy for the treatment of stormy and fatal outbreaks of this pandemic virus in the world is still in the hypothesis level, and final corroboration is in dire need of supportive clinical evidence.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
We would like to especially thank Dr.Bizhan Solimani, and Dr. Hamid Reza Mohammadi motlagh for their valuable discussions and help with manuscript preparation.
References
- 1.Ashour H.M., Elkhatib W.F., Rahman M.M., Elshabrawy H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9:186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uddin M., Mustafa F., Rizvi T.A., Loney T., Suwaidi H.A., Al-Marzouqi A.H., et al. SARS-CoV-2/COVID-19: viral genomics, epidemiology, vaccines, and therapeutic interventions. Viruses. 2020;12:526. doi: 10.3390/v12050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Situation updates on 12 August, 2020. Available at https://www.who.int/emergencies/diseases/novel-coronavirus-2019 2020 (Accessed 12 August 2020).
- 4.Sanchez E.L., Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;479:609–618. doi: 10.1016/j.virol.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer K.A., Stöckl J., Zlabinger G.J., Gualdoni G.A. Hijacking the supplies: metabolism as a novel facet of virus-host interaction. Front. Immunol. 2019;10:1533. doi: 10.3389/fimmu.2019.01533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;17:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan B., Chu H., Yang D., Sze K.H., Lai P.M., Yuan S., et al. Characterization of the lipidomic profile of human coronavirus-infected cells: implications for lipid metabolism remodeling upon coronavirus replication. Viruses. 2019;11:73. doi: 10.3390/v11010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikolajczyk S., Brody S. De novo fatty acid synthesis mediated by acyl‐carrier protein in Neurospora crassa mitochondria. Eur. J. Biochem. 1990;187:431–437. doi: 10.1111/j.1432-1033.1990.tb15322.x. [DOI] [PubMed] [Google Scholar]
- 9.Wakil S.J. Enzymatic synthesis of fatty acids. Comp. Biochem. Physiol. 1962;4:123–158. doi: 10.1016/0010-406X(62)90002-6. [DOI] [PubMed] [Google Scholar]
- 10.Varnum S.M., Streblow D.N., Monroe M.E., Smith P., Auberry K.J., Pasa-Tolic L., et al. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 2004;78:10960–10966. doi: 10.1128/JVI.78.20.10960-10966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bechtel J.T., Winant R.C., Ganem D. Host and viral proteins in the virion of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2005;79:4952–4964. doi: 10.1128/JVI.79.8.4952-4964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chertova E., Chertov O., Coren L.V., Roser J.D., Trubey C.M., Bess J.W., et al. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J. Virol. 2006;80:9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Segura M.M., Garnier A., Di Falco M.R., Whissell G., Meneses-Acosta A., Arcand N., et al. Identification of host proteins associated with retroviral vector particles by proteomic analysis of highly purified vector preparations. J. Virol. 2008;82:1107–1117. doi: 10.1128/JVI.01909-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw M.L., Stone K.L., Colangelo C.M., Gulcicek E.E., Palese P. Cellular proteins in influenza virus particles. PLoS Pathog. 2008;4(6) doi: 10.1371/journal.ppat.1000085. e1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong Q., Xue C., Ren X., Zhang C., Li L., Shu D., et al. Proteomic analysis of purified coronavirus infectious bronchitis virus particles. Proteome Sci. 2010;8:29. doi: 10.1186/1477-5956-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan S., Chu H., Chan J.F.W., Ye Z.W., Wen L., Yan B., et al. SREBP-dependent lipidomic reprogramming as a broad-spectrum antiviral target. Nat. Commun. 2019;10:1–15. doi: 10.1038/s41467-018-08015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gualdoni G.A., Mayer K.A., Kapsch A.M., Kreuzberg K., Puck A., Kienzl P., et al. Rhinovirus induces an anabolic reprogramming in host cell metabolism essential for viral replication. Proc Natl Acad Sci. 2018;115:E7158. doi: 10.1073/pnas.1800525115. E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McArdle J., Schafer X.L., Munger J. Inhibition of calmodulin-dependent kinase kinase blocks human cytomegalovirus-induced glycolytic activation and severely attenuates production of viral progeny. J. Virol. 2011;85:705–714. doi: 10.1128/JVI.01557-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McArdle J., Moorman N.J., Munger J. HCMV targets the metabolic stress response through activation of AMPK whose activity is important for viral replication. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thai M., Graham N.A., Braas D., Nehil M., Komisopoulou E., Kurdistani S.K., et al. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19:694–701. doi: 10.1016/j.cmet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thai M., Thaker S.K., Feng J., Du Y., Hu H., Wu T.T., et al. MYC-induced reprogramming of glutamine catabolism supports optimal virus replication. Nat. Commun. 2015;6:1–9. doi: 10.1038/ncomms9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spencer C.M., Schafer X.L., Moorman N.J., Munger J. Human cytomegalovirus induces the activity and expression of acetyl-coenzyme A carboxylase, a fatty acid biosynthetic enzyme whose inhibition attenuates viral replication. J. Virol. 2011;85:5814–5824. doi: 10.1128/JVI.02630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purdy J.G., Shenk T., Rabinowitz J.D. Fatty acid elongase 7 catalyzes lipidome remodeling essential for human cytomegalovirus replication. Cell Rep. 2015;10:1375–1385. doi: 10.1016/j.celrep.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y., Maguire T.G., Alwine J.C. Human cytomegalovirus infection induces adipocyte-like lipogenesis through activation of sterol regulatory element binding protein 1. J. Virol. 2012;86:2942–2949. doi: 10.1128/JVI.06467-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y., Jr., F.J. Pierciey, Maguire T.G., Alwine J.C. PKR-like endoplasmic reticulum kinase is necessary for lipogenic activation during HCMV infection. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Y., Maguire T.G., Alwine J.C. ChREBP, a glucose-responsive transcriptional factor, enhances glucose metabolism to support biosynthesis in human cytomegalovirus-infected cells. Proc Natl Acad Sci. 2014;111:1951–1956. doi: 10.1073/pnas.1310779111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neufeldt C.J., Joyce M.A., Van Buuren N., Levin A., Kirkegaard K., Gale Jr.M., et al. The hepatitis C virus-induced membranous web and associated nuclear transport machinery limit access of pattern recognition receptors to viral replication sites. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Overby A.K., Popov V.L., Niedrig M., Weber F. Tick-borne encephalitis virus delays interferon induction and hides its double-stranded RNA in intracellular membrane vesicles. J. Virol. 2010;84:8470–8483. doi: 10.1128/JVI.00176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arita M. Phosphatidylinositol‐4 kinase III beta and oxysterol‐binding protein accumulate unesterified cholesterol on poliovirus‐induced membrane structure. Microbiol. Immunol. 2014;58:239–256. doi: 10.1111/1348-0421.12144. [DOI] [PubMed] [Google Scholar]
- 30.Dorobantu C.M., Albulescu L., Harak C., Feng Q., van Kampen M., Strating J.R., et al. Modulation of the host lipid landscape to promote RNA virus replication: the picornavirus encephalomyocarditis virus converges on the pathway used by hepatitis C virus. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roulin P.S., Lotzerich M., Torta F., Tanner L.B., van Kuppeveld F.J., Wenk M.R., et al. Rhinovirus uses a phosphatidylinositol 4-phosphate/cholesterol counter-current for the formation of replication compartments at the ER-Golgi interface. Cell Host Microbe. 2014;16:677–690. doi: 10.1016/j.chom.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Strating J.R., van der Linden L., Albulescu L., Bigay J., Arita M., Delang L., et al. Itraconazole inhibits enterovirus replication by targeting the oxysterol-binding protein. Cell Rep. 2015;10:600–615. doi: 10.1016/j.celrep.2014.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., Perry J.W., Lauring A.S., Neddermann P., De Francesco R., Tai A.W. Oxysterol-binding protein is a phosphatidylinositol 4-kinase effector required for HCV replication membrane integrity and cholesterol trafficking. Gastroenterology. 2014;146:1373–1385. doi: 10.1053/j.gastro.2014.02.002. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan B., Chu H., Yang D., Sze K.H., Lai P.M., Yuan S., et al. Characterization of the lipidomic profile of human coronavirus-infected cells: implications for lipid metabolism remodeling upon coronavirus replication. Viruses. 2019;11:73. doi: 10.3390/v11010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikolajczyk S., Brody S. De novo fatty acid synthesis mediated by acyl‐carrier protein in Neurospora crassa mitochondria. Eur. J. Biochem. 1990;187:431–437. doi: 10.1111/j.1432-1033.1990.tb15322.x. [DOI] [PubMed] [Google Scholar]
- 36.Wakil S.J. Enzymatic synthesis of fatty acids. Comp. Biochem. Physiol. 1962;4:123–158. doi: 10.1016/0010-406X(62)90002-6. [DOI] [PubMed] [Google Scholar]
- 37.Varnum S.M., Streblow D.N., Monroe M.E., Smith P., Auberry K.J., Pasa-Tolic L., et al. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 2004;78:10960–10966. doi: 10.1128/JVI.78.20.10960-10966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bechtel J.T., Winant R.C., Ganem D. Host and viral proteins in the virion of Kaposi’s sarcoma-associated herpesvirus. J. Virol. 2005;79:4952–4964. doi: 10.1128/JVI.79.8.4952-4964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chertova E., Chertov O., Coren L.V., Roser J.D., Trubey C.M., Bess J.W., et al. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J. Virol. 2006;80:9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segura M.M., Garnier A., Di Falco M.R., Whissell G., Meneses-Acosta A., Arcand N., et al. Identification of host proteins associated with retroviral vector particles by proteomic analysis of highly purified vector preparationsm. J. Virol. 2008;82:1107–1117. doi: 10.1128/JVI.01909-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw M.L., Stone K.L., Colangelo C.M., Gulcicek E.E., Palese P. Cellular proteins in influenza virus particles. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong Q., Xue C., Ren X., Zhang C., Li L., Shu D., et al. Proteomic analysis of purified coronavirus infectious bronchitis virus particles. Proteome Sci. 2010;8:29. doi: 10.1186/1477-5956-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kindrachuk J., Ork B., Hart B.J., Mazur S., Holbrook M.R., Frieman M.B., et al. Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrobial Antimicrob Agents Chemother. 2015;59:1088–1099. doi: 10.1128/AAC.03659-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubenfeld G., Thompson T., Ferguson N., Caldwell E., Fan E., Camporota L., et al. Acute respiratory distress syndrome, the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 46.Han S., Mallampalli R.K. The acute respiratory distress syndrome: from mechanism to translation. J. Immunol. 2015;194:855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biswas S.K., Rahman I. Environmental toxicity, redox signaling and lung inflammation: the role of glutathione. Mol. Aspects Med. 2009;30:60–76. doi: 10.1016/j.mam.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y.H., Tseng C.P., Cheng M.L., Ho H.Y., Shih S.R., Chiu D.T.Y. Glucose-6-phosphate dehydrogenase deficiency enhances human coronavirus 229E infection. Int. J. Infect. Dis. 2008;197:812–816. doi: 10.1086/528377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Y.H., Chiu D.T.Y., Lin H.R., Tang H.Y., Cheng M.L., Ho H.Y. Glucose-6-phosphate dehydrogenase enhances antiviral response through downregulation of NADPH sensor HSCARG and upregulation of NF-κB signaling. Viruses. 2015;7:6689–6706. doi: 10.3390/v7122966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riganti C., Gazzano E., Polimeni M., Aldieri E., Ghigo D. The pentose phosphate pathway: an antioxidant defense and a crossroad in tumor cell fate. Free Radic. Biol. Med. 2012;53:421–436. doi: 10.1016/j.freeradbiomed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Prentice E., Jerome W.G., Yoshimori T., Mizushima N., Denison M.R. Coronavirus replication complex formation utilizes components of cellular autophagy. J. Biol. Chem. 2004;279:10136–10141. doi: 10.1074/jbc.M306124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mele L., la Noce M., Paino F., Regad T., Wagner S., Liccardo D., et al. Glucose-6-phosphate dehydrogenase blockade potentiates tyrosine kinase inhibitor effect on breast cancer cells through autophagy perturbation. J. Exp. Clin. Cancer Res. 2019;38:160. doi: 10.1186/s13046-019-1164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pillat M.M., Krüger A., Guimarães L.M., Lameu C., de Souza E.E., Wrenger C., Ulrich H. Insights in chloroquine action: perspectives and implications in Malaria and COVID‐19. Cytom. Part A. 2020 doi: 10.1002/cyto.a.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 55.Bonam S.R., Muller S., Bayry J., Klionsky D.J. Autophagy as an emerging target for COVID-19: lessons from an old friend, chloroquine. Autophagy. 2020 doi: 10.1080/15548627.2020.1779467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu Q., Choksi S., Qu J., Jang J., Choe M., Banfi B., et al. NADPH oxidases are essential for macrophage differentiation. J. Biol. Chem. 2016;291:20030–20041. doi: 10.1074/jbc.M116.731216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peiro C., Romacho T., Azcutia V., Villalobos L., Fernandez E., Bolanos J.P., et al. Inflammation, glucose, and vascular cell damage: the role of the pentose phosphate pathway. Cardiovasc. Diabetol. 2016;15:82. doi: 10.1186/s12933-017-0502-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taube S., Jiang M., Wobus C.E. Glycosphingolipids as receptors for non-enveloped viruses. Viruses. 2010;2:1011–1049. doi: 10.3390/v2041011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chazal N., Gerlier D. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 2003;67:226–237. doi: 10.1128/MMBR.67.2.226-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagy P.D., Strating J.R., van Kuppeveld F.J. Building, viral replication organelles: close encounters of the membrane types. PLoS Pathog. 2016;12 doi: 10.1371/journal.ppat.1005912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hsu N.Y., Ilnytska O., Belov G., Santiana M., Chen Y.H., Takvorian P.M., et al. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diamond D.L., Syder A.J., Jacobs J.M., Sorensen C.M., Walters K.A., Proll S.C., et al. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ono A., Ablan S.D., Lockett S.J., Nagashima K., Freed E.O. Phosphatidylinositol \(4., 5) bisphosphate regulates HIV-1 gag targeting to the plasma membrane. PNAS. 2004;101:14889–14894. doi: 10.1073/pnas.0405596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang J., Pekosz A., Lamb R.A. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 2000;74:4634–4644. doi: 10.1128/JVI.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xintaropoulou C., Ward C., Wise A., Marston H., Turnbull A., Langdon S.P. A comparative analysis of inhibitors of the glycolysis pathway in breast and ovarian cancer cell line models. Oncotarget. 2015;6:25677. doi: 10.18632/oncotarget.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong D., Xiong L., Liu T., Liu X., Liu X., Chen J., et al. The glycolytic inhibitor 2-deoxyglucose activates multiple prosurvival pathways through IGF1R. J. Biol. 2009;284:23225–23233. doi: 10.1074/jbc.M109.005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gambino R., Piscitelli J., Ackattupathil T.A., Theriault J.L., Andrin R.D., Sanfilippo M.L., et al. Acidification of blood is superior to sodium fluoride alone as an inhibitor of glycolysis. Clin. Chem. 2009;55:1019–1021. doi: 10.1373/clinchem.2008.121707. [DOI] [PubMed] [Google Scholar]
- 68.Waring W., Evans L., Kirkpatrick C. Glycolysis inhibitors negatively bias blood glucose measurements: potential impact on the reported prevalence of diabetes mellitus. J. Clin. Pathol. 2007;60:820–923. doi: 10.1136/jcp.2006.039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gonzalez A.M., Sochor M., Hothersall J., McLean P. Effect of aldose reductase inhibitor (sorbinil) on integration of polyol pathway, pentose phosphate pathway, and glycolytic route in diabetic rat lens. Diabetes. 1986;35:1200–1205. doi: 10.2337/diab.35.11.1200. [DOI] [PubMed] [Google Scholar]
- 70.Farabegoli F., Vettraino M., Manerba M., Fiume L., Roberti M., Di Stefano Galloflavin G. A new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur. J. Pharm. Sci. 2012;47:729–738. doi: 10.1016/j.ejps.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 71.Obrosova I., Faller A., Burgan J., Ostrow E., Williamson J.R. Glycolytic pathway, redox state of NAD (P)-couples and energy metabolism in lens in galactose-fed rats: effect of an aldose reductase inhibitor. Curr. Eye Res. 1997;16:34–43. doi: 10.1076/ceyr.16.1.34.5113. [DOI] [PubMed] [Google Scholar]
- 72.Gambino R. Sodium fluoride: an ineffective inhibitor of glycolysis. Ann. Clin. Biochem. 2013;50:3–5. doi: 10.1258/acb.2012.012135. [DOI] [PubMed] [Google Scholar]
- 73.Floridi A., Paggi M.G., Marcante M.L., Silvestrini B., Caputo A., de Martino C. Lonidamine, a selective inhibitor of aerobic glycolysis of murine tumor cells. J. Natl. Cancer Inst. 1981;66:497–499. doi: 10.1093/jnci/66.3.497. [DOI] [PubMed] [Google Scholar]
- 74.Levy A.G., Zage P.E., Akers L.J., Ghisoli M.L., Chen Z., Fang W., et al. The combination of the novel glycolysis inhibitor 3-BrOP and rapamycin is effective against neuroblastoma. INVEST NEW DRUG. 2012;30:191–199. doi: 10.1007/s10637-010-9551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonfils C., Beaulieu N., Fournel M., Ste-Croix H., Besterman J.M., Maroun C.R. The combination of MGCD265, a Met/VEGFR inhibitor in clinical development, and erlotinib potently inhibits tumor growth by altering multiple pathways including glycolysis. AACR. 2012;72:1790. doi: 10.1158/1538-7445.AM2012-1790. [DOI] [Google Scholar]
- 76.Mi Yj., Geng Gj., Zou Zz., Gao J., Luo Xy., Liu Y., et al. Dihydroartemisinin inhibits glucose uptake and cooperates with glycolysis inhibitor to induce apoptosis in non-small cell lung carcinoma cells. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S., Li Y., Hu R., Li W., Qiu H., Cai H., et al. The mTOR inhibitor AZD8055 inhibits proliferation and glycolysis in cervical cancer cells. Oncol. Lett. 2013;5:717–721. doi: 10.3892/ol.2012.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nagodawithana T., Whitt J., Cutaia A. Study of the feedback effect of ethanol on selected enzymes of the glycolytic pathway. J. Am. Soc. Brew. Chem. 1977;35:179–183. doi: 10.1094/ASBCJ-35-0179. [DOI] [Google Scholar]
- 79.Hartmann S., Neeff J., Heer U., Mecke D. Arenaemycin (pentalenolactone): a specific inhibitor of glycolysis. FEBS Lett. 1978;93:339–342. doi: 10.1016/0014-5793(78)81135-1. [DOI] [PubMed] [Google Scholar]
- 80.Tesori V., Piscaglia A.C., Samengo D., Barba M., Bernardini C., Scatena R., et al. The multikinase inhibitor Sorafenib enhances glycolysis and synergizes with glycolysis blockade for cancer cell killing. Sci. Rep. 2015;5:9149. doi: 10.1038/srep09149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eleftheriadis T., Pissas G., Karioti A., Antoniadi G., Liakopoulos V., Dafopoulou K., et al. The indoleamine 2, 3-dioxygenase inhibitor 1-methyl-tryptophan suppresses mitochondrial function, induces aerobic glycolysis and decreases interleukin-10 production in human lymphocytes. Immunol. Invest. 2012;41:507–520. doi: 10.3109/08820139.2012.682244. [DOI] [PubMed] [Google Scholar]
- 82.Massieu L., Gomez-Roman N., Montiel T. In vivo potentiation of glutamate-mediated neuronal damage after chronic administration of the glycolysis inhibitor iodoacetate. Exp. Neurol. 2000;165:257–267. doi: 10.1006/exnr.2000.7481. [DOI] [PubMed] [Google Scholar]
- 83.Vuyyuri S.B., Rinkinen J., Worden E., Shim H., Lee S., Davis K.R. Ascorbic acid and a cytostatic inhibitor of glycolysis synergistically induce apoptosis in non-small cell lung cancer cells. PLoS One. 2013;8:e67081. doi: 10.1371/journal.pone.0067081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mallawaaratchy D.M., Mactier S., Kaufman K.L., Blomfield K., Christopherson R.I. The phosphoinositide 3-kinase inhibitor LY294002, decreases aminoacyl-tRNA synthetases, chaperones and glycolytic enzymes in human HT-29 colorectal cancer cells. J. Proteomics. 2012;75:1590–1599. doi: 10.1016/j.jprot.2011.11.032. [DOI] [PubMed] [Google Scholar]
- 85.Board M., Colquhoun A., Newsholme E.A. High Km glucose-phosphorylating (glucokinase) activities in a range of tumor cell lines and inhibition of rates of tumor growth by the specific enzyme inhibitor mannoheptulose. Cancer Res. 1995;55:3278–3285. [PubMed] [Google Scholar]
- 86.Lapchak P., Maher P., Schubert D., Zivin J. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150:585–591. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 87.Wang G., Fu X.L., Wang J.J., Guan R., Sun Y., To Ss.Tony. Inhibition of glycolytic metabolism in glioblastoma cells by Pt3glc combinated with PI3K inhibitor via SIRT3‐mediated mitochondrial and PI3K/Akt–MAPK pathway. J. Cell. Physiol. 2019;234:5888–5903. doi: 10.1002/jcp.26474. [DOI] [PubMed] [Google Scholar]
- 88.Black M., Kleiner I., Bolkerm H. Glycolytic enzyme inhibitor therapy in human malignant neoplasia. Cancer Res. 1949;9:314–319. [PubMed] [Google Scholar]
- 89.Mondal S., Roy D., Sarkar Bhattacharya S., Jin L., Jung D., Zhang S., et al. Therapeutic targeting of PFKFB3 with a novel glycolytic inhibitor PFK158 promotes lipophagy and chemosensitivity in gynecologic cancers. Int. J. Cancer. 2019;144:178–189. doi: 10.1002/ijc.31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blum R., Jacob-Hirsch J., Amariglio N., Rechavi G., Kloog Y. Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1α, causing glycolysis shutdown and cell death. Cancer Res. 2005;65:999–1006. [PubMed] [Google Scholar]
- 91.Beckner M.E., Gobbel G.T., Abounader R., Burovic F., Agostino N.R., Laterra J., et al. Glycolytic glioma cells with active glycogen synthase are sensitive to PTEN and inhibitors of PI3K and gluconeogenesis. Lab. Invest. 2005;85:1457–1470. doi: 10.1038/labinvest.3700355. [DOI] [PubMed] [Google Scholar]
- 92.Tan B., Young D.A., Lu Z.H., Wang T., Meier T.I., Shepard R.L., et al. Pharmacological inhibition of nicotinamide phosphoribosyltransferase (NAMPT), an enzyme essential for NAD+ biosynthesis, in human cancer cells metabolic basis and potential clinical implications. J. Biol. 2013;288:3500–3511. doi: 10.1074/jbc.M112.394510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Egler V., Korur S., Failly M., Boulay J.L., Imber R., Lino M.M., et al. Histone deacetylase inhibition and blockade of the glycolytic pathway synergistically induce glioblastoma cell death. Clin. Cancer Res. 2008;14 doi: 10.1158/1078-0432.CCR-07-4182. 3132-1340. [DOI] [PubMed] [Google Scholar]
- 94.Nagle W.A., Moss A., Henle K.J. Sensitization of cultured Chinese hamster cells to 42° C hyperthermia by pentalenolactone, an inhibitor of glycolytic ATP synthesis. Int. J. Radiat. Biol. 1985;48:821–835. doi: 10.1080/09553008514551921. [DOI] [PubMed] [Google Scholar]
- 95.Redman R.A., Pohlmann P.R., Kurman M.R., Tapolsky G., Chesney J.A. A phase I, dose-escalation, multi-center study of PFK-158 in patients with advanced solid malignancies explores a first-in-man inhibitor of glycolysis. Am. J. Clin. Oncol. 2015;33 [Google Scholar]
- 96.Shen Q.W., Gerrard D.E., Du M. Compound C, an inhibitor of AMP-activated protein kinase, inhibits glycolysis in mouse longissimus dorsi postmortem. Meat Sci. 2008;78:323–330. doi: 10.1016/j.meatsci.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 97.Ebata T., Kuttner R., Apantaku F., Schumer W. Effect of a new synthetic complement inhibitor on hepatic glycolytic intermediates in septic rats. Adv. Shock Res. 1983;9:275–282. [PubMed] [Google Scholar]
- 98.Du G.J., Song Z.H., Lin H.H., Han Xf., Zhang S., Yan Ym. Luteolin as a glycolysis inhibitor offers superior efficacy and lesser toxicity of doxorubicin in breast cancer cells. Biochem. Biophys. Res. Commun. 2008;372:497–502. doi: 10.1016/j.bbrc.2008.05.080. [DOI] [PubMed] [Google Scholar]
- 99.Billiard J., Dennison J.B., Briand J., Annan R.S., Chai D., Colon M., et al. Quinoline 3-sulfonamides inhibit lactate dehydrogenase A and reverse aerobic glycolysis in cancer cells. Cancer Metab. 2013;1:19. doi: 10.1186/2049-3002-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kudchodkar S.B., Del Prete G.Q., Maguire T.G., Alwine J.C. AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J. Virol. 2007;81:3649–3651. doi: 10.1128/JVI.02079-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanchez E.L., Pulliam T.H., Dimaio T.A., Thalhofer A.B., Delgado T., Lagunoff M. Glycolysis, glutaminolysis, and fatty acid synthesis are required for distinct stages of Kaposi’s sarcoma-associated herpesvirus lytic replication. J. Virol. 2017;91:e02237–16. doi: 10.1128/JVI.02237-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brown H.A., Thomas P.G., Lindsley C.W. Targeting phospholipase D in cancer, infection and neurodegenerative disorders. Nat. Rev. Drug Discov. 2017;16:351. doi: 10.1038/nrd.2016.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lin S.C., Chen M.C., Liu S., Callahan V.M., Bracci N.R., Lehman C.W., et al. Phloretin inhibits Zika virus infection by interfering with cellular glucose utilisation. Int. J. Antimicrob. Agents. 2019;54:80–84. doi: 10.1016/j.ijantimicag.2019.03.01. [DOI] [PubMed] [Google Scholar]
- 104.Yao C., Xi C., Hu K., Gao W., Cai X., Qin J., et al. Inhibition of enterovirus 71 replication and viral 3C protease by quercetin. Virol. J. 2018;15:116. doi: 10.1186/s12985-018-1023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu W., Li R., Li X., He J., Jiang S., Liu S., et al. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses. 2016;8:6. doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kaul T.N., Middleton Jr.E., Ogra P.L. Antiviral effect of flavonoids on human viruses. J. Med. Virol. 1985;15:71–79. doi: 10.1002/jmv.1890150110. [DOI] [PubMed] [Google Scholar]
- 107.Wang M., Firrman J., Liu L., Yam K. A review on flavonoid apigenin: dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. Biomed Res. Int. 2019;9:1–18. doi: 10.1155/2019/7010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.LeCher J.C., Diep N., Krug P.W., Hilliard J.K. Genistein has antiviral activity against herpes B virus and acts synergistically with antiviral treatments to reduce effective dose. Viruses. 2019;11:499. doi: 10.3390/v11060499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chandwani A., Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther. Clin. Risk Manag. 2008;4:1023. doi: 10.3390/v11060499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu C.H., Jassey A., Hsu H.Y., Lin L.T. Antiviral activities of silymarin and derivatives. Molecules. 2019;24:1552. doi: 10.3390/molecules24081552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen X., Yang L., Zhang N., Turpin J.A., Buckheit R.W., Osterling C., et al. Shikonin, a component of Chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 2003;47:2810–2816. doi: 10.1128/AAC.47.9.2810-2816.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gao H., Liu L., Qu Zy., Wei Fx., Wang Sq., Chen G., et al. Anti-adenovirus activities of shikonin, a component of Chinese herbal medicine in vitro. Biol. Pharm. Bull. 2011;34:197–202. doi: 10.1248/bpb.34.197. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Y., Han H., Qiu H., Lin H., Yu L., Zhu W., et al. Antiviral activity of a synthesized shikonin ester against influenza a (H1N1) virus and insights into its mechanism. Biomed. Pharmacother. 2017;93:636–645. doi: 10.1016/j.biopha.2017.06.076. [DOI] [PubMed] [Google Scholar]
- 114.Prahoveanu E., Esanu V. The effect of sodium fluoride on experimental influenza virus infection in mice. Virologie. 1981;32:293–295. [PubMed] [Google Scholar]
- 115.Bui N., Wong-Sefidan I. Reactivation of hepatitis B virus after withdrawal of erlotinib. Curr. Oncol. 2015;22:430. doi: 10.3747/co.22.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lundberg L., Brahms A., Hooper I., Carey B., Lin S.C., Dahal B., et al. Repurposed FDA-Approved drug sorafenib reduces replication of Venezuelan equine encephalitis virus and other alphaviruses. ANTIVIR RES. 2018;157:57–67. doi: 10.1016/j.antiviral.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 117.Brahms A., Mudhasani R., Pinkham C., Kota K., Nasar F., Zamani R., et al. Sorafenib impedes rift valley fever virus egress by inhibiting valosin-containing protein function in the cellular secretory pathway. J. Virol. 2017;91:e00968–17. doi: 10.1128/JVI.00968-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cho H.K., Kim J.R., Kim S.Y., Kyaw Y.Y., Win A.A., Cheong J.H. Sorafenib suppresses hepatitis B virus gene expression via inhibiting JNK pathway. Hepatoma Res. 2015;1:97–103. doi: 10.4103/2394-5079.158391. [DOI] [Google Scholar]
- 119.Hosry J., Kaseb A., Economides M.P., Hassan M., Torres H. Effect of sorafenib on hepatitis C viremia in cirrhotic patients with hepatocellular carcinoma. Open Forum Infect. Dis. 2016;3:459. doi: 10.1093/ofid/ofw172.323. [DOI] [Google Scholar]
- 120.Boasso A., Herbeuval J.P., Hardy A.W., Anderson S.A., Dolan M.J., Fuchs D., et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2, 3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mehraj V., Routy J.P. Tryptophan catabolism in chronic viral infections: handling uninvited guests. IJTR. 2015;8:S26862. doi: 10.4137/IJTR.S26862. IJTR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagai Y. Metabolic requirements for the development of hemadsorption activity and virus formation in BHK-21 cells infected with Newcastle disease virus. J. Virol. 1973;11:479–486. doi: 10.1128/jvi.11.4.479-486.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koenig R. Effect of organic mercury compounds in serological tests on some isometric plant viruses. J. Gen. Virol. 1970;7:257–261. doi: 10.1099/0022-1317-7-3-257. [DOI] [PubMed] [Google Scholar]
- 124.Callebaut I., Burny A., Portetelle D. Iodoacetamide treatment of bovine leukemia virus glycoprotein gp51 enhances the Western blotting reactivity of anti-peptide antibodies. FEBS Lett. 1991;292:148–150. doi: 10.1016/0014-5793(91)80854-V. [DOI] [PubMed] [Google Scholar]
- 125.Anson M., Stanley W. Some effects of iodine and other reagents on the structure and activity of tobacco mosaic virus. J. Gen. Physiol. 1941;24:679–690. doi: 10.1085/jgp.24.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Routledge E., Willcocks M., Morgan L., Samson A., Scott R., Toms G. Heterogeneity of the respiratory syncytial virus 22K protein revealed by Western blotting with monoclonal antibodies. J. Gen. Virol. 1987;68:1209–12015. doi: 10.1099/0022-1317-68-4-1209. [DOI] [PubMed] [Google Scholar]
- 127.Molla A., Hellen C., Wimmer E. Inhibition of proteolytic activity of poliovirus and rhinovirus 2A proteinases by elastase-specific inhibitors. J. Virol. 1993;67:4688–4695. doi: 10.1128/jvi.67.8.4688-4695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Burney T.E., Golub O.J. The effect of certain enzyme inhibitors on the activity and growth of psittacosis virus. J. Immunol. 1948;60:213–221. [PubMed] [Google Scholar]
- 129.Xiang K., Wang B. Role of the PI3K‑AKT‑mTOR pathway in hepatitis B virus infection and replication. Mol. Med. Rep. 2018;17:4713–4719. doi: 10.3892/mmr.2018.8395. [DOI] [PubMed] [Google Scholar]
- 130.Shih C.M., Wu C.H., Wu W.J., Hsiao Y.M., Ko J.L. Hypericin inhibits hepatitis C virus replication via deacetylation and down-regulation of heme oxygenase-1. Phytomedicine. 2018;46:193–198. doi: 10.1016/j.phymed.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 131.Beatrice S.T., Wagner R.R. Effect of sulfhydryl reagents on the infectivity of vesicular stomatitis virus. Virology. 1980;100:246–251. doi: 10.1016/0042-6822(80)90517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhuo C., Zheng D., He Z., Jin J., Ren Z., Jin F., et al. HSV-1 enhances the energy metabolism of human umbilical cord mesenchymal stem cells to promote virus infection. Future Virol. 2017;12:349–360. doi: 10.2217/fvl-2017-0038. [DOI] [Google Scholar]
- 133.Prahoveanu E., Gruia M., Lupu R., Cajal N. The effect of malonate on succinic dehydrogenase (SDH) activity during the multiplication of influenza and herpes viruses in the embryonate hen egg. Rev. Roum. Virol. 1974;25:247–253. [PubMed] [Google Scholar]
- 134.Chen Z., Li P., Hu D., Dong L., Pan J., Luo L., et al. Synthesis, antiviral activity, and 3D-QSAR study of novel chalcone derivatives containing malonate and pyridine moieties. Arab. J. Chem. 2015;12:2685–2696. doi: 10.1016/j.arabjc.2015.05.003. [DOI] [Google Scholar]
- 135.Mizutani M., El-Fotoh M., Mori M., Ono K., Doi K., Awaya A., et al. In vivo administration of serum thymic factor (FTS) prevents EMC-D virus-induced diabetes and myocarditis in BALB/cAJcl mice. Arch. Virol. 1996;141:73–83. doi: 10.1007/BF01718589. [DOI] [PubMed] [Google Scholar]
- 136.Lee S.H. Further observations on the effect of 6-Aminonicotinamide on chick embryo tissue cultures infected with vaccinia and other viruses. Nature. 1965;205:1242–1243. doi: 10.1038/2051242b0. [DOI] [Google Scholar]
- 137.Nakagawa A., Tomoda H., Van Hao M., Okano K., Iwai Y., Omura S. Antiviral activities of pentalenolactones. J. Antibiot. Res. 1985;38:1114–1115. doi: 10.7164/antibiotics.38.1114. [DOI] [PubMed] [Google Scholar]
- 138.Terry L.J., Vastag L., Rabinowitz J.D., Shenk T. Human kinome profiling identifies a requirement for AMP-activated protein kinase during human cytomegalovirus infection. PNAS. 2012;109:3071–3076. doi: 10.1073/pnas.1200494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mehla R., Bivalkar-Mehla S., Chauhan A. A flavonoid, luteolin, cripples HIV-1 by abrogation of tat function. PLoS One. 2011;6:e27915. doi: 10.1371/journal.pone.0027915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Peng M., Watanabe S., Chan K.W.K., He Q., Zhao Y., Zhang Z., et al. Luteolin restricts dengue virus replication through inhibition of the proprotein convertase furin. ANTIVIR RES. 2017;143:176–185. doi: 10.1016/j.antiviral.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 141.Yan H., Ma L., Wang H., Wu S., Huang H., Gu Z., et al. Luteolin decreases the yield of influenza A virus in vitro by interfering with the coat protein I complex expression. J. Nat. Med. 2019;73:487–496. doi: 10.1007/s11418-019-01287-7. [DOI] [PubMed] [Google Scholar]
- 142.Bai L., Nong Y., Shi Y., Liu M., Yan L., Shang J., et al. Luteolin inhibits hepatitis B virus replication through extracellular signal-regulated kinase-mediated down-regulation of hepatocyte nuclear factor 4α expression. Mol. Pharm. 2016;13:568–577. doi: 10.1021/acs.molpharmaceut.5b00789. [DOI] [PubMed] [Google Scholar]
- 143.Lin H.W., Chang T.J., Yang D.J., Chen Y.C., Wang M., Chang Y.Y. Regulation of virus-induced inflammatory response by β-carotene in RAW264. 7 cells. Food Chem. 2012;134:2169–2175. doi: 10.1016/j.foodchem.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 144.Wu C.C., Fang C.Y., Hsu H.Y., Chuang H.Y., Cheng Y.J., Chen Y.J., et al. EBV reactivation as a target of luteolin to repress NPC tumorigenesis. Oncotarget. 2016;7:18999. doi: 10.18632/oncotarget.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fan W., Qian S., Qian P., Li X. Antiviral activity of luteolin against Japanese encephalitis virus. Virus Res. 2016;220:112–116. doi: 10.1016/j.virusres.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 146.Murali K.S., Sivasubramanian S., Vincent S., Murugan S.B., Giridaran B., Dinesh S., et al. Anti—chikungunya activity of luteolin and apigenin rich fraction from Cynodon dactylon. Asian Pac. J. Trop. Med. 2015;8:352–358. doi: 10.1016/S1995-7645(14)60343-6. [DOI] [PubMed] [Google Scholar]
- 147.Park S., Ahn S., Shin Y., Yang Y., Yeom C.H. Vitamin C in cancer: a metabolomics perspective. Front. Physiol. 2018;9:762. doi: 10.3389/fphys.2018.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jariwalla R.J., Harakeh S. Antiviral and immunomodulatory activities of ascorbic acid. Subcell. Biochem. 1996;25:213–231. doi: 10.1007/978-1-4613-0325-1_11. [DOI] [PubMed] [Google Scholar]
- 149.Byun S.H., Jeon Y. Administration of vitamin C in a patient with herpes zoster-a case report. Korean J. Pain. 2011;24:108. doi: 10.3344/kjp.2011.24.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Harakeh S., Jariwalla R.J., Pauling L. Suppression of human immunodeficiency virus replication by ascorbate in chronically and acutely infected cells. PNAS. 1990;87:7245–7249. doi: 10.1073/pnas.87.18.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hemila H., Herman Z.S. Vitamin C and the common cold: a retrospective analysis of Chalmers’ review. J. Am. Coll. Nutr. 1995;14:116–123. doi: 10.1080/07315724.1995.10718483. [DOI] [PubMed] [Google Scholar]
- 152.Ely J.T. Ascorbic acid role in containment of the world avian flu pandemic. Exp. Biol. Med. 2007;232:847–851. doi: 10.3181/00379727-232-2320847. [DOI] [PubMed] [Google Scholar]
- 153.Levy T.E. Vitamin C and severe influenza: a case report. TLDP. 2005;258:109–111. [Google Scholar]
- 154.Duconge J., Rodríguez-López J.L., Pedro A., Adrover-López B. High dose intravenous vitamin c treatment for zika fever. JOM. 2016;31:19. [PMC free article] [PubMed] [Google Scholar]
- 155.Gonzalez M.J., Miranda-Massari J.R., Berdiel M.J., Duconge J., Rodríguez-López J.L., Hunninghake R., et al. High dose intraveneous vitamin C and chikungunya fever: a case report. J. Orthomol. Med. 2014;29:154. [PMC free article] [PubMed] [Google Scholar]
- 156.Marcial-Vega V., Idxian G.G.T., Levy T.E. Intravenous ascorbic acid and hydrogen peroxide in the management of patients with chikungunya. Bol. Asoc. Med. P. R. 2015;107:20–24. [PubMed] [Google Scholar]
- 157.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9:1211. doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yun J., Mullarky E., Lu C., Bosch K.N., Kavalier A., Rivera K., et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wohlrab C., Phillips E., Dachs G.U. Vitamin C transporters in cancer: current understanding and gaps in knowledge. Front. Oncol. 2017;7:74. doi: 10.3389/fonc.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gale T.V., Horton T.M., Hoffmann A.R., Branco L.M., Garry R.F. Host proteins identified in extracellular viral particles as targets for broad-spectrum antiviral inhibitors. J. Proteome Res. 2018;18:7–17. doi: 10.1021/acs.jproteome.8b00204. [DOI] [PubMed] [Google Scholar]
- 161.Qiu M., Chen Y., Song S., Song H., Chu Y., Yuan Z., et al. Poly (4-styrenesulfonic acid-co-maleic acid) is an entry inhibitor against both HIV-1 and HSV infections–Potential as a dual functional microbicide. ANTIVIR RES. 2012;96:138–147. doi: 10.1016/j.antiviral.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 162.Pirrone V., Passic S., Wigdahl B., Rando R.F., Labib M., Krebs F.C. A styrene-alt-maleic acid copolymer is an effective inhibitor of R5 and X4 human immunodeficiency virus type 1 infection. Biomed Res. Int. 2010;2010 doi: 10.1155/2010/548749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Link J.O., Taylor J.G., Xu L., Mitchell M., Guo H., Liu H., et al. Discovery of ledipasvir (GS-5885): a potent, once-daily oral NS5A inhibitor for the treatment of hepatitis C virus infection. J. Med. Chem. 2014;57:2033–2046. doi: 10.1021/jm401499g. [DOI] [PubMed] [Google Scholar]
- 164.Xu W., Xia S., Pu J., Wang Q., Li P., Lu L., et al. The antihistamine drugs carbinoxamine maleate and chlorpheniramine maleate exhibit potent antiviral activity against a broad spectrum of influenza viruses. Front. Microbiol. 2018;9:2643. doi: 10.3389/fmicb.2018.02643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Horie M., Ogawa H., Yoshida Y., Yamada K., Hara A., Ozawa K., et al. Inactivation and morphological changes of avian influenza virus by copper ions. Arch. Virol. 2008;153:1467. doi: 10.1007/s00705-008-0154-2. [DOI] [PubMed] [Google Scholar]
- 166.Pang H., Chen D., Cui Q.C., Dou Q.P. Sodium diethyldithiocarbamate, an AIDS progression inhibitor and a copper-binding compound, has proteasome-inhibitory and apoptosis-inducing activities in cancer cells. Int. J. Mol. Med. 2007;19:809–816. doi: 10.3892/ijmm.19.5.809. [DOI] [PubMed] [Google Scholar]
- 167.Quastel J. Vol. 2. 2012. pp. 473–502. (Inhibitions in the Citric Acid Cycle, Metabolic Inhibitors–A Comprehensive Treatise). [Google Scholar]
- 168.Beeckmans S., Van Driessche E. Pig heart fumarase contains two distinct substrate-binding sites differing in affinity. J. Biol. Chem. 1998;273:31661–31669. doi: 10.1074/jbc.273.48.31661. [DOI] [PubMed] [Google Scholar]
- 169.Janzer A., German N.J., Gonzalez-Herrera K.N., Asara J.M., Haigis M.C., Struhl K. Metformin and phenformin deplete tricarboxylic acid cycle and glycolytic intermediates during cell transformation and NTPs in cancer stem cells. PNAS. 2014;111:10574–10579. doi: 10.1073/pnas.1409844111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Owen M.R., Doran E., Halestrap A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000;348:607–614. doi: 10.1042/bj3480607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Khaperskyy D.A., Emara M.M., Johnston B.P., Anderson P., Hatchette T.F., McCormick C. Influenza a virus host shutoff disables antiviral stress-induced translation arrest. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Petrick J.S., Jagadish B., Mash E.A., Aposhian H.V. Monomethylarsonous acid (MMAIII) and arsenite: LD50 in hamsters and in vitro inhibition of pyruvate dehydrogenase. Chem. Res. Toxicol. 2001;14:651–656. doi: 10.1021/tx000264z. [DOI] [PubMed] [Google Scholar]
- 173.Ruffo A., Testa E., Adinolfi A., Pelizza G., Moratti R. Control of the citric acid cycle by glyoxylate: mechanism of the inhibition by oxalomalate and γ-hydroxy-α-oxoglutarate. Biochem. J. 1967;103:19. doi: 10.1042/bj1030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Koenig H., Patel A. Biochemical basis for fluorouracil neurotoxicity: the role of Krebs cycle inhibition by fluoroacetate. Arch. Neurol. 1970;23:155–160. doi: 10.1001/archneur.1970.00480260061008. [DOI] [PubMed] [Google Scholar]
- 175.Jardim-Messeder D., Moreira-Pacheco F. 3-Bromopyruvic acid inhibits tricarboxylic acid cycle and glutaminolysis in HepG2 cells. Anticancer Res. 2016;36:2233–2241. [PubMed] [Google Scholar]
- 176.Ackermann W., Kleinschmidt Wt.T.Ao.E., Kurtz H. The relation of the krebs cycle to viral synthesis: ii. The effect of sodium fluoroacetate on the propagation of influenza virus in mice. J. Exp. Med. 1951;93:635–642. doi: 10.1084/jem.93.6.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Goncharov N.V., Jenkins R.O., Radilov A.S. Toxicology of fluoroacetate: a review, with possible directions for therapy research. J. Appl. Toxicol. 2006;26:148–161. doi: 10.1002/jat.1118. [DOI] [PubMed] [Google Scholar]
- 178.Davies D., Ribereau-Gayon G. Inhibition of the Krebs cycle by glyoxylic acid. Phytochemistry. 1969;8:1101–1108. doi: 10.1016/S0031-9422(00)85544-2. [DOI] [Google Scholar]
- 179.Ruffo A., Testa E., Adinolfi A., Pelizza G. Control of the citric acid cycle by glyoxylate. 1. A new inhibitor of aconitase formed by the condensation of glyoxylate with oxaloacetate. Biochem. J. 1962;85:588. doi: 10.1042/bj0850588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Galkin M., Jonas B.A. Enasidenib in the treatment of relapsed/refractory acute myeloid leukemia: an evidence-based review of its place in therapy. Core Evid. 2019;14:3. doi: 10.2147/CE.S172912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Liu X., Gong Y. Isocitrate dehydrogenase inhibitors in acute myeloid leukemia. Biomark. Res. 2019;7:22. doi: 10.1186/s40364-019-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Stein E.M., DiNardo C.D., Pollyea D.A., Fathi A.T., Roboz G.J., Altman J.K., et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia, Blood. Am. J. Hematol. 2017;130:722–731. doi: 10.1182/blood-2017-04-779405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Anderson N.M., Mucka P., Kern J.G., Feng H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell. 2018;9:216–237. doi: 10.1007/s13238-017-0451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Song M., Kim S.H., Im C.Y., Hwang H.J. Recent development of small molecule glutaminase inhibitors. Curr. Top. Med. Chem. 2018;18:432–443. doi: 10.2174/1568026618666180525100830. [DOI] [PubMed] [Google Scholar]
- 185.Cinatl J., Vogel J.U., Cinatl J., Kabickova H., Kornhuber B., Doerr H.W. Antiviral effects of 6-diazo-5-oxo-l-norleucin on replication of herpes simplex virus type 1. ANTIVIR RES. 1997;33:165–175. doi: 10.1016/S0166-3542(96)01012-1. [DOI] [PubMed] [Google Scholar]
- 186.Cervantes-Madrid D., Romero Y., Duenas-Gonzalez A. Reviving lonidamine and 6-diazo-5-oxo-L-norleucine to be used in combination for metabolic cancer therapy. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/690492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhao J., Yu H.Y., Zhao Y., Li F.H., Zhou W., Xia B.B., et al. Soluble expression, rapid purification, biological identification of chicken interferon-alpha using a thioredoxin fusion system in E. Coli and its antiviral effects to H9N2 avian influenza virus. Prep. Biochem. Biotechnol. 2019;49:192–201. doi: 10.1080/10826068.2019.1566150. [DOI] [PubMed] [Google Scholar]
- 188.Daloso D.M., Müller K., Obata T., Florian A., Tohge T., Bottcher A., et al. Thioredoxin, a master regulator of the tricarboxylic acid cycle in plant mitochondria. PNAS. 2015;112:E1392–E1400. doi: 10.1073/pnas.1424840112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Goldner M.G., Gomori G. Studies on the mechanism of alloxan diabetes. Endocrinology. 1944;35:241–248. doi: 10.1210/endo-35-4-241. [DOI] [PubMed] [Google Scholar]
- 190.Berry L.J., Ehlers K.H., Mitchell R.B. The relation of the tricarboxylic acid cycle to bacterial infection: IV. The effect of three metabolic inhibitors and Salmonella typhimurium on the citric acid content of mouse tissues. J. Infect. Dis. 1954;94:152–158. doi: 10.1093/infdis/94.2.152. [DOI] [PubMed] [Google Scholar]
- 191.McDowell E.M. Light and electron microscopic studies of the rat kidney after administration of inhibitors of the citric acid cycle in vivo: I. Effects of sodium fluoroacetate on the proximal convoluted tubule. Am. J. Pathol. 1972;66:513. doi: 10.1002/path.1711080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xu W., Yang H., Liu Y., Yang Y., Wang P., Kim S.H., et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hewitson K.S., Lienard B.M., McDonough M.A., Clifton I.J., Butler D., Soares A.S., et al. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J. Biol. Chem. 2007;282:3293–3301. doi: 10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- 194.Kim C., Jadhav R.R., Gustafson C.E., Smithey M.J., Hirsch A.J., Uhrlaub J.L., et al. Defects in antiviral t cell responses inflicted by aging-associated miR-181a deficiency. Cell Rep. 2019;29:2202–2216. doi: 10.1016/j.celrep.2019.10.044. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Singaravelu R., Ahmed N., Quan C., Srinivasan P., Ablenas C.J., Roy D.G., et al. A conserved miRNA-183 cluster regulates the innate antiviral response. J. Biol. Chem. 2019;294:19785–19794. doi: 10.1074/jbc.RA119.010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Li W., Cheng P., Nie S., Cui W. miR-370 mimic inhibits replication of Japanese encephalitis virus in glioblastoma cells. Neuropsychiatr. Dis. Treat. 2016;12:2411. doi: 10.2147/NDT.S113236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fan H., Lv P., Lv J., Zhao X., Liu M., Zhang G., et al. miR‐370 suppresses HBV gene expression and replication by targeting nuclear factor IA. J. Med. Virol. 2017;89:834–844. doi: 10.1002/jmv.24695. [DOI] [PubMed] [Google Scholar]
- 198.Shen C., Yang H., Liu H., Wang X., Zhang Y., Xu R. Inhibitory effect and mechanisms of microRNA-146b-5p on the proliferation and metastatic potential of Caski human cervical cancer cells. Mol. Med. Rep. 2015;11:3955–3961. doi: 10.3892/mmr.2015.3151. [DOI] [PubMed] [Google Scholar]
- 199.Gao S., Li J., Song L., Wu J., Huang W. Influenza A virus-induced downregulation of miR-26a contributes to reduced IFNα/β production. Virol. Sin. 2017;32:261–270. doi: 10.1007/s12250-017-4004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jia X., Bi Y., Li J., Xie Q., Yang H., Liu W. Cellular microRNA miR-26a suppresses replication of porcine reproductive and respiratory syndrome virus by activating innate antiviral immunity. Sci. Rep. 2015;5:10651. doi: 10.1038/srep10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang J., Li Z., Huang J., Yin H., Tian J., Qu L. miR-26a inhibits feline herpesvirus 1 replication by targeting SOCS5 and promoting type I interferon signaling. Viruses. 2020;12:2. doi: 10.3390/v12010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Raimundo N., Baysal B.E., Shadel G.S. Revisiting the TCA cycle: signaling to tumor formation. Trends Mol. Med. 2011;17:641–649. doi: 10.1016/j.molmed.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Marquez J., Flores J., Kim A.H., Nyamaa B., Nguyen A.T.T., Park N., et al. Rescue of TCA cycle dysfunction for Cancer therapy. J. Clin. Med. 2019;8:2161. doi: 10.3390/jcm8122161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yen K., Travins J., Wang F., David M.D., Artin E., Straley K., et al. AG-221, a first-in-class therapy targeting acute myeloid leukemia harboring oncogenic IDH2 mutations. Cancer Discov. 2017;7:478–493. doi: 10.1158/2159-8290.CD-16-1034. [DOI] [PubMed] [Google Scholar]
- 205.Popovici-Muller J., Lemieux R.M., Artin E., Saunders J.O., Salituro F.G., Travins J., et al. Discovery of AG-120 (Ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med. Chem. Lett. 2018;9:300–305. doi: 10.1021/acsmedchemlett.7b00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tretter L., Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: a key role of α-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J. Neurosci. Res. 2000;20:8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Essam K., Mohsen R., Ismail E.A., Mohamed A.F. In vitro preparation of H2O2 inactivated rabies vaccine and related immunogenicity. IJOPRS. 2018;3:1–8. doi: 10.19080/IJOPRS.2018.03.555619. [DOI] [Google Scholar]
- 208.Yu J.W., Sun L.J., Zhao Y.H., Kang P., Yan B.Z. The effect of metformin on the efficacy of antiviral therapy in patients with genotype 1 chronic hepatitis C and insulin resistance. Int. J. Infect. Dis. 2012;16 doi: 10.1016/j.ijid.2012.02.004. e436-e41. [DOI] [PubMed] [Google Scholar]
- 209.Tsai W.L., Chang T.H., Sun W.C., Chan H.H., Wu C.C., Hsu P.I., et al. Metformin activates type I interferon signaling against HCV via activation of adenosine monophosphate-activated protein kinase. Oncotarget. 2017;8:91928. doi: 10.18632/oncotarget.20248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Alexopoulou A., Karayiannis P. Interferon-based combination treatment for chronic hepatitis C in the era of direct acting antivirals. Ann. Gastroenterol. 2015;28:55. [PMC free article] [PubMed] [Google Scholar]
- 211.Cheng F., da Silva S.R., Huang I.C., Jung J.U., Gao S.J. Suppression of Zika virus infection and replication in endothelial cells and astrocytes by PKA inhibitor PKI 14-22. J. Virol. 2018;92:e02019–17. doi: 10.1128/JVI.02019-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Htun H.L., Yeo T.W., Tam C.C., Pang J., Leo Y.S., Lye D.C. Metformin use and severe dengue in diabetic adults. Sci. Rep. 2018;8:1–9. doi: 10.1038/s41598-018-21612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Routy J.P., Isnard S., Mehraj V., Ostrowski M., Chomont N., Ancuta P., et al. Effect of metformin on the size of the HIV reservoir in non-diabetic ART-treated individuals: single-arm non-randomised Lilac pilot study protocol. BMJ Open. 2019;9:e028444. doi: 10.1136/bmjopen-2018-028444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vera I.M., Ruivo M.T.G., Rocha L.F.L., Marques S., Bhatia S.N., Mota M.M., et al. Targeting liver stage malaria with metformin. JCI Insight. 2019;4:e127441. doi: 10.1172/jci.insight.127441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Li H., Ning X., Liu H., Chen Y., Ding X., Zhang H. Metformin suppressed human cytomegalovirus (hCMV) replication and its potential molecular mechanisms in human fibroblasts. Am Assoc Immnol. 2017;198 [Google Scholar]
- 216.Li M., Li X., Zhang H., Lu Y. Molecular mechanisms of metformin for diabetes and cancer treatment. Front. Physiol. 2018;9:1039. doi: 10.3389/fphys.2018.01039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bramante C., Ingraham N., Murray T., Marmor S., Hoversten S., Gronski J., et al. Observational study of metformin and risk of mortality in patients hospitalized with Covid-19. MedRxiv. 2020 doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Griss T., Vincent E.E., Egnatchik R., Chen J., Ma E.H., Faubert B., et al. Metformin antagonizes cancer cell prolifeation by suppressing mitochondrial-dependent biosynthesis. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Andrzejewski S., Siegel P.M., St-Pierre J. Metabolic profiles associated with metformin efficacy in cancer. Front. Endocrinol. 2018;9:372. doi: 10.3389/fendo.2018.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cantoria M.J., Patel H., Boros L.G., Meuillet E.J. In: Metformin and Pancreatic Cancer Metabolism, Pancreatic Cancer-Insights into Molecular Mechanisms and Novel Approaches to Early Detection and Treatment. McCall Dr.Kelly., editor. IntechOpen, Publisher: InTech; 2014. pp. 159–187. Chapter: 7. [DOI] [Google Scholar]
- 221.Nguyen T.T., Ung T.T., Li S., Lian S., Xia Y., Park S.Y., et al. Metformin inhibits lithocholic acid-induced interleukin 8 upregulation in colorectal cancer cells by suppressing ROS production and NF-kB activity. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-38778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020 doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 223.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls Publishing; StatPearls. Treasure Island (FL): 2020. Features, Evaluation and Treatment Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 224.Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., Black S.M. Pulmonary Vasculature Redox Signaling in Health and Disease. Springer; 2017. ROS signaling in the pathogenesis of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) pp. 105–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Liu Q., Gao Y., Ci X. Role of Nrf2 and its activators in respiratory diseases. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/7090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Fowler A.A., III, Kim C., Lepler L., Malhotra R., Debesa O., Natarajan R., et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J. Crit. Care Med. 2017;6:85. doi: 10.5492/wjccm.v6.i1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hemila H., Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. 2019;11:708. doi: 10.3390/nu11040708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marik P.E., Khangoora V., Rivera R., Hooper M.H., Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151:1229–1238. doi: 10.3390/jcm8040478. [DOI] [PubMed] [Google Scholar]
- 229.Patel V., Dial K., Wu J., Gauthier A.G., Wu W., Lin M., et al. Dietary antioxidants significantly attenuate hyperoxia-induced acute inflammatory lung injury by enhancing macrophage function via reducing the accumulation of airway HMGB1. Int. J. Mol. Sci. 2020;21:977. doi: 10.3390/ijms21030977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang B., Swamy S., Balijepalli S., Panicker S., Mooliyil J., Sherman M.A., et al. Direct pulmonary delivery of solubilized curcumin reduces severity of lethal pneumonia. FASEB J. 2019;33:13294. doi: 10.1096/fj.201901047RR. 12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hasanpour Dehkordi A., Abbaszadeh A., Mir S., Hasanvand A. Metformin and its anti-inflammatory and anti-oxidative effects; new concepts. J. Renal Inj. Prev. 2019;8:54–61. doi: 10.15171/jrip.2019.11. [DOI] [Google Scholar]
- 232.Fiorillo M., Tóth F., Sotgia F., Lisanti M.P. Doxycycline, Azithromycin and Vitamin C (DAV): a potent combination therapy for targeting mitochondria and eradicating cancer stem cells (CSCs) Aging. 2019;11:2202. doi: 10.18632/aging.101905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010;36:646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 234.Menzel M., Akbarshahi H., Bjermer L., Uller L. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Iannetta M., Ippolito G., Nicastri E. Azithromycin shows anti-Zika virus activity in human glial cells, Antimicrob. Agents Chemother. 2017;61:e01152–17. doi: 10.1128/AAC.01152-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zeng S., Meng X., Huang Q., Lei N., Zeng L., Jiang X., et al. Spiramycin and azithromycin, safe for administration to children, exert antiviral activity against enterovirus A71 in vitro and in vivo. Int. J. Antimicrob. Agents. 2019;53:362–369. doi: 10.1016/j.ijantimicag.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 237.Ishaqui A.A., Khan A.H., Sulaiman S.A.S., Alsultan M.T., Khan I., Naqvi A.A. Assessment of efficacy of oseltamivir-azithromycin combination therapy in prevention of influenza-a (H1N1) infection complications and rapidity of symptom relief. Expert Rev. Respir. Med. 2020;14:533–541. doi: 10.1080/17476348.2020.1730180. [DOI] [PubMed] [Google Scholar]
- 238.Pino M.G. 2016. Azithromycin for Recurrent Respiratory Tract Infections in Pediatric Populations, Theses, Dissertations and Capstone Projects. [Google Scholar]
- 239.Wu Zc., Wang X., Wei Jc., Li Bb., Shao Dh., Li Ym., et al. Antiviral activity of doxycycline against vesicular stomatitis virus in vitro. FEMS Microbiol. Lett. 2015;362:fnv195. doi: 10.1093/femsle/fnv195. [DOI] [PubMed] [Google Scholar]
- 240.Rothan H.A., Mohamed Z., Paydar M., Rahman N.A., Yusof R. Inhibitory effect of doxycycline against dengue virus replication in vitro. Arch. Virol. 2014;159:711–718. doi: 10.1007/s00705-013-1880-7. [DOI] [PubMed] [Google Scholar]
- 241.Rothan H.A., Bahrani H., Mohamed Z., Teoh T.C., Shankar E.M., Rahman N.A., et al. A combination of doxycycline and ribavirin alleviated chikungunya infection. PLoS One. 2015;10:e0126360. doi: 10.1371/journal.pone.0126360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chang Y.C., Tung Y.A., Lee K.H., Chen T.F., Hsiao Y.C., Chang H.C., et al. Potential therapeutic agents for COVID-19 based on the analysis of protease and RNA polymerase docking. Preprints. 2020 doi: 10.20944/preprints202002.0242.v1. [DOI] [Google Scholar]
- 243.Das J.K., Bilal H., Salam R.A., Bhutta Z.A. Vol. 2018. 2018. Vitamin C supplementation for prevention and treatment of pneumonia. (The Cochrane Database of Systematic Reviews). (9) [DOI] [Google Scholar]
- 244.Hemilä H., Louhiala P. Vitamin C may affect lung infections. J ROY SOC MED. 2007;100:495–498. doi: 10.1258/jrsm.100.11.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Thaker S.K., Chng J., Christofk H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019;17:59. doi: 10.1186/s12915-019-0678-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shutt D., O’Dorisio M.S., Aykin-Burns N., Spitz D. 2-deoxy-D-glucose induces oxidative stress and cell killing in human neuroblastoma cells. Cancer Biol. Ther. 2010;9:853–861. doi: 10.4161/cbt.9.11.11632. [DOI] [PMC free article] [PubMed] [Google Scholar]